The Regulatory Role of Long Non-Coding RNAs in the Development and Progression of Osteoporosis
Abstract
1. Introduction
2. Long Non-Coding RNAs: Their Role in Bone Metabolism Diseases
2.1. Classification of LncRNAs
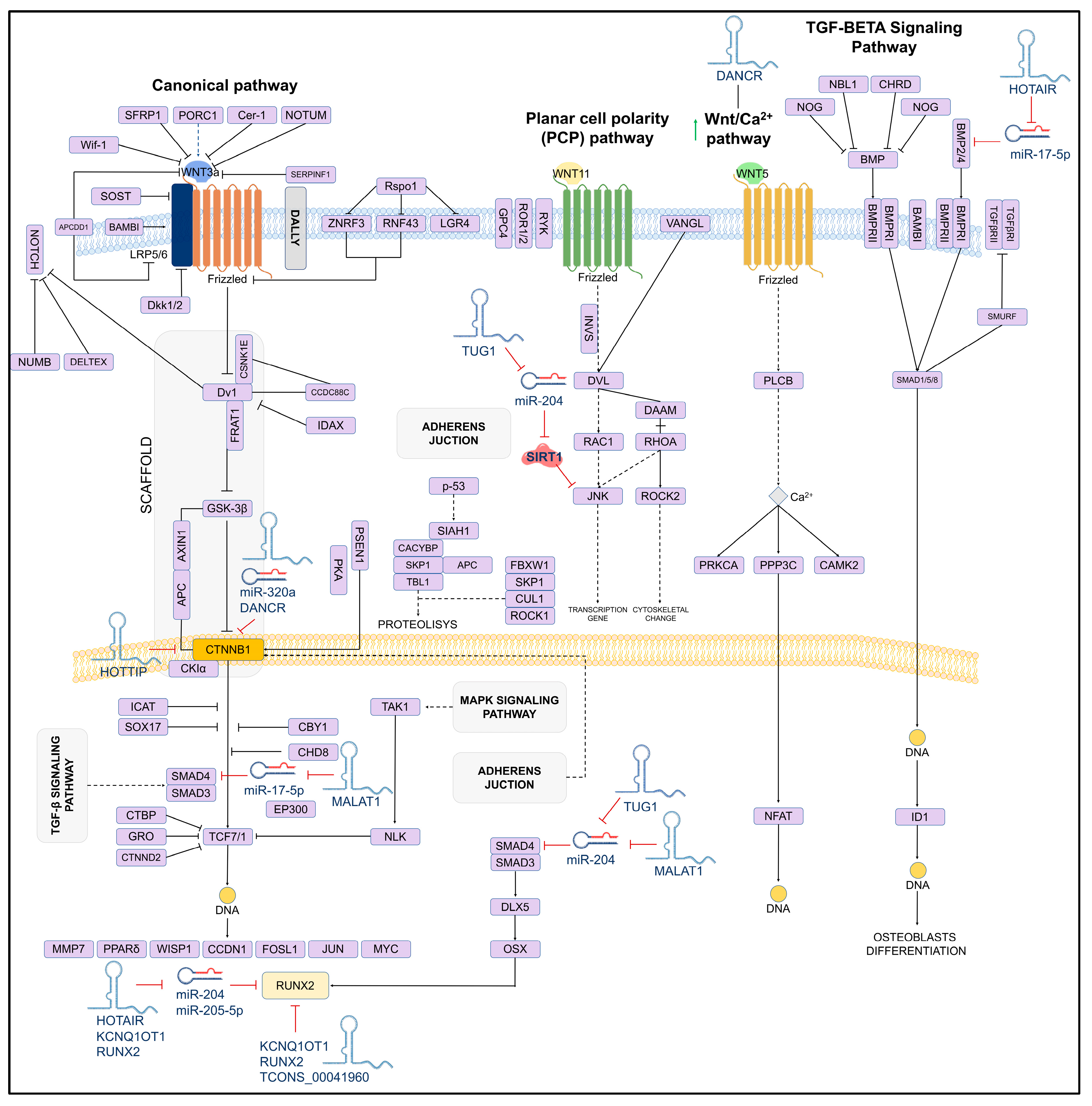
2.2. Mechanisms of Action of LncRNAs
2.3. The Role of LncRNAs in Bone Resorption
2.4. The Role of LncRNAs in Bone Formation
2.5. The Role of LncRNA in Osteocytes
3. LncRNAs Involved in the Development of Osteoporosis
4. Perspectives of Clinical Applications of LncRNAs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva, A.M.; Moura, S.R.; Teixeira, J.H.; Barbosa, M.A.; Santos, S.G.; Almeida, M.I. Long noncoding RNAs: A missing link in osteoporosis. Bone Res. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Williams, S.A.; Daigle, S.G.; Weiss, R.; Wang, Y.; Arora, T.; Curtis, J.R. Economic Burden of Osteoporosis-Related Fractures in the US Medicare Population. Ann. Pharmacother. 2021, 55, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Aziziyeh, R.; Amin, M.; Habib, M.; Garcia Perlaza, J.; Szafranski, K.; McTavish, R.K.; Disher, T.; Lüdke, A.; Cameron, C. The burden of osteoporosis in four Latin American countries: Brazil, Mexico, Colombia, and Argentina. J. Med. Econ. 2019, 22, 638–644. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Arias, C.F.; Herrero, M.A.; Echeverri, L.F.; Oleaga, G.E.; López, J.M. Bone remodeling: A tissue-level process emerging from cell-level molecular algorithms. PLoS ONE 2018, 13, e0204171. [Google Scholar] [CrossRef]
- Del Fattore, A.; Cappariello, A.; Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone 2008, 42, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Cianferotti, L.; Brandi, M.L. Epigenetic Mechanisms in Bone Biology and Osteoporosis: Can They Drive Therapeutic Choices? Int. J. Mol. Sci. 2016, 17, 1329. [Google Scholar] [CrossRef]
- Huynh, N.P.; Anderson, B.A.; Guilak, F.; McAlinden, A. Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect. Tissue Res. 2017, 58, 116–141. [Google Scholar] [CrossRef]
- Choudhuri, S. Long noncoding RNAs: Biogenesis, regulation, function, and their emerging significance in toxicology. Toxicol. Mech. Methods. 2023, 33, 541–551. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Yu, W.; Zhang, Y.; Ao, X.; Wang, J. Long non-coding RNAs: Biogenesis, functions, and clinical significance in gastric cancer. Mol. Ther. Oncolytics 2021, 2, 458–476. [Google Scholar] [CrossRef]
- Aurilia, C.; Donati, S.; Palmini, G.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. The Involvement of Long Non-Coding RNAs in Bone. Int. J. Mol. Sci. 2021, 22, 3909. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Long non-coding RNAs in osteosarcoma. Oncotarget 2017, 8, 20462–20475. [Google Scholar] [CrossRef] [PubMed]
