The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance
Abstract
1. Introduction
2. Anti-Efflux Potentials of Pure Polyphenols
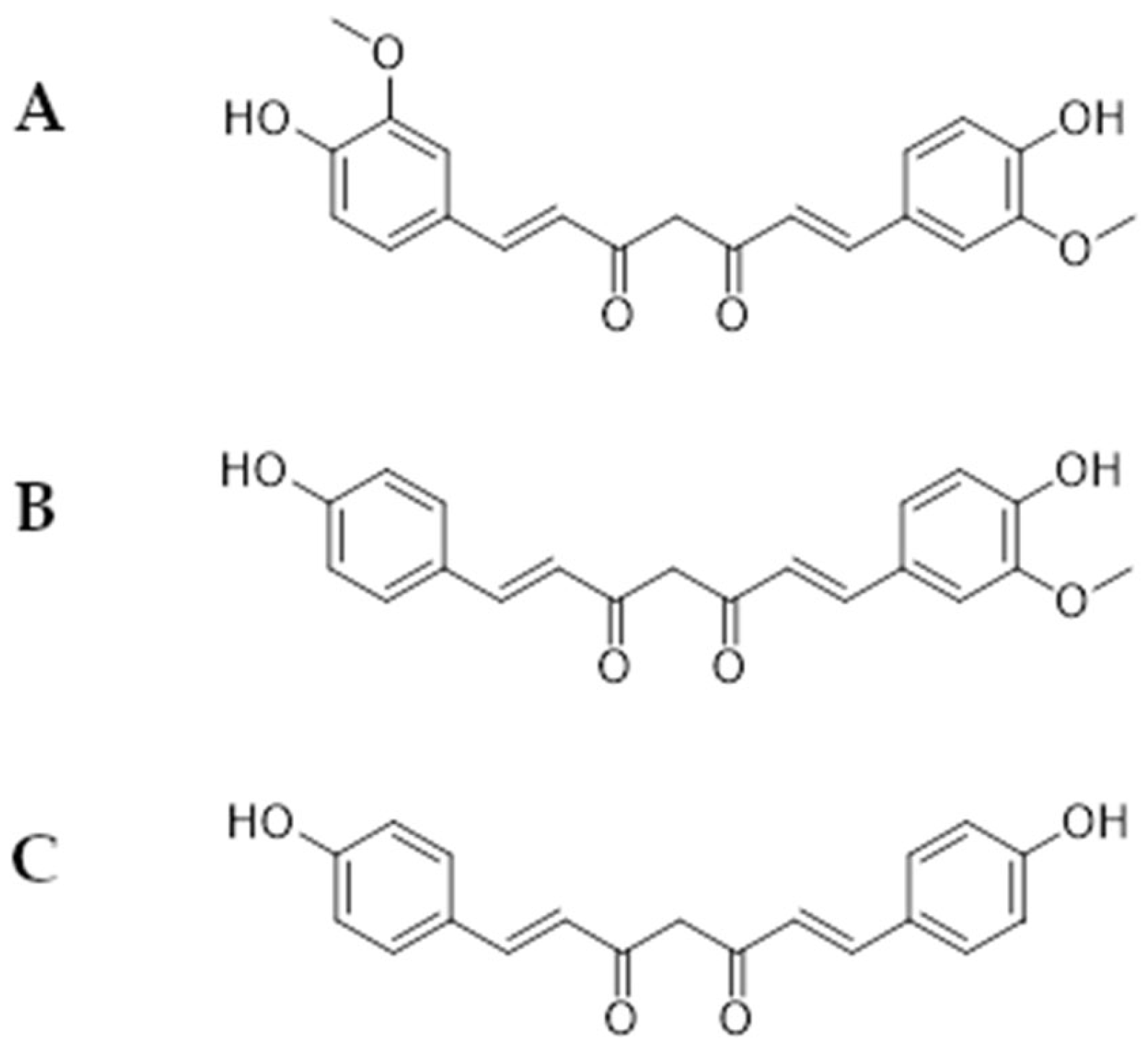
2.1. Curcuminoids Activity as an Efflux Pump Inhibitor
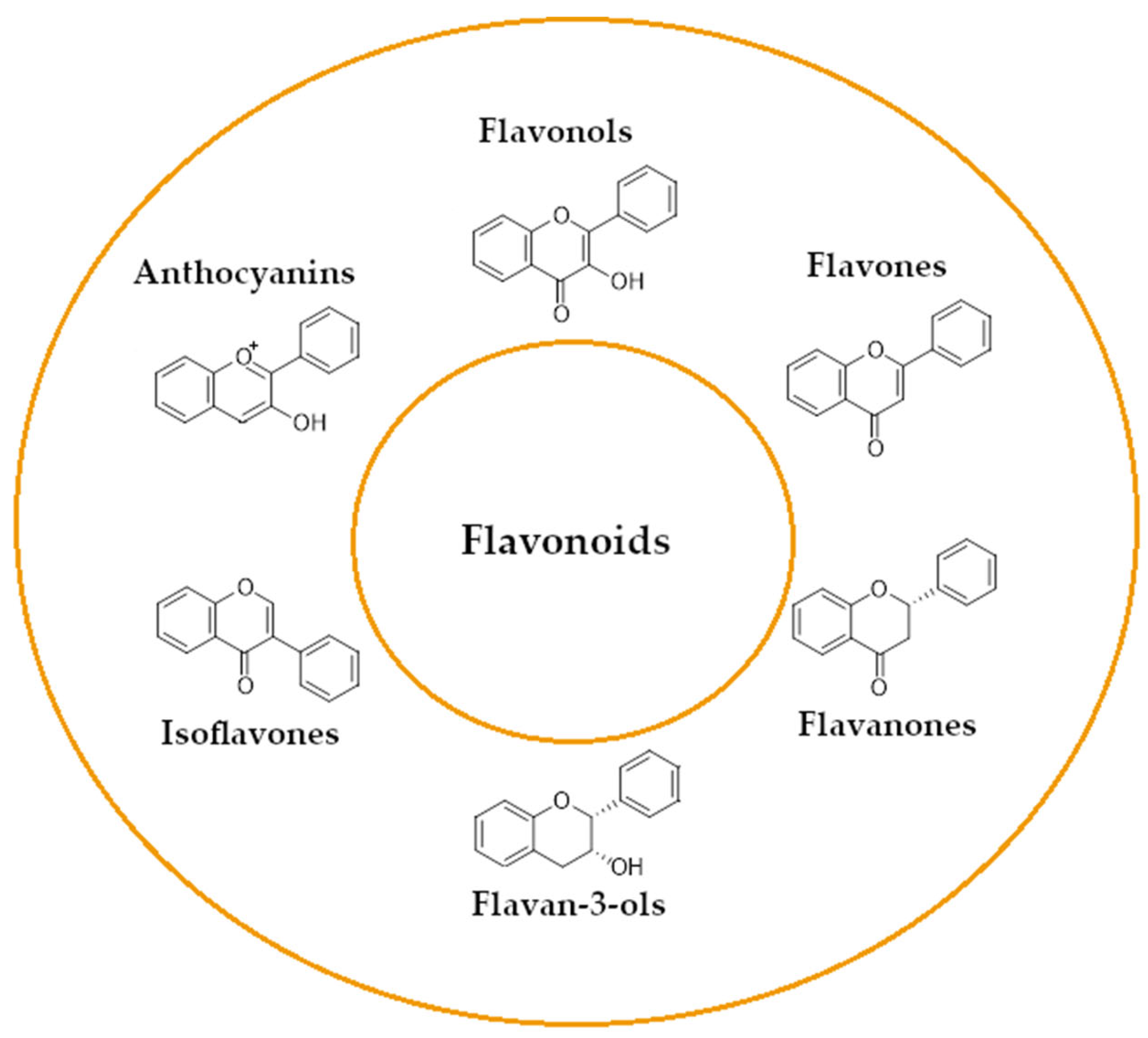
2.2. Flavonoids Activity as an Efflux Pump Inhibitor
2.2.1. Quercetin Activity as an Efflux Pump Inhibitor
2.2.2. Epigallocatechin-3-Gallate (EGCG) as an Efflux Pump Inhibitor
2.2.3. Luteolin Activity as an Efflux Pump Inhibitor
2.2.4. Naringenin Activity as an Efflux Pump Inhibitor
2.3. Non-Flavonoids Activity as an Efflux Pump Inhibitor
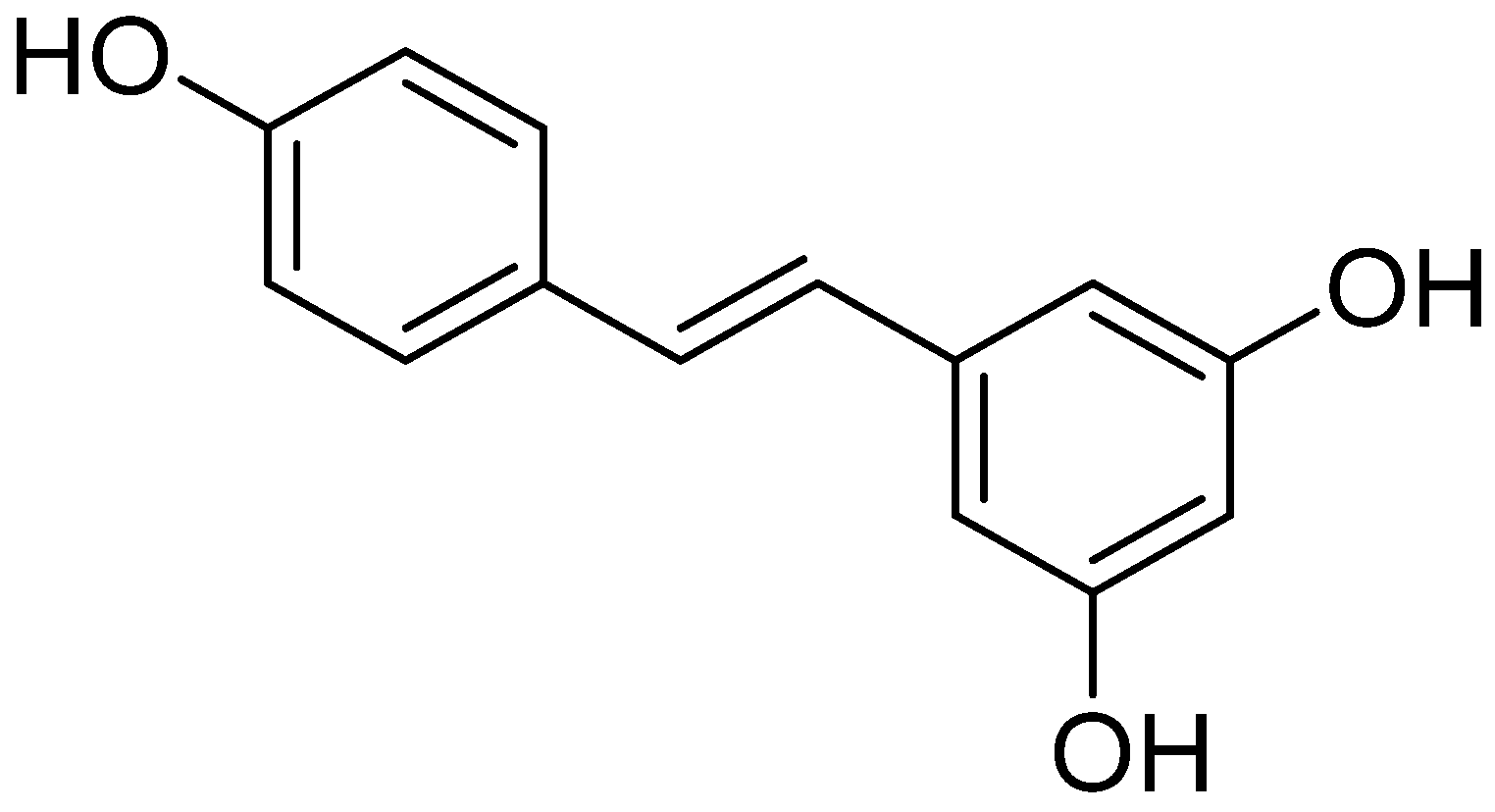
Stilbenoids
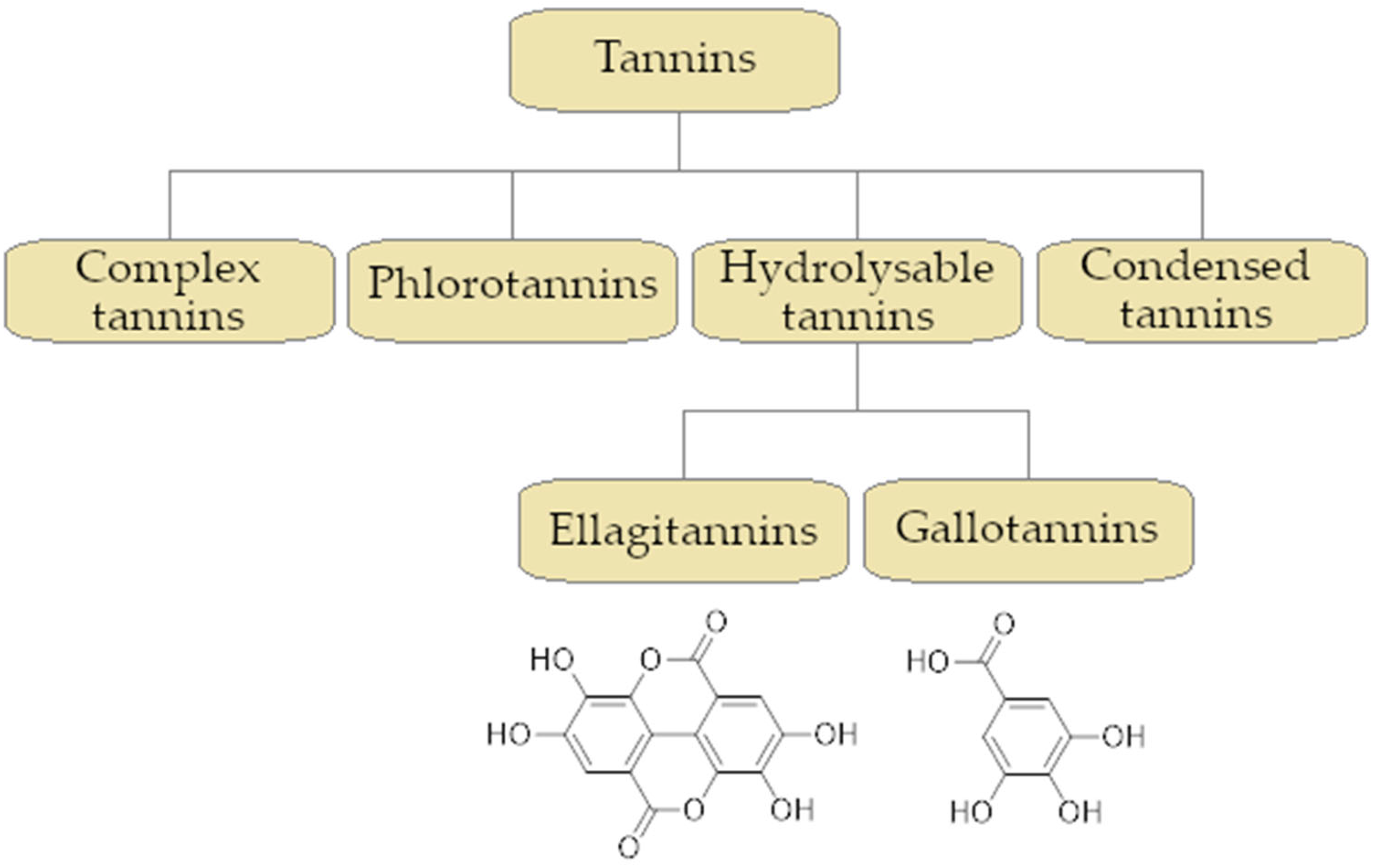
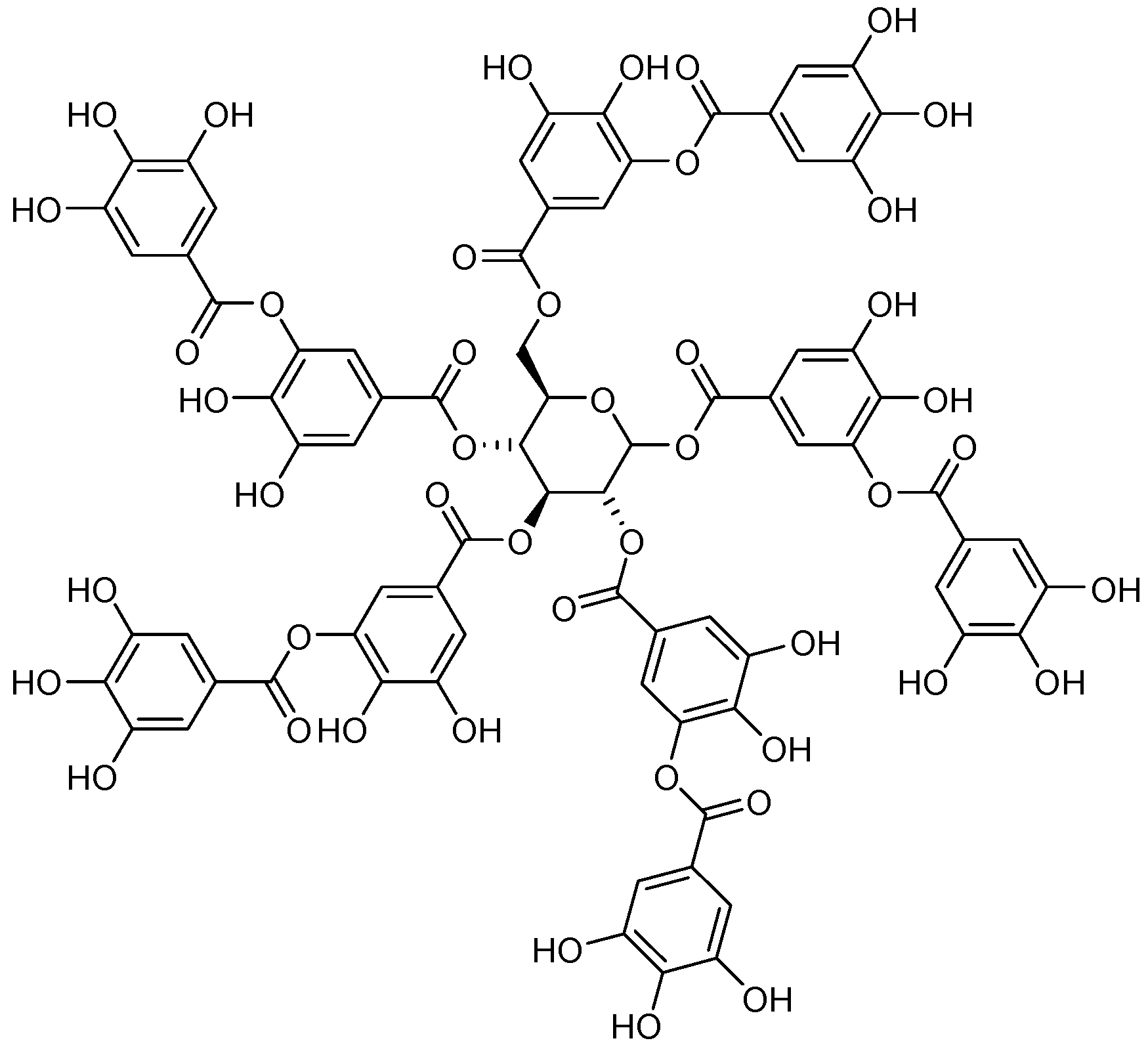
2.4. Tannins Activity as an Efflux Pump Inhibitor
Tannic Acid as an Efflux Pump Inhibitor
2.5. Phenolic Acids
Ellagic Acid as an Efflux Pump
3. Cutting-Edge Technologies Enhancing the Efficiency of Plant-Derived Drugs
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seukep, A.J.; Mbuntcha, H.G.; Kuete, V.; Chu, Y.; Fan, E.; Guo, M.Q. What Approaches to Thwart Bacterial Efflux Pumps-Mediated Resistance? Antibiotics 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Active Efflux, a Common Mechanism for Biocide and Antibiotic Resistance. Symp. Ser. Soc. Appl. Microbiol. 2002, 92, 65–71. [Google Scholar] [CrossRef]
- Gilbert, P.; McBain, A.J. Potential Impact of Increased Use of Biocides in Consumer Products on Prevalence of Antibiotic Resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef]
- Poole, K. Efflux-Mediated Antimicrobial Resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ortiz-Alegria, A.; Kumar, S.; Varela, M.F. Major Facilitator Superfamily Efflux Pumps in Human Pathogens: Role in Multidrug Resistance and Beyond. Curr. Res. Microb. Sci. 2024, 7, 100248. [Google Scholar] [CrossRef]
- Pasqua, M.; Grossi, M.; Zennaro, A.; Fanelli, G.; Micheli, G.; Barras, F.; Colonna, B.; Prosseda, G. The Varied Role of Efflux Pumps of the Mfs Family in the Interplay of Bacteria with Animal and Plant Cells. Microorganisms 2019, 7, 285. [Google Scholar] [CrossRef]
- Stephen, J.; Salam, F.; Lekshmi, M.; Kumar, S.H.; Varela, M.F. The Major Facilitator Superfamily and Antimicrobial Resistance Efflux Pumps of the ESKAPEE Pathogen Staphylococcus aureus. Antibiotics 2023, 12, 343. [Google Scholar] [CrossRef]
- Bay, D.C.; Rommens, K.L.; Turner, R.J. Small Multidrug Resistance Proteins: A Multidrug Transporter Family That Continues to Grow. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1814–1838. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Nikaido, H. Structure and Mechanism of RND-Type Multidrug Efflux Pumps. Adv. Enzymol. Relat. Areas Mol. Biol. 2010, 77, 1–60. [Google Scholar] [CrossRef]
- Novelli, M.; Bolla, J.M. RND Efflux Pump Induction: A Crucial Network Unveiling Adaptive Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Antibiotics 2024, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Auda, I.G.; Ali Salman, I.M.; Odah, J.G. Efflux Pumps of Gram-Negative Bacteria in Brief. Gene Rep. 2020, 20, 100666. [Google Scholar] [CrossRef]
- Gurvic, D.; Zachariae, U. Multidrug Efflux in Gram-Negative Bacteria: Structural Modifications in Active Compounds Leading to Efflux Pump Avoidance. npj Antimicrob. Resist. 2024, 2, 6. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Richmond, G.E.; Piddock, L.J.V. Multidrug Efflux Pumps in Gram-Negative Bacteria and Their Role in Antibiotic Resistance. Future Microbiol. 2014, 9, 1165–1177. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug Efflux Pumps of Gram-Positive Bacteria. Drug Resist. Updat. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef]
- Cho, H.H.; Sung, J.Y.; Kwon, K.C.; Koo, S.H. Expression of Sme Efflux Pumps and Multilocus Sequence Typing in Clinical Isolates of Stenotrophomonas Maltophilia. Ann. Lab. Med. 2012, 32, 38–43. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, X.; Guo, Q.; Zhao, X.; Ye, X.; Guo, Y.; Wang, M. Prevalence of the OqxAB Gene Complex in Klebsiella pneumoniae and Escherichia coli Clinical Isolates. J. Antimicrob. Chemother. 2012, 67, 1655–1659. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Jones, R.N.; Farrell, D.J. Evaluation of Quinolone Resistance–Determining Region Mutations and Efflux Pump Expression in Neisseria Meningitidis Resistant to Fluoroquinolones. Diagn. Microbiol. Infect. Dis. 2012, 72, 263–266. [Google Scholar] [CrossRef]
- Dreier, J.; Ruggerone, P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 660. [Google Scholar] [CrossRef] [PubMed]
- Leus, I.V.; Weeks, J.W.; Bonifay, V.; Smith, L.; Richardson, S.; Zgurskaya, H.I. Substrate Specificities and Efflux Efficiencies of RND Efflux Pumps of Acinetobacter baumannii. J. Bacteriol. 2018, 200, e00049-18. [Google Scholar] [CrossRef] [PubMed]
- Saw, H.T.H.; Webber, M.A.; Mushtaq, S.; Woodford, N.; Piddock, L.J.V. Inactivation or Inhibition of AcrAB-TolC Increases Resistance of Carbapenemase-Producing Enterobacteriaceae to Carbapenems. J. Antimicrob. Chemother. 2016, 71, 1510–1519. [Google Scholar] [CrossRef]
- Du, D.; van Veen, H.W.; Murakami, S.; Pos, K.M.; Luisi, B.F. Structure, Mechanism and Cooperation of Bacterial Multidrug Transporters. Curr. Opin. Struct. Biol. 2015, 33, 76–91. [Google Scholar] [CrossRef]
- Rouquette-Loughlin, C.E.; Dhulipala, V.; Reimche, J.L.; Raterman, E.; Begum, A.A.; Jerse, A.E.; Shafer, W.M. Cis- and Trans-Acting Factors Influence Expression of the NorM -Encoded Efflux Pump of Neisseria Gonorrhoeae and Levels of Gonococcal Susceptibility to Substrate Antimicrobials. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Banchs, C.; Poulos, S.; Nimjareansuk, W.S.; Joo, Y.E.; Faham, S. Substrate Binding to the Multidrug Transporter MepA. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2539–2546. [Google Scholar] [CrossRef]
- Kuroda, T.; Tsuchiya, T. Multidrug Efflux Transporters in the MATE Family. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 763–768. [Google Scholar] [CrossRef]
- Tocci, N.; Iannelli, F.; Bidossi, A.; Ciusa, M.L.; Decorosi, F.; Viti, C.; Pozzi, G.; Ricci, S.; Oggioni, M.R. Functional Analysis of Pneumococcal Drug Efflux Pumps Associates the MATE DinF Transporter with Quinolone Susceptibility. Antimicrob. Agents Chemother. 2013, 57, 248–253. [Google Scholar] [CrossRef]
- Lu, S.; Zgurskaya, H.I. MacA, a Periplasmic Membrane Fusion Protein of the Macrolide Transporter MacAB-TolC, Binds Lipopolysaccharide Core Specifically and with High Affinity. J. Bacteriol. 2013, 195, 4865–4872. [Google Scholar] [CrossRef]
