Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics
Abstract
1. Introduction
2. Drug Combinations in Cancer Treatment
2.1. Targeting Tumor Heterogeneity
2.2. Blocking Alternative Survival Pathways
2.3. Modulation of Tumor Microenvironment
2.4. Targeting Tumor-Associated Inflammatory Microenvironment
2.5. Reduced Toxicity
2.6. Improved Tumoral Drug Distribution
3. Drug Delivery Systems for Multiple Drug Therapy
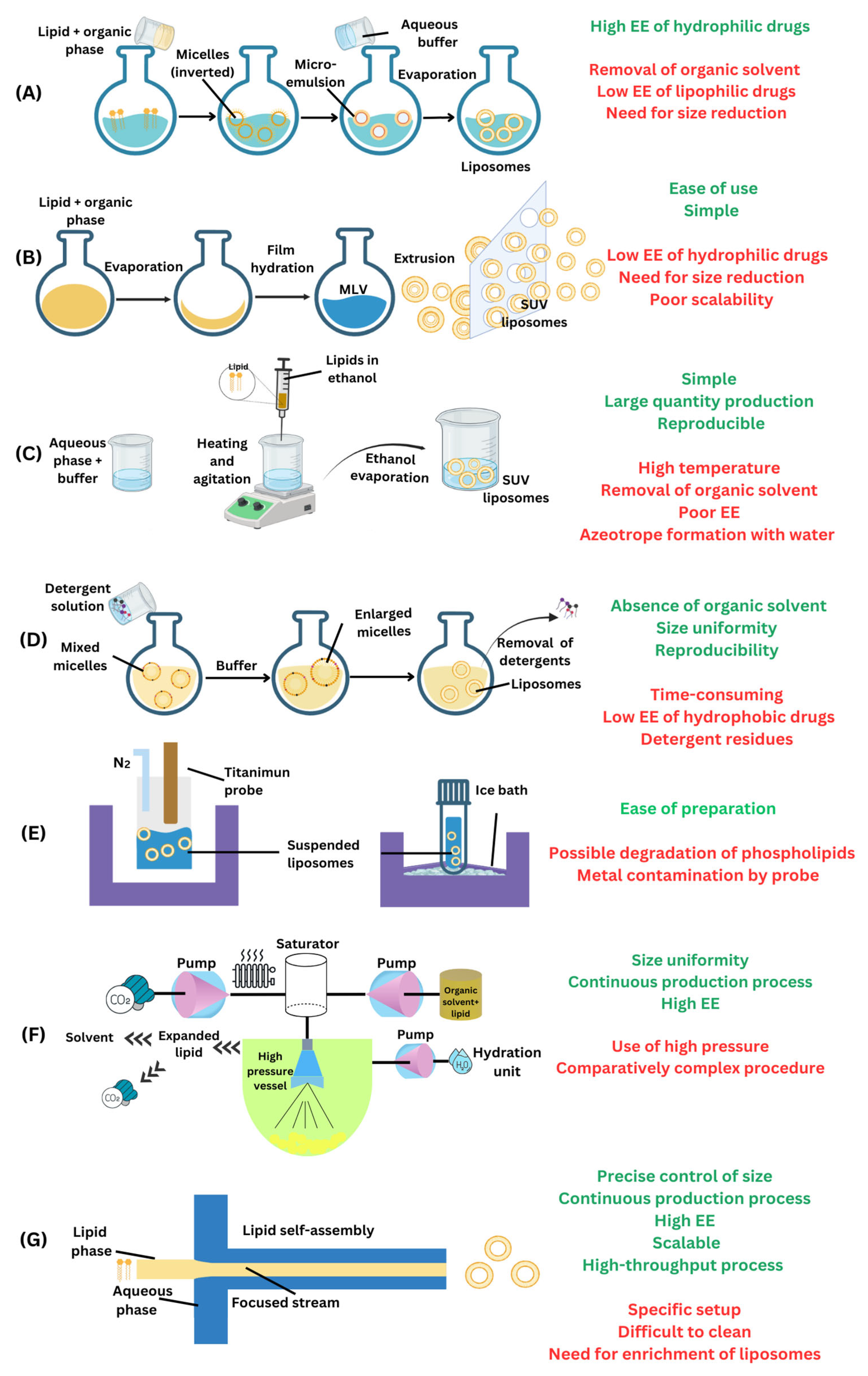
3.1. Liposomes
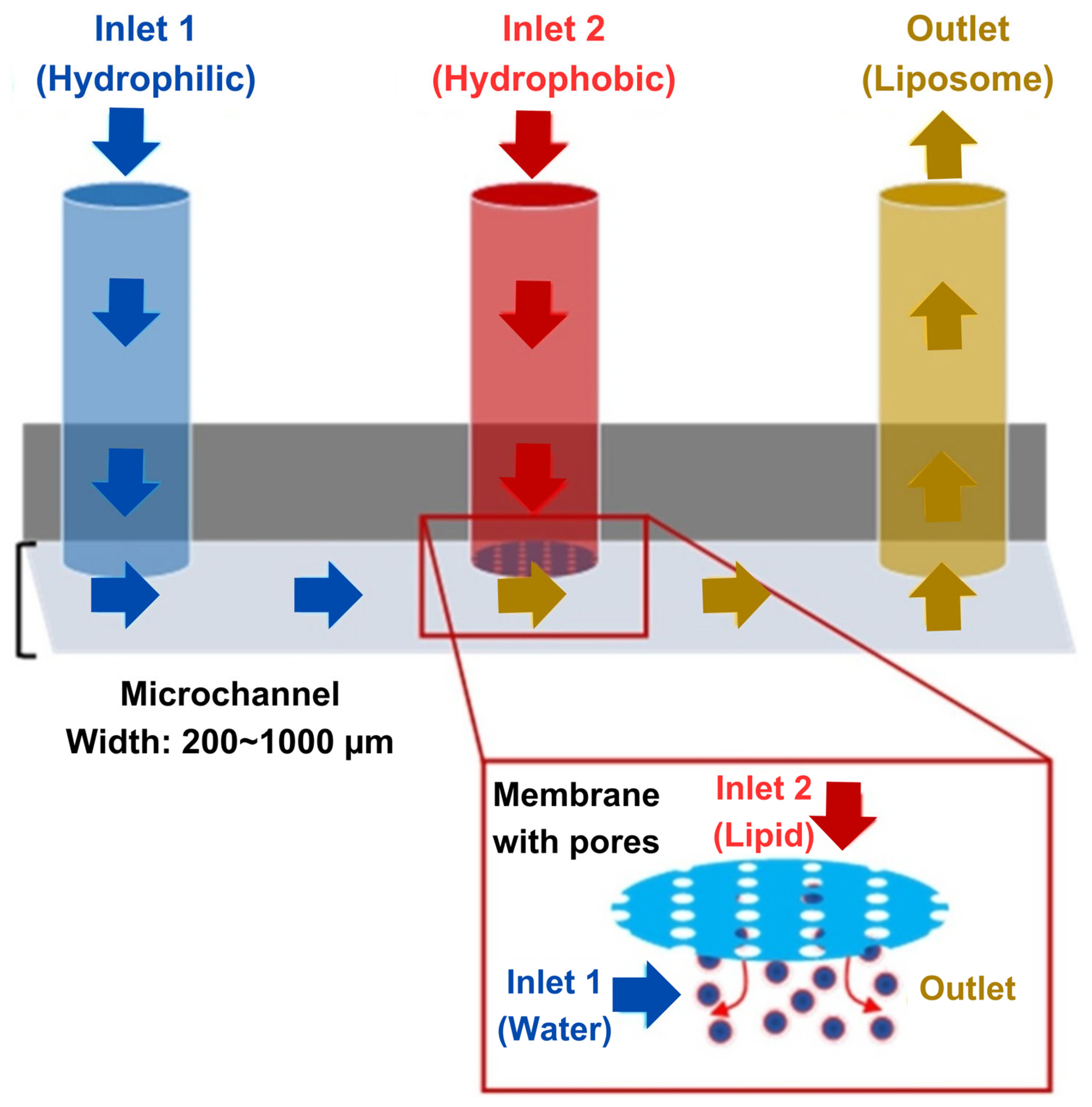
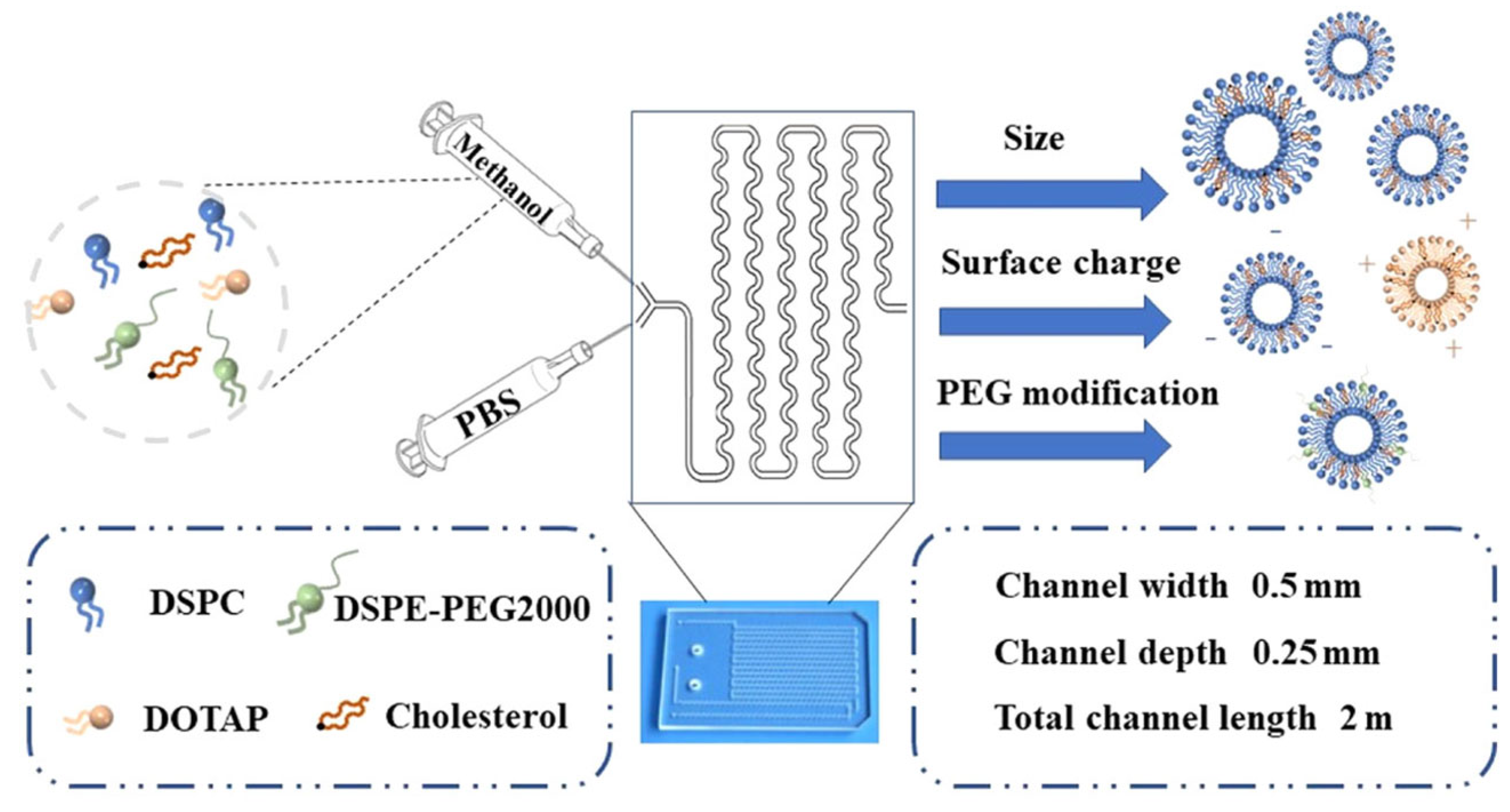
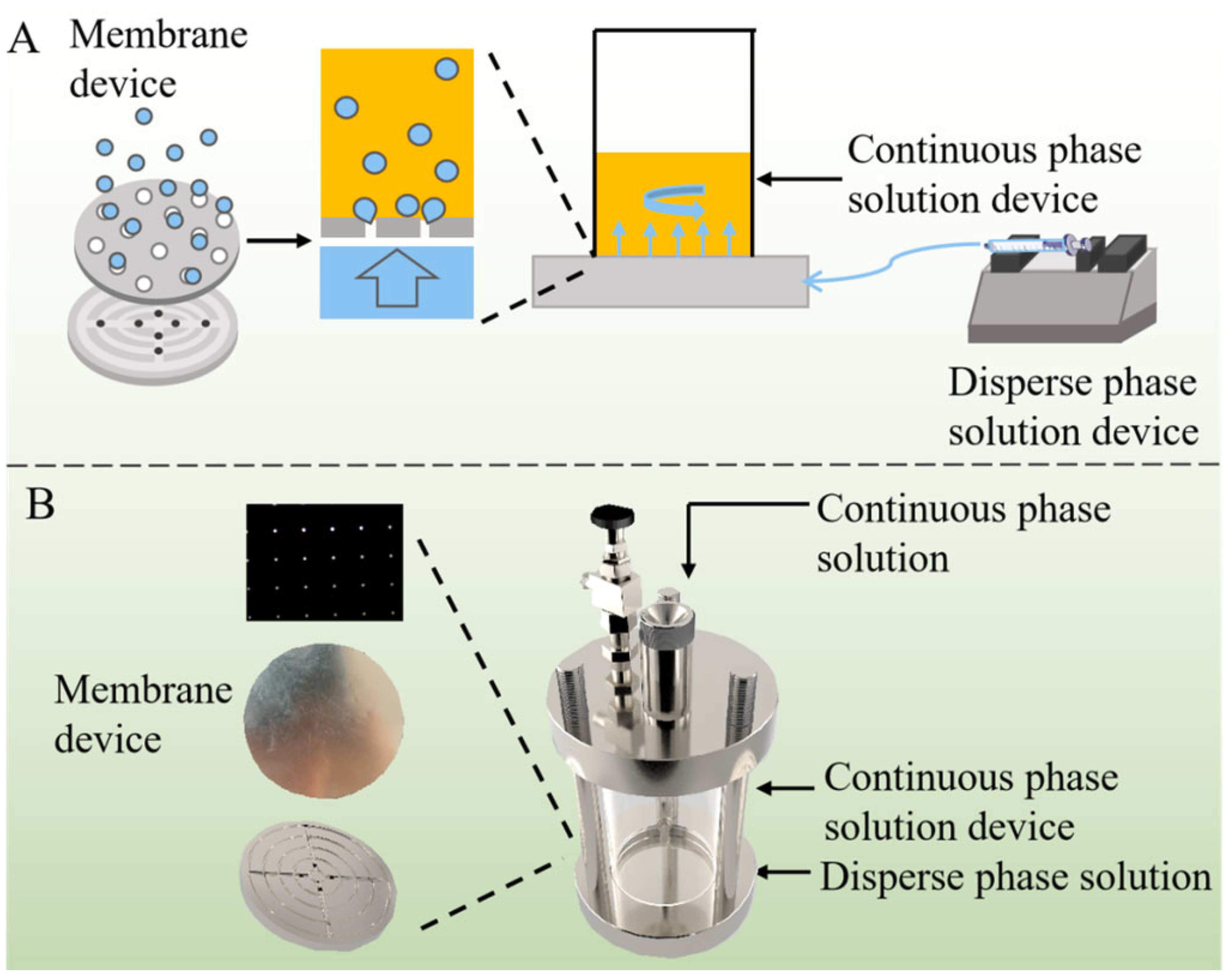
3.2. Exploring Microfluidics for Co-Encapsulation of Antineoplastic Agents
3.2.1. Technical Considerations
Micromixer Geometry
Flow Rates
Lipid Composition and Choice of Solvent
Drug Encapsulation Method
- 1.