- Chodurska, B.; Kunej, T. Long non-coding RNAs in humans: Classification, genomic organization and function. Noncoding RNA Res. 2025, 11, 313–327. [Google Scholar] [CrossRef]
- Chen, L.L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Y. The potential role of lncRNAs in osteoporosis. J. Bone Min. Metab. 2021, 39, 341–352. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Noncoding RNA 2021, 7, 3. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Li, W.H. Transposable elements are found in a large number of human protein-coding genes. Trends Genet. 2001, 17, 619–621. [Google Scholar] [CrossRef]
- Dhanoa, J.K.; Sethi, R.S.; Verma, R.; Arora, J.S.; Mukhopadhyay, C.S. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J. Anim. Sci. Technol. 2018, 60, 25. [Google Scholar] [CrossRef]
- Ahmad, P.; Bensaoud, C.; Mekki, I.; Rehman, M.U.; Kotsyfakis, M. Long Non-Coding RNAs and Their Potential Roles in the Vector-Host-Pathogen Triad. Life 2021, 11, 56. [Google Scholar] [CrossRef]
- Graf, J.; Kretz, M. From structure to function: Route to understanding lncRNA mechanism. Bioessays 2020, 42, e2000027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Min, L.; Qiu, X.; Wu, X.; Liu, C.; Ma, J.; Zhang, D.; Zhu, L. Biological Function of Long Non-coding RNA (LncRNA) Xist. Front. Cell Dev. Biol. 2021, 9, 645647. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Cech, T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA 2015, 21, 2007–2022. [Google Scholar] [CrossRef]
- Luo, J.; Qu, L.; Gao, F.; Lin, J.; Liu, J.; Lin, A. LncRNAs: Architectural Scaffolds or More Potential Roles in Phase Separation. Front. Genet. 2021, 12, 626234. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, R.; Sun, X. Enhancer LncRNAs Influence Chromatin Interactions in Different Ways. Front. Genet. 2019, 10, 936. [Google Scholar] [CrossRef]
- Dou, C.; Cao, Z.; Yang, B.; Ding, N.; Hou, T.; Luo, F.; Kang, F.; Li, J.; Yang, X.; Jiang, H.; et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci. Rep. 2016, 6, 21499. [Google Scholar] [CrossRef]
- Cong, C.; Tian, J.; Gao, T.; Zhou, C.; Wang, Y.; Cui, X.; Zhu, L. lncRNA GAS5 Is Upregulated in Osteoporosis and Downregulates miR-21 to Promote Apoptosis of Osteoclasts. Clin. Interv. Aging 2020, 15, 1163–1169. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, W.; Yan, W.; Xu, Z.; Xie, Y.; Zhang, P. LncRNA CASC11 is upregulated in postmenopausal osteoporosis and is correlated with TNF-α. Clin. Interv. Aging 2019, 14, 1663–1669. [Google Scholar] [CrossRef]
