Ferroptosis in Cancer: Mechanism and Therapeutic Potential
Abstract
1. Introduction
2. Types of Drug-Induced Cell Death
2.1. Apoptosis
2.2. Autophagy
2.3. Necrosis
2.4. Necroptosis
2.5. Ferroptosis
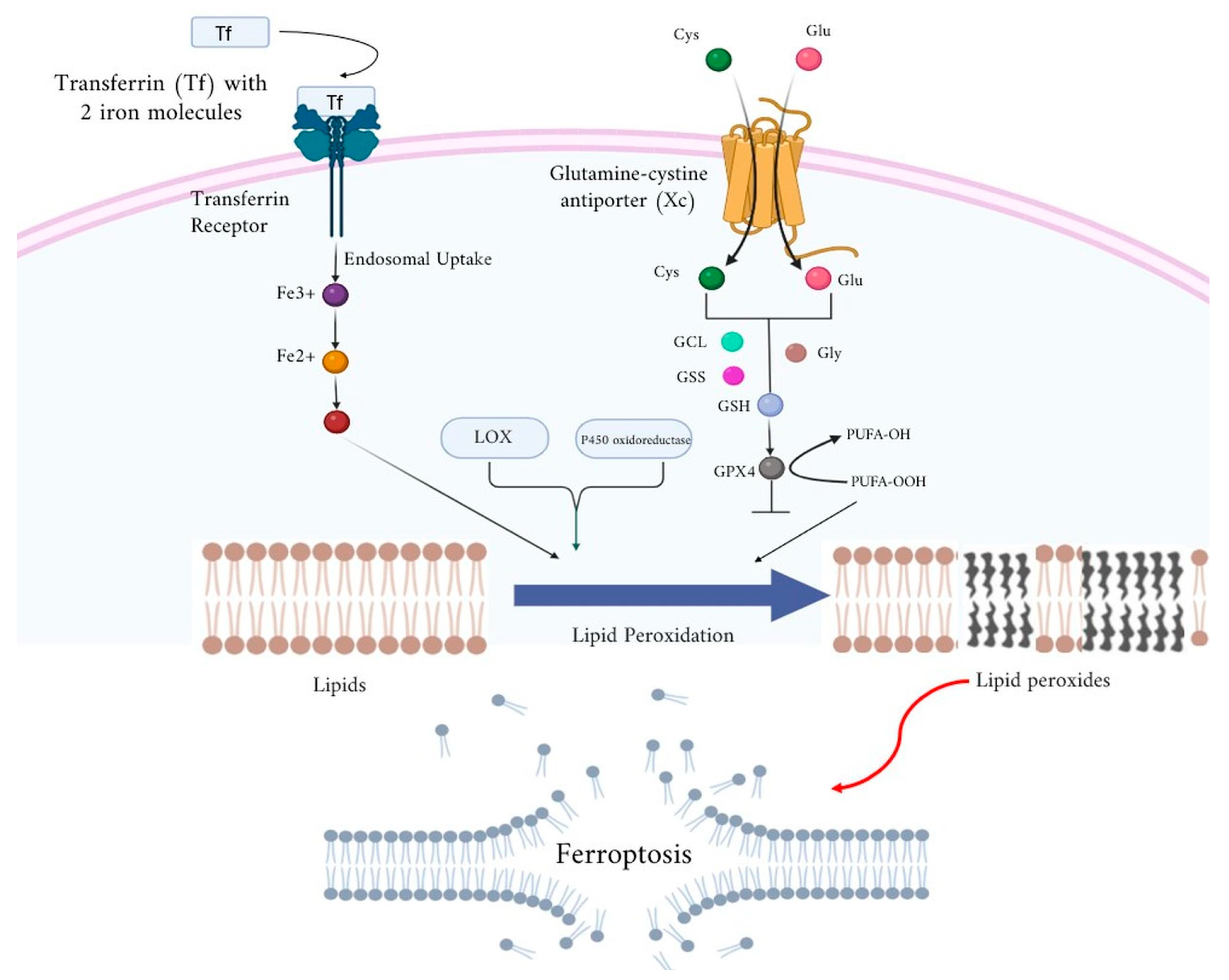
2.6. Ferroptosis: Molecular Mechanism
3. Types of Ferroptosis
3.1. Ferroptosis: Detection, Estimation, and Biomarkers of Ferroptosis
3.2. Ferroptosis: Preclinical Evidence
3.3. Ferroptosis: Clinical Evidence
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Wang, Z.; Cheng, L. Tumor microenvironment heterogeneity: An important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer. Mol. Cancer 2024, 23, 261. [Google Scholar]
- Yang, S.; Hu, C.; Chen, X.; Tang, Y.; Li, J.; Yang, H.; Yang, Y.; Ying, B.; Xiao, X.; Li, S.Z.; et al. Crosstalk between metabolism and cell death in tumorigenesis. Mol. Cancer 2024, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Abdul Razak, S.R.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a potential target for cancer therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Parlani, M.; Jorgez, C.; Friedl, P. Plasticity of cancer invasion and energy metabolism. Trends Cell Biol. 2023, 33, 388–402. [Google Scholar] [CrossRef]
- Mao, C.; Wang, M.; Zhuang, L.; Gan, B. Metabolic cell death in cancer: Ferroptosis, cuproptosis, disulfidptosis, and beyond. Protein Cell 2024, 15, 642–660. [Google Scholar] [CrossRef] [PubMed]
- NaveenKumar, S.K.; SharathBabu, B.N.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S.; Mugesh, G. The role of reactive oxygen species and ferroptosis in heme-mediated activation of human platelets. ACS Chem. Biol. 2018, 13, 1996–2002. [Google Scholar] [CrossRef]
- Lyamzaev, K.G.; Panteleeva, A.A.; Simonyan, R.A.; Avetisyan, A.V.; Chernyak, B.V. Mitochondrial lipid peroxidation is responsible for ferroptosis. Cells 2023, 12, 611. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.L. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med. Hypotheses 2017, 101, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar]
- Morales, M.; Xue, X. Targeting Iron Metabolism in Cancer Therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting Cell Death Pathways for Cancer Therapy: Recent Developments in Necroptosis, Pyroptosis, Ferroptosis, and Cuproptosis Research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in Cancer Therapy: A Novel Approach to Reversing Drug Resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Pranzini, E.; Pardella, E.; Paoli, P.; Fendt, S.M.; Taddei, M.L. Metabolic Reprogramming in Anticancer Drug Resistance: A Focus on Amino Acids. Trends Cancer 2021, 7, 682–699. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Rabbani, S.A.; El-Tanani, Y.; Matalka, I.I. Metabolic Vulnerabilities in Cancer: A New Therapeutic Strategy. Crit. Rev. Oncol. Hematol. 2024, 201, 104438. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting Ferroptosis Opens New Avenues for the Development of Novel Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Yang, Y.; Gu, C. Iron Metabolism and Its Contribution to Cancer. Int. J. Oncol. 2019, 54, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Kazan, H.H.; Urfali-Mamatoglu, C.; Gunduz, U. Iron Metabolism and Drug Resistance in Cancer. Biometals 2017, 30, 629–641. [Google Scholar] [CrossRef]
- Kawahara, I.; Yoshino, H.; Fukumoto, W.; Arima, J.; Saito, S.; Li, G.; Fukuda, I.; Mitsuke, A.; Sakaguchi, T.; Inoguchi, S.; et al. Targeting Metabolic Reprogramming to Overcome Drug Resistance in Advanced Bladder Cancer: Insights from Gemcitabine- and Cisplatin-Resistant Models. Mol. Oncol. 2024, 18, 2196–2211. [Google Scholar] [CrossRef]
