Inflammatory Responses to Zn/Cu-Containing Welding Fume in Human Alveolar Epithelial and Macrophage Cell Lines, with MIP-1β/CCL4 as a Much More Sensitive Macrophage Activation Marker than IL-8 and TNF-α
Abstract
1. Introduction
2. Results
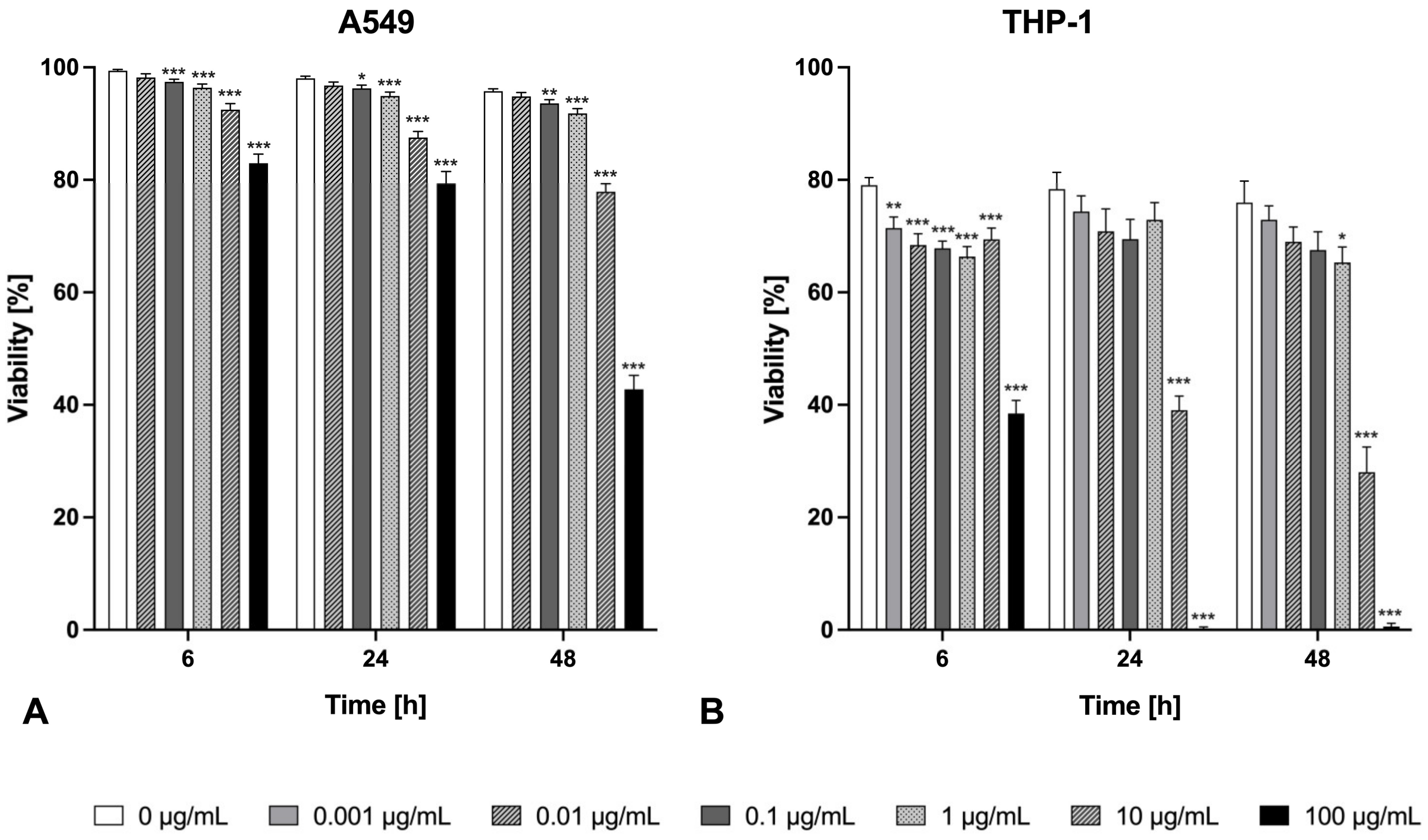
2.1. Cytotoxicity
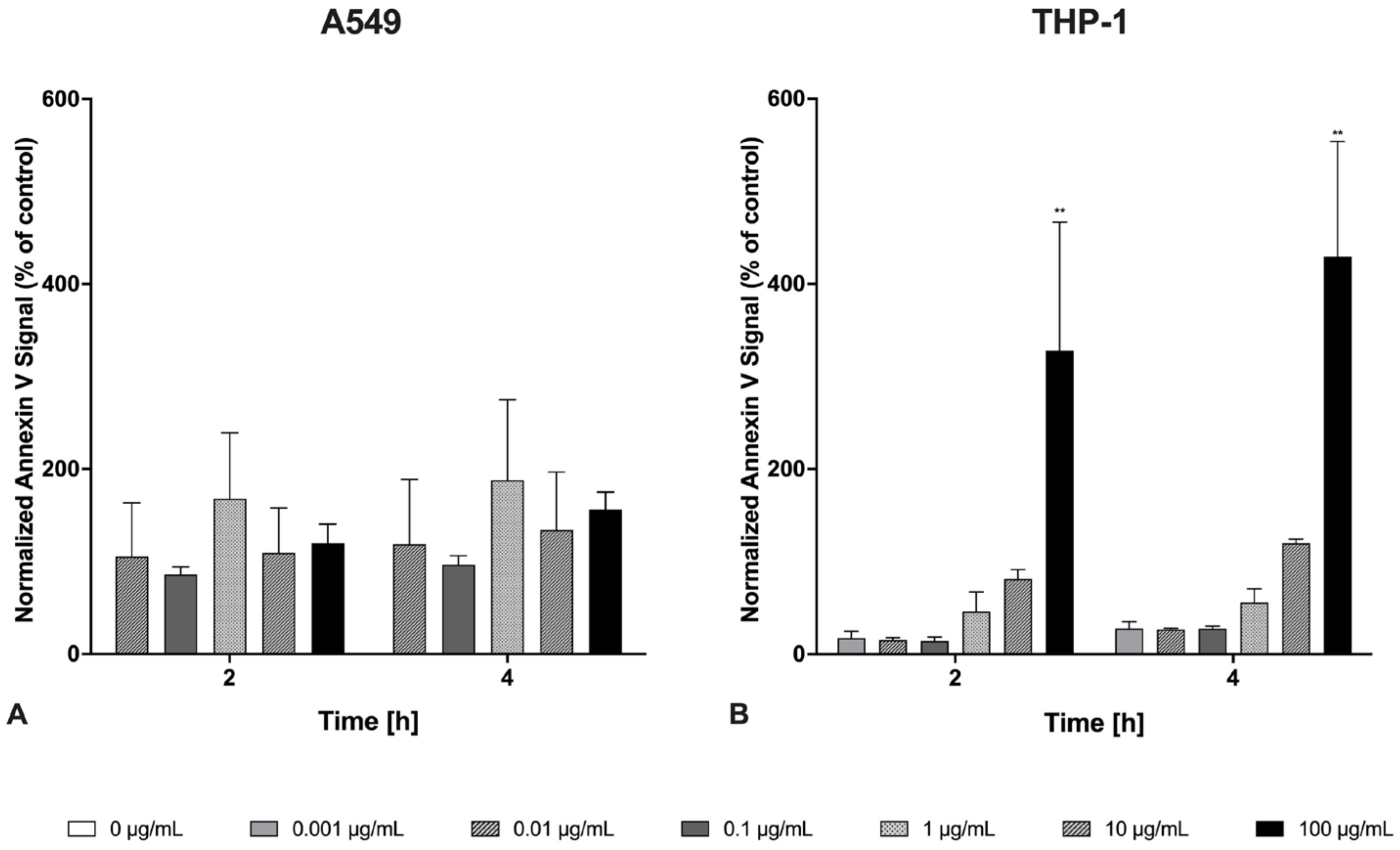
2.2. Apoptosis
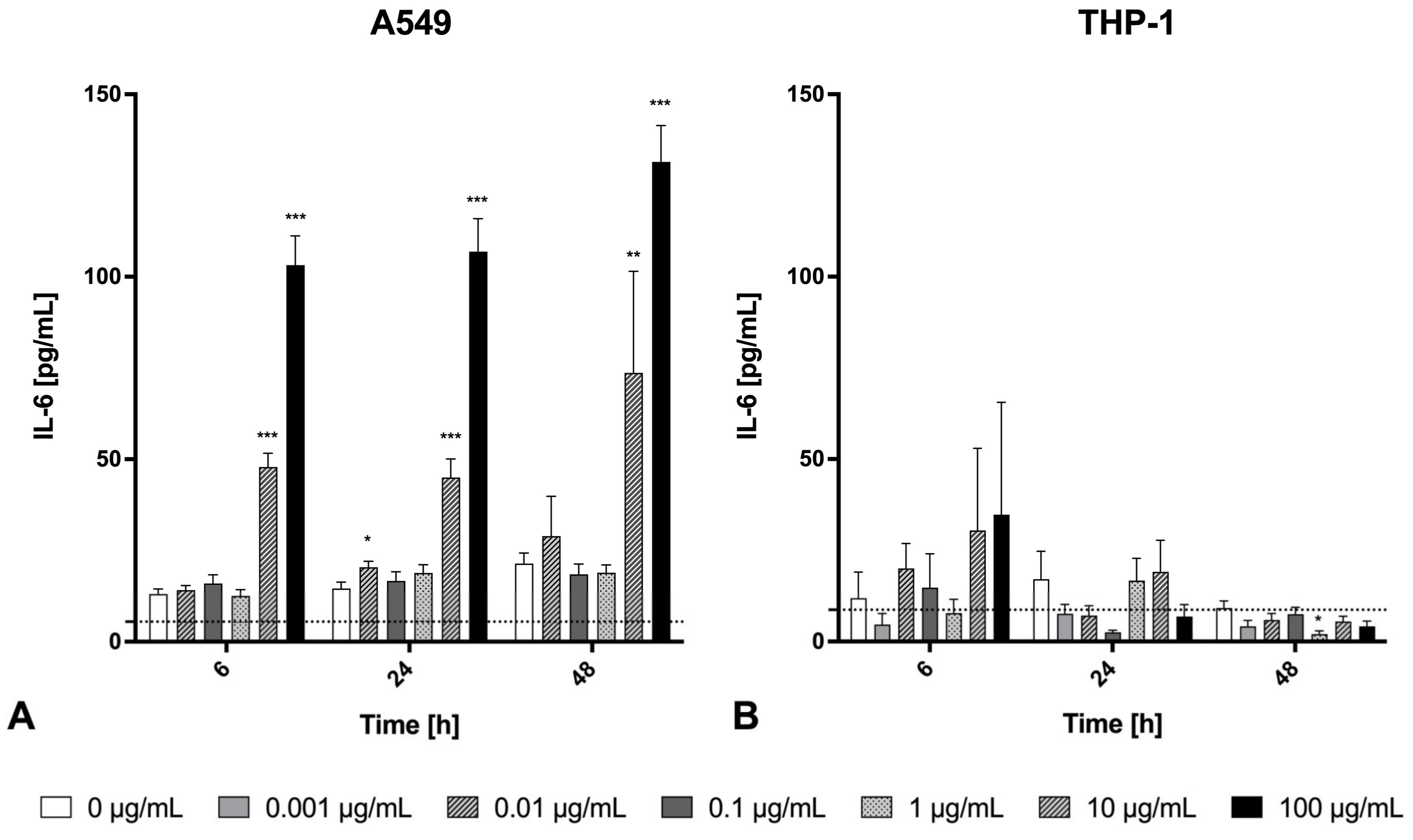
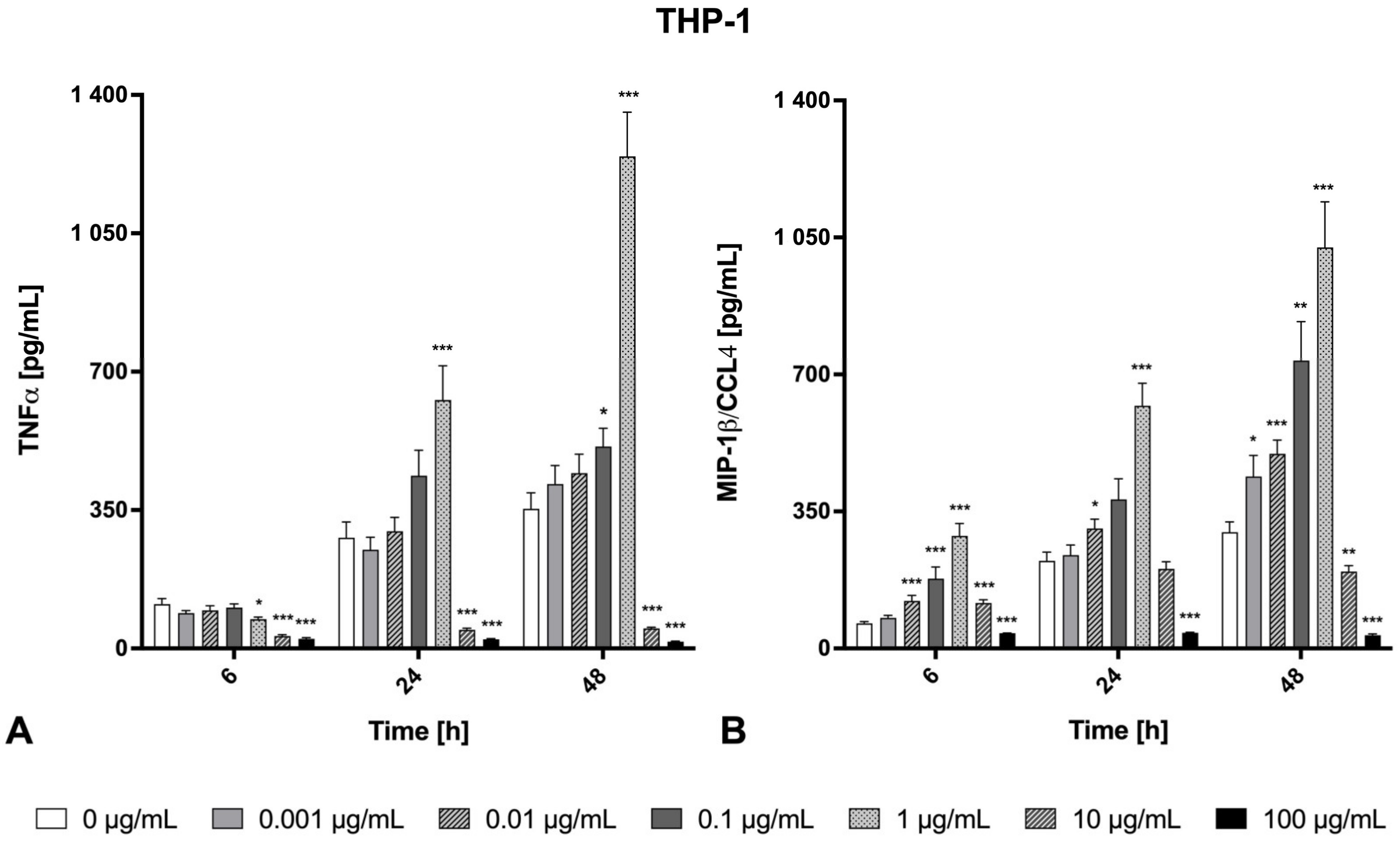
2.3. Inflammatory Effects
2.4. Genotoxicity
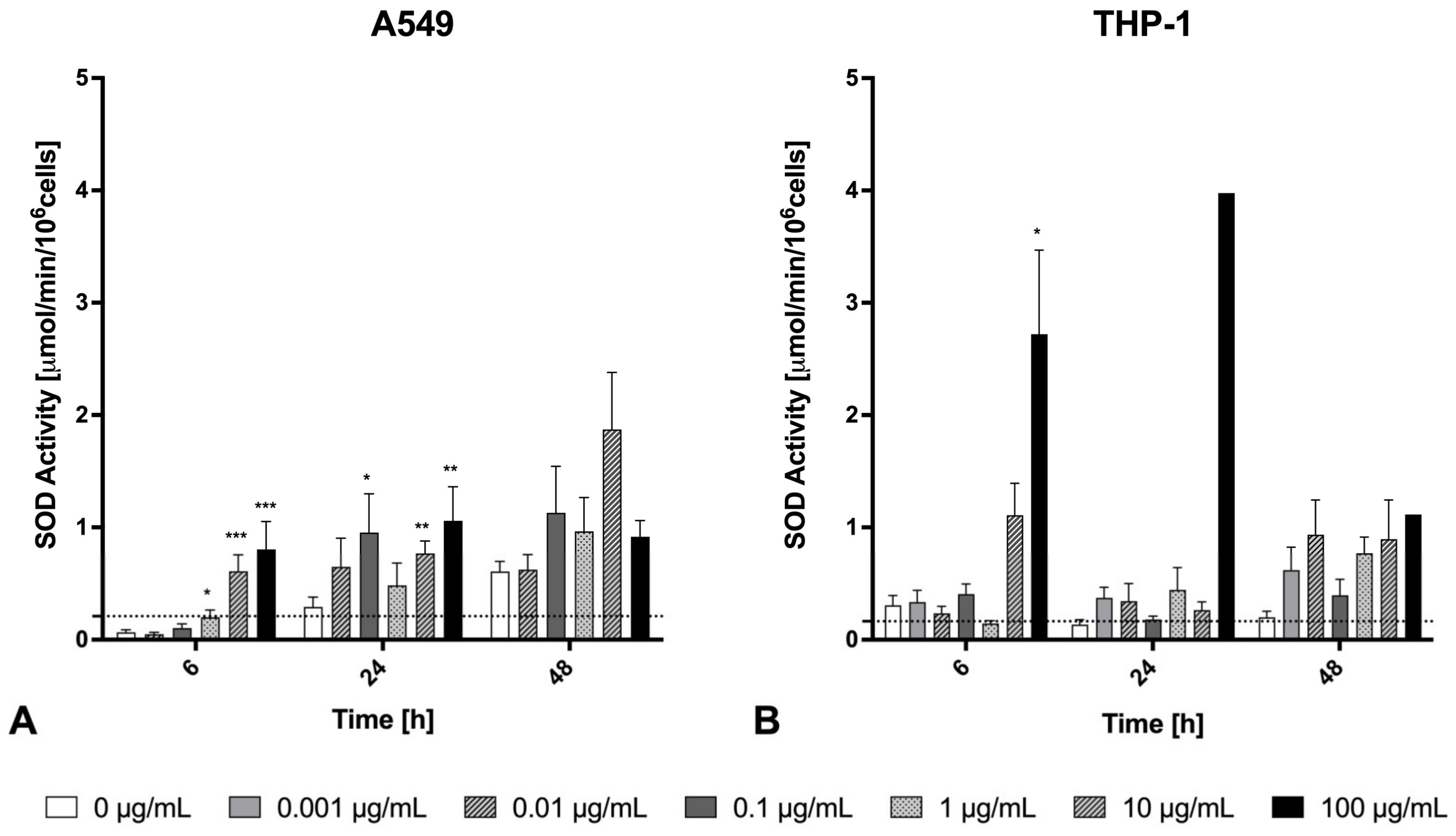
2.5. Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Welding Fume Generation
4.2. Welding Fume Characterization via Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.3. Preparation of the Welding Fume Extracts and Exposure Solutions
4.4. Controlled Experimental In Vitro Exposure to Welding Fumes Containing Zinc and Copper on A549 Lung Epithelial Cells and THP-1 Macrophages
4.4.1. Cell Culture and Culture Conditions
4.4.2. Experimental In Vitro Exposure Procedure and Sample Preparation
4.4.3. Cytotoxicity by Assessing the Cell Viability
4.4.4. Cytokines and Chemokines Detection Cell Culture Supernatant
4.4.5. Genotoxicity Assessment Due to Exposure to Welding Fumes Containing Zinc and Copper
4.4.6. Annexin V Detection Using the Sartorius Incucyte® SX5 Live-Cell Analysis System for Live-Cell Imaging
4.4.7. Determination of Oxidative Stress via Superoxide Dismutase (SOD)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
| In Vitro vs. Human Inhalation | In Vitro vs. Human Inhalation | Exposure Classification | ||||
|---|---|---|---|---|---|---|
| In Vitro | In Vitro | 1-Week TBD | 1-Week ALD | 1-Year TBD | 1-Year ALD | |
| [µg/mL] | [µg/cm2] | (0.102 µg/cm2) | (0.083 µg/cm2) | (1.15 µg/cm2) | (2.85 µg/cm2) | |
| - | - | 0.102 | 0.083 | 1.15 | 2.85 | OEL 6 h as baseline (5 mg/m3) |
| 0.001 | 0.00024 | 0.24% | 0.29% | 0.021% | 0.0084% | Realistic short-term exposure |
| 0.01 | 0.0024 | 2.4% | 2.9% | 0.21% | 0.084% | Realistic short-term exposure |
| 0.1 | 0.024 | 23.5% | 28.9% | 2.1% | 0.84% | Upper limit of short-term exposure |
| 1 | 0.24 | 2.35-fold | 2.89-fold | 20.9% | 8.4% | Most representative for long-term exposure |
| 10 | 2.4 | 23.5-fold | 28.9-fold | 2.09-fold | 16% | Exceeds occupational thresholds |