- Du, Y.J.; Yu, Q.Q.; Zheng, X.F.; Wang, S.P. LncRNA TUG1 positively regulates osteoclast differentiation by targeting v-maf musculoaponeurotic fibrosarcoma oncogene homolog B. Autoimmunity 2020, 53, 443–449. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Xing, Z.; Lin, A.; Liang, K.; Song, J.; Hu, Q.; Yao, J.; Chen, Z.; Park, P.K.; et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat. Cell Biol. 2017, 19, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Hu, H.L.; Liu, K.Y.; Ram, Y.I.; Gao, J.L.; Cao, Y.M. Long noncoding RNA MIRG induces osteoclastogenesis and bone resorption in osteoporosis through negative regulation of miR-1897. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10195–10203. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.; Cai, Z.; Xie, Z.; Li, J.; Li, M.; Cen, S.; Tang, S.; Zheng, G.; Ye, G.; et al. Perfiles de expresión de LncRNA-mRNA y redes funcionales en diferenciación osteoclasta. J. Cell. Mol. Med. 2020, 24, 9786–9797. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fu, S.; Sun, D.; Xing, J.; Hou, T.; Wu, X. EPC-derived exosomes promote osteoclastogenesis through LncRNA-MALAT1. J. Cell. Mol. Med. 2019, 23, 3843–3854. [Google Scholar] [CrossRef]
- Dou, C.; Zhang, C.; Kang, F.; Yang, X.; Jiang, H.; Bai, Y.; Xiang, J.; Xu, J.; Dong, S. MiR-7b directly targets DC-STAMP causing suppression of NFATc1 and c-Fos signaling during osteoclast fusion and differentiation. Biochim. Biophys. Acta 2014, 1839, 1084–1096. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Li, D.; Zhou, X.; Chen, Z. LncRNA NEAT1/miR-29b-3p/BMP1 axis promotes osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Pathol. Res. Pract. 2019, 215, 525–531. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, W.; Zhang, Q.; Wu, Y.; Ni, S.; Tan, C.; Xu, M.; Sun, H.; Liu, C.; Wei, P.; et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J. Hematol. Oncol. 2018, 11, 113. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Z.; Bai, Y.; Dou, C.; Gong, X.; Liang, M.; Dong, R.; Quan, H.; Li, J.; Dai, J.; et al. LncRNA AK077216 promotes RANKL-induced osteoclastogenesis and bone resorption via NFATc1 by inhibition of NIP45. J. Cell Physiol. 2019, 234, 1606–1617. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Li, G.; Jin, F.; Wang, Z.; Yue, R.; Wang, Y.; Wang, X.; Sun, Y. LncRNA Nron regulates osteoclastogenesis during orthodontic bone resorption. Int. J. Oral. Sci. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, C.; Ipseiz, N.; Böhm, C.; Krishnacoumar, B.; Stenzel, M.; Czerwinski, T.; Palumbo-Zerr, K.; Rothe, T.; Weidner, D.; Klej, A.; et al. NR4A1 Regulates Motility of Osteoclast Precursors and Serves as Target for the Modulation of Systemic Bone Turnover. J. Bone Min. Miner. Res. 2018, 33, 2035–2047. [Google Scholar] [CrossRef]