- Burz, C.; Berindan-Neagoe, I.; Balacescu, O.; Irimie, A. Apoptosis in Cancer: Key Molecular Signaling Pathways and Therapy Targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, G. Apoptosis Genes and Resistance to Cancer Therapy: What Does the Experimental and Clinical Data Tell Us? Cancer Biol. Ther. 2003, 2, 477–490. [Google Scholar] [CrossRef]
- Bhat, G.R.; Sethi, I.; Sadida, H.Q.; Rah, B.; Mir, R.; Algehainy, N.; Albalawi, I.A.; Masoodi, T.; Subbaraj, G.K.; Jamal, F.; et al. Cancer Cell Plasticity: From Cellular, Molecular, and Genetic Mechanisms to Tumor Heterogeneity and Drug Resistance. Cancer Metastasis Rev. 2024, 43, 197–228. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of Cell Death Signaling Pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Ande, S.R.; Panigrahi, S.; Paranjothy, T.; Weglarczyk, K.; Zuse, A.; Eshraghi, M.; Manda, K.D.; Wiechec, E.; Los, M. Cell Survival, Cell Death and Cell Cycle Pathways Are Interconnected: Implications for Cancer Therapy. Drug Resist. Updates 2007, 10, 13–29. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiao, D. Necroptosis, Tumor Necrosis and Tumorigenesis. Cell Stress 2019, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and Cancer—Role and Therapeutic Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and Autophagy-Related Pathways in Cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- McGee, M.M. Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediat. Inflamm. 2015, 2015, 146282. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Agarwal, S.; Singh, A.; Bajaj, A.; Dasgupta, U. Insights into molecular mechanisms of chemotherapy resistance in cancer. Transl. Oncol. 2024, 42, 101901. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, E.V.; Yapryntseva, M.A.; Pervushin, N.V.; Tsvetcov, R.I.; Zhivotovsky, B.; Kopeina, G.S. Cancer Drug Resistance: Targeting Proliferation or Programmed Cell Death. Cells 2024, 13, 388. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis: Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, F.; Li, C.Y.; Xiong, Y.; Liu, X. Caspase-3 promotes oncogene-induced malignant transformation via EndoG-dependent Src-STAT3 phosphorylation. Cell Death Dis. 2024, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar]
- Zhang, J.; Lou, X.; Jin, L.; Zhou, R.; Liu, S.; Xu, N.; Liao, D.J. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: Clearance of a few misconceptions. Oncoscience 2014, 1, 407–422. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis—Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Shahar, N.; Larisch, S. Inhibiting the inhibitors: Targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resist. Updates 2020, 52, 100712. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Ryter, S.W.; Cloonan, S.M.; Choi, A.M. Autophagy: A critical regulator of cellular metabolism and homeostasis. Mol. Cells 2013, 36, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef]
- Chang, H.; Zou, Z. Targeting autophagy to overcome drug resistance: Further developments. J. Hematol. Oncol. 2020, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR Pathways in Cancer and Autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Mukhopadhyay, S.; Sinha, N.; Das, D.N.; Panda, P.K.; Patra, S.K.; Maiti, T.K.; Mandal, M.; Dent, P.; Wang, X.Y.; et al. Autophagy: Cancer’s Friend or Foe? Adv. Cancer Res. 2013, 118, 61–95. [Google Scholar]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Altman, B.J.; Rathmell, J.C. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a008763. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, A.; Song, Y. Propofol Triggers Cell Death in Lung Cancer Cells by Increasing PANX1 Expression, Activating the Mitochondrial Cell Death Pathway, and Enhancing ROS Levels. Disc Med. 2024, 36, 2231–2243. [Google Scholar] [CrossRef]