| 100 | 24 | 235-fold | 289-fold | 20.9-fold | 8.4-fold | Exceeds occupational thresholds |
References
- Ali, N.; Mattsson, K.; Rissler, J.; Karlsson, H.M.; Svensson, C.R.; Gudmundsson, A.; Lindh, C.H.; Jonsson, B.A.; Cedervall, T.; Karedal, M. Analysis of nanoparticle-protein coronas formed in vitro between nanosized welding particles and nasal lavage proteins. Nanotoxicology 2016, 10, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M. Health effects of welding. Crit. Rev. Toxicol. 2003, 33, 61–103. [Google Scholar] [CrossRef]
- Antonini, J.M.; Lewis, A.B.; Roberts, J.R.; Whaley, D.A. Pulmonary effects of welding fumes: Review of worker and experimental animal studies. Am. J. Ind. Med. 2003, 43, 350–360. [Google Scholar] [CrossRef]
- Ibfelt, E.; Bonde, J.P.; Hansen, J. Exposure to metal welding fume particles and risk for cardiovascular disease in Denmark: A prospective cohort study. Occup. Environ. Med. 2010, 67, 772–777. [Google Scholar] [CrossRef]
- Zeidler-Erdely, P.C.; Kashon, M.L.; Battelli, L.A.; Young, S.H.; Erdely, A.; Roberts, J.R.; Reynolds, S.H.; Antonini, J.M. Pulmonary inflammation and tumor induction in lung tumor susceptible A/J and resistant C57BL/6J mice exposed to welding fume. Part. Fibre Toxicol. 2008, 5, 12. [Google Scholar] [CrossRef]
- Raulf, M.; Weiss, T.; Lotz, A.; Lehnert, M.; Hoffmeyer, F.; Liebers, V.; Van Gelder, R.; Udo Kafferlein, H.; Hartwig, A.; Pesch, B.; et al. Analysis of inflammatory markers and metals in nasal lavage fluid of welders. J. Toxicol. Environ. Health A 2016, 79, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, C.G.; Berg, P.; Bryngelsson, I.L.; Elihn, K.; Ngo, Y.; Westberg, H.; Sjogren, B. Inflammatory markers and exposure to occupational air pollutants. Inhal. Toxicol. 2010, 22, 1083–1090. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Lim, G.B. Coronary artery disease: IL-6 signaling linked with CHD. Nat. Rev. Cardiol. 2012, 9, 313. [Google Scholar] [CrossRef]
- Reisgen, U.; Geffers, C.; Willms, K.; Angerhausen, M.; Deckert, K.; Hof, S.; Prenger, F.; Wisniewski, J. Low-energy thermal joining with zinc and tin base solder for application in vehicle construction. In Brazing, High Temperature Brazing and Diffusion Bonding; DVS Media: Düsseldorf, Germany, 2013; pp. 272–277. [Google Scholar]