- Chen, R.S.; Zhang, X.B.; Zhu, X.T.; Wang, C.S. LncRNA Bmncr alleviates the progression of osteoporosis by inhibiting RANML-induced osteoclast differentiation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9199–9206. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hou, Y.; Liu, Y.; Zheng, J. LncRNA SNHG15 contributes to proliferation, invasion and autophagy in osteosarcoma cells by sponging miR-141. J. Biomed. Sci. 2017, 24, 46. [Google Scholar] [CrossRef]
- Lee, C.P.; Huang, Y.N.; Nithiyanantham, S.; Huang, C.M.; Ko, Y.C. LncRNA-Jak3: Jak3 coexpressed pattern regulates monosodium urate crystal-induced osteoclast differentiation through Nfatc1/Ctsk expression. Environ. Toxicol. 2019, 34, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, P.C. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem. Biophys. Res. Commun. 2013, 432, 612–617. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, H.G.; Zhang, K.B.; Guo, Y.Q.; Yang, L.J.; Lv, H. LncRNA XIST facilita la diferenciación osteoclata mediada por S1P mediante la interacción con FUS. J. Bone Min. Metab. 2022, 40, 240–250. [Google Scholar] [CrossRef]
- Xu, J.; Li, D.; Cai, Z.; Sun, H.; Su, B.; Qiu, M.; Ma, R. Exosomal lncRNAs NONMMUT000375.2 and NONMMUT071578.2 derived from titanium particle treated RAW264.7 cells regulate osteogenic differentiation of MC3T3-E1 cells. J. Biomed. Mater. Res. A. 2020, 108, 2251–2262. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Xie, F.; Guo, S.; Liu, F.; Dong, N.; Wang, Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc. Res. 2018, 114, 168–179. [Google Scholar] [CrossRef]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Miao, Y.X.; Hirokazu, T.; Zhu, S.Z.; Lu, J.S. Effects of lncRNA DANCR on proliferation and differentiation of osteoblasts by regulating the Wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5558–5566. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Hu, Y.H.; Su, S.L.; Zhong, D. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 2020, 52, 1310–1325. [Google Scholar] [CrossRef]
- Wei, B.; Wei, W.; Zhao, B.; Guo, X.; Liu, S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS ONE 2017, 12, e0169097. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Liu, S.C.; Qiao, X.F.; Kong, Y.; Liu, J.G.; Peng, X.M.; Wang, Y.X.; Abdulkarim Mohammed Al-Mohana, R.A. LncRNA MEG3 promotes proliferation and differentiation of osteoblasts through Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4521–4529. [Google Scholar] [CrossRef]