- Lin, H.T.; Bavro, V.N.; Barrera, N.P.; Frankish, H.M.; Velamakanni, S.; van Veen, H.W.; Robinson, C.V.; Borges-Walmsley, M.I.; Walmsley, A.R. MacB ABC Transporter Is a Dimer Whose ATPase Activity and Macrolide-Binding Capacity Are Regulated by the Membrane Fusion Protein MacA*. J. Biol. Chem. 2009, 284, 1145–1154. [Google Scholar] [CrossRef]
- Lerma, L.L.; Benomar, N.; Valenzuela, A.S.; Muñoz, M.d.C.C.; Gálvez, A.; Abriouel, H. Role of EfrAB Efflux Pump in Biocide Tolerance and Antibiotic Resistance of Enterococcus faecalis and Enterococcus faecium Isolated from Traditional Fermented Foods and the Effect of EDTA as EfrAB Inhibitor. Food Microbiol. 2014, 44, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, I.; Isaka, M.; Masuno, K.; Hata, N.; Matsumoto, M.; Hasegawa, T. Functional Predominance of Msr (D), Which Is More Effective as Mef(A)-Associated Than Mef(E)-Associated, Over Mef (A)/ Mef (E) in Macrolide Resistance in Streptococcus pyogenes. Microb. Drug Resist. 2018, 24, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.I.; Baylay, A.J.; Wong, R.L.; Piddock, L.J.V. Overexpression of PatA and PatB, Which Encode ABC Transporters, Is Associated with Fluoroquinolone Resistance in Clinical Isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Shirshikova, T.V.; Sierra-Bakhshi, C.G.; Kamaletdinova, L.K.; Matrosova, L.E.; Khabipova, N.N.; Evtugyn, V.G.; Khilyas, I.V.; Danilova, I.V.; Mardanova, A.M.; Sharipova, M.R.; et al. The ABC-Type Efflux Pump MacAB Is Involved in Protection of Serratia marcescens against Aminoglycoside Antibiotics, Polymyxins, and Oxidative Stress. mSphere 2021, 6. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug Efflux Pumps in Staphylococcus aureus: An Update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Chancey, S.T.; Bai, X.; Kumar, N.; Drabek, E.F.; Daugherty, S.C.; Colon, T.; Ott, S.; Sengamalay, N.; Sadzewicz, L.; Tallon, L.J.; et al. Transcriptional Attenuation Controls Macrolide Inducible Efflux and Resistance in Streptococcus pneumoniae and in Other Gram-Positive Bacteria Containing Mef/Mel(Msr(D)) Elements. PLoS ONE 2015, 10, e0116254. [Google Scholar] [CrossRef]
- Nunez-Samudio, V.; Chesneau, O. Functional Interplay between the ATP Binding Cassette Msr(D) Protein and the Membrane Facilitator Superfamily Mef(E) Transporter for Macrolide Resistance in Escherichia coli. Res. Microbiol. 2013, 164, 226–235. [Google Scholar] [CrossRef]
- Wright, D.J.; Tate, C.G. Isolation and Characterisation of Transport-Defective Substrate-Binding Mutants of the Tetracycline Antiporter TetA(B). Biochim. Biophys. Acta Biomembr. 2015, 1848, 2261–2270. [Google Scholar] [CrossRef]
- Fluman, N.; Bibi, E. Bacterial Multidrug Transport through the Lens of the Major Facilitator Superfamily. Biochim. Biophys. Acta Proteins Proteomics 2009, 1794, 738–747. [Google Scholar] [CrossRef]
- Bay, D.C.; Turner, R.J. Diversity and Evolution of the Small Multidrug Resistance Protein Family. BMC Evol. Biol. 2009, 9, 140. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lin, Y.-Y.; Tu, C.-C.; Lan, C.-Y. Distribution of Different Efflux Pump Genes in Clinical Isolates of Multidrug-Resistant Acinetobacter baumannii and Their Correlation with Antimicrobial Resistance. J. Microbiol. Immunol. Infect. 2017, 50, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Padariya, M.; Kalathiya, U.; Baginski, M. Structural and Dynamic Insights on the EmrE Protein with TPP + and Related Substrates through Molecular Dynamics Simulations. Chem. Phys. Lipids 2018, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.A.; Piddock, L.J.V. The Importance of Efflux Pumps in Bacterial Antibiotic Resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Z.; Yan, A. Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Vaez, H.; Faghri, J.; Isfahani, B.; Moghim, S.; Yadegari, S.; Fazeli, H.; Moghofeei, M.; Safaei, H. Efflux Pump Regulatory Genes Mutations in Multidrug Resistance Pseudomonas aeruginosa Isolated from Wound Infections in Isfahan Hospitals. Adv. Biomed. Res. 2014, 3, 117. [Google Scholar] [CrossRef]
- Roy, S.; Chatterjee, S.; Bhattacharjee, A.; Chattopadhyay, P.; Saha, B.; Dutta, S.; Basu, S. Overexpression of Efflux Pumps, Mutations in the Pumps’ Regulators, Chromosomal Mutations, and AAC(6′)-Ib-Cr Are Associated With Fluoroquinolone Resistance in Diverse Sequence Types of Neonatal Septicaemic Acinetobacter baumannii: A 7-Year Single Center S. Front. Microbiol. 2021, 12, 602724. [Google Scholar] [CrossRef]
- Shaheen, A.; Afridi, W.A.; Mahboob, S.; Sana, M.; Zeeshan, N.; Ismat, F.; Mirza, O.; Iqbal, M.; Rahman, M. Reserpine Is the New Addition into the Repertoire of AcrB Efflux Pump Inhibitors. Mol. Biol. 2019, 53, 596–605. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X. Role of Berberine as a Potential Efflux Pump Inhibitor against MdfA from Escherichia coli: In Vitro and In Silico Studies. Microbiol. Spectr. 2023, 11, e03324-22. [Google Scholar] [CrossRef]
- Morita, Y.; Nakashima, K.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 1223. [Google Scholar] [CrossRef]
- PTegos, G.P.; Haynes, M.; Strouse, J.J.; Khan, M.M.T.; Bologa, C.G.; Oprea, T.I.; Sklar, L.A. Microbial Efflux Pump Inhibition: Tactics and Strategies. Curr. Pharm. Des. 2012, 17, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Mahamoud, A.; Chevalier, J.; Davin-Regli, A.; Barbe, J.; Pages, J.-M. Quinoline Derivatives as Promising Inhibitors of Antibiotic Efflux Pump in Multidrug Resistant Enterobacter aerogenes Isolates. Curr. Drug Targets 2006, 7, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Compagne, N.; Jiménez-Castellanos, J.C.; Meurillon, V.; Pradel, E.; Vieira Da Cruz, A.; Piveteau, C.; Biela, A.; Eveque, M.; Leroux, F.; Deprez, B.; et al. Optimization of Pyridylpiperazine-Based Inhibitors of the Escherichia coli AcrAB-TolC Efflux Pump. Eur. J. Med. Chem. 2023, 259, 115630. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T. Fast Antioxidant Reaction of Polyphenols and Their Metabolites. Antioxidants 2021, 10, 1297. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Hedayati, N.; Yaghoobi, A.; Salami, M.; Gholinezhad, Y.; Aghadavood, F.; Eshraghi, R.; Aarabi, M.H.; Homayoonfal, M.; Asemi, Z.; Mirzaei, H.; et al. Impact of Polyphenols on Heart Failure and Cardiac Hypertrophy: Clinical Effects and Molecular Mechanisms. Front. Cardiovasc. Med. 2023, 10, 1174816. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive Polyphenols and Cardiovascular Disease: Chemical Antagonists, Pharmacological Agents or Xenobiotics That Drive an Adaptive Response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in Health and Food Processing: Antibacterial, Anti-Inflammatory, and Antioxidant Insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Witek-Krowiak, A.; Mikula, K.; Szopa, D. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Mandal, M.K.; Domb, A.J. Antimicrobial Activities of Natural Bioactive Polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Ji, Y.; Yang, Y.; Zheng, X.; Xiao, D.; Wang, M.; Ai, B.; Sheng, Z. Enhancing the Antioxidant and Antifungal Properties of Polyphenols through Encapsulation in β-Lactoglobulin Nanoparticles Using Emulsification-Evaporation Technique. LWT 2023, 185, 115198. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Di Ff Erent Matrices of Vitis Vinifera. Molecules 2020, 25, 3478. [Google Scholar] [CrossRef] [PubMed]