- Blanc, P.D.; Boushey, H.A.; Wong, H.; Wintermeyer, S.F.; Bernstein, M.S. Cytokines in metal fume fever. Am. Rev. Respir. Dis. 1993, 147, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kuschner, W.G.; D’Alessandro, A.; Wong, H.; Blanc, P.D. Early pulmonary cytokine responses to zinc oxide fume inhalation. Environ. Res. 1997, 75, 7–11. [Google Scholar] [CrossRef]
- Fine, J.M.; Gordon, T.; Chen, L.C.; Kinney, P.; Falcone, G.; Beckett, W.S. Metal fume fever: Characterization of clinical and plasma IL-6 responses in controlled human exposures to zinc oxide fume at and below the threshold limit value. J. Occup. Environ. Med. 1997, 39, 722–726. [Google Scholar] [CrossRef]
- Baumann, R.; Brand, P.; Chaker, A.; Markert, A.; Rack, I.; Davatgarbenam, S.; Joraslafsky, S.; Gerhards, B.; Kraus, T.; Gube, M. Human nasal mucosal C-reactive protein responses after inhalation of ultrafine welding fume particles: Positive correlation to systemic C-reactive protein responses. Nanotoxicology 2018, 12, 1130–1147. [Google Scholar] [CrossRef]
- Baumann, R.; Gube, M.; Markert, A.; Davatgarbenam, S.; Kossack, V.; Gerhards, B.; Kraus, T.; Brand, P. Systemic serum amyloid A as a biomarker for exposure to zinc and/or copper-containing metal fumes. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 84–91. [Google Scholar] [CrossRef]
- Baumann, R.; Joraslafsky, S.; Markert, A.; Rack, I.; Davatgarbenam, S.; Kossack, V.; Gerhards, B.; Kraus, T.; Brand, P.; Gube, M. IL-6, a central acute-phase mediator, as an early biomarker for exposure to zinc-based metal fumes. Toxicology 2016, 373, 63–73. [Google Scholar] [CrossRef]
- Markert, A.; Baumann, R.; Gerhards, B.; Gube, M.; Kossack, V.; Kraus, T.; Brand, P. Single and Combined Exposure to Zinc- and Copper-Containing Welding Fumes Lead to Asymptomatic Systemic Inflammation. J. Occup. Environ. Med. 2016, 58, 127–132. [Google Scholar] [CrossRef]
- Krabbe, J.; Beilmann, V.; Gerhards, B.; Markert, A.; Thomas, K.; Kraus, T.; Brand, P. The Effects of Repeated Exposure to Zinc- and Copper-Containing Welding Fumes on Healthy Volunteers. J. Occup. Environ. Med. 2019, 61, 8–15. [Google Scholar] [CrossRef]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef]