- Optimization of microfluidic parametersMicrofluidic techniques allow precise control over mixing, flow rates, and liposome formation, which is essential for co-encapsulation.
- i.
- Flow rate ratio (FRR):
- Adjusting the ratio of the lipid-containing organic phase to the aqueous phase influences the liposome size and drug encapsulation efficiency. Higher FRRs enhance lipid precipitation, promoting simultaneous encapsulation of hydrophilic and hydrophobic drugs into the aqueous core and lipid bilayer, respectively.
- ii.
- Total flow rate (TFR):
- Increasing the TFR improves mixing efficiency, leading to a uniform drug distribution within liposomes. TFR adjustments can help fine-tune drug loading while minimizing variability.
- iii.
- Microfluidic mixer geometry:
- Mixers with optimized channel geometries (e.g., staggered herringbone or chaotic mixers) enhance the uniform distribution of both drugs during liposome synthesis.
- Rapid mixing at microfluidic junctions ensures simultaneous encapsulation without competitive displacement.
- 2.
- Use of drug-specific partitioningThe physicochemical properties of the drugs dictate their partitioning between the aqueous core and the lipid bilayer during co-encapsulation.
- i.
- Hydrophilic and hydrophobic drug combinations:
- Hydrophilic drugs are encapsulated within the aqueous core, while hydrophobic drugs partition into the lipid bilayer.
- Proper solubilization of both drugs in their respective phases before mixing is critical to achieving the desired ratio.
- Solvents with high miscibility (e.g., ethanol) are preferred for the lipid phase to achieve efficient hydrophobic drug encapsulation and maintain the integrity of the aqueous drug.
- ii.
- Lipid composition:
- Using lipids with optimal hydrophobicity and flexibility enhances bilayer stability and loading capacity for hydrophobic drugs.
- Incorporating cholesterol increases membrane rigidity, improving the retention of both drug types.
- 3.
- Remote loading for dual drugs
- This technique utilizes preformed liposomes with gradients (e.g., pH, ion, or charge) to actively load both drugs after liposome formation.
- A pH gradient can drive hydrophilic drug molecules into the aqueous core. Simultaneously, lipophilic molecules interact with the lipid bilayer.
Drug Release Behavior
Long-Term Storage Stability
3.2.2. Microfluidic-Assisted Design of Antineoplastic Agents Co-Encapsulated in Liposomes
3.2.3. Biological, Clinical, Safety, and Translational Considerations of Liposomal Cancer Therapy
Biological Efficacy Considerations
Immunogenicity and Safety Considerations
Clinical Considerations
Translational Considerations
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadam, U.T.; Roberts, I.; White, S.; Bednall, R.; Khunti, K.; Nilsson, P.M.; Lawson, C.A. Conceptualizing multiple drug use in patients with comorbidity and multimorbidity: Proposal for standard definitions beyond the term polypharmacy. J. Clin. Epidemiol. 2019, 106, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Majidpoor, J. Checkpoint inhibitor/interleukin-based combination therapy of cancer. Cancer Med. 2022, 11, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Chen, R.; Li, T.; Chen, M. Active Ingredients from Chinese Medicine for Combination Cancer Therapy. Int. J. Biol. Sci. 2023, 19, 3499–3525. [Google Scholar] [CrossRef] [PubMed]
- Blumer, V.; Vaduganathan, M. A rationale for dedicated trials of combination therapy in heart failure. Eur. Heart J. Suppl. 2022, 24, L49–L52. [Google Scholar] [CrossRef]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef]
- Xie, X.; Wu, C.; Hao, Y.; Wang, T.; Yang, Y.; Cai, P.; Zhang, Y.; Huang, J.; Deng, K.; Yan, D.; et al. Benefits and risks of drug combination therapy for diabetes mellitus and its complications: A comprehensive review. Front. Endocrinol. 2023, 14, 1301093. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Yan, D. Editorial: Benefits and risks of drug combination therapy for chronic metabolic diseases. Front. Endocrinol. 2024, 15, 1390248. [Google Scholar] [CrossRef]
- Huuskes, B.M.; Wise, A.F.; Cox, A.J.; Lim, E.X.; Payne, N.L.; Kelly, D.J.; Samuel, C.S.; Ricardo, S.D. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 2015, 29, 540–553. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Lagerberg, T.; Sjölander, A.; Gibbons, R.D.; Quinn, P.D.; D’Onofrio, B.M.; Hellner, C.; Lichtenstein, P.; Fazel, S.; Chang, Z. Use of central nervous system drugs in combination with selective serotonin reuptake inhibitor treatment: A Bayesian screening study for risk of suicidal behavior. Front. Psychiatry 2022, 13, 1012650. [Google Scholar] [CrossRef]
- Chrastina, M.; Poništ, S.; Tóth, J.; Czigle, S.; Pašková, Ľ.; Vyletelová, V.; Švík, K.; Bauerová, K. Combination Therapy of Carnosic Acid and Methotrexate Effectively Suppressed the Inflammatory Markers and Oxidative Stress in Experimental Arthritis. Molecules 2022, 27, 7115. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, C.; Bu, Y.; Li, Z.; Zheng, X.; Qiu, S.; Machuki, J.O.a.; Zhang, L.; Yang, Y.; Guo, K.; et al. A multifunctional nano-therapeutic platform based on octahedral yolk-shell Au NR@CuS: Photothermal/photodynamic and targeted drug delivery tri-combined therapy for rheumatoid arthritis. Biomaterials 2021, 277, 121088. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.E.; Bueno, R.V.; de Souza, J.O.; Zanini, C.L.; Cruz, F.C.; Oliva, G.; Guido, R.V.C.; Aguiar, A.C.C. Antiplasmodial profile of selected compounds from Malaria Box: In vitro evaluation, speed of action and drug combination studies. Malar. J. 2019, 18, 447. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Maeda, K. HIV Reservoirs and Treatment Strategies toward Curing HIV Infection. Int. J. Mol. Sci. 2024, 25, 2621. [Google Scholar] [CrossRef]
- Nath, B.J.; Sadri, K.; Sarmah, H.K.; Hosseini, K. An optimal combination of antiretroviral treatment and immunotherapy for controlling HIV infection. Math. Comput. Simul. 2024, 217, 226–243. [Google Scholar] [CrossRef]
- Dartois, V.; Dick, T. Therapeutic developments for tuberculosis and nontuberculous mycobacterial lung disease. Nat. Rev. Drug Discov. 2024, 23, 381–403. [Google Scholar] [CrossRef]
- Dawson, R.; Diacon, A.H.; Takuva, S.; Liu, Y.; Zheng, B.; Karwe, V.; Hafkin, J. Quabodepistat in combination with delamanid and bedaquiline in participants with drug-susceptible pulmonary tuberculosis: Protocol for a multicenter, phase 2b/c, open-label, randomized, dose-finding trial to evaluate safety and efficacy. Trials 2024, 25, 70. [Google Scholar] [CrossRef]
- Smith, C.S.; Aerts, A.; Saunderson, P.; Kawuma, J.; Kita, E.; Virmond, M. Multidrug therapy for leprosy: A game changer on the path to elimination. Lancet Infect. Dis. 2017, 17, e293–e297. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Boshuizen, J.; Peeper, D.S. Rational Cancer Treatment Combinations: An Urgent Clinical Need. Mol. Cell 2020, 78, 1002–1018. [Google Scholar] [CrossRef]
- National Cancer Institute. Drugs Approved for Different Types of Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/cancer-type (accessed on 5 April 2024).