- Sabin, O.; Pop, R.M.; Bocsan, I.C.; Chedea, V.S.; Grozav, A.; Levai’, A.-M.; Buzoianu, A.D. The Anti-Inflammatory, Analgesic, and Antioxidant Effects of Polyphenols from Brassica oleracea Var. capitata Extract on Induced Inflammation in Rodents. Molecules 2024, 29, 3448. [Google Scholar] [CrossRef]
- Ghannadi, A.; Hajhashemi, V.; Jafarabadi, H. An Investigation of the Analgesic and Anti-Inflammatory Effects of Nigella Sativa Seed Polyphenols. J. Med. Food 2005, 8, 488–493. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A Novel Gene, OptrA, that Confers Transferable Resistance to Oxazolidinones and Phenicols and Its Presence in Enterococcus faecalis and Enterococcus faecium of Human and Animal Origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef]
- Antonelli, A.; D’Andrea, M.M.; Brenciani, A.; Galeotti, C.L.; Morroni, G.; Pollini, S.; Varaldo, P.E.; Rossolini, G.M. Characterization of PoxtA, a Novel Phenicol-Oxazolidinone-Tetracycline Resistance Gene from an MRSA of Clinical Origin. J. Antimicrob. Chemother. 2018, 73, 1763–1769. [Google Scholar] [CrossRef]
- Sharkey, L.K.R.; Edwards, T.A.; O’Neill, A.J. ABC-F Proteins Mediate Antibiotic Resistance through Ribosomal Protection. MBio 2016, 7, e01975-15. [Google Scholar] [CrossRef]
- Anandaraj, M.; Prasath, D.; Kandiannan, K.; Zachariah, T.J.; Srinivasan, V.; Jha, A.K.; Singh, B.K.; Singh, A.K.; Pandey, V.P.; Singh, S.P.; et al. Genotype by Environment Interaction Effects on Yield and Curcumin in Turmeric (Curcuma Longa L.). Ind. Crops Prod. 2014, 53, 358–364. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Neyestani, Z.; Ebrahimi, S.A.; Ghazaghi, A.; Jalili, A.; Sahebkar, A.; Rahimi, H.R. Review of Anti-Bacterial Activities of Curcumin against Pseudomonas aeruginosa. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Negi, N. Possible Role of Curcumin as Ection an Efflux Pump Inhibitor in Multi Drug Resistant Clinical Isolates of Pseudomonas aeruginosa. J. Clin. Diagn. Res. 2014, 8, DC04–DC07. [Google Scholar] [CrossRef]
- Charkhi, P.; Haghshenas, M.R.; Mirzaei, B.; Davoodi, L.; Norouzi Bazgir, Z.; Goli, H.R. Comparison of the Effect of Phenylalanine Arginine Beta Naphthylamide (PAβN) and Curcumin on Minimum Inhibitory Concentration of Aminoglycosides on Pseudomonas aeruginosa Clinical Isolates. J. Adv. Med. Biomed. Res. 2020, 28, 105–110. [Google Scholar] [CrossRef]
- Sulaiman, S.D.; Abdulhasan, G.A. Curcumin as Efflux Pump Inhibitor Agent for Enhancement Treatment Against Multidrug Resistant Pseudomonas aeruginosa Isolates. Iraqi J. Sci. 2020, 61, 59–67. [Google Scholar] [CrossRef]
- Yaseen, N.N.; Ahmed, D.A. Detection of MexB Multidrug Efflux Gene in Some Local Isolates of Pseudomonas aeruginosa. Iraqi J. Sci. 2023, 64, 111–118. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, P.; Capalash, N. Curcumin Alleviates Persistence of Acinetobacter baumannii against Colistin. Sci. Rep. 2018, 8, 11029. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.S.; Sivasubramanian, A.; Nagarajan, S. Simultaneous Inhibition of MarR by Salicylate and Efflux Pumps by Curcumin Sensitizes Colistin Resistant Clinical Isolates of Enterobacteriaceae. Microb. Pathog. 2020, 148, 104445. [Google Scholar] [CrossRef]
- Qin, C.; Tang, N.; Gan, Y.; Zhao, H.; Li, Y.; Tian, G.; Yang, Y.Y.; Yuan, P.; Ding, X. Liposomes Co-Delivering Curcumin and Colistin to Overcome Colistin Resistance in Bacterial Infections. Adv. Healthc. Mater. 2023, 12, e2202903. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, X.; Qin, Z.; Li, J.; Yu, J.; He, X.; Zhu, L.; Fan, H.; Hu, Y.; Yao, J.; et al. Photodynamic Inactivation of Shigella flexneri by Curcumin. LWT 2022, 153, 112491. [Google Scholar] [CrossRef]
- Joshi, P.; Singh, S.; Wani, A.; Sharma, S.; Jain, S.K.; Singh, B.; Gupta, B.D.; Satti, N.K.; Koul, S.; Khan, I.A.; et al. Osthol and Curcumin as Inhibitors of Human Pgp and Multidrug Efflux Pumps of Staphylococcus aureus: Reversing the Resistance against Frontline Antibacterial Drugs. Med. Chem. Commun. 2014, 5, 1540–1547. [Google Scholar] [CrossRef]
- Jaberi, S.; Fallah, F.; Hashemi, A.; Karimi, A.; Azimi, L. Inhibitory Effects of Curcumin on the Expression of NorA Efflux Pump and Reduce Antibiotic Resistance in Staphylococcus aureus. J. Pure Appl. Microbiol. 2018, 12, 95–102. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- de la Lastra, A.; Martin, M.J.; Motilva, V. Antiulcer and Gastroprotective Effects of Quercetin: A Gross and Histologic Study. Pharmacology 1994, 48, 56–62. [Google Scholar] [CrossRef]
- Han, M.; Song, Y.; Zhang, X. Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells through Regulating Toll-like Receptor 4/Nuclear Factor-Kappa B Pathway. Pharmacogn. Mag. 2016, 12, 237. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The Effect of Quercetin Supplementation on Selected Markers of Inflammation and Oxidative Stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar]
- Amin, I.; Majid, S.; Farooq, A.; Wani, H.A.; Noor, F.; Khan, R.; Shakeel, S.; Bhat, S.A.; Ahmad, A.; Madkhali, H.; et al. Naringenin (4,5,7-Trihydroxyflavanone) as a Potent Neuroprotective Agent: From Chemistry to Medicine. In Studies in Natural Products Chemistry; Atta Ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 65, pp. 271–300. [Google Scholar]
- Adedokun, K.A.; Imodoye, S.O.; Bello, I.O.; Lanihun, A.-A.; Bello, I.O. Therapeutic Potentials of Medicinal Plants and Significance of Computational Tools in Anti-Cancer Drug Discovery. In Phytochemistry, Computational Tools and Databases in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 393–455. [Google Scholar]
- Singhal, S.; Singh, M.; Singh, R.K.; Tiwari, V.K.; Bajpai, S. Molecular Mechanisms Underlying Breast Cancer and Role of Plant Products in Targeted Therapy. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–351. [Google Scholar]
