Identification of CDK1 as a Biomarker for the Treatment of Liver Fibrosis and Hepatocellular Carcinoma Through Bioinformatics Analysis
Abstract
1. Introduction
2. Results
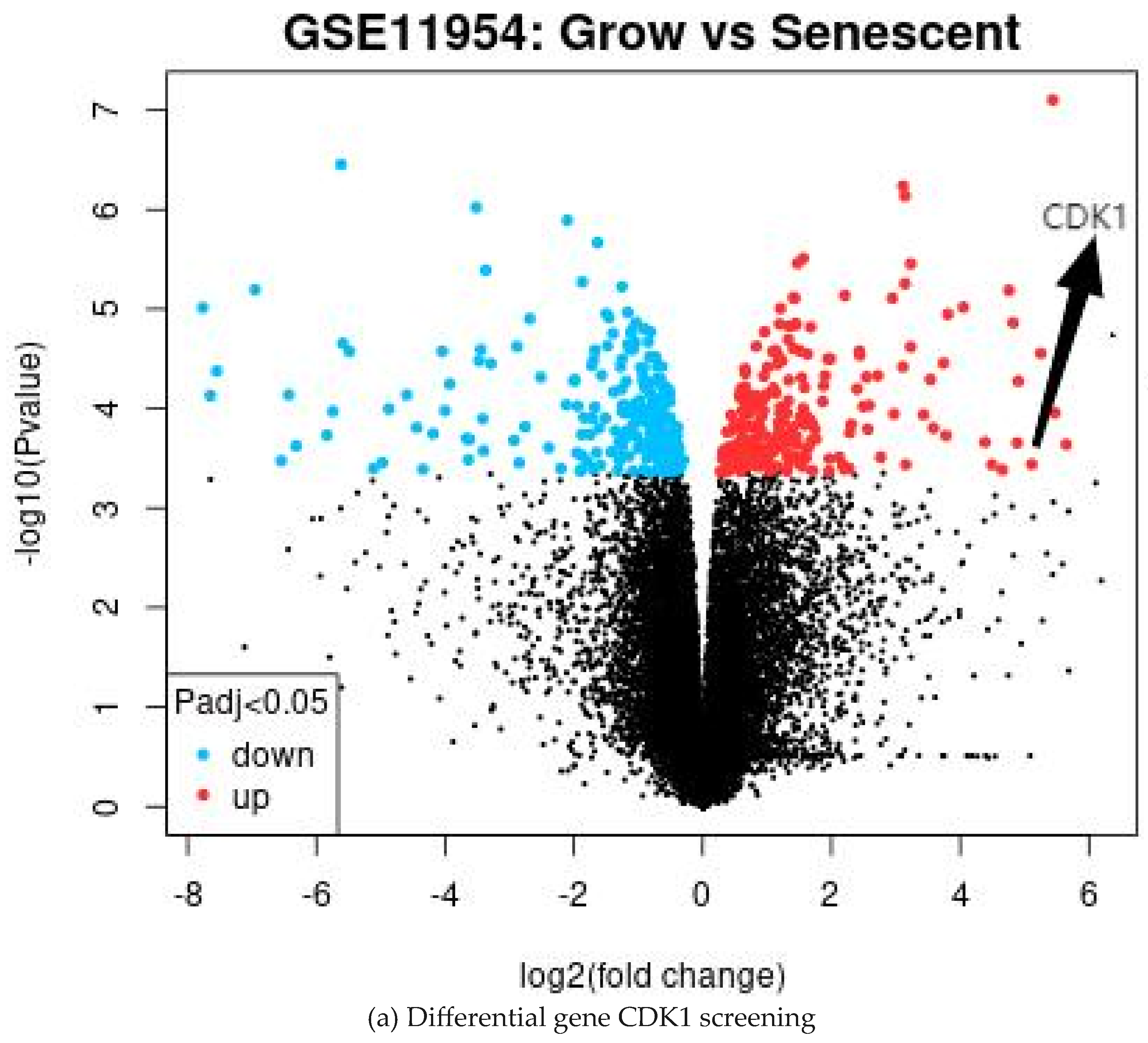