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021, 184, 2239–2254.e2239. [Google Scholar] [CrossRef]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef]
- Gómez-Valenzuela, F.; Escobar, E.; Pérez-Tomás, R.; Montecinos, V.P. The Inflammatory Profile of the Tumor Microenvironment, Orchestrated by Cyclooxygenase-2, Promotes Epithelial-Mesenchymal Transition. Front. Oncol. 2021, 11, 686792. [Google Scholar] [CrossRef]
- Kolawole, O.R.; Kashfi, K. NSAIDs and Cancer Resolution: New Paradigms beyond Cyclooxygenase. Int. J. Mol. Sci. 2022, 23, 1432. [Google Scholar] [CrossRef]
- Rodrigues, P.; Bangali, H.; Hammoud, A.; Mustafa, Y.F.; Al-Hetty, H.R.A.K.; Alkhafaji, A.T.; Deorari, M.M.; Al-Taee, M.M.; Zabibah, R.S.; Alsalamy, A. COX 2-inhibitors; a thorough and updated survey into combinational therapies in cancers. Med. Oncol. 2024, 41, 41. [Google Scholar] [CrossRef]
- Changou, C.A.; Shiah, H.S.; Chen, L.T.; Liu, S.; Luh, F.; Liu, S.H.; Cheng, Y.C.; Yen, Y. A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma. Oncologist 2020, 26, e367–e373. [Google Scholar] [CrossRef]
- Marcucci, F.; Berenson, R.; Corti, A. Improving drug uptake and penetration into tumors: Current and forthcoming opportunities. Front. Oncol. 2013, 3, 161. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Barzegar-Fallah, A.; Alshaibani, L.; Pittalà, V. Nitric oxide-releasing nanoparticles improve doxorubicin anticancer activity. Int. J. Nanomed. 2018, 13, 7771–7787. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef]
- Beyer, I.; van Rensburg, R.; Strauss, R.; Li, Z.; Wang, H.; Persson, J.; Yumul, R.; Feng, Q.; Song, H.; Bartek, J.; et al. Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res. 2011, 71, 7080–7090. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Velez, I.; Belardi, B. Storming the gate: New approaches for targeting the dynamic tight junction for improved drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114905. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhong, D.; Gong, P.Y. Synergistic effect of paclitaxel and verapamil to overcome multi-drug resistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 516, 183–188. [Google Scholar] [CrossRef]
- Ocaña-Arakachi, K.; Martínez-Herculano, J.; Jurado, R.; Llaguno-Munive, M.; Garcia-Lopez, P. Pharmacokinetics and Anti-Tumor Efficacy of PEGylated Liposomes Co-Loaded with Cisplatin and Mifepristone. Pharmaceuticals 2023, 16, 1337. [Google Scholar] [CrossRef]
- Yan, V.C.; Butterfield, H.E.; Poral, A.H.; Yan, M.J.; Yang, K.L.; Pham, C.D.; Muller, F.L. Why Great Mitotic Inhibitors Make Poor Cancer Drugs. Trends Cancer 2020, 6, 924–941. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Mayer, L.D. Improving combination cancer therapy: The CombiPlex® development platform. Future Oncol. 2018, 14, 1317–1332. [Google Scholar] [CrossRef]
- Liboiron, B.D.; Mayer, L.D. Nanoscale particulate systems for multidrug delivery: Towards improved combination chemotherapy. Ther. Deliv. 2014, 5, 149–171. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, M.; Wang, H.; Sun, H.; Zhu, J.; Zhao, W.; Fang, Q.; Yu, J.; Chen, P.; Wu, S.; et al. A narrative review of tumor heterogeneity and challenges to tumor drug therapy. Ann. Transl. Med. 2021, 9, 1351. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, S.; Sun, M.; Shi, J.; Zhang, H.; Yu, H.; Yang, Z. Recent advances and clinical translation of liposomal delivery systems in cancer therapy. Eur. J. Pharm. Sci. 2024, 193, 106688. [Google Scholar] [CrossRef]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review. Adv. Pharm. Bull. 2023, 13, 7–23. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Lelkes, P.I. Clinical prospects for liposomes. Med. Phys. 1982, 9, 149–175. [Google Scholar] [CrossRef]
- Sanati, M.; Afshari, A.R.; Ahmadi, S.S.; Kesharwani, P.; Sahebkar, A. Advances in liposome-based delivery of RNA therapeutics for cancer treatment. Prog. Mol. Biol. Transl. Sci. 2024, 204, 177–218. [Google Scholar] [CrossRef]
- Miatmoko, A.; Octavia, R.T.; Araki, T.; Annoura, T.; Sari, R. Advancing liposome technology for innovative strategies against malaria. Saudi Pharm. J. 2024, 32, 102085. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, 1904673. [Google Scholar] [CrossRef] [PubMed]
- Kotouček, J.; Hubatka, F.; Mašek, J.; Kulich, P.; Velínská, K.; Bezděková, J.; Fojtíková, M.; Bartheldyová, E.; Tomečková, A.; Stráská, J.; et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci. Rep. 2020, 10, 5595. [Google Scholar] [CrossRef]
- Gbian, D.L.; Omri, A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines 2022, 10, 2137. [Google Scholar] [CrossRef]
- Sebastian, V. Toward continuous production of high-quality nanomaterials using microfluidics: Nanoengineering the shape, structure and chemical composition. Nanoscale 2022, 14, 4411–4447. [Google Scholar] [CrossRef]
- Naghib, S.M.; Mohammad-Jafari, K. Microfluidics-mediated Liposomal Nanoparticles for Cancer Therapy: Recent Developments on Advanced Devices and Technologies. Curr. Top. Med. Chem. 2024, 24, 1185–1211. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef]
- Akar, S.; Fardindoost, S.; Hoorfar, M. High throughput microfluidics-based synthesis of PEGylated liposomes for precise size control and efficient drug encapsulation. Colloids Surf. B Biointerfaces 2024, 238, 113926. [Google Scholar] [CrossRef]
- Han, J.Y.; La Fiandra, J.N.; DeVoe, D.L. Microfluidic vortex focusing for high throughput synthesis of size-tunable liposomes. Nat. Commun. 2022, 13, 6997. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; Horst, J.H.t.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bianco, M.; Carbone, L.; Perrone, E.; Amato, F.; Maruccio, G.; Rendina, F.; Arima, V. Continuous-Flow Production of Injectable Liposomes via a Microfluidic Approach. Materials 2017, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Michelon, M.; Oliveira, D.R.B.; de Figueiredo Furtado, G.; Gaziola de la Torre, L.; Cunha, R.L. High-throughput continuous production of liposomes using hydrodynamic flow-focusing microfluidic devices. Colloids Surf. B Biointerfaces 2017, 156, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Mochizuki, A.; Sou, K.; Takeoka, S. Evaluation of a static mixer as a new microfluidic method for liposome formulation. Front. Bioeng. Biotechnol. 2023, 11, 1229829. [Google Scholar] [CrossRef]