- Sachan, N.; Singh Chauhan, B.; Srikrishna, S. Plant Bioactives as Promising Therapeutic Agents in Parkinson’s Disease Targeting Oxidative Stress during Aging. In Plant Bioactives as Natural Panacea Against Age-Induced Diseases; Elsevier: Amsterdam, The Netherlands, 2023; pp. 329–357. [Google Scholar]
- Gupta, M.K.; Gouda, G.; Sultana, S.; Punekar, S.M.; Vadde, R.; Ravikiran, T. Structure-Related Relationship: Plant-Derived Antidiabetic Compounds. Stud. Nat. Prod. Chem. 2023, 77, 241–295. [Google Scholar]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus aureus and Listeria Monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Sangeetha, R. Luteolin in the Management of Type 2 Diabetes Mellitus. Curr. Res. Nutr. Food Sci. J. 2019, 7, 393–398. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.; Gao, Z. Luteolin Exerts an Anticancer Effect on Gastric Cancer Cells through Multiple Signaling Pathways and Regulating MiRNAs. J. Cancer 2018, 9, 3669–3675. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Choi, J.H.; Kim, W.; Nam, S.M.; Jung, H.Y.; Kim, J.H.; Won, M.-H.; Yoon, Y.S.; Hwang, I.K. Effects of Luteolin on Spatial Memory, Cell Proliferation, and Neuroblast Differentiation in the Hippocampal Dentate Gyrus in a Scopolamine-Induced Amnesia Model. Neurol. Res. 2013, 35, 813–820. [Google Scholar] [CrossRef]
- Si, H.; Wyeth, R.P.; Liu, D. The Flavonoid Luteolin Induces Nitric Oxide Production and Arterial Relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic Effects of EGCG: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial Activities of Green Tea Crude Extracts and Synergistic Effects of Epigallocatechingallate (EGCG) with Gentamicin against MDR Pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.-A. Green Tea (−)Epigallocatechin-3-Gallate Reverses Oxidative Stress and Reduces Acetylcholinesterase Activity in a Streptozotocin-Induced Model of Dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Rahardiyan, D. Antibacterial Potential of Catechin of Tea (Camellia sinensis) and Its Applications. Food Res. 2018, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of Antioxidant Activity of Ten Compounds in Different Tea Samples by Means of an On-Line HPLC–DPPH Assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Farzaei, M.; Rahimi, R.; Abdollahi, M. The Role of Dietary Polyphenols in the Management of Inflammatory Bowel Disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-Inflammatory Properties of Oolong Tea (Camellia sinensis) Ethanol Extract and Epigallocatechin Gallate in LPS-Induced RAW 264.7 Cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-Gallate: From Green Tea to Cancer Therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Kumazoe, M.; Tachibana, H. Anti-Cancer Effect of EGCG and Its Mechanisms. Funct. Foods Health Dis. 2016, 6, 70. [Google Scholar] [CrossRef]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A Mass Spectrometry-Based Assay for Improved Quantitative Measurements of Efflux Pump Inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simões, M. Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Abreu, A.C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Combinatorial Approaches with Selected Phytochemicals to Increase Antibiotic Efficacy against Staphylococcus aureus Biofilms. Biofouling 2016, 32, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.F.S.; Tintino, S.R.; da Silva, A.R.P.; Barbosa, C.R.d.S.; Scherf, J.R.; Silveira, Z.d.S.; de Freitas, T.S.; Neto, L.J.d.L.; Barros, L.M.; Menezes, I.R.d.A.; et al. Enhancement of the Antibiotic Activity by Quercetin against Staphylococcus aureus Efflux Pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Tripathi, A. Quercetin Inhibits Carbapenemase and Efflux Pump Activities among Carbapenem-resistant Gram-negative Bacteria. APMIS 2020, 128, 251–259. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin Potentiates Meropenem Activity among Pathogenic Carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef]
- de Carvalho Matias, E.G.; Bezerra, K.S.; Costa, A.H.L.; Clemente Junior, W.S.; Oliveira, J.I.N.; Ribeiro Junior, L.A.; Galvão, D.S.; Fulco, U.L. Quantum Biochemical Analysis of the TtgR Regulator and Effectors. Sci. Rep. 2024, 14, 8519. [Google Scholar] [CrossRef]
- Majumdar, G.; Mandal, S. Evaluation of Broad-Spectrum Antibacterial Efficacy of Quercetin by Molecular Docking, Molecular Dynamics Simulation and in Vitro Studies. Chem. Phys. Impact 2024, 8, 100501. [Google Scholar] [CrossRef]
- Sahoo, M.; Behera, D.U.; Gaur, M.; Subudhi, E. Molecular Docking, Molecular Dynamics Simulation, and MM/PBSA Analysis of Ginger Phytocompounds as a Potential Inhibitor of AcrB for Treating Multidrug-Resistant Klebsiella pneumoniae Infections. J. Biomol. Struct. Dyn. 2024, 43, 3585–3601. [Google Scholar] [CrossRef]
- Liu, T.; Xu, H.; Huang, T.; Liu, G.; Cao, H.; Lin, Y.; Li, Y.; Li, Y.; Yao, X. Fuzheng Touxie Jiedu Huayu Decoction Inhibits the MexAB-OprM Efflux Pump and Quorum Sensing-Mediated Biofilm Formation in Difficult-to-Treat Multidrug Resistance Pseudomonas aeruginosa. J. Ethnopharmacol. 2024, 332, 118365. [Google Scholar] [CrossRef]
- Ohene-Agyei, T.; Mowla, R.; Rahman, T.; Venter, H. Phytochemicals Increase the Antibacterial Activity of Antibiotics by Acting on a Drug Efflux Pump. Microbiologyopen 2014, 3, 885–896. [Google Scholar] [CrossRef]
- Lee, S.; Al Razqan, G.S.; Kwon, D.H. Antibacterial Activity of Epigallocatechin-3-Gallate (EGCG) and Its Synergism with β-Lactam Antibiotics Sensitizing Carbapenem-Associated Multidrug Resistant Clinical Isolates of Acinetobacter baumannii. Phytomedicine 2017, 24, 49–55. [Google Scholar] [CrossRef]
- Kanagaratnam, R.; Sheikh, R.; Alharbi, F.; Kwon, D.H. An Efflux Pump (MexAB-OprM) of Pseudomonas aeruginosa Is Associated with Antibacterial Activity of Epigallocatechin-3-Gallate (EGCG). Phytomedicine 2017, 36, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kurinčič, M.; Klančnik, A.; Smole Možina, S. Epigallocatechin Gallate as a Modulator of Campylobacter Resistance to Macrolide Antibiotics. Int. J. Antimicrob. Agents 2012, 40, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Kurinčič, M.; Klančnik, A.; Smole Možina, S. Effects of Efflux Pump Inhibitors on Erythromycin, Ciprofloxacin, and Tetracycline Resistance in Campylobacter Spp. Isolates. Microb. Drug Resist. 2012, 18, 492–501. [Google Scholar] [CrossRef]