- Scheurer, T.; Steffens, J.; Markert, A.; Du Marchie Sarvaas, M.; Roderburg, C.; Rink, L.; Tacke, F.; Luedde, T.; Kraus, T.; Baumann, R. The human long noncoding RNAs CoroMarker, MALAT1, CDR1as, and LINC00460 in whole blood of individuals after controlled short-term exposure with ultrafine metal fume particles at workplace conditions, and in human macrophages in vitro. J. Occup. Med. Toxicol. 2022, 17, 15. [Google Scholar] [CrossRef]
- Steffens, J.; Michael, S.; Kuth, K.; Hollert, H.; Du Marchie Sarvaas, M.; Nesic, A.; Kraus, T.; Baumann, R. Occupationally Relevant Zinc- and Copper-Containing Metal Fumes Inhibit Human THP-1 Macrophage TNF and IL-6 Responses to Bacterial Stimuli. Glob. Chall. 2024, 2400302. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef]
- Krabbe, J.; Kraus, T.; Krabbe, H.; Martin, C.; Ziegler, P. Welding Fume Instillation in Isolated Perfused Mouse Lungs-Effects of Zinc- and Copper-Containing Welding Fumes. Int. J. Mol. Sci. 2022, 23, 9052. [Google Scholar] [CrossRef]
- Tokac, D.; Anlar, H.G.; Bacanli, M.; Dilsiz, S.A.; Iritas, S.; Basaran, N. Oxidative stress status of Turkish welders. Toxicol. Ind. Health 2020, 36, 263–271. [Google Scholar] [CrossRef]
- Bayat, M.; Daei, S.; Ziamajidi, N.; Abbasalipourkabir, R.; Nourian, A. The protective effects of vitamins A, C, and E on zinc oxide nanoparticles (ZnO NPs)-induced liver oxidative stress in male Wistar rats. Drug Chem. Toxicol. 2023, 46, 209–218. [Google Scholar] [CrossRef]
- Gupta, G.; Cappellini, F.; Farcal, L.; Gornati, R.; Bernardini, G.; Fadeel, B. Copper oxide nanoparticles trigger macrophage cell death with misfolding of Cu/Zn superoxide dismutase 1 (SOD1). Part. Fibre Toxicol. 2022, 19, 33. [Google Scholar] [CrossRef]
- Castell, J.V.; Gomez-Lechon, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Wolpe, S.D.; Davatelis, G.; Sherry, B.; Beutler, B.; Hesse, D.G.; Nguyen, H.T.; Moldawer, L.L.; Nathan, C.F.; Lowry, S.F.; Cerami, A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J. Exp. Med. 1988, 167, 570–581. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef]
- Capelli, A.; Di Stefano, A.; Gnemmi, I.; Balbo, P.; Cerutti, C.G.; Balbi, B.; Lusuardi, M.; Donner, C.F. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur. Respir. J. 1999, 14, 160–165. [Google Scholar] [CrossRef]