- Bing, L.; Hao, M.; Zhenbo, T. Preparation of lipid nanoparticles by micro-mixer Process simulation and experimental study. BIO Web Conf. 2024, 111, 01009. [Google Scholar] [CrossRef]
- Ceccato, B.T.; Vianna, S.S.V.; de la Torre, L.G. Numerical and experimental investigation of chaotic advection and diffusion mixing effects in 3D multihelical microfluidics for liposome synthesis. Chem. Eng. Sci. 2024, 295, 120190. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine 2019, 18, 146–156. [Google Scholar] [CrossRef]
- López, R.R.; Ocampo, I.; Font de Rubinat, P.G.; Sánchez, L.M.; Alazzam, A.; Tsering, T.; Bergeron, K.F.; Camacho-Léon, S.; Burnier, J.V.; Mounier, C.; et al. Parametric Study of the Factors Influencing Liposome Physicochemical Characteristics in a Periodic Disturbance Mixer. Langmuir 2021, 37, 8544–8556. [Google Scholar] [CrossRef]
- Weaver, E.; Mathew, E.; Caldwell, J.; Hooker, A.; Uddin, S.; Lamprou, D.A. The manufacturing of 3D-printed microfluidic chips to analyse the effect upon particle size during the synthesis of lipid nanoparticles. J. Pharm. Pharmacol. 2023, 75, 245–252. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.S.; Jung, H.S. Precise control of liposome size using characteristic time depends on solvent type and membrane properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Kimura, N.; Okada, Y.; Shimizu, K.; Shibata, K.; Miyazaki, Y.; Ishida, A.; Yonezawa, K.; Shimizu, N.; Shinoda, W.; et al. Understanding the effects of ethanol on the liposome bilayer structure using microfluidic-based time-resolved small-angle X-ray scattering and molecular dynamics simulations. Nanoscale Adv. 2024, 6, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgess, D.J. Predicting hydrophilic drug encapsulation inside unilamellar liposomes. Int. J. Pharm. 2012, 423, 410–418. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Saorin, A.; Saorin, G.; Duzagac, F.; Parisse, P.; Cao, N.; Corona, G.; Cavarzerani, E.; Rizzolio, F. Microfluidic production of amiodarone loaded nanoparticles and application in drug repositioning in ovarian cancer. Sci. Rep. 2024, 14, 6280. [Google Scholar] [CrossRef]
- Li, T.; Cipolla, D.; Rades, T.; Boyd, B.J. Drug nanocrystallisation within liposomes. J. Control. Release 2018, 288, 96–110. [Google Scholar] [CrossRef]
- Pisani, S.; Di Martino, D.; Cerri, S.; Genta, I.; Dorati, R.; Bertino, G.; Benazzo, M.; Conti, B. Investigation and Comparison of Active and Passive Encapsulation Methods for Loading Proteins into Liposomes. Int. J. Mol. Sci. 2023, 24, 13542. [Google Scholar] [CrossRef]
- Nam, J.H.; Kim, S.Y.; Seong, H. Investigation on Physicochemical Characteristics of a Nanoliposome-Based System for Dual Drug Delivery. Nanoscale Res. Lett. 2018, 13, 101. [Google Scholar] [CrossRef]
- Jaradat, E.; Meziane, A.; Lamprou, D.A. Conventional vs PEGylated loaded liposomal formulations by microfluidics for delivering hydrophilic chemotherapy. Int. J. Pharm. 2024, 655, 124077. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy-Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lin, X.; Tan, X.; Li, J.; Wei, Z.; Zhang, D.; Zheng, Y.; Zheng, A.X.; Zhao, B.; Zeng, Y.; et al. Photoresponsive Nanovehicle for Two Independent Wavelength Light-Triggered Sequential Release of P-gp shRNA and Doxorubicin To Optimize and Enhance Synergistic Therapy of Multidrug-Resistant Cancer. ACS Appl. Mater. Interfaces 2018, 10, 19416–19427. [Google Scholar] [CrossRef]
- Andra, V.; Pammi, S.V.N.; Bhatraju, L.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Nounou, M.M.; El-Khordagui, L.K.; Khalafallah, N.A.; Khalil, S.A. In vitro release of hydrophilic and hydrophobic drugs from liposomal dispersions and gels. Acta Pharm. 2006, 56, 311–324. [Google Scholar]
- Song, F.; Yang, G.; Wang, Y.; Tian, S. Effect of phospholipids on membrane characteristics and storage stability of liposomes. Innov. Food Sci. Emerg. Technol. 2022, 81, 103155. [Google Scholar] [CrossRef]
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D.N. Effect of Lipid Composition on In Vitro Release and Skin Deposition of Curcumin Encapsulated Liposomes. J. Nanomater. 2016, 2016, 4535790. [Google Scholar] [CrossRef]
- Sheikholeslami, B.; Lam, N.W.; Dua, K.; Haghi, M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022, 300, 120574. [Google Scholar] [CrossRef]
- Liu, Y.; Tamam, H.; Yeo, Y. Mixed Liposome Approach for Ratiometric and Sequential Delivery of Paclitaxel and Gemcitabine. AAPS PharmSciTech 2018, 19, 693–699. [Google Scholar] [CrossRef]
- Tang, T.; Gong, Y.; Gao, Y.; Pang, X.; Liu, S.; Xia, Y.; Liu, D.; Zhu, L.; Fan, Q.; Sun, X. A pH-responsive liposomal nanoplatform for co-delivery of a Pt(IV) prodrug and cinnamaldehyde for effective tumor therapy. Front. Bioeng. Biotechnol. 2023, 11, 1191534. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, F.; Steffen, K.J.; Mallik, S. Enzyme-Responsive Liposomes for the Delivery of Anticancer Drugs. Bioconjug. Chem. 2017, 28, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.J.; Wang, Y.Y.; Li, S.T.; Kong, L.; Li, X.T.; Ma, L.L.; Liu, X.X. Construction of ROS-Responsive Hyaluronic Acid Modified Paclitaxel and Diosgenin Liposomes and Study on Synergistic Enhancement of Anti-Ovarian Cancer Efficacy. Int. J. Nanomed. 2024, 19, 5193–5211. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Preis, E.; Dayyih, A.A.; Alawak, M.; El-Said Azzazy, H.M.; Bakowsky, U.; Shoeib, T. Thermosensitive Liposomes Encapsulating Nedaplatin and Picoplatin Demonstrate Enhanced Cytotoxicity against Breast Cancer Cells. ACS Omega 2022, 7, 42115–42125. [Google Scholar] [CrossRef]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; He, J.; Yu, L.; Li, X.; Zhang, J.; Li, S.; Zhang, C.; Kagan, J.C.; Karp, J.M.; et al. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity. Nat. Commun. 2023, 14, 3877. [Google Scholar] [CrossRef]