- Fiedler, S.; Bender, J.K.; Klare, I.; Halbedel, S.; Grohmann, E.; Szewzyk, U.; Werner, G. Tigecycline Resistance in Clinical Isolates of Enterococcus faecium Is Mediated by an Upregulation of Plasmid-Encoded Tetracycline Determinants Tet (L) and Tet (M). J. Antimicrob. Chemother. 2016, 71, 871–881. [Google Scholar] [CrossRef]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-Gallate Enhances the Activity of Tetracycline in Staphylococci by Inhibiting Its Efflux from Bacterial Cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, G.; Wei, X.; Zhou, H.; Li, C.; Luo, Z.; Ou, D.; Yang, J.; Song, X. Antibacterial Activity and Mechanism of Luteolin Isolated from Lophatherum Gracile Brongn. against Multidrug-Resistant Escherichia coli. Front. Pharmacol. 2024, 15, 1430564. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant Phenolic Compounds as Ethidium Bromide Efflux Inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, C.; Su, H.; Zhang, Z.; Chen, M.; Wang, R.; Zhang, D.; Zhang, L.; Liu, M. Luteolin Increases Susceptibility to Macrolides by Inhibiting MsrA Efflux Pump in Trueperella pyogenes. Vet. Res. 2022, 53, 3. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Song, X.; Zhou, W.; Hong, W.; Tian, C.; Liu, Y.; Liu, M. Luteolin Showed a Resistance Elimination Effect on Gentamicin by Decreasing MATE MRNA Expression in Trueperella pyogenes. Microb. Drug Resist. 2018, 25, 619–626. [Google Scholar] [CrossRef]
- Terán, W.; Felipe, A.; Segura, A.; Rojas, A.; Ramos, J.-L.; Gallegos, M.-T. Antibiotic-Dependent Induction of Pseudomonas Putida DOT-T1E TtgABC Efflux Pump Is Mediated by the Drug Binding Repressor TtgR. Antimicrob. Agents Chemother. 2003, 47, 3067–3072. [Google Scholar] [CrossRef]
- Alguel, Y.; Meng, C.; Terán, W.; Krell, T.; Ramos, J.L.; Gallegos, M.-T.; Zhang, X. Crystal Structures of Multidrug Binding Protein TtgR in Complex with Antibiotics and Plant Antimicrobials. J. Mol. Biol. 2007, 369, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escamilla, A.M.; Fernandez-Ballester, G.; Morel, B.; Casares-Atienza, S.; Ramos, J.L. Molecular Binding Mechanism of TtgR Repressor to Antibiotics and Antimicrobials. PLoS ONE 2015, 10, e0138469. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas Thouarsii R.Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yao, Z.; Zhao, Y.; Shi, S.; Sun, Y.; Feng, L.; Zhou, C.; Zhang, X.; Cao, J.; Zhou, T. Naringenin Restores Colistin Activation against Colistin-Resistant Gram-Negative Bacteria in Vitro and in Vivo. Front. Microbiol. 2022, 13, 916587. [Google Scholar] [CrossRef]
- Oyedara, O.O.; Fadare, O.A.; Franco-Frías, E.; Heredia, N.; García, S. Computational Assessment of Phytochemicals of Medicinal Plants from Mexico as Potential Inhibitors of Salmonella enterica Efflux Pump AcrB Protein. J. Biomol. Struct. Dyn. 2023, 41, 1776–1789. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Magnani, M.; de Siqueira, S.; de Souza, E.L.; de Siqueira-Júnior, J.P. Fruit Flavonoids as Modulators of Norfloxacin Resistance in Staphylococcus aureus That Overexpresses NorA. LWT Food Sci. Technol. 2017, 85, 324–326. [Google Scholar] [CrossRef]
- Aggarwal, S.; Singh, D.V. Efflux Pumps and Biofilm Formation by Both Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Strains. Indian J. Exp. Biol. 2020, 58, 527–538. [Google Scholar]
- Medeiros Barreto, H.; Cerqueira Fontinele, F.; Pereira de Oliveira, A.; Arcanjo, D.D.R.; Cavalcanti dos Santos, B.H.; de Abreu, A.P.L.; Douglas Melo Coutinho, H.; Alves Carvalho da Silva, R.; Oliveira de Sousa, T.; Freire de Medeiros, M.d.G.; et al. Phytochemical Prospection and Modulation of Antibiotic Activity In Vitro by Lippia origanoides H.B.K. in Methicillin Resistant Staphylococcus aureus. Biomed Res. Int. 2014, 2014, 305610. [Google Scholar] [CrossRef]
- Johari, S.A. Efflux Inhibitory Activity of Flavonoids from Chromolaena Odorata against Selected Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates. African J. Microbiol. Res. 2012, 6, 5631–5635. [Google Scholar] [CrossRef]
- Biswas, S.S.; Roy, J.D. Phytocompounds as Potential Inhibitors of Mycobacterial Multidrug Efflux Pump Rv1258c: An in Silico Approach. AMB Express 2024, 14, 25. [Google Scholar] [CrossRef]
- Lucas Tenório, C.J.; Assunção Ferreira, M.R.; Lira Soares, L.A. Recent Advances on Preparative LC Approaches for Polyphenol Separation and Purification: Their Sources and Main Activities. Trends Food Sci. Technol. 2022, 128, 129–146. [Google Scholar] [CrossRef]
- Kotkar, G.D.; Shetgaonkar, A.D.; Tilve, S.G. Diversity of Chemical Skeletons. In New Horizons in Natural Compound Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 75–132. [Google Scholar]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application—A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Xie, C.-F.; Wang, X.-N.; Lou, H.-X. Stilbenoids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 1901–1949. ISBN 978-3-642-22144-6. [Google Scholar]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective Inhibition of MERS-CoV Infection by Resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Franciosoa, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Raj, P.; Zieroth, S.; Netticadan, T. An Overview of the Efficacy of Resveratrol in the Management of Ischemic Heart Disease. Ann. N. Y. Acad. Sci. 2015, 1348, 55–67. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, T.; Ma, Y.; Huang, S.; Lei, L.; Wen, A.; Ding, Y. Resveratrol: Multi-Targets Mechanism on Neurodegenerative Diseases Based on Network Pharmacology. Front. Pharmacol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Zhu, L.; Jiang, J.-G. Active Ingredients from Natural Botanicals in the Treatment of Obesity. Obes. Rev. 2014, 15, 957–967. [Google Scholar] [CrossRef]
- Santos, M.; Santos, R.; Ferreira, S. Resveratrol, A Novel Inhibitor of the NorA Efflux Pump and Resistance Modulator in Staphylococcus aureus. In Proceedings of the 2nd International Electronic Conference on Antibiotics—Drugs for Superbugs: Antibiotic Discovery, Modes of Action and Mechanisms of Resistance, Online, 15–30 June 2022; MDPI: Basel, Switzerland, 2022; p. 16. [Google Scholar]
- Seukep, J.A.; Sandjo, L.P.; Ngadjui, B.T.; Kuete, V. Antibacterial and Antibiotic-Resistance Modifying Activity of the Extracts and Compounds from Nauclea Pobeguinii against Gram-Negative Multi-Drug Resistant Phenotypes. BMC Complement. Altern. Med. 2016, 16, 193. [Google Scholar] [CrossRef]
- Abdulkareem, M.A.; Mubarak, K.I. Detection of Antibiotics Resistance Andefflux Pumps Production in Clinical Acinetobacter baumannii Isolates. J. Appl. Nat. Sci. 2023, 15, 1347–1353. [Google Scholar] [CrossRef]