- Elfsmark, L.; Ekstrand-Hammarstrom, B.; Forsgren, N.; Lejon, C.; Hagglund, L.; Wingfors, H. Characterization of toxicological effects of complex nano-sized metal particles using in vitro human cell and whole blood model systems. J. Appl. Toxicol. 2022, 42, 203–215. [Google Scholar] [CrossRef]
- McCarrick, S.; Karlsson, H.L.; Carlander, U. Modelled lung deposition and retention of welding fume particles in occupational scenarios: A comparison to doses used in vitro. Arch. Toxicol. 2022, 96, 969–985. [Google Scholar] [CrossRef]
- IARC. Welding, Molybdenum Trioxide, and Indium Tin Oxide; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Uski, O.J.; Rankin, G.D.; Wingfors, H.; Magnusson, R.; Boman, C.; Muala, A.; Blomberg, A.; Bosson, J.; Sandstrom, T. In vitro toxicity evaluation in A549 cells of diesel particulate matter from two different particle sampling systems and several resuspension media. J. Appl. Toxicol. 2024, 44, 1269–1278. [Google Scholar] [CrossRef]
- Kumarathasan, P.; Nazemof, N.; Blais, E.; Syama, K.P.; Breznan, D.; Dirieh, Y.; Aoki, H.; Phanse, S.; Tayabali, A.; Babu, M. In Vitro Exposure of A549 and J774A.1 Cells to SiO2 and TiO2 Nanoforms and Related Cellular- and Molecular-Level Effects: Application of Proteomics. J. Proteome Res. 2025, 24, 1672–1687. [Google Scholar] [CrossRef]
- Nazemof, N.; Breznan, D.; Dirieh, Y.; Blais, E.; Johnston, L.J.; Tayabali, A.F.; Gomes, J.; Kumarathasan, P. Cytotoxic Potencies of Zinc Oxide Nanoforms in A549 and J774 Cells. Nanomaterials 2024, 14, 1601. [Google Scholar] [CrossRef]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS ONE 2016, 11, e0164438. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Arodin Selenius, L.; Wallenberg Lundgren, M.; Jawad, R.; Danielsson, O.; Bjornstedt, M. The Cell Culture Medium Affects Growth, Phenotype Expression and the Response to Selenium Cytotoxicity in A549 and HepG2 Cells. Antioxidants 2019, 8, 130. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Nazem, M.R.; Asadi, M.; Jabbari, N.; Allameh, A. Effects of zinc supplementation on superoxide dismutase activity and gene expression, and metabolic parameters in overweight type 2 diabetes patients: A randomized, double-blind, controlled trial. Clin. Biochem. 2019, 69, 15–20. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Eleutherio, E.C.A.; Silva Magalhaes, R.S.; de Araujo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef]