- Che, M.; Gong, W.; Zhao, Y.; Liu, M. Long noncoding RNA HCG18 inhibits the differentiation of human bone marrow-derived mesenchymal stem cells in osteoporosis by targeting miR-30a-5p/NOTCH1 axis. Mol. Med. 2020, 26, 106. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Q.; Huang, Y.; Jia, L.; Zheng, Y.; Li, W. Long non-coding RNA SNHG5 promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the miR-212-3p/GDF5/SMAD pathway. Stem Cell Res. Ther. 2022, 13, 130. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.; Guo, S.; Guo, C.; Zhang, Q.; Dong, N.; Wang, Y. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int. J. Cardiol. 2017, 243, 404–412. [Google Scholar] [CrossRef]
- Pan, K.; Lu, Y.; Cao, D.; Peng, J.; Zhang, Y.; Li, X. Long Non-coding RNA SNHG1 Suppresses the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Binding with HMGB1. Biochem. Genet. 2024, 62, 2869–2883. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, R.; Sun, H.; Long, D.; Li, Z.; Liao, H.; Xue, T.; Zhang, Z.; Kang, Y.; Mao, G. Long Non-coding RNAs LOC100126784 and POM121L9P Derived from Bone Marrow Mesenchymal Stem Cells Enhance Osteogenic Differentiation via the miR-503-5p/SORBS1 Axis. Front. Cell Dev. Biol. 2021, 9, 723759. [Google Scholar] [CrossRef]
- Ming, Y.; Liu, Z.P. Overexpression of lncRNA-NEF regulates the miR-155/PTEN axis to inhibit adipogenesis and promote osteogenesis. Kaohsiung J. Med. Sci. 2021, 37, 930–939. [Google Scholar] [CrossRef]
- Liu, R.; Li, Z.; Song, E.; Hu, P.; Yang, Q.; Hu, Y.; Liu, H.; Jin, A. LncRNA HOTTIP enhances human osteogenic BMSCs differentiation via interaction with WDR5 and activation of Wnt/β-catenin signalling pathway. Biochem. Biophys. Res. Commun. 2020, 524, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. LncRNA NKILA integrates RXFP1/AKT and NF-κB signalling to regulate osteogenesis of mesenchymal stem cells. J. Cell. Mol. Med. 2020, 24, 521–529. [Google Scholar] [CrossRef]
- Wang, F.; Deng, H.; Chen, J.; Wang, Z.; Yin, R. LncRNA MIAT can regulate the proliferation, apoptosis, and osteogenic differentiation of bone marrow-derived mesenchymal stem cells by targeting miR-150-5p. Bioengineered 2022, 13, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Peng, W.X.; Zhang, M.B. LncRNA KCNQ1OT1 promotes osteogenic differentiation via miR-205-5p/RICTOR axis. Exp. Cell Res. 2022, 415, 113119. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Liu, Q.; Liu, C.Z. lncRNA H19 promotes matrix mineralization through up-regulating IGF1 by sponging miR-185-5p in osteoblasts. BMC Mol. Cell Biol. 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, F.; Song, Y.; Li, X.; Wu, Q.; Duan, Y.; Jin, Z. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis. 2016, 7, e2327. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Wang, W.; Yu, J.; Wang, Y.; Wu, X.; He, Y. Long non-coding HCG18 promotes intervertebral disc degeneration by sponging miR-146a-5p and regulating TRAF6 expression. Sci. Rep. 2017, 7, 13234. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yan, Y.W.; Chen, D.; Ai, C.Z.; Lu, X.; Xu, S.S.; Jiang, S.; Zhong, G.S.; Chen, D.B.; Jiang, Y.Z. Long non-coding RNA HoxA-AS3 interacts with EZH2 to regulate lineage commitment of mesenchymal stem cells. Oncotarget 2016, 7, 63561–63570. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Han, H.; Song, S.; Zhang, X.; Ouyang, L.; Qian, C.; Hong, Y.; Qiu, Y.; Zhou, W.; et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene 2018, 37, 5508–5519. [Google Scholar] [CrossRef]
- Weng, J.; Peng, W.; Zhu, S.; Chen, S. Long Noncoding RNA Sponges miR-454 to Promote Osteogenic Differentiation in Maxillary Sinus Membrane Stem Cells. Implant. Dent. 2017, 26, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, L.; Liu, L.; Gong, Q.; Zheng, J.; Peng, C.; Deng, J. Comparison of HIF1A AS1 and HIF1A AS2 in regulating HIF 1α and the osteogenic differentiation of PDLCs under hypoxia. Int. J. Mol. Med. 2017, 40, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Wang, Y.; Xu, Y.; Zhang, S.; Sun, X.; Guan, H.; Zhao, X.; Wang, Y.; Li, Y.; Zhao, G. Long non-coding RNA TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cell by targeting miR-204-5p and miR-125a-3p. J. Cell Physiol. 2018, 233, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Peng, S.; Wu, W.; Ouyang, Y.; Tan, D.; Fu, X. LncRNA HOTAIRM1 promotes osteogenesis by controlling JNK/AP-1 signalling-mediated RUNX2 expression. J. Cell. Mol. Med. 2019, 23, 7517–7524. [Google Scholar] [CrossRef]
- Yu, X.; Rong, P.Z.; Song, M.S.; Shi, Z.W.; Feng, G.; Chen, X.J.; Shi, L.; Wang, C.H.; Pang, Q.J. lncRNA SNHG1 induced by SP1 regulates bone remodeling and angiogenesis via sponging miR-181c-5p and modulating SFRP1/Wnt signaling pathway. Mol. Med. 2021, 27, 141. [Google Scholar] [CrossRef]
- Arai, M.; Ochi, H.; Sunamura, S.; Ito, N.; Nangaku, M.; Takeda, S.; Sato, S. A Novel Long Noncoding RNA in Osteocytes Regulates Bone Formation through the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 13633. [Google Scholar] [CrossRef]
- Zhao, Y.; Ning, J.; Teng, H.; Deng, Y.; Sheldon, M.; Shi, L.; Martinez, C.; Zhang, J.; Tian, A.; Sun, Y.; et al. Long noncoding RNA Malat1 protects against osteoporosis and bone metastasis. Nat. Commun. 2024, 15, 2384, Erratum in Nat. Commun. 2024, 15, 4937. https://doi.org/10.1038/s41467-024-49356-0. [Google Scholar] [CrossRef]
- Ouyang, X.; Ding, Y.; Yu, L.; Xin, F.; Yang, X. LncRNA TUG regulates osteogenic differentiation of bone marrow mesenchymal stem cells via miRNA-204/SIRT 1. J. Musculoskelet. Neuronal Interact. 2022, 22, 401–410. [Google Scholar]