- Shen, S.; Shao, Y.; Li, C. Different types of cell death and their shift in shaping disease. Cell Death Discov. 2023, 9, 284. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Brault, M.; Olsen, T.M.; Martinez, J.; Stetson, D.B.; Oberst, A. Intracellular Nucleic Acid Sensing Triggers Necroptosis through Synergistic Type I IFN and TNF Signaling. J. Immunol. 2018, 200, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kwon, H.K.; Lee, J.H.; Gui, X.; Achek, A.; Kim, J.H.; Choi, S. Doxorubicin-induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Sci. Rep. 2015, 5, 15798. [Google Scholar] [CrossRef]
- Garay, R.P.; Viens, P.; Bauer, J.; Normier, G.; Bardou, M.; Jeannin, J.F.; Chiavaroli, C. Cancer relapse under chemotherapy: Why TLR2/4 receptor agonists can help. Eur. J. Pharmacol. 2007, 563, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The Mechanisms of Graphene-Based Materials-Induced Programmed Cell Death: A Review of Apoptosis, Autophagy, and Programmed Necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef] [PubMed]
- Ploumi, C.; Papandreou, M.E.; Tavernarakis, N. The Complex Interplay between Autophagy and Cell Death Pathways. Biochem. J. 2022, 479, 75–90. [Google Scholar] [CrossRef]
- Christofferson, D.E.; Yuan, J. Necroptosis as an Alternative Form of Programmed Cell Death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef]
- Bansal, N.; Sciabola, S.; Bhisetti, G. Understanding Allosteric Interactions in hMLKL Protein That Modulate Necroptosis and Its Inhibition. Sci. Rep. 2019, 9, 16853. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell Death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between Apoptosis, Necrosis and Autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Chen, F.; Zhang, Z.; Shi, D.; Zhong, B.; Lv, X.; Tucker, A.B.; Fan, J.; Li, A.J.; Qin, K.; et al. Ferroptosis as a Novel Form of Regulated Cell Death: Implications in the Pathogenesis, Oncometabolism and Treatment of Human Cancer. Genes Dis. 2020, 9, 347–357. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Tang, J.; Qiu, X.; Nie, X.; Ou, S.; Wu, G.; Zhang, R.; Zhu, J. Ferroptosis: Emerging Mechanisms, Biological Function, and Therapeutic Potential in Cancer and Inflammation. Cell Death Discov. 2024, 10, 45. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative Stress Induces Mitochondrial Iron Overload and Ferroptotic Cell Death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.K.; Pereira, M.; Rajavelu, I.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Oxidative Stress: Fundamentals and Advances in Quantification Techniques. Front. Chem. 2024, 12, 1470458. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Xu, Y.; Li, K.; Zhao, Y.; Qiao, H.; Xu, Q.; Zhao, J. Heat Shock Proteins and Ferroptosis. Front. Cell Dev. Biol. 2022, 10, 864635. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Mbah, N.E.; Lyssiotis, C.A. Metabolic Regulation of Ferroptosis in the Tumor Microenvironment. J. Biol. Chem. 2022, 298, 101617. [Google Scholar] [CrossRef]
- Vana, F.; Szabo, Z.; Masarik, M.; Kratochvilova, M. The Interplay of Transition Metals in Ferroptosis and Pyroptosis. Cell Div. 2024, 19, 24. [Google Scholar] [CrossRef]