- Pilkington, C.P.; Gispert, I.; Chui, S.Y.; Seddon, J.M.; Elani, Y. Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release. Nat. Chem. 2024, 16, 1612–1620. [Google Scholar] [CrossRef]
- Sainaga Jyothi, V.G.S.; Bulusu, R.; Venkata Krishna Rao, B.; Pranothi, M.; Banda, S.; Kumar Bolla, P.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tian, F.; Pan, Y.; Zhang, T.; Deng, L.; Jiang, H.; Han, J.; Liu, J.; Zhao, Y.; Liu, W. Emerging mechanistic insights into liposomal stability: Full process management from production and storage to food application. Chem. Eng. J. 2025, 505, 159552. [Google Scholar] [CrossRef]
- Lehman, S.E.; Benkstein, K.D.; Cleveland, T.E.I.V.; Anderson, K.W.; Carrier, M.J.; Vreeland, W.N. Particle Metrology Approach to Understanding How Storage Conditions Affect Long-Term Liposome Stability. Langmuir 2023, 39, 12313–12323. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Oude Blenke, E.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J.A. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Andreana, I.; Bincoletto, V.; Manzoli, M.; Rodà, F.; Giarraputo, V.; Milla, P.; Arpicco, S.; Stella, B. Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches. Materials 2023, 16, 1212. [Google Scholar] [CrossRef]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef]
- Sydykov, B.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Storage stability of liposomes stored at elevated subzero temperatures in DMSO/sucrose mixtures. PLoS ONE 2018, 13, e0199867. [Google Scholar] [CrossRef]
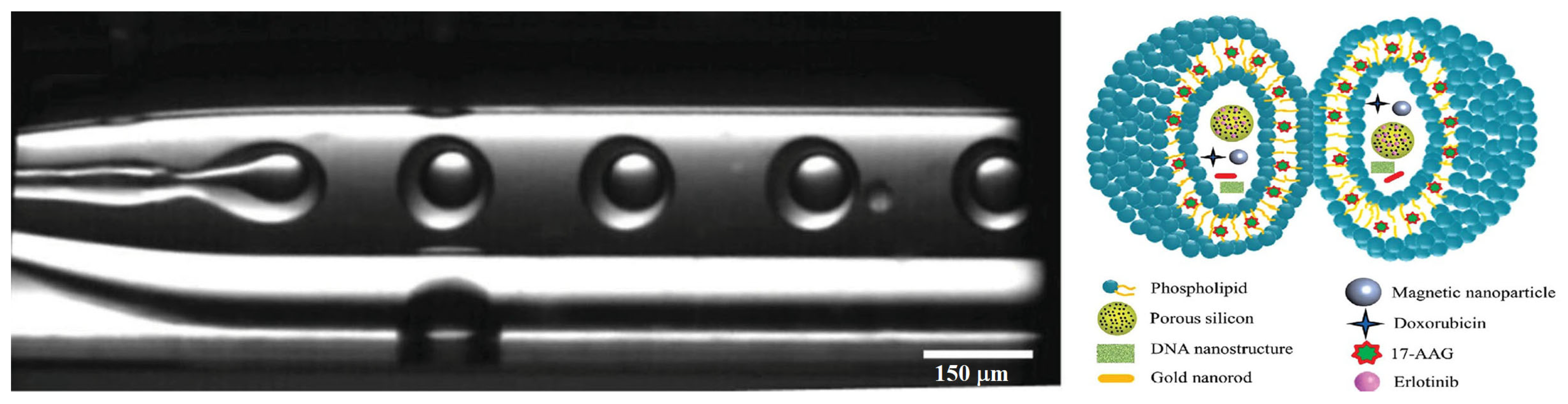
- Kong, F.; Zhang, X.; Zhang, H.; Qu, X.; Chen, D.; Servos, M.; Mäkilä, E.; Salonen, J.; Santos, H.A.; Hai, M.; et al. Inhibition of Multidrug Resistance of Cancer Cells by Co-Delivery of DNA Nanostructures and Drugs Using Porous Silicon Nanoparticles@Giant Liposomes. Adv. Funct. Mater. 2015, 25, 3330–3340. [Google Scholar] [CrossRef]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic potential and limitations of curcumin as antimetastatic agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-C.; Park, K.-M.; Hong, C.R.; Kim, J.-C.; Yang, S.-H.; Yu, H.-S.; Paik, H.-D.; Pan, C.-H.; Chang, P.-S. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf. Physicochem. Eng. Asp. 2020, 594, 124670. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Charest, G.; Tippayamontri, T.; Shi, M.; Wehbe, M.; Anantha, M.; Bally, M.; Sanche, L. Concomitant Chemoradiation Therapy with Gold Nanoparticles and Platinum Drugs Co-Encapsulated in Liposomes. Int. J. Mol. Sci. 2020, 21, 4848. [Google Scholar] [CrossRef]
- Lv, S.; Jing, R.; Liu, X.; Shi, H.; Shi, Y.; Wang, X.; Zhao, X.; Cao, K.; Lv, Z. One-Step Microfluidic Fabrication of Multi-Responsive Liposomes for Targeted Delivery of Doxorubicin Synergism with Photothermal Effect. Int. J. Nanomed. 2021, 16, 7759–7772. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Yin, J.; Liu, G.; Chen, D. Single-Step Synthesis of Highly Tunable Multifunctional Nanoliposomes for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 21301–21309. [Google Scholar] [CrossRef]
- Donkor, M.; Jones, H.P. The Proposition of the Pulmonary Route as an Attractive Drug Delivery Approach of Nano-Based Immune Therapies and Cancer Vaccines to Treat Lung Tumors. Front. Nanotechnol. 2021, 3, 635194. [Google Scholar] [CrossRef]
- Rong, A.; Han, Z.; Wang, T.; Zhu, M.; Zhou, M.; Sun, X. Pulmonary delivery of nano-particles for lung cancer diagnosis and therapy: Recent advances and future prospects. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1933. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Xu, M.; Luo, Y.; Wang, B.; Kuang, M.; Liu, X.; Sun, M.; Guo, Y.; Teng, L.; et al. Pulmonary delivery of liposomes co-loaded with SN38 prodrug and curcumin for the treatment of lung cancer. Eur. J. Pharm. Biopharm. 2022, 179, 156–165. [Google Scholar] [CrossRef]
- Jin, Y.; Tomeh, M.A.; Zhang, P.; Su, M.; Zhao, X.; Cai, Z. Microfluidic fabrication of photo-responsive Ansamitocin P-3 loaded liposomes for the treatment of breast cancer. Nanoscale 2023, 15, 3780–3795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, H.; Ye, P.; Zhu, L.; Ren, Y.; Lei, J. Controlled production of liposomes with novel microfluidic membrane emulsification for application of entrapping hydrophilic and lipophilic drugs. J. Ind. Eng. Chem. 2024, 131, 470–480. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Middelberg, A.P.J.; Zhao, C.X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef]
- Dacos, M.; Immordino, B.; Diroff, E.; Sicard, G.; Kosta, A.; Rodallec, A.; Giacometti, S.; Ciccolini, J.; Fanciullino, R. Pegylated liposome encapsulating docetaxel using microfluidic mixing technique: Process optimization and results in breast cancer models. Int. J. Pharm. 2024, 656, 124091. [Google Scholar] [CrossRef]
- Arduino, I.; Di Fonte, R.; Sommonte, F.; Lopedota, A.A.; Porcelli, L.; Li, J.; Serrati, S.; Bártolo, R.; Santos, H.A.; Iacobazzi, R.M.; et al. Fabrication of Biomimetic Hybrid Liposomes via Microfluidic Technology: Homotypic Targeting and Antitumor Efficacy Studies in Glioma Cells. Int. J. Nanomed. 2024, 19, 13217–13233. [Google Scholar] [CrossRef]