- Qian, T.Y.; Wan, H.; Huang, C.Y.; Hu, X.J.; Yao, W.F. Plasma LncRNA MALAT1 Expressions Are Negatively Associated with Disease Severity of Postmenopausal Osteoporosis. Lab. Med. 2022, 53, 446–452. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Z.; Gao, W.; Wang, J.; Liu, Y.; Gao, F.; Sun, S.; Zhou, X.; Yang, Z.; Zheng, W. LncRNA-NEF is downregulated in postmenopausal osteoporosis and is related to course of treatment and recurrence. J. Int. Med. Res. 2019, 47, 3299–3306. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Xiao, D.; Zhuang, J.; Liang, G.; Liang, C.; Zheng, X.; Ke, Y.; Chang, Y. LncRNA SNHG1 was down-regulated after menopause and participates in postmenopausal osteoporosis. Biosci. Rep. 2019, 39, BSR20190445. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, C.; Xiao, B.; Li, S.; Sheng, Y.; Wang, Q.; Tao, J.; Zhang, Y.; Jiang, X. Integration analysis of lncRNA and mRNA expression data identifies DOCK4 as a potential biomarker for elderly osteoporosis. BMC Med. Genom. 2024, 17, 70. [Google Scholar] [CrossRef] [PubMed]
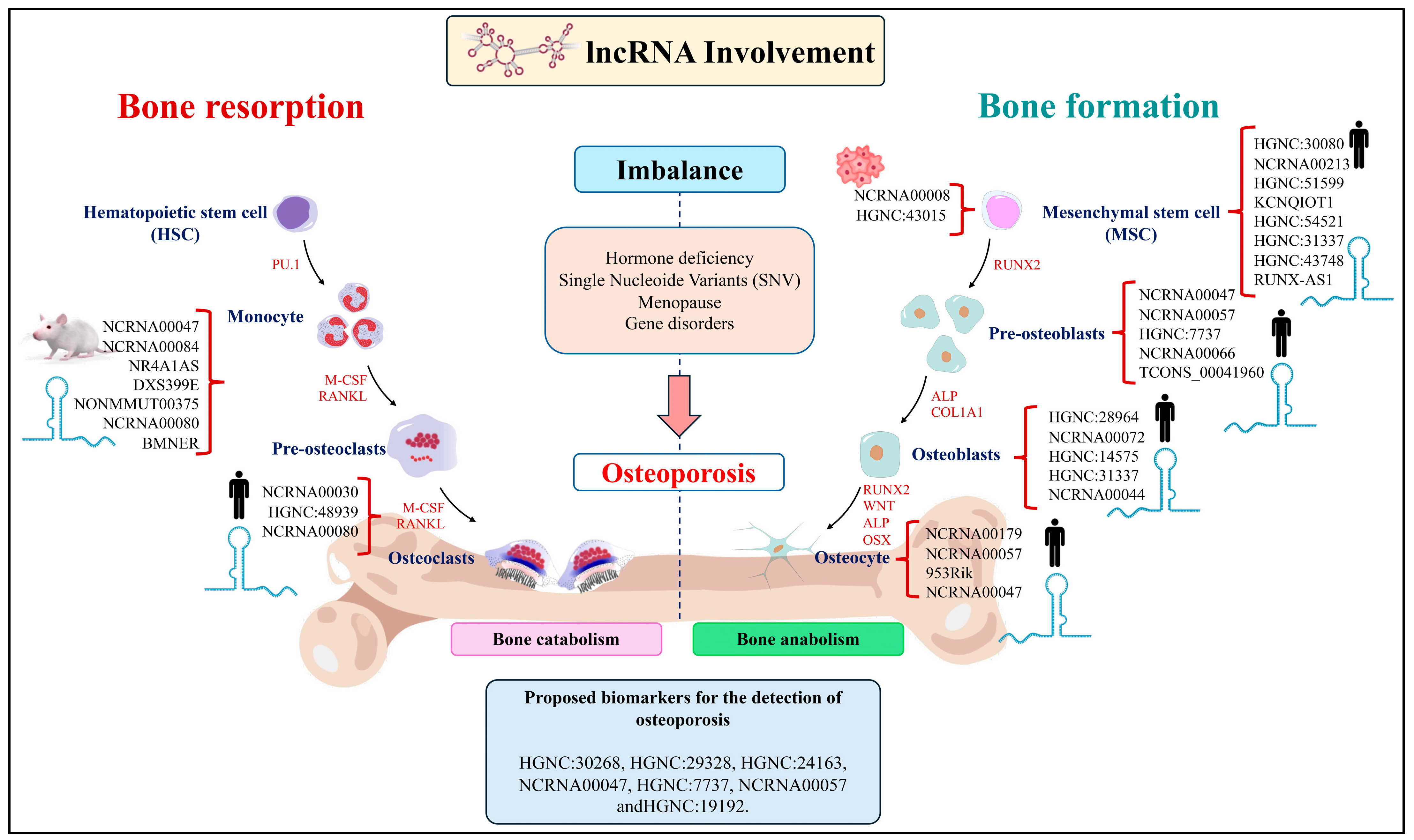

| LncRNA ID | Study Model | Target | Effects on Bone Remodeling | Reference |
|---|---|---|---|---|
| NCRNA00047 (MALAT1/NEAT2) | Exosomes derived of EPC | miR-124/ITGB1 | Promotes recruitment and differentiation of osteoclast precursors | [37] |
| NCRNA00084 (NEAT1) | Mice | miR-7b/PTK2 BMP1/miR-29b-3p DDX5/Wnt/β-catenin | Promotes osteoclastogenesis | [38,39,40] |
| AK077216 | Mice | NIP45 | Promotes osteoclastogenesis | [41] |
| NCRNA00194 (NRON) | Mice | NFATC1 | Inhibits osteoclastogenesis | [42] |
| NR4A1AS | Mice | UPF1 | Control of migration and recruitment of osteoclast precursors | [43] |
| HGNC:54188 (Bmncr) | RAW264.7 | RANKL | Inhibits osteoclastogenesis | [44] |
| HGNC:54188 (Bmncr) | RAW264.7 | RANKL | Promotes osteoclastogenesis | [44] |
| FLJ38860 (SNHG15) | HFOB1.19 | miR-14/RANK/RANKL | Regulates osteoclastogenesis | [45] |
| LncRNA-Jack3 | RAW264.7 | Jack3/Nfatc1/Ctsk | Regulates osteoclastogenesis | [46] |
| HGNC:482 (ANCR) | HFOB1.19 | RUNX2 | Inhibits osteoclastogenesis | [47] |
| DXS399E (XIST) | RAW264.7/BMMs | FUS/SPHK1/S1P/ERK | Promotes osteoclastogenesis | [48] |
| lncRNA-NONMMUT000375.2 | RAW264.7/MC3T3-E1 | Bcl2, Wnt11, TGFB1, and Pdk1 | Promotes osteoclastogenesis | [49] |
| NCRNA00080 (TUG1) | VECs | miR-204-5p/PTEN | Promotes osteoclastogenesis | [50] |
| LncRNA ID | Study Model | Target | Effects on Bone Remodeling | Reference |
|---|---|---|---|---|
| HGNC:29665 (MALAT1) | ICAV | miR-204/SMAD4 | Promotes differentiation of osteoblasts and osteocytes | [58] |
| NCRNA00057 (SNHG1) | Osteoblasts and serum samples | OCN ALP | Inhibits osteoblast differentiation | [59] |
| HGNC:30080 (POM121L9P) | MSC | miR-503-5p/SORBS1 | Inhibits osteoblast differentiation | [60] |
| HGNC:7737 (NEFH) | Serum samples | miR-155/PTEN | Promotes differentiation of osteoblasts | [61] |