- Abdulkareem, M.A.; Mubarak, K.I. Activity of Ciprofloxacin and Resveratrol On Expression Of AdeI J Genes In Clinical Acinetobacter baumannii Isolates. Acad. Sci. J. 2024, 2, 139–160. [Google Scholar] [CrossRef]
- Cheng, P.; Sun, Y.; Wang, B.; Liang, S.; Yang, Y.; Gui, S.; Zhang, K.; Qu, S.; Li, L. Mechanism of Synergistic Action of Colistin with Resveratrol and Baicalin against Mcr-1-Positive Escherichia coli. Biomed. Pharmacother. 2024, 180, 117487. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lim, Y.-H. Resveratrol Controls Escherichia coli Growth by Inhibiting the AcrAB-TolC Efflux Pump. FEMS Microbiol. Lett. 2019, 366, fnz030. [Google Scholar] [CrossRef]
- Singkham-in, U.; Higgins, P.G.; Wannigama, D.L.; Hongsing, P.; Chatsuwan, T. Rescued Chlorhexidine Activity by Resveratrol against Carbapenem-Resistant Acinetobacter baumannii via down-Regulation of AdeB Efflux Pump. PLoS ONE 2020, 15, e0243082. [Google Scholar] [CrossRef]
- Migliaccio, A.; Esposito, E.P.; Bagattini, M.; Berisio, R.; Triassi, M.; De Gregorio, E.; Zarrilli, R. Inhibition of AdeB, AceI, and AmvA Efflux Pumps Restores Chlorhexidine and Benzalkonium Susceptibility in Acinetobacter baumannii ATCC 19606. Front. Microbiol. 2022, 12, 790263. [Google Scholar] [CrossRef]
- Migliaccio, A.; Stabile, M.; Bagattini, M.; Triassi, M.; Berisio, R.; De Gregorio, E.; Zarrilli, R. Resveratrol Reverts Tolerance and Restores Susceptibility to Chlorhexidine and Benzalkonium in Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts. Antibiotics 2022, 11, 961. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health Effects, Sources, Utilization and Safety of Tannins: A Critical Review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Tannins Characterisation in New and Historic Vegetable Tanned Leathers Fibres by Spot Tests. J. Cult. Herit. 2011, 12, 149–156. [Google Scholar] [CrossRef]
- D’Mello, J.F.; Duffus, C.M.; Duffus, J.H. (Eds.) Toxic Substances in Crop Plants. In Toxic Substances in Crop Plants; The Royal Society of Chemistry: Edinburgh, Scotland, 1991; pp. 180–201. [Google Scholar]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Bacon, J.R.; Rhodes, M.J.C. Binding Affinity of Hydrolyzable Tannins to Parotid Saliva and to Proline-Rich Proteins Derived from It. J. Agric. Food Chem. 2000, 48, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. Wine Tannins, Saliva Proteins and Membrane Lipids. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183670. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Jourdes, M.; Pouységu, L.; Deffieux, D.; Teissedre, P.-L.; Quideau, S. Hydrolyzable Tannins: Gallotannins and Ellagitannins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 1975–2010. ISBN 978-3-642-22144-6. [Google Scholar]
- Porter, L.J. Structure and Chemical Properties of the Condensed Tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer US: Boston, MA, USA, 1992; pp. 245–258. ISBN 978-1-4615-3476-1. [Google Scholar]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Qualitative Analysis for Evaluation of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–149. [Google Scholar]
- Cabezas, R.; Fidel Avila, M.; Torrente, D.; Gonzalez, J.; Santos El-Bachá, R.; Guedes, R.; Barreto, G.E. Natural Antioxidants in Dementia. In Diet and Nutrition in Dementia and Cognitive Decline; Elsevier: Amsterdam, The Netherlands, 2015; pp. 827–836. [Google Scholar]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Costa, M.d.S.; Menezes, I.R.A.; de Matos, Y.M.L.S.; Calixto-Júnior, J.T.; Pereira, P.S.; Siqueira-Junior, J.P.; Leal-Balbino, T.C.; et al. Tannic Acid Affects the Phenotype of Staphylococcus aureus Resistant to Tetracycline and Erythromycin by Inhibition of Efflux Pumps. Bioorg. Chem. 2017, 74, 197–200. [Google Scholar] [CrossRef]
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 474–475. [Google Scholar]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic Acid: A Green Crosslinker for Biopolymer-Based Food Packaging Films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Nejad, F.S.; Karami, H.; Darvish, M. Triggering of Endoplasmic Reticulum Stress by Tannic Acid Inhibits the Proliferation and Migration of Colorectal Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 2705. [Google Scholar]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Tintino, S.R.; Wilairatana, P.; de Souza, V.C.A.; da Silva, J.M.A.; Pereira, P.S.; de Morais Oliveira-Tintino, C.D.; de Matos, Y.M.L.S.; Júnior, J.T.C.; de Queiroz Balbino, V.; Siqueira-Junior, J.P.; et al. Inhibition of the NorA Gene Expression and the NorA Efflux Pump by the Tannic Acid. Sci. Rep. 2023, 13, 17394. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Silva, R.L.P.; Costa, M.d.S.; Menezes, I.R.A.; Calixto-Júnior, J.T.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Leal-Balbino, T.C.; et al. Evaluation of the Tannic Acid Inhibitory Effect against the NorA Efflux Pump of Staphylococcus aureus. Microb. Pathog. 2016, 97, 9–13. [Google Scholar] [CrossRef]
- Myint, K.B.; Sing, L.C.; Wei, Z. Tannic Acid as Phytochemical Potentiator for Antibiotic Resistance Adaptation. APCBEE Procedia 2013, 7, 175–181. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Cirino, I.C.d.S.; Falcão-Silva, V.d.S.; Magnani, M.; de Souza, E.L.; Siqueira-Júnior, J.P. Tannic Acid as a Potential Modulator of Norfloxacin Resistance in Staphylococcus aureus Overexpressing norA. Chemotherapy 2016, 61, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; Villanueva, I.; Voravuthikunchai, S.P.; Davies, J. Enhancing Antibiotic Activity: A Strategy to Control Acinetobacter Infections. J. Antimicrob. Chemother. 2009, 64, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-F.; Lin, Y.-Y.; Lan, C.-Y. The Role of the Two-Component System BaeSR in Disposing Chemicals through Regulating Transporter Systems in Acinetobacter baumannii. PLoS ONE 2015, 10, e0132843. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Chandrasekara, A. Phenolic Acids. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 535–545. [Google Scholar]
- Ratnavathi, C.V. Grain Structure, Quality, and Nutrition. In Breeding Sorghum for Diverse End Uses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–207. [Google Scholar]
- Peng, S.; Wang, Y.; Sun, Z.; Zhao, L.; Huang, Y.; Fu, X.; Luo, R.; Xue, J.; Yang, S.; Ling, L.; et al. Nanoparticles Loaded with Pharmacologically Active Plant-Derived Natural Products: Biomedical Applications and Toxicity. Colloids Surf. B Biointerfaces 2023, 225, 113214. [Google Scholar] [CrossRef]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. White Biotechnology in Cosmetics. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar]