- Grishaeva, A.; Ponezheva, Z.; Chanyshev, M.; Ploskireva, A.; Usenko, D.; Tsvetkova, N.; Omarova, K.; Pshenichnaya, N. MIP-1a and MIP-1b in serum as potential markers of the severe course COVID-19. Int. J. Infect. Dis. 2022, 116, S44. [Google Scholar] [CrossRef]
- Hirota, M.; Moro, O. MIP-1beta, a novel biomarker for in vitro sensitization test using human monocytic cell line. Toxicol. Vitr. 2006, 20, 736–742. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-α and Palmitate Requires MyD88 and Involves MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef]
- Kochumon, S.; Wilson, A.; Chandy, B.; Shenouda, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. Palmitate Activates CCL4 Expression in Human Monocytic Cells via TLR4/MyD88 Dependent Activation of NF-κB/MAPK/ PI3K Signaling Systems. Cell. Physiol. Biochem. 2018, 46, 953–964. [Google Scholar] [CrossRef]
- Ahmad, R.; Kochumon, S.; Chandy, B.; Shenouda, S.; Koshy, M.; Hasan, A.; Arefanian, H.; Al-Mulla, F.; Sindhu, S. TNF-α Drives the CCL4 Expression in Human Monocytic Cells: Involvement of the SAPK/JNK and NF-κB Signaling Pathways. Cell. Physiol. Biochem. 2019, 52, 908–921. [Google Scholar] [CrossRef]
- Bocca, B.; Battistini, B. Biomarkers of exposure and effect in human biomonitoring of metal-based nanomaterials: Their use in primary prevention and health surveillance. Nanotoxicology 2024, 18, 1–35. [Google Scholar] [CrossRef]
- Buonaurio, F.; Astolfi, M.L.; Pigini, D.; Tranfo, G.; Canepari, S.; Pietroiusti, A.; D’Alessandro, I.; Sisto, R. Oxidative Stress Biomarkers in Urine of Metal Carpentry Workers Can Be Diagnostic for Occupational Exposure to Low Level of Welding Fumes from Associated Metals. Cancers 2021, 13, 3167. [Google Scholar] [CrossRef]
- Corradi, M.; Gergelova, P.; Mutti, A. Use of exhaled breath condensate to investigate occupational lung diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 93–98. [Google Scholar] [CrossRef]
- Palmer, K.T.; McNeill Love, R.M.; Poole, J.R.; Coggon, D.; Frew, A.J.; Linaker, C.H.; Shute, J.K. Inflammatory responses to the occupational inhalation of metal fume. Eur. Respir. J. 2006, 27, 366–373. [Google Scholar] [CrossRef]