- Qi, D.; Peng, M. Ferroptosis-Mediated Immune Responses in Cancer. Front. Immunol. 2023, 14, 1188365. [Google Scholar] [CrossRef]
- Vucetic, M.; Daher, B.; Cassim, S.; Meira, W.; Pouyssegur, J. Together We Stand, Apart We Fall: How Cell-to-Cell Contact/Interplay Provides Resistance to Ferroptosis. Cell Death Dis. 2020, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Horky, P.; Abbas, S.Q.; Majid, M.; Bilal, A.H.M.; Ali, F.; Behl, T.; Hassan, S.S.U.; Bungau, S. Ferroptosis: A New Road towards Cancer Management. Molecules 2022, 27, 2129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, H.; Wang, X.; Li, G.; Liu, N.; Zhang, M.; Shen, Y. The Application of Approaches in Detecting Ferroptosis. Heliyon 2023, 10, e23507. [Google Scholar] [CrossRef] [PubMed]
- Veglia Tranchese, R.; Battista, S.; Cerchia, L.; Fedele, M. Ferroptosis in Cancer: Epigenetic Control and Therapeutic Opportunities. Biomolecules 2024, 14, 1443. [Google Scholar] [CrossRef]
- Bebber, C.M.; Müller, F.; Prieto Clemente, L.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef]
- Gooz, M.; Maldonado, E.N. Fluorescence Microscopy Imaging of Mitochondrial Metabolism in Cancer Cells. Front. Oncol. 2023, 13, 1152553. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A. Chromatin and Cell Death. Biochim. Biophys. Acta 2004, 1677, 181–186. [Google Scholar] [CrossRef]
- Sharma, A.; Flora, S.J.S. Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis. Oxid. Med. Cell. Longev. 2021, 2021, 9074206. [Google Scholar] [CrossRef]
- Kroemer, G.; Martin, S.J. Caspase-Independent Cell Death. Nat. Med. 2005, 11, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, Z.G. Execution of RIPK3-Regulated Necrosis. Mol. Cell. Oncol. 2014, 1, e960759. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, Ferroptosis, Pyroptosis, and Necroptosis in Tumor Immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. The Roles of Ferroptosis in Cancer: Tumor Suppression, Tumor Microenvironment, and Therapeutic Interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Li, X.; Huang, R.; Luo, L. Recent Progress on Targeting Ferroptosis for Cancer Therapy. Biochem. Pharmacol. 2021, 190, 114584. [Google Scholar] [CrossRef]
- Luo, L.; Wang, H.; Tian, W.; Zeng, J.; Huang, Y.; Luo, H. Targeting Ferroptosis for Cancer Therapy: Iron Metabolism and Anticancer Immunity. Am. J. Cancer Res. 2021, 11, 5508–5525. [Google Scholar]
- Ye, L.; Wen, X.; Qin, J.; Zhang, X.; Wang, Y.; Wang, Z.; Zhou, T.; Di, Y.; He, W. Metabolism-Regulated Ferroptosis in Cancer Progression and Therapy. Cell Death Dis. 2024, 15, 196. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, Ferroptosis, and Diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-CoA Synthetase ACSL4: An Essential Target in Ferroptosis and Fatty Acid Metabolism. Chin. Med. J. 2023, 136, 2521–2537. [Google Scholar]
- Sha, R.; Xu, Y.; Yuan, C.; Sheng, X.; Wu, Z.; Peng, J.; Wang, Y.; Lin, Y.; Zhou, L.; Xu, S.; et al. Predictive and Prognostic Impact of Ferroptosis-Related Genes ACSL4 and GPX4 on Breast Cancer Treated with Neoadjuvant Chemotherapy. EBioMedicine 2021, 71, 103560. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, J.; Lin, R.; Lu, S.; Rong, L.; Xue, Y.; Fang, Y. Understanding the Unique Mechanism of Ferroptosis: A Promising Therapeutic Target. Front. Cell Dev. Biol. 2024, 11, 1329147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Ma, T.; Du, J.; Zhang, Y.; Wang, Y.; Wang, B.; Zhang, T. GPX4-Independent Ferroptosis—A New Strategy in Disease’s Therapy. Cell Death Discov. 2022, 8, 434. [Google Scholar] [CrossRef]