- Shan, H.; Sun, X.; Liu, X.; Sun, Q.; He, Y.; Chen, Z.; Lin, Q.; Jiang, Z.; Chen, X.; Chen, Z.; et al. One-Step Formation of Targeted Liposomes in a Versatile Microfluidic Mixing Device. Small 2023, 19, 2205498. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Sani, G.M.; Donnelley, M.A.; Figueroa, J.K.; Ciuffetelli, R.; Trigg, K.; Arredondo, J.; Koff, A.; Nearing, M.; Loque, I.C.; et al. Liposomal amphotericin B and complement activation-related pseudoallergy (CARPA). Antimicrob. Agents Chemother. 2025, 69, e0169224. [Google Scholar] [CrossRef]
- Gordon, S. The Mononuclear Phagocyte System: Features Relevant to Interactions with Liposomes. In Targeting of Drugs 6: Strategies for Stealth Therapeutic Systems; Gregoriadis, G., McCormack, B., Eds.; Springer US: Boston, MA, USA, 1998; pp. 15–23. [Google Scholar]
- Belyaev, I.B.; Mirkasymov, A.B.; Rodionov, V.I.; Babkova, J.S.; Nikitin, P.I.; Deyev, S.M.; Zelepukin, I.V. MPS blockade with liposomes controls pharmacokinetics of nanoparticles in a size-dependent manner. Biomed. Mater. 2024, 19, 065022. [Google Scholar] [CrossRef]
- Taher, M.; Susanti, D.; Haris, M.S.; Rushdan, A.A.; Widodo, R.T.; Syukri, Y.; Khotib, J. PEGylated liposomes enhance the effect of cytotoxic drug: A review. Heliyon 2023, 9, e13823. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yuan, C.; Xu, X.; Zhou, W.; Huang, Y.; Lu, H.; Zheng, Y.; Luo, G.; Shang, J.; et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines 2023, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, H.; Giannino, J.; Wu, Y.; Cheng, C. Biodegradable zwitterionic polymers as PEG alternatives for drug delivery. J. Polym. Sci. 2024, 62, 2231–2250. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qi, C.; Sun, C.; Huo, F.; Jiang, X. Poly(ethylene glycol) alternatives in biomedical applications. Nano Today 2023, 48, 101738. [Google Scholar] [CrossRef]
- Kurzrock, R.; Lin, C.C.; Wu, T.C.; Hobbs, B.P.; Pestana, R.M.; Hong, D.S. Moving Beyond 3+3: The Future of Clinical Trial Design. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e133–e144. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.Y.; Kim, J.H.; Kim, D.Y.; An, H.J.; Bae, S.; Park, H.-S.; Kang, J.H. Precision Oncology Clinical Trials: A Systematic Review of Phase II Clinical Trials with Biomarker-Driven, Adaptive Design. Cancer Res. Treat. 2024, 56, 991–1013. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Lopez, A.; Sandborn, W. Head-to-head Compsarative Studies: Challenges and Opportunities? J. Crohn's Colitis 2016, 11, S567–S575. [Google Scholar] [CrossRef][Green Version]
- Dagenais, S.; Russo, L.; Madsen, A.; Webster, J.; Becnel, L. Use of Real-World Evidence to Drive Drug Development Strategy and Inform Clinical Trial Design. Clin. Pharmacol. Ther. 2022, 111, 77–89. [Google Scholar] [CrossRef]
- Crestan, D.; Trojniak, M.P.; Francescon, S.; Fornasier, G.; Baldo, P. Pharmacovigilance of anti-cancer medicines: Opportunities and challenges. Expert Opin. Drug Saf. 2020, 19, 849–860. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef]
- Blair, H.A. Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903–1910. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef] [PubMed]
- Sealy, A. Manufacturing Moonshot: How Pfizer Makes Its Millions of Covid-19 Vaccine Doses; CNN Health: Atlanta, GA, USA, 2021; Available online: https://edition.cnn.com/2021/03/31/health/pfizer-vaccine-manufacturing/index.html (accessed on 5 April 2024).

| Sr No. | Cancer | Drug Combinations |
|---|---|---|
| 1 | Acute Lymphoblastic Leukemia | Cyclophosphamide–Vincristine–Doxorubicin–Dexamethasone |
| 2 | Acute Myeloid Leukemia | Cytarabine–Daunorubicin–Etoposide |
| 3 | Bladder | Gemcitabine–Cisplatin |
| 4 | Methotrexate–Vinblastine–Doxorubicin–Cisplatin | |
| 5 | Brain | Procarbazine–Lomustine–Vincristine |
| 6 | Breast | Doxorubicin–Cyclophosphamide |
| 7 | Doxorubicin–Cyclophosphamide–Paclitaxel | |
| 8 | Doxorubicin–Cyclophosphamide–Fluorouracil | |
| 9 | Methotrexate–Cyclophosphamide–Fluorouracil | |
| 10 | Epirubicin–Cyclophosphamide–Fluorouracil | |
| 11 | Doxorubicin–Cyclophosphamide–Docetaxel | |
| 12 | Cervical | Gemcitabine–Cisplatin |
| 13 | Carboplatin–Paclitaxel | |
| 14 | Chronic Lymphocytic Leukemia | Chlorambucil–Prednisone |
| 15 | Cyclophosphamide–Vincristine–Prednisone | |
| 16 | Colorectal | Capecitabine–Oxaliplatin |
| 17 | Capecitabine–Irinotecan | |
| 18 | Leucovorin–Fluorouracil–Irinotecan | |
| 19 | Leucovorin–Fluorouracil–Irinotecan–Bevacizumab | |
| 20 | Leucovorin–Fluorouracil–Irinotecan–Cetuximab | |
| 21 | Leucovorin–Fluorouracil–Oxaliplatin | |
| 22 | Endometrial | Carboplatin–Paclitaxel |
| 23 | Esophageal | Capecitabine–Irinotecan |
| 24 | Gastric | Leucovorin–Fluorouracil |
| 25 | Capecitabine–Irinotecan | |
| 26 | Docetaxel–Cisplatin–Fluorouracil | |
| 27 | Head and Neck | Carboplatin–Paclitaxel |
| 28 | Docetaxel–Cisplatin–Fluorouracil | |
| 29 | Hodgkin Lymphoma | Ifosfamide–Carboplatin–Etoposide |
| 30 | Doxorubicin–Bleomycin–Vinblastine–Etoposide | |
| 31 | Doxorubicin–Bleomycin–Vinblastine–Dacarbazine | |
| 32 | Doxorubicin–Vincristine–Procarbazine–Prednisone | |
| 33 | Mechlorethamine–Vincristine–Prednisone–Dacarbazine | |
| 34 | Vincristine–Etoposide–Prednisone–Doxorubicin | |
| 35 | Cyclophosphamide–Vincristine–Procarbazine–Prednisone | |
| 36 | Cyclophosphamide–Vincristine–Procarbazine–Prednisone–Doxorubicin–Bleomycin–Vinblastine | |
| 37 | Doxorubicin–Bleomycin–Vinblastine–Etoposide–Prednisone–Cyclophosphamide | |
| 38 | Bleomycin–Etoposide–Doxorubicin–Cyclophosphamide–Vincristine–Procarbazine–Prednisone | |
| 39 | Mechlorethamine–Doxorubicin–Vinblastine–Vincristine–Bleomycin–Etoposide–Prednisone | |
| 40 | Vincristine–Doxorubicin–Methotrexate–Prednisone | |
| 41 | Malignant Mesothelioma | Gemcitabine–Cisplatin |
| 42 | Multiple Myeloma | Bortezomib–Doxorubicin–Dexamethasone |
| 43 | Myeloproliferative Neoplasms | Cytarabine–Daunorubicin–Etoposide |
| 44 | Neuroblastoma | Busulfan–Melphalan |
| 45 | Carboplatin–Etoposide–Melphalan | |
| 46 | Non-Hodgkin Lymphoma | Ifosfamide–Carboplatin–Etoposide |