- Hartmann, L.; Bauer, M.; Bertram, J.; Gube, M.; Lenz, K.; Reisgen, U.; Schettgen, T.; Kraus, T.; Brand, P. Assessment of the biological effects of welding fumes emitted from metal inert gas welding processes of aluminium and zinc-plated materials in humans. Int. J. Hyg. Environ. Health 2014, 217, 160–168. [Google Scholar] [CrossRef]
- Koh, D.H.; Kim, J.I.; Kim, K.H.; Yoo, S.W.; Korea Welders Cohort, G. Welding fume exposure and chronic obstructive pulmonary disease in welders. Occup. Med. 2015, 65, 72–77. [Google Scholar] [CrossRef]
- Grahn, K.; Gustavsson, P.; Andersson, T.; Linden, A.; Hemmingsson, T.; Selander, J.; Wiebert, P. Occupational exposure to particles and increased risk of developing chronic obstructive pulmonary disease (COPD): A population-based cohort study in Stockholm, Sweden. Environ. Res. 2021, 200, 111739. [Google Scholar] [CrossRef]
- Krabbe, J.; Esser, A.; Kanzler, S.; Braunschweig, T.; Kintsler, S.; Spillner, J.; Schroder, T.; Kalverkamp, S.; Balakirski, G.; Gerhards, B.; et al. The effects of zinc- and copper-containing welding fumes on murine, rat and human precision-cut lung slices. J. Trace Elem. Med. Biol. 2018, 49, 192–201. [Google Scholar] [CrossRef]
- Johnson, B.D.; Kip, K.E.; Marroquin, O.C.; Ridker, P.M.; Kelsey, S.F.; Shaw, L.J.; Pepine, C.J.; Sharaf, B.; Bairey Merz, C.N.; Sopko, G.; et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: The National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004, 109, 726–732. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, T.; Qin, W.; An, C.; Wang, Z.; Zhang, M.; Zhang, Y.; Zhang, C.; An, F. Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol. Med. 2011, 17, 1357–1364. [Google Scholar] [CrossRef]
- Thompson, J.C.; Jayne, C.; Thompson, J.; Wilson, P.G.; Yoder, M.H.; Webb, N.; Tannock, L.R. A brief elevation of serum amyloid A is sufficient to increase atherosclerosis. J. Lipid Res. 2015, 56, 286–293. [Google Scholar] [CrossRef]
- Taj, T.; Gliga, A.R.; Hedmer, M.; Wahlberg, K.; Assarsson, E.; Lundh, T.; Tinnerberg, H.; Albin, M.; Broberg, K. Effect of welding fumes on the cardiovascular system: A six-year longitudinal study. Scand. J. Work Environ. Health 2021, 47, 52–61. [Google Scholar] [CrossRef] [PubMed]
- GESTIS. Institut fur Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung IFA GESTIS International Limit Values. Available online: https://ilv.ifa.dguv.de/substances (accessed on 1 March 2025).