- Bayır, H.; Anthonymuthu, T.S.; Tyurina, Y.Y.; Patel, S.J.; Amoscato, A.A.; Lamade, A.M.; Yang, Q.; Vladimirov, G.K.; Philpott, C.C.; Kagan, V.E. Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem. Biol. 2020, 27, 387–408. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. System Xc-/GSH/GPX4 Axis: An Important Antioxidant System for the Ferroptosis in Drug-Resistant Solid Tumor Therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, G.; Chen, X. Mechanism of Ferroptosis Resistance in Cancer Cells. Cancer Drug Resist. 2024, 7, 47. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Raju, M.V.; Chandrasekaran, M.K.; Ahalliya, R.M.; Kanniappan, G.V. Reconnoitering the Role of Lipid Metabolites in Ferroptosis. Adv. Redox Res. 2024, 14, 100117. [Google Scholar]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A Cell Death Connecting Oxidative Stress, Inflammation and Cardiovascular Diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Villalón García, I.; Povea Cabello, S.; Álvarez Córdoba, M.; Talaverón Rey, M.; Suárez Rivero, J.M.; Suárez Carrillo, A.; Munuera Cabeza, M.; Reche López, D.; Cilleros Holgado, P.; Piñero Pérez, R.; et al. Vicious Cycle of Lipid Peroxidation and Iron Accumulation in Neurodegeneration. Neural Regen. Res. 2023, 18, 1196–1202. [Google Scholar] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Feng, F.; He, S.; Li, X.; He, J.; Luo, L. Mitochondria Mediated Ferroptosis in Diseases Therapy: From Molecular Mechanisms to Implications. Aging Dis. 2024, 15, 714–738. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Li, W.; Liang, L.; Liu, S.; Yi, H.; Zhou, Y. FSP1: A Key Regulator of Ferroptosis. Trends Mol. Med. 2023, 29, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Yang, W.H.; Huang, Z.; Wu, J.; Ding, C.C.; Murphy, S.K.; Chi, J.T. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol. Cancer Res. 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Salimi, Z.; Afsharinasab, M.; Rostami, M.; Eshaghi Milasi, Y.; Mousavi Ezmareh, S.F.; Sakhaei, F.; Mohammad-Sadeghipour, M.; Rasooli Manesh, S.M.; Asemi, Z. Iron chelators: As therapeutic agents in diseases. Ann. Med. Surg. 2024, 86, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Liu, Z.; He, X.; Tang, W.; He, L.; Feng, Y.; Liu, D.; Yin, Y.; Li, T. Ferroptosis and its multifaceted role in cancer: Mechanisms and therapeutic approach. Antioxidants 2022, 11, 1504. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R. The selenoprotein glutathione peroxidase 4: From molecular mechanisms to novel therapeutic opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef]
- Beretta, G.L.; Zaffaroni, N. Radiotherapy-induced ferroptosis for cancer treatment. Front. Mol. Biosci. 2023, 10, 1216733. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, X.; Ye, F.; Liu, X.; Yu, B.; Li, Z.; Zhao, T.; Chen, W.; Liu, X.; Di, C.; et al. Ferroptosis: A novel regulated cell death participating in cellular stress response, radiotherapy, and immunotherapy. Exp. Hematol. Oncol. 2023, 12, 65. [Google Scholar] [CrossRef]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Jin, X.; Wang, Q.; Su, J.; Guo, L. Dual ratio and ultraprecision quantification of mitochondrial viscosity in ferroptosis enabled by a vibration-based triple-emission fluorescent probe. Anal. Chem. 2023, 95, 17003–17010. [Google Scholar] [CrossRef]
- Wu, S.; Yan, Y.; Hou, H.; Huang, Z.; Li, D.; Zhang, X.; Xiao, Y. Polarity-sensitive and membrane-specific probe quantitatively monitoring ferroptosis through fluorescence lifetime imaging. Anal. Chem. 2022, 94, 11238–11247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef] [PubMed]