- De La Rosa-Esteban, K.; Sepúlveda, L.; Chávez-González, M.; Torres-León, C.; Estrada-Gil, L.; Aguilar, C.; Ascacio-Valdés, J. Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast. Fermentation 2023, 9, 723. [Google Scholar] [CrossRef]
- Athiappan, M.; Srinivasan, S.; Anandan, R.; Rajaram, J. Novel Process of Ellagic Acid Synthesis from Waste Generated from Mango Pulp Processing Industries. In Emerging Technologies in Environmental Bioremediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–454. [Google Scholar]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and Apoptosis-Inducing Activities of Ellagic Acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Norouzalinia, F.; Asadpour, L.; Mokhtary, M. Anti-Microbial, Anti-Biofilm, and Efflux Pump Inhibitory Effects of Ellagic Acid–Bonded Magnetic Nanoparticles against Escherichia coli Isolates. Int. Microbiol. 2024, 28, 563–573. [Google Scholar] [CrossRef]
- Macêdo, N.S.; Barbosa, C.R.d.S.; Bezerra, A.H.; Silveira, Z.d.S.; da Silva, L.; Coutinho, H.D.M.; Dashti, S.; Kim, B.; da Cunha, F.A.B.; da Silva, M.V. Evaluation of Ellagic Acid and Gallic Acid as Efflux Pump Inhibitors in Strains of Staphylococcus aureus. Biol. Open 2022, 11, bio059434. [Google Scholar] [CrossRef]
- Nirmal, C.R.; Rajadas, S.E.; Balasubramanian, M.; Mohanvel, S.K.; Aathi, M.S.; Munishankar, S.; Chilamakuru, N.B.; Thiruvenkadam, K.; Pandiya Raj, A.K.; Paraman, R.; et al. Myoinositol and Methyl Stearate Increases Rifampicin Susceptibility among Drug-resistant Mycobacterium Tuberculosis Expressing Rv1819c. Chem. Biol. Drug Des. 2023, 101, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Raj Dwivedi, G.; Khwaja, S.; Singh Negi, A.; Panda, S.S.; Swaroop Sanket, A.; Pati, S.; Chand Gupta, A.; Bawankule, D.U.; Chanda, D.; Kant, R.; et al. Design, Synthesis and Drug Resistance Reversal Potential of Novel Curcumin Mimics Van D. Bioorg. Chem. 2021, 106, 104454. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.; Santos, H.S.; Coutinho, H.D.M.; Bandeira, P.N.; da Silva, P.T.; Freitas, T.S.; Rocha, J.E.; Xavier, J.C.; Campina, F.F.; Barbosa, C.R.S.; et al. Spectroscopic Characterization and Efflux Pump Modulation of a Thiophene Curcumin Derivative. J. Mol. Struct. 2020, 1215, 128291. [Google Scholar] [CrossRef]
- Park, K.; Choo, H.; Kim, M.K.; Chong, Y. Quercetin 7-O-Glutamate Potentiates Staphylococcus aureus to Fluoroquinolone Antibiotics. Bull. Korean Chem. Soc. 2016, 37, 1515–1517. [Google Scholar] [CrossRef]
- Lee, T.; Lee, S.; Kim, M.K.; Ahn, J.H.; Park, J.S.; Seo, H.W.; Park, K.-H.; Chong, Y. 3-O-Substituted Quercetin: An Antibiotic-Potentiating Agent against Multidrug-Resistant Gram-Negative Enterobacteriaceae through Simultaneous Inhibition of Efflux Pump and Broad-Spectrum Carbapenemases. ACS Infect. Dis. 2024, 10, 1624–1643. [Google Scholar] [CrossRef]
- Xiao, Z.-P.; Wang, X.-D.; Wang, P.-F.; Zhou, Y.; Zhang, J.-W.; Zhang, L.; Zhou, J.; Zhou, S.-S.; Ouyang, H.; Lin, X.-Y.; et al. Design, Synthesis, and Evaluation of Novel Fluoroquinolone–Flavonoid Hybrids as Potent Antibiotics against Drug-Resistant Microorganisms. Eur. J. Med. Chem. 2014, 80, 92–100. [Google Scholar] [CrossRef]
- Verma, P.; Tiwari, M.; Tiwari, V. Potentiate the Activity of Current Antibiotics by Naringin Dihydrochalcone Targeting the AdeABC Efflux Pump of Multidrug-Resistant Acinetobacter baumannii. Int. J. Biol. Macromol. 2022, 217, 592–605. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Andreani, T.; Cheng, R.; Elbadri, K.; Ferro, C.; Menezes, T.; dos Santos, M.R.; Pereira, C.M.; Santos, H.A. Natural Compounds-Based Nanomedicines for Cancer Treatment: Future Directions and Challenges. Drug Deliv. Transl. Res. 2024, 14, 2845–2916. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Yasbolaghi Sharahi, J.; Taati Moghadam, M. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef]
- Rahbar Takrami, S.; Ranji, N.; Sadeghizadeh, M. Antibacterial Effects of Curcumin Encapsulated in Nanoparticles on Clinical Isolates of Pseudomonas aeruginosa through Downregulation of Efflux Pumps. Mol. Biol. Rep. 2019, 46, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Huang, Y.; Jin, Q.; Ji, J. Inhibiting Quorum Sensing by Active Targeted PH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections. ACS Nano 2023, 17, 10019–10032. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, H.; Yadav, R.P. Boosting Gentamicin Activity Against Pseudomonas aeruginosa Using Nano-Formulation of Curcumin. Bionanoscience 2024, 14, 4606–4623. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, X.; Ruan, H.; Yang, J.; Wei, W.; Wu, Y.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Curcumin-Stabilized Silver Nanoparticles Encapsulated in Biocompatible Electrospun Nanofibrous Scaffold for Sustained Eradication of Drug-Resistant Bacteria. J. Hazard. Mater. 2023, 452, 131290. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh, H.; Mirpour, M.; Zamani, H.; Rasti, B. Effect of Samarium Oxide Nanoparticles Fabricated by Curcumin on Efflux Pump and Virulence Genes Expression in MDR Pseudomonas aeruginosa and Staphylococcus aureus. J. Clust. Sci. 2023, 34, 1227–1235. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In Vivo and In Vitro Antimicrobial Activity of Biogenic Silver Nanoparticles against Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- Srichaiyapol, O.; Thammawithan, S.; Siritongsuk, P.; Nasompag, S.; Daduang, S.; Klaynongsruang, S.; Kulchat, S.; Patramanon, R. Tannic Acid-Stabilized Silver Nanoparticles Used in Biomedical Application as an Effective Antimelioidosis and Prolonged Efflux Pump Inhibitor against Melioidosis Causative Pathogen. Molecules 2021, 26, 1004. [Google Scholar] [CrossRef]
- Elhassan, E.; Omolo, C.A.; Gafar, M.A.; Kiruri, L.W.; Ibrahim, U.H.; Ismail, E.A.; Devnarain, N.; Govender, T. Disease-Inspired Design of Biomimetic Tannic Acid–Based Hybrid Nanocarriers for Enhancing the Treatment of Bacterial-Induced Sepsis. Mol. Pharm. 2024, 21, 4924–4946. [Google Scholar] [CrossRef]
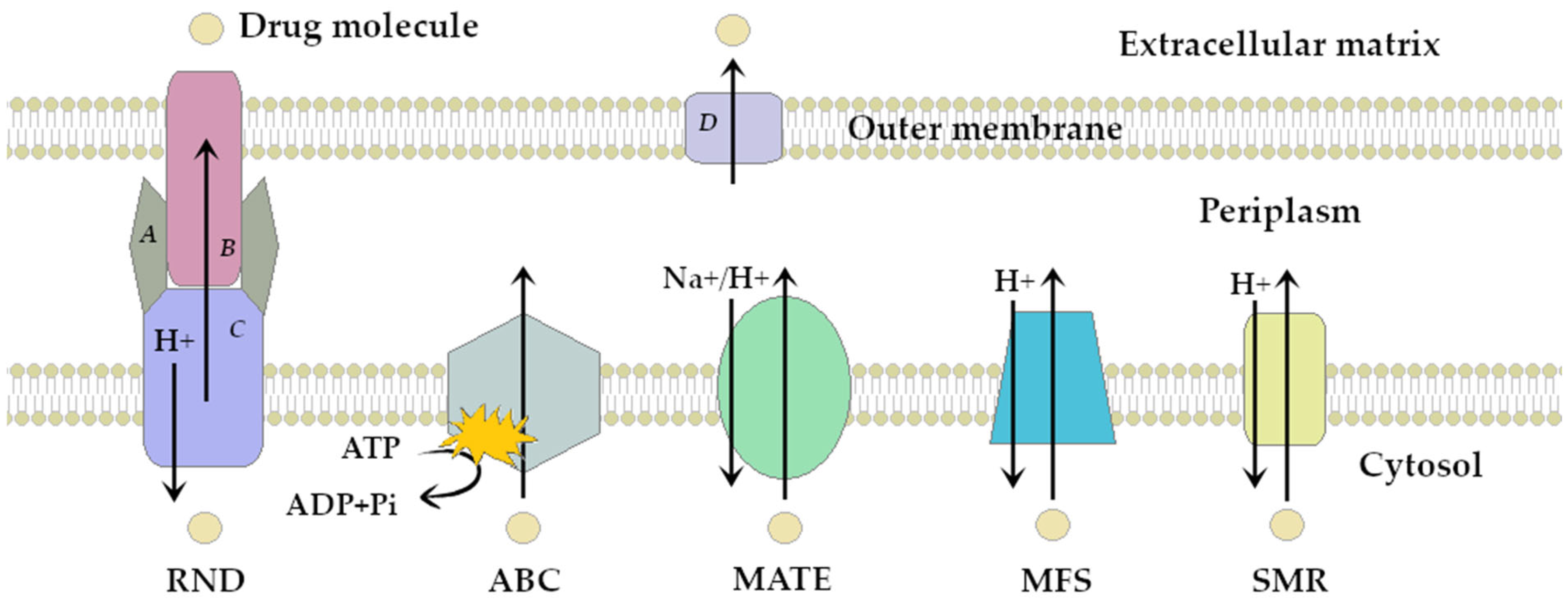
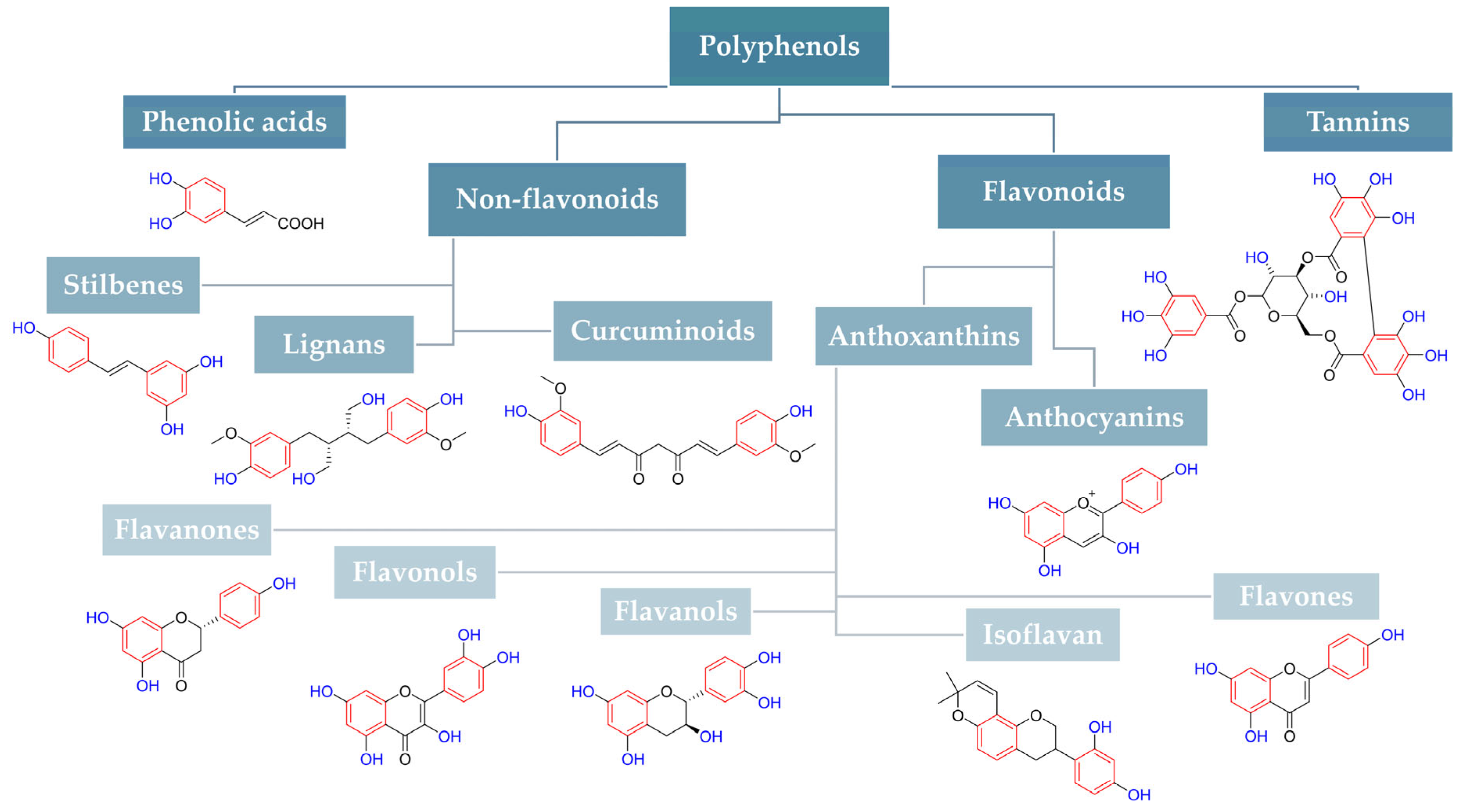

| Efflux Pump Superfamily | Pumps Examples | Main Producers | Antibiotic Resistance Profile | References |
|---|---|---|---|---|
| RND (Resistance- Nodulation-Cell Division) | AcrAB-TolC, AdeABC, MexAB-OprM, MexCD-OprJ, MmpL5, MtrCDE, OqxAB, SmeABC | M. tuberculosis GNB: N. gonorrhoeae, Enterobacteriaceae, N-F: P. aeruginosa, A. baumannii, S. maltophilia | AMG, CPL, FLQ, LNC, MCL, PLX, RIF, SLF, TET, BL | [17,18,19,20,21,22,23] |