- Dauter, U.M.; Gliga, A.R.; Albin, M.; Broberg, K. Longitudinal changes in cardiovascular disease-related proteins in welders. Int. Arch. Occup. Environ. Health 2024, 97, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Tatara, Y.; Ohishi, M.; Yamamoto, K.; Shiota, A.; Hayashi, N.; Iwamoto, Y.; Takeda, M.; Takagi, T.; Katsuya, T.; Ogihara, T.; et al. Macrophage inflammatory protein-1beta induced cell adhesion with increased intracellular reactive oxygen species. J. Mol. Cell. Cardiol. 2009, 47, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-T.; Yang, H.-Y.; Chen, C.; Chen, J.-W. CCL4 Inhibition in Atherosclerosis: Effects on Plaque Stability, Endothelial Cell Adhesiveness, and Macrophages Activation. Int. J. Mol. Sci. 2020, 21, 6567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Sheng, H.; Li, H.; Wang, R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Chem. 2019, 90, 25–80. [Google Scholar] [CrossRef]
- German Version EN 10346:2015; Continuously Hot-Dip Coated Steel Flat Products for Cold Forming—Technical Delivery Conditions. DINMedia: Berlin, Germany, 2015. Available online: https://www.dinmedia.de/de/norm/din-en-10346/226007287 (accessed on 1 March 2025).
- German version EN ISO 24373:2018; Welding Consumables—Solid Wires and Rods For Fusion Welding of Copper and Copper Alloys—Classification (ISO 24373:2018). DINMedia: Berlin, Germany, 2018. Available online: https://www.dinmedia.de/de/norm/din-en-iso-24373/295232973 (accessed on 1 March 2025).
- Oda, Y.; Nakamura, S.; Oki, I.; Kato, T.; Shinagawa, H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res. Environ. Mutagen. Relat. Subj. 1985, 147, 219–229. [Google Scholar] [CrossRef]
- DIN 38415-3:1996-12; German Standard Methods for the Examination of Water, Waste Water and Sludge—Sub-Animal Testing (Group T)—Part 3: Determination of the Genotype Potential of Water and Waste Water Components with the Umu-Test (T 3). DINMedia: Berlin, Germany, 1996. Available online: https://www.dinmedia.de/de/norm/din-38415-3/2902871 (accessed on 1 March 2025).
- ISO 13829:2000-03; Water Quality—Determination of the Genotoxicity of Water and Waste Water Using the Umu-Test. DINMedia: Berlin, Germany, 2000. Available online: https://www.dinmedia.de/de/norm/iso-13829/33008417 (accessed on 1 March 2025).

| Without Metabolic Activation | With Metabolic Activation | |||||||
|---|---|---|---|---|---|---|---|---|
| DLi | IR | GF | Genotoxicity | DLi | IR | GF | Genotoxicity | |
| Blank (Assay medium) | 100 | 1.11 ± 0.23 | 0.99 ± 0.1 | - | 100 | 1.03 ± 0.11 | 0.97 ± 0.06 | - |
| Welding fume [µg/mL] | ||||||||
| 0.001 | 100 | 1.06 ± 0.08 | 1.01 ± 0.11 | - | 100 | 0.88 ± 0.03 | 1.09 ± 0.05 | - |
| 0.01 | 100 | 1.06 ± 0.08 | 1.24 ± 0.11 | - | 100 | 1.06 ± 0.06 | 1.10 ± 0.06 | - |
| 0.1 | 100 | 1.0 ± 0.08 | 1.12 ± 0.13 | - | 100 | 1.0 ± 0.05 | 1.16 ± 0.03 | - |
| 1.0 | 100 | 1.07 ± 0.10 | 1.07 ± 0.11 | - | 100 | 0.89 ± 0.17 | 1.13 ± 0.04 | - |
| 10 | 100 | 1.24 ± 0.05 | 0.95 ± 0.11 | - | 100 | 1.07 ± 0.05 | 0.89 ± 0.08 | - |
| 100 | 100 | 1.05 ± 0.06 | 1.15 ± 0.15 | - | 100 | 1.24 ± 0.12 | 0.83 ± 0.11 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffens, J.; Kuth, K.; Kraus, T.; Dott, W.; Michael, S.; Baumann, R. Inflammatory Responses to Zn/Cu-Containing Welding Fume in Human Alveolar Epithelial and Macrophage Cell Lines, with MIP-1β/CCL4 as a Much More Sensitive Macrophage Activation Marker than IL-8 and TNF-α. Int. J. Mol. Sci. 2025, 26, 3843. https://doi.org/10.3390/ijms26083843
Steffens J, Kuth K, Kraus T, Dott W, Michael S, Baumann R. Inflammatory Responses to Zn/Cu-Containing Welding Fume in Human Alveolar Epithelial and Macrophage Cell Lines, with MIP-1β/CCL4 as a Much More Sensitive Macrophage Activation Marker than IL-8 and TNF-α. International Journal of Molecular Sciences. 2025; 26(8):3843. https://doi.org/10.3390/ijms26083843
Chicago/Turabian StyleSteffens, Jan, Katharina Kuth, Thomas Kraus, Wolfgang Dott, Sabrina Michael, and Ralf Baumann. 2025. "Inflammatory Responses to Zn/Cu-Containing Welding Fume in Human Alveolar Epithelial and Macrophage Cell Lines, with MIP-1β/CCL4 as a Much More Sensitive Macrophage Activation Marker than IL-8 and TNF-α" International Journal of Molecular Sciences 26, no. 8: 3843. https://doi.org/10.3390/ijms26083843
APA StyleSteffens, J., Kuth, K., Kraus, T., Dott, W., Michael, S., & Baumann, R. (2025). Inflammatory Responses to Zn/Cu-Containing Welding Fume in Human Alveolar Epithelial and Macrophage Cell Lines, with MIP-1β/CCL4 as a Much More Sensitive Macrophage Activation Marker than IL-8 and TNF-α. International Journal of Molecular Sciences, 26(8), 3843. https://doi.org/10.3390/ijms26083843