| NCRNA00213 (HOTTIP) | MSC | Wnt/β-catenin signaling pathway | Promotes differentiation of osteoblasts | [62] |
| HGNC:51599 (NKILA) | MSC | RXFP1/PI3K-AKT/NF-kB | Promotes differentiation of osteoblasts | [63] |
| NCRNA00066 (MIAT) | Serum samples | miR-150-5p | Inhibits osteoblast differentiation | [64] |
| NCRNA00012 (KCNQ1OT1) | MSC | miR-205-5p/RICTOR OPN, RUNX2, OCN | Promotes differentiation of osteoblasts | [65] |
| NCRNA00008 (H19) | MC3T3-E1 | miR-185-5p/IGF1 | Modulating matrix mineralization of osteoblasts | [66] |
| HGNC:54521 (POIR) | MSC | miR-182/FoxO1 | Promotes differentiation of osteoblasts | [67] |
| HGNC:31337 (HCG18) | NP | miR-146a-5p/TRAF6/NF-kB | Regulates differentiation of osteoblasts | [68] |
| HGNC:43748 (HOXA-AS3) | MSC | EZH2/Runx2/H3K27me3 | Inhibits osteoblast differentiation | [69] |
| lncRUNX2-AS1 | MSC | RUNX2 | Inhibits osteoblast differentiation | [70] |
| MODR | MSC | miR-454 | Promotes differentiation of osteoblasts | [71] |
| HGNC:43015 (HIF1A-AS2) | hpPDLSC | HIF-1α | Osteogenic differentiation of periodontal ligament cells | [72] |
| TCONS_00041960 | rBMC | miR-204-5p/miR-125a-3p/Runx2/GILZ | Promotes/osteogenesis | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ortega, R.F.; Aparicio-Bautista, D.I.; Becerra-Cervera, A.; Ortega-Meléndez, A.I.; Patiño, N.; Rivera-Paredez, B.; Hidalgo-Bravo, A.; Velázquez-Cruz, R. The Regulatory Role of Long Non-Coding RNAs in the Development and Progression of Osteoporosis. Int. J. Mol. Sci. 2025, 26, 4273. https://doi.org/10.3390/ijms26094273
Jiménez-Ortega RF, Aparicio-Bautista DI, Becerra-Cervera A, Ortega-Meléndez AI, Patiño N, Rivera-Paredez B, Hidalgo-Bravo A, Velázquez-Cruz R. The Regulatory Role of Long Non-Coding RNAs in the Development and Progression of Osteoporosis. International Journal of Molecular Sciences. 2025; 26(9):4273. https://doi.org/10.3390/ijms26094273
Chicago/Turabian StyleJiménez-Ortega, Rogelio F., Diana I. Aparicio-Bautista, Adriana Becerra-Cervera, Alejandra I. Ortega-Meléndez, Nelly Patiño, Berenice Rivera-Paredez, Alberto Hidalgo-Bravo, and Rafael Velázquez-Cruz. 2025. "The Regulatory Role of Long Non-Coding RNAs in the Development and Progression of Osteoporosis" International Journal of Molecular Sciences 26, no. 9: 4273. https://doi.org/10.3390/ijms26094273
APA StyleJiménez-Ortega, R. F., Aparicio-Bautista, D. I., Becerra-Cervera, A., Ortega-Meléndez, A. I., Patiño, N., Rivera-Paredez, B., Hidalgo-Bravo, A., & Velázquez-Cruz, R. (2025). The Regulatory Role of Long Non-Coding RNAs in the Development and Progression of Osteoporosis. International Journal of Molecular Sciences, 26(9), 4273. https://doi.org/10.3390/ijms26094273