| MATE (Multidrug and toxic compound extrusion) | AbeM, DinF, EmmdR, FepA, MepA, NorM, KetM, PmpM, Rv2836c | M. tuberculosis GNB: N. gonorrhoeae, K. pneumoniae GPB: S. aureus, L. monocytogenes, E. faecalis N-F: A. baumannii, P. aeruginosa | AMG, CPL, FLQ, TGC, TRM | [17,24,25,26,27,28] |
| ABC (ATP-binding cassette) | AbcA, EfrAB, LmrA, macAB-TolC, MsrA, PatA/B, Rv1218c | M. tuberculosis, GPB: E. faecalis, S. pneumoniae, S. aureus GNB: Enterobacteriaceae | AMG, CPL, FLQ, LNC, MCL, RIF, TET | [17,29,30,31,32,33,34] |
| MFS (Major facilitator superfamily) | AbaQ, EmrAB-TolC, Lde, MdeA, MdfA, Mef, NorA-D, QepA, Rv1258c, TetK | M. tuberculosis GPB: S. aureus, S. pneumoniae, L. monocytogenes, GNB: Enterobacteriaceae N-F: P. aeruginosa, A. baumannii | AMG, CPL, COT, FLQ, LNC, MCL, PLX, RIF, SLF, TET, TGC, BL | [17,35,36,37,38,39] |
| SMR (Small multidrug resistance family) | AbeS, EmrE, KpnEF, Mmr, QacC-J, SepA | M. tuberculosis GPB: S. aureus, E. faecalis GNB: Enterobacteriaceae N-F: P. aeruginosa, A. baumannii | AMG, MCL, PLX, RIF, SLF, TET, BL | [8,17,40,41,42] |
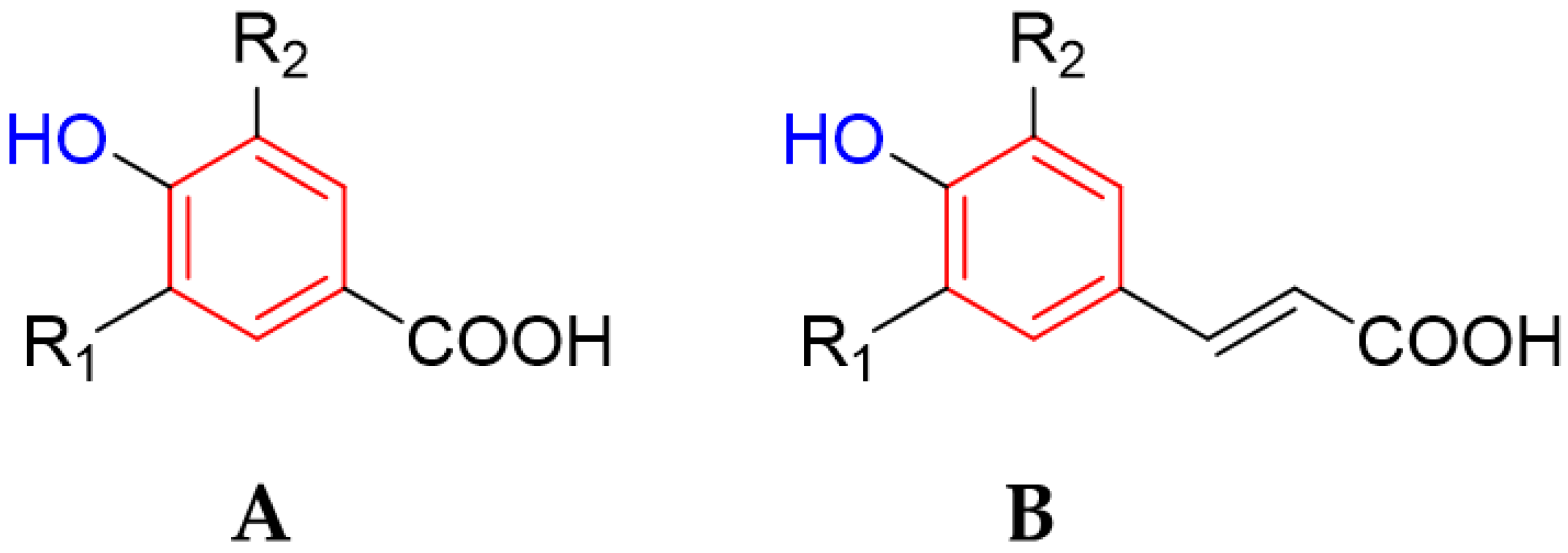
| Structure and Name | Flavonoid Group | Plant Cultivar | Biological Activity | References |
|---|---|---|---|---|
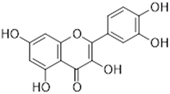 Quercetin | Flavonol | Moraceae, Theaceae, Apiaceae, Brassicaceae, Asteraceae, Rosaceae | anti-inflammatory anti-microbial anti-neoplasm gastroprotective | [95,96,97,98,99] |
 Naringenin | Flavanone | Main source: Citrus aurantium, Citrus clementia, Citrus reticulata | antidiabetic anti-estrogenic anti-inflammatory anti-neoplasm antioxidant hypolipidemic neuroprotective | [100,101,102,103,104] |
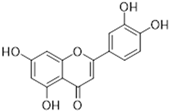 Luteolin | Flavone | Various fruits and vegetables e.g., Apium graveolens L., Chrysanthemum L. and Daucus carota Hoffm. | antidiabetic anti-inflammatory anti-microbial anti-neoplasm antioxidant cardioprotective | [105,106,107,108,109,110] |
 Epigallocatechin-3-gallate | Flavan-3-ol | Main source: Camellia sinensis (L.) Kuntze | anti-inflammatory anti-microbial anti-neoplasm antioxidant anti-viral neuroprotective | [111,112,113,114,115,116,117,118,119,120,121,122] |
| Name of Derivative | Precursor Polyphenol | Pathogens | Mechanism of Antimicrobial Activity | References |
|---|---|---|---|---|
| 1,3-Bis-(E/E)-[3-(3-Methoxy,4-hydroxyphenyl)- 1-oxo-2-en-yl]- benzene | CU | P. aeruginosa (MBL positive) | Synergy with TET | [209] |
| ↓ expression of mexA, mexB and oprM genes (separately and combined with TET) | ||||
| Direct inhibition of pumps subunits: MexA, MexB, OprM | ||||
| (1E,4E)-1,5-Di (thiophen-2-yl) penta-1,4-dien-3-one | S. aureus 1199B and K2068 | Synergy with NOR | [210] | |
| Direct inhibition of pumps: NorA (1199B) and MepA (K2068) | ||||
| quercetin 7-O-glutamate | QR | S. aureus | Inhibition of NorA pump (superior to QR and comparable to reserpine) | [211] |
| Potentiating of antibiotics activity for: TET (2-fold MIC reduction), CYP and GEN (4-fold) | ||||
| 3-O-substituted quercetin | CRE | Direct inhibiton of AcrB pump subunit | [212] | |
| Inhibition of MBL e.g., NDM-1 | ||||
| Synergy with MER (>4-fold MIC reduction) | ||||
| Naringenin- ethylidene- fluoroquinolone hybrid | NG | E. coli Bacillus subtilis S. aureus | Synergy with CYP (8–88-fold MIC reduction) | [213] |
| Enhancing bacterial DNA-gyrase inhibition | ||||
| Reduction in CYP efflux ratio in tested strains | ||||
| Naringin dihydrochalcone | NG | CRAB | Direct inhibition of AdeB protein | [214] |
| Inhibition of MER efflux |
| Polyphenol Precursor | Nanotechnology Application | Pathogens | Mechanism of Antibacterial Action | References |
|---|---|---|---|---|
| CU | Polyphenol-based NPs | MDR P. aeruginosa and PA01 strain | ↓ expression of mexB, mexT, mexD genes | [217] |
| ↑ expression of nfxB gene (negative regulator of MexCD-OprJ) | ||||
| CYP-NS P. aeruginosa | Concentration-dependent enhancing activity of CYP by NPs | [218] | ||
| Increased accumulation of CYP in pathogen cells | ||||
| ↓ expression of mexX and oprM genes (mexXY-oprM and mexAB-oprM pumps) | ||||
| CU-based NPs combined with 2,3-dimethyl maleic anhydride | MDR P. aeruginosa | ↓ expression of mexA-F and oprM genes | [219] | |
| Synergy with TOB | ||||
| Polyphenol-based NPs | P. aeruginosa | Synergy with GEN (combination with CU formed ZOI comparable to combination with verapamil) | [220] | |
| CU-stabilized silver NPs (green synthesis) | S. aureus MRSA | ↓ expression of sdrM gene | [221] | |
| CU-synthesized samar oxide-based NPs (green synthesis) | MDR S. aureus | ↓ expression of norA-B genes | [222] | |
| MDR P. aeruginosa | ↓ expression of mexA-B genes | |||
| NG | naringin dihydrochalcone based silver NPs | CRAB | Generating of ROS and RNS | [214] |
| Increasing susceptibility to MER via disrupting transmembrane proton gradient (malfunction of RND type pumps e.g., AdeABC) | ||||
| QR | QR-stabilized silver NPs (green synthesis) | S. aureus | Enhanced EtBr accumulation | [223] |
| ↓ expression of norA-C genes | ||||
| TA | TA-stabilized silver NPs (green synthesis) | Burkoholderia pseudomallei MDR Enterobacter cloacae | ↑ permeability of cell membrane via silver NPs | [224] |
| Interference with acrAB-TolC efflux pump | ||||
| TA-based hybrid NPs | S. aureus MRSA, P. aeruginosa | NorA pump inhibition via metal ions chelating | [225] | |
| Inhibition of QS | ||||
| EA | Polyphenol-bonded magnetic Fe3O4 NPs | ESβL E. coli | Eradication both planktonic and biofilm form of pathogen | [206] |
| ↓ expression of acrB and tolC genes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Viscardi, S.; Niezgódka, P.; Szewczyk, W.; Wińska, K. The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 4030. https://doi.org/10.3390/ijms26094030
Duda-Madej A, Viscardi S, Niezgódka P, Szewczyk W, Wińska K. The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. International Journal of Molecular Sciences. 2025; 26(9):4030. https://doi.org/10.3390/ijms26094030
Chicago/Turabian StyleDuda-Madej, Anna, Szymon Viscardi, Piotr Niezgódka, Wiktoria Szewczyk, and Katarzyna Wińska. 2025. "The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance" International Journal of Molecular Sciences 26, no. 9: 4030. https://doi.org/10.3390/ijms26094030
APA StyleDuda-Madej, A., Viscardi, S., Niezgódka, P., Szewczyk, W., & Wińska, K. (2025). The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. International Journal of Molecular Sciences, 26(9), 4030. https://doi.org/10.3390/ijms26094030