| 47 | Rituximab–Ifosfamide–Carboplatin–Etoposide | |
| 48 | Cyclophosphamide–Vincristine–Prednisone | |
| 49 | Rituximab–Cyclophosphamide–Vincristine–Prednisone | |
| 50 | Cyclophosphamide–Vincristine–Procarbazine–Prednisone | |
| 51 | Cyclophosphamide–Doxorubicin–Vincristine–Prednisone | |
| 52 | Cyclophosphamide–Vincristine–Doxorubicin–Dexamethasone | |
| 53 | Etoposide–Prednisone–Vincristine–Cyclophosphamide–Doxorubicin | |
| 54 | Rituximab–Etoposide–Prednisone–Vincristine–Cyclophosphamide–Doxorubicin | |
| 55 | Rituximab–Cyclophosphamide–Doxorubicin–Vincristine–Prednisone | |
| 56 | Non-Small-Cell Lung Cancer | Carboplatin–Paclitaxel |
| 57 | Gemcitabine–Cisplatin | |
| 58 | Ovarian, Fallopian Tube, or Primary Peritoneal | Carboplatin–Paclitaxel |
| 59 | Gemcitabine–Cisplatin | |
| 60 | Bleomycin–Etoposide–Carboplatin | |
| 61 | Bleomycin–Etoposide–Cisplatin | |
| 62 | Vincristine–Dactinomycin–Cyclophosphamide | |
| 63 | Vinblastine–Ifosfamide–Cisplatin | |
| 64 | Pancreatic | Gemcitabine–Cisplatin |
| 65 | Gemcitabine–Oxaliplatin | |
| 66 | Leucovorin–Fluorouracil–Oxaliplatin | |
| 67 | Leucovorin–Fluorouracil–Irinotecan–Oxaliplatin | |
| 68 | Retinoblastoma | Carboplatin–Etoposide–Vincristine |
| 69 | Soft Tissue Sarcoma | Vincristine–Dactinomycin–Cyclophosphamide |
| 70 | Testicular | Bleomycin–Etoposide–Cisplatin |
| 71 | Vinblastine–Ifosfamide–Cisplatin | |
| 72 | Etoposide–Ifosfamide–Cisplatin | |
| 73 | Bleomycin–Etoposide–Carboplatin |
| Feature | Microfluidic Techniques | Conventional Techniques (Thin-Film Hydration, Solvent Injection, Extrusion, Sonication) |
|---|---|---|
| Size Control | Excellent control (10–200 nm) with narrow size distribution due to precise fluid dynamics. | Size variability (50–500 nm) that requires post-processing (e.g., extrusion) for uniformity. |
| Encapsulation Efficiency (EE%) | Active loading is needed for the encapsulation of hydrophilic drugs. | Active loading is needed for the encapsulation of hydrophilic drugs. |
| Reproducibility | Highly reproducible due to automated, continuous-flow processing. | Batch-to-batch variability due to dependence on manual preparation and process conditions. |
| Scalability | Scalable via parallelized microfluidic chips and continuous-flow systems. | Limited scalability as batch-based production increases variability and processing time. |
| Processing Time | Rapid (< minutes per batch) continuous processing is possible. | Time-consuming (hours per batch) due to multiple steps (hydration, extrusion, filtration). |
| Solvent Use and Removal | Efficient solvent use with rapid removal; minimal residual organic solvents. | Require solvent evaporation, increasing processing time and potential toxicity risks. |
| Clinical Feasibility | Emerging in good manufacturing practice (GMP) manufacturing, potential for automated and sterile production. | Widely used in commercial formulations, but limited by batch-to-batch inconsistency. |
| Cost & Infrastructure | Requires specialized microfluidic chips and automation, the initial cost is high but decreases with scaling. | Lower initial costs, but higher long-term costs due to labor-intensive processing. |
| Sr. No. | Lipids | Hydrophilic Drug | Lipophilic Drug | Method | Size (nm) | Z.P. (mV) | PDI | EE (%) | Cancer | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DPPC, DSPC, POPC, DOPC | Doxorubicin, Nanomagnetite, Gold nanorods, DNA nanostructures | 17-AAG, Erlotinib | w/o/w double emulsion via glass capillary microfluidic device | 30,000–50,000 | – | – | – | HeLa, MCF-7, MCF-7/ADR, M28 | [118] |
| 2 | DSPC, Chol, DSPE-PEG2000 | Doxorubicin | Umbelliprenin | Hydrodynamic flow-focusing | 227 | −2.5 | 0.2 | 74 (Doxorubicin)/47 (Umbelliprenin) | MCF-7, MDA-MB 231, BT-474 | [119] |
| 3 | DPPC, Chol, HDA | Catechin | Curcumin | Hydrodynamic flow-focusing | ~200 | ~35 | ~0.2 | 16.2 (Catechin)/100 (Curcumin) | HT-29, Caco-2 | [124] |
| 4 | DOTAP, DSPC, Chol | Cisplatin | Gold nanoparticles | Staggered herringbone micromixer | 134 | – | 0.199 | – | HCT-116 | [126] |
| 5 | DPPC, Chol, DSPE-PEG2000, DSPE-PEG2000-FA | Doxorubicin, Gold nanorods | Magnetite nanoparticles | Microfluidic hybrid chip | 234 | – | – | 28.6 (Doxorubicin) | Human bladder cancer cell line 5637 | [127] |
| 6 | SPC, Chol | Curcumin, TAT-PEG-SN38 | Hydrodynamic flow-focusing | 171 | −5.94 | 0.124 | 88.4 (Curcumin) | A549 | [131] | |
| 7 | DOTAP, DSPE-PEG2000, DSPC, Chol | Copper complexed Chlorin e6 (Cu-Ce6) | Serpentine mixer | 50–100 | −15 to 35 | 0.1–0.6 | – | HeLa | [128] | |
| 8 | DPPC, DSPC, chol, DSPE-MPEG2000 | Indocyanine green | Ansamitocin P-3 | Microfluidic swirl mixer | 125 | −8.3 | 0.04 | 51 (Indocyanine green)/81 (Ansamitocin P-3) | MCF-7 | [132] |
| 9 | JK-102-CA, Chol, DSPC, PEG2000-DMG | Nicotinamide mononucleotide | Honokiol | Microfluidic membrane emulsification device | 164 | – | >0.2 | 28.4 (Nicotinamide mononucleotide)/99.2 (Honokiol) | HCT-116 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, S.; Iliescu, R.; Stiufiuc, R.-I.; Dragoi, B. Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics. Int. J. Mol. Sci. 2025, 26, 3820. https://doi.org/10.3390/ijms26083820
Asghar S, Iliescu R, Stiufiuc R-I, Dragoi B. Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics. International Journal of Molecular Sciences. 2025; 26(8):3820. https://doi.org/10.3390/ijms26083820
Chicago/Turabian StyleAsghar, Sajid, Radu Iliescu, Rares-Ionut Stiufiuc, and Brindusa Dragoi. 2025. "Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics" International Journal of Molecular Sciences 26, no. 8: 3820. https://doi.org/10.3390/ijms26083820
APA StyleAsghar, S., Iliescu, R., Stiufiuc, R.-I., & Dragoi, B. (2025). Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics. International Journal of Molecular Sciences, 26(8), 3820. https://doi.org/10.3390/ijms26083820