- Houglum, K.; Filip, M.; Witztum, J.L.; Chojkier, M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J. Clin. Investig. 1990, 86, 1991–1998. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef]
- Kirkwood-Donelson, K.I.; Jarmusch, A.K.; Bortner, C.D.; Merrick, B.A.; Sinha, B.K. Metabolic consequences of erastin-induced ferroptosis in human ovarian cancer cells: An untargeted metabolomics study. Front. Mol. Biosci. 2025, 11, 1520876. [Google Scholar] [CrossRef]
- Sinha, B.K.; Bortner, C.D.; Jarmusch, A.K.; Tokar, E.J.; Murphy, C.; Wu, X.; Winter, H.; Cannon, R.E. Ferroptosis-mediated cell death induced by NCX4040, the non-steroidal nitric oxide donor, in human colorectal cancer cells: Implications in therapy. Cells 2023, 12, 1626. [Google Scholar] [CrossRef]
- Sun, J.; Ren, H.; Wang, J.; Xiao, X.; Zhu, L.; Wang, Y.; Yang, L. CHAC1: A master regulator of oxidative stress and ferroptosis in human diseases and cancers. Front. Cell Dev. Biol. 2024, 12, 1458716. [Google Scholar] [CrossRef]
- Sinha, B.K.; Murphy, C.; Brown, S.M.; Silver, B.B.; Tokar, E.J.; Bortner, C.D. Mechanisms of cell death induced by erastin in human ovarian tumor cells. Int. J. Mol. Sci. 2024, 25, 8666. [Google Scholar] [CrossRef]
- Qi, Y.L.; Wang, H.R.; Chen, L.L.; Duan, Y.T.; Yang, S.Y.; Zhu, H.L. Recent advances in small-molecule fluorescent probes for studying ferroptosis. Chem. Soc. Rev. 2022, 51, 7752–7778. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, J.; Hu, Q.; Huang, S.; Lin, W. Fluorescent probes for ferroptosis bioimaging: Advances, challenges, and prospects. Chem. Soc. Rev. 2023, 52, 2011–2030. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, J.; Yang, L.; Li, Z.; Yu, M. A NIR dual-channel fluorescent probe for fluctuations of intracellular polarity and H2O2 and its applications for the visualization of inflammation and ferroptosis. Chem. Biomed. Imaging 2024, 2, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Nizami, Z.N.; Aburawi, H.E.; Semlali, A.; Muhammad, K.; Iratni, R. Oxidative stress inducers in cancer therapy: Preclinical and clinical evidence. Antioxidants 2023, 12, 1159. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, W.; Li, X.; Wang, L.; Meng, L.; Yu, R. Cisplatin synergizes with PRLX93936 to induce ferroptosis in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2021, 569, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Andia, A.A.; Liu, H.; Csuka, J.M.; Hurlocker, B.; Vaiana, C.A.; Heindel, D.W.; Zuckerman, D.S.; Bos, P.H.; Reznik, E.; et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 2018, 14, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Perera, L.; Brown, S.M.; Silver, B.B.; Tokar, E.J.; Sinha, B.K. Ferroptosis inducers erastin and RSL3 enhance adriamycin and topotecan sensitivity in ABCB1/ABCG2-expressing tumor cells. Int. J. Mol. Sci. 2025, 26, 635. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Xia, W.; Mai, Z.; Ye, Y.; Zhao, B.; Song, Y. CHAC1 promotes cell ferroptosis and enhances radiation sensitivity in thyroid carcinoma. Neoplasma 2023, 70, 777–786. [Google Scholar] [CrossRef]
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arnér, E.S.J. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef]
- Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis inducers upregulate PD-L1 in recurrent triple-negative breast cancer. Cancers 2023, 16, 155. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-mediated ferroptosis in the sensitivity of triple-negative breast cancer cells to gefitinib. Front. Oncol. 2020, 10, 597434. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhong, Y.; Chen, R.; Zhan, X.; Huang, G.; Xiong, Y.; Tan, B. Tripterygium glycosides reverse chemotherapy resistance in ovarian cancer by targeting the NRF2/GPX4 signal axis to induce ferroptosis of drug-resistant human epithelial ovarian cancer cells. Biochem. Biophys. Res. Commun. 2023, 665, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, X.; Zhang, Q.; Huang, Y.; Wei, J.; Zhang, H.; Luo, H. Synergistic suppression of ovarian cancer by combining NRF2 and GPX4 inhibitors: In vitro and in vivo evidence. J. Ovarian Res. 2024, 17, 49. [Google Scholar] [CrossRef]
- Yan, G.; Huang, H.; Lu, Z.; Chen, M.; Wang, X.; Zhong, P.; Qin, C.; Mo, S.; Han, C.; Luo, X.; et al. Comprehensive pan-cancer analysis and functional studies reveal SLC2A6 as a ferroptosis modulator in hepatocellular carcinoma. Sci. Rep. 2025, 15, 2545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lee, C.H.; Hsieh, Y.H.; Chiou, H.L.; Hung, M.C.; Lee, H.L. Sorafenib, a tyrosine kinase inhibitor, synergistically enhances the ferroptosis effects of asiatic acid in hepatocellular carcinoma cells. Environ. Toxicol. 2025, 40, 79–87. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Xu, Y.; Liu, X.; Kang, X.; Zhu, J.; Long, S.; Han, Y.; Xue, C.; Sun, Z.; et al. SLC7A11 protects luminal A breast cancer cells against ferroptosis induced by CDK4/6 inhibitors. Redox Biol. 2024, 76, 103304. [Google Scholar] [CrossRef]
- Skeie, B.S.; Bragstad, S.; Sarowar, S.; Behbahani, M.; Filippi, C.; Knisely, J.; Schulder, M.; Goplen, D.; Eide, G.E.; Heggdal, J.I.; et al. CTNI-40. Phase I Trial of Sulfasalazine Combined with Stereotactic Radiosurgery or Recurrent Glioblastoma: Study Protocol for NCT04205357. Neuro Oncol. 2022, 24, vii80–vii81. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID NCT06048367. Carbon Nanoparticle-Loaded Iron [CNSI-Fe(II)] in the Treatment of Advanced Solid Tumor (CNSI-Fe(II)). Available online: https://clinicaltrials.gov/ (accessed on 11 February 2025).
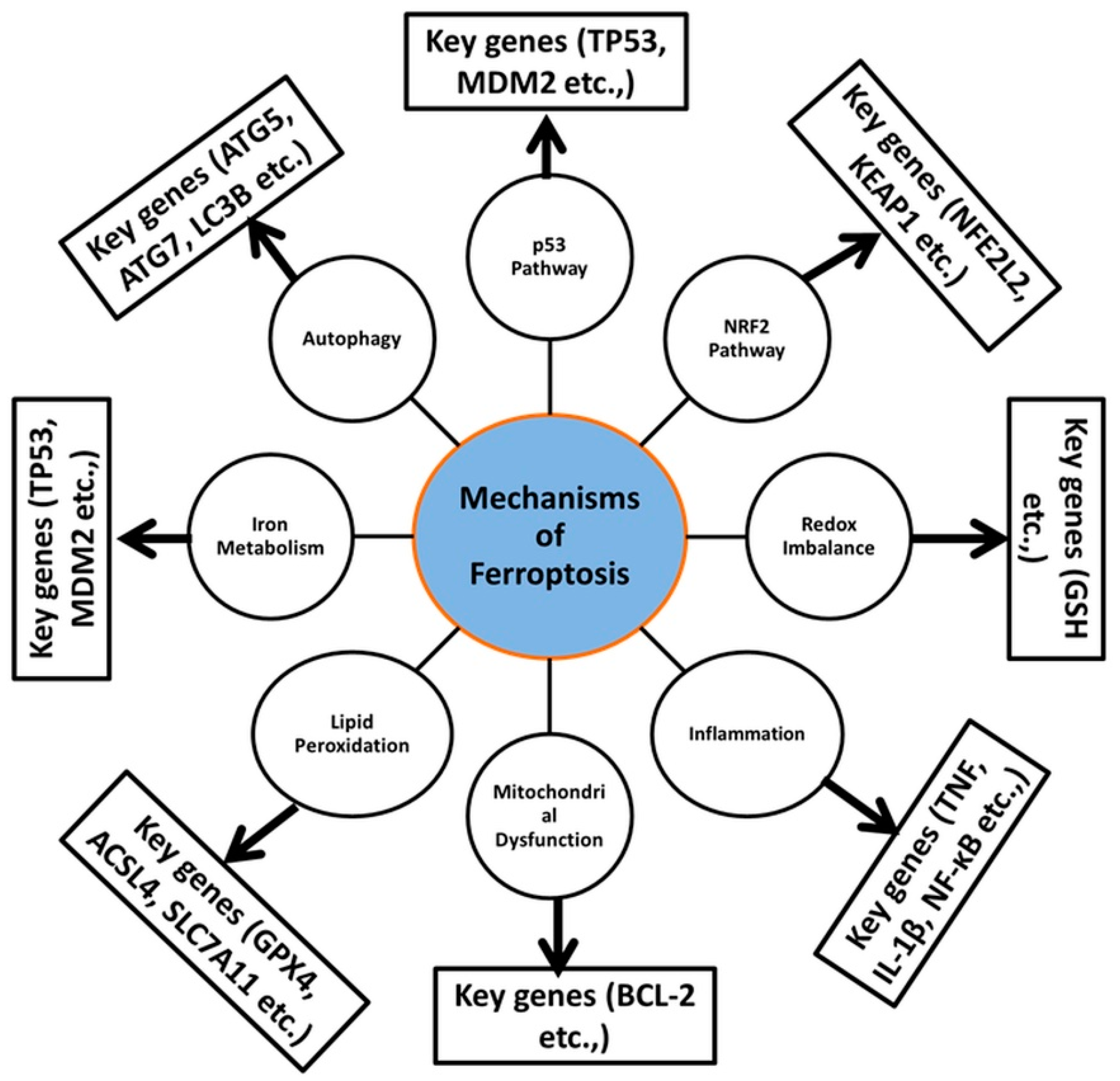
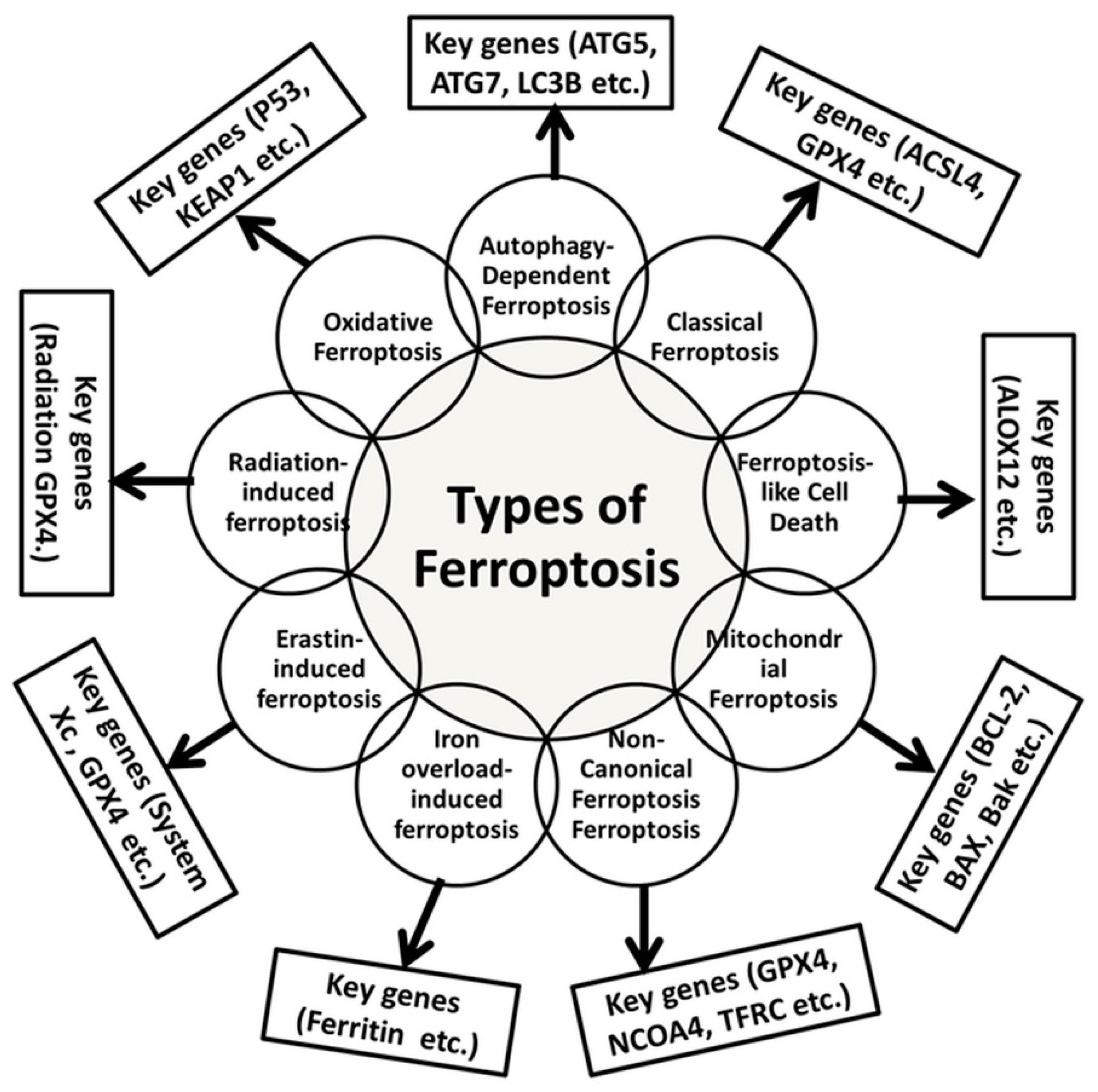
| Mechanism | Molecular Genes/Proteins | Pathways | Therapeutic Implications | References |
|---|---|---|---|---|
| Autophagy | ATG5, ATG7, LC3B | Autophagic degradation | Autophagy inhibition | [38] |
| p53 Pathway | TP53, MDM2 | Tumor suppressor regulation | p53-reactivating therapies | [45] |
| NRF2 Pathway | NFE2L2, KEAP1 | Antioxidant response element (ARE) regulation | NRF2 activation | [113] |
| Redox Imbalance | GSH | Reactive oxygen species (ROS) regulation | Antioxidant therapies | [123] |
| Inflammation | TNF, IL-1β, NF-κB | Cytokine signaling | Anti-inflammatory therapies | [130,131] |
| Mitochondrial Dysfunction | BCL-2 | Mitochondrial outer membrane permeabilization | Mitochondria-targeting therapies | [132,133] |
| Lipid Peroxidation | GPX4, ACSL4, SLC7A11 | Fatty acid synthesis, antioxidant defenses | Targeting lipid metabolism | [134,135,136,137,138] |
| Iron Metabolism | NCOA4, TFRC, SLC40A1 | Ferritinophagy, iron uptake | Iron chelation therapy | [139,140,141] |
| Types | Molecular Mechanisms | Key Genes Involved | Relevant Cancer Hallmarks | References |
|---|---|---|---|---|
| Oxidative Ferroptosis | Accumulation of oxygen species (ROS) and mitochondrial damage | P53, KEAP1, NFE2L2 | Cancer cell sensitivity | [100] |
| Autophagy-Dependent Ferroptosis | Involves autophagic degradation of damaged cellular components | ATG5, ATG7, LC3B | Cancer cell survival | [121] |
| Classical Ferroptosis | Regulated cell death driven by iron-dependent lipid peroxidation | ACSL4, GPX4, SLC7A11 | Cancer cell vulnerability | [124,125] |
| Ferroptosis-like Cell Death | Shares features with ferroptosis but lacks lipid peroxidation | ALOX12 | Cancer therapy potential | [127] |
| Mitochondrial Ferroptosis | Mitochondrial dysfunction, including changes in membrane potential | BCL-2, BAX, Bak | Cancer therapy target | [133] |
| Non-Canonical Ferroptosis | Independent of GPX4 | NCOA4, TFRC, SLC40A1 | Resistance in cancer cells | [135,136] |
| Iron Overload-Induced Ferroptosis | Fenton reaction and lipid peroxidation | Ferritin | Resistance in cancer cells | [141] |
| Erastin-Induced Ferroptosis | System Xc−, an antiporter of cystine/glutamate | GPX4 | Cancer therapy target | [144,145] |
| Radiation-Induced Ferroptosis | Radiation leads to the generation of ROS | GPX4 | Cancer cell sensitivity | [147,148] |
| Inducers of Ferroptosis | Molecular Target/ Pathway | Combinatorial Anticancer Drug Approach | Preclinical/Clinical Evidence | References |
|---|---|---|---|---|
| Selenite | GPX4/SELENOP | Anticancer drug | Enhanced antitumor effect in ovarian cancer via induction of ferroptosis and inhibition of GPX4-mediated antioxidant defenses | [143] |
| PRLX93936 inhibitor of GPX4 | GPX4 | Cisplatin | upregulation of ROS, lipid peroxidation, and Fe2+ | [165] |
| FINO2 | GPX4/SLC7A11 | Anticancer drug | FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation | [166] |
| ML162, ML210 | Selenoprotein, TXNRD1 | Anticancer drug | Inhibition of selenoprotein, TXNRD1 | [169] |
| Lentivirus sh-GPX4 | GPX4 | Gefitinib | Increased antitumor efficacy in breast cancer via inhibition of GPX4-mediated ferroptosis and induction of SELENOP-mediated selenium depletion | [171] |
| Tripterygium glycosides | GPX4/NRF2 | Cisplatin | Synergistic antitumor effect in ovarian cancer via induction of ferroptosis and inhibition of NRF2-mediated GPX4 expression | [172] |
| ML385 | GPX4/NRF2 | Anticancer drugs | Enhanced antitumor efficacy in ovarian cancer via inhibition of NRF2-mediated GPX4 expression and induction of ferroptosis | [173] |
| GPX4 inhibitor (RSL3) | GPX4/NRF2 | Sorafenib | Synergistic antitumor effect in HCC via inhibition of NRF2-mediated GPX4 expression and induction of ferroptosis | [175] |
| FINO2 | GPX4/SLC7A11 | Paclitaxel | Increased antitumor efficacy in breast cancer via inhibition of GPX4-mediated ferroptosis and induction of SLC7A11-mediated glutathione depletion | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Arora, H.L.; Naik, R.; Joshi, S.; Sonawane, K.; Sharma, N.K.; Sinha, B.K. Ferroptosis in Cancer: Mechanism and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 3852. https://doi.org/10.3390/ijms26083852
Singh M, Arora HL, Naik R, Joshi S, Sonawane K, Sharma NK, Sinha BK. Ferroptosis in Cancer: Mechanism and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(8):3852. https://doi.org/10.3390/ijms26083852
Chicago/Turabian StyleSingh, Mansaa, Hasmiq L. Arora, Rutuja Naik, Shravani Joshi, Kaveri Sonawane, Nilesh Kumar Sharma, and Birandra K. Sinha. 2025. "Ferroptosis in Cancer: Mechanism and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 8: 3852. https://doi.org/10.3390/ijms26083852
APA StyleSingh, M., Arora, H. L., Naik, R., Joshi, S., Sonawane, K., Sharma, N. K., & Sinha, B. K. (2025). Ferroptosis in Cancer: Mechanism and Therapeutic Potential. International Journal of Molecular Sciences, 26(8), 3852. https://doi.org/10.3390/ijms26083852