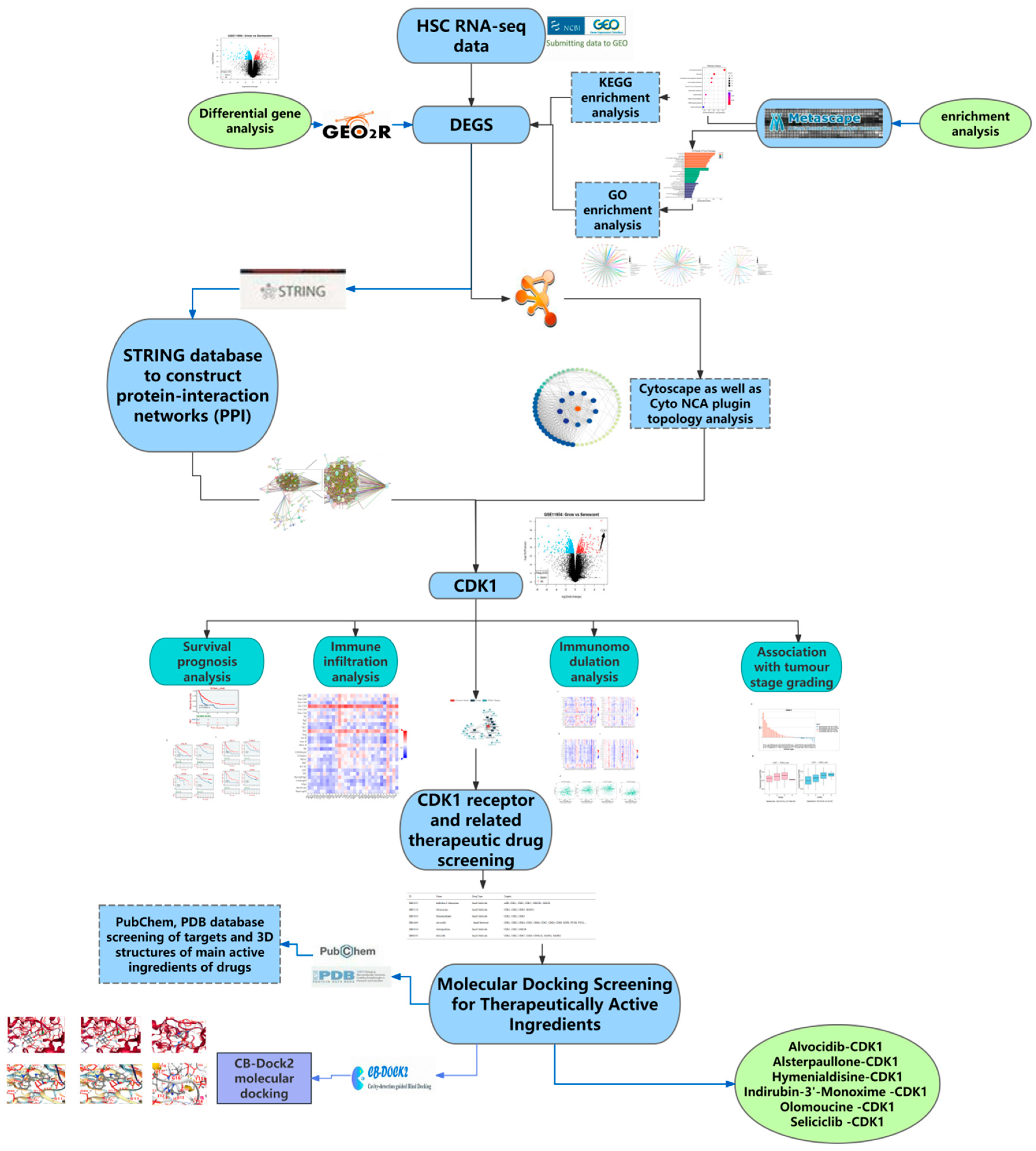
2.1. Acquisition of DEGs and the GEO2R Analysis
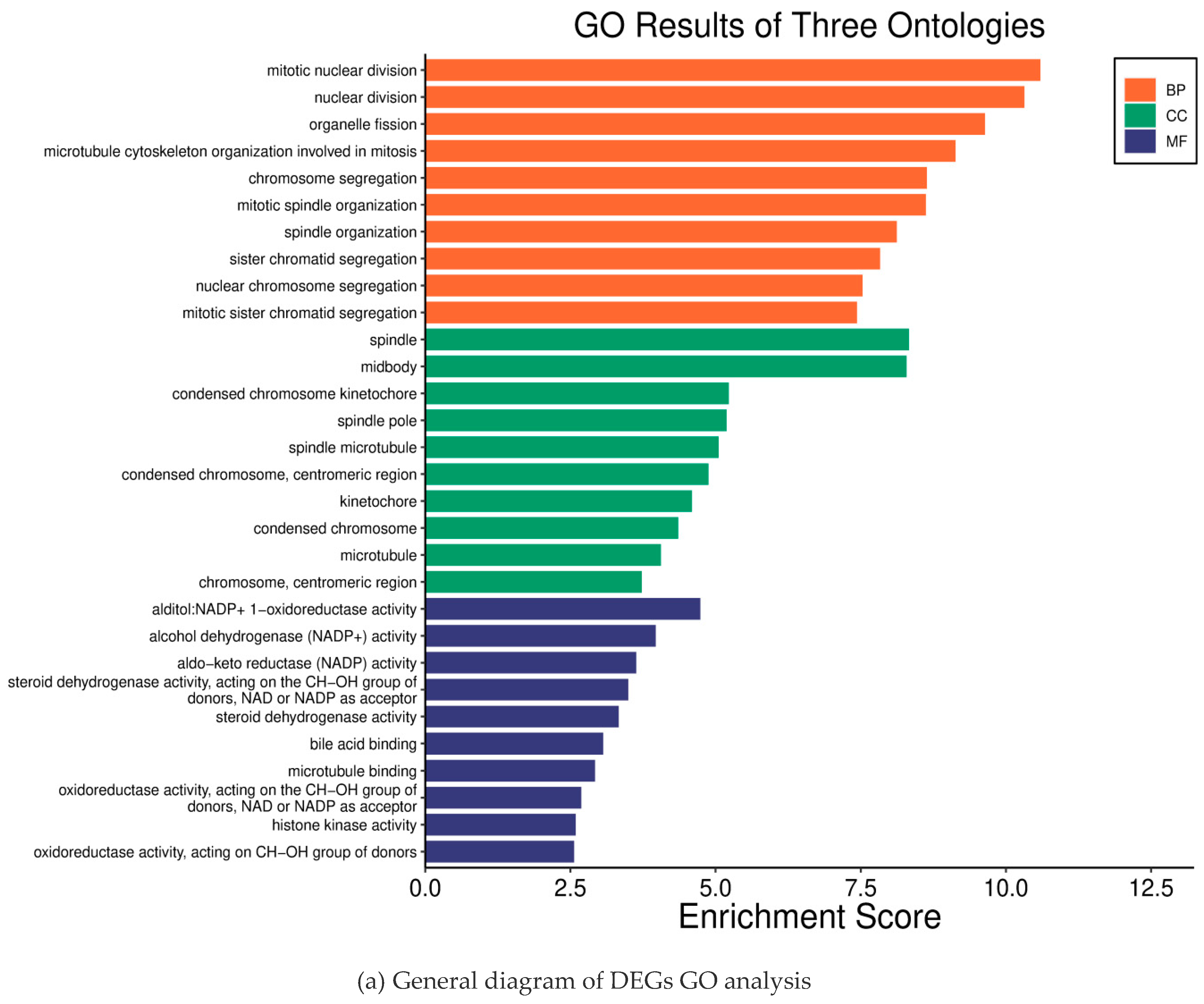
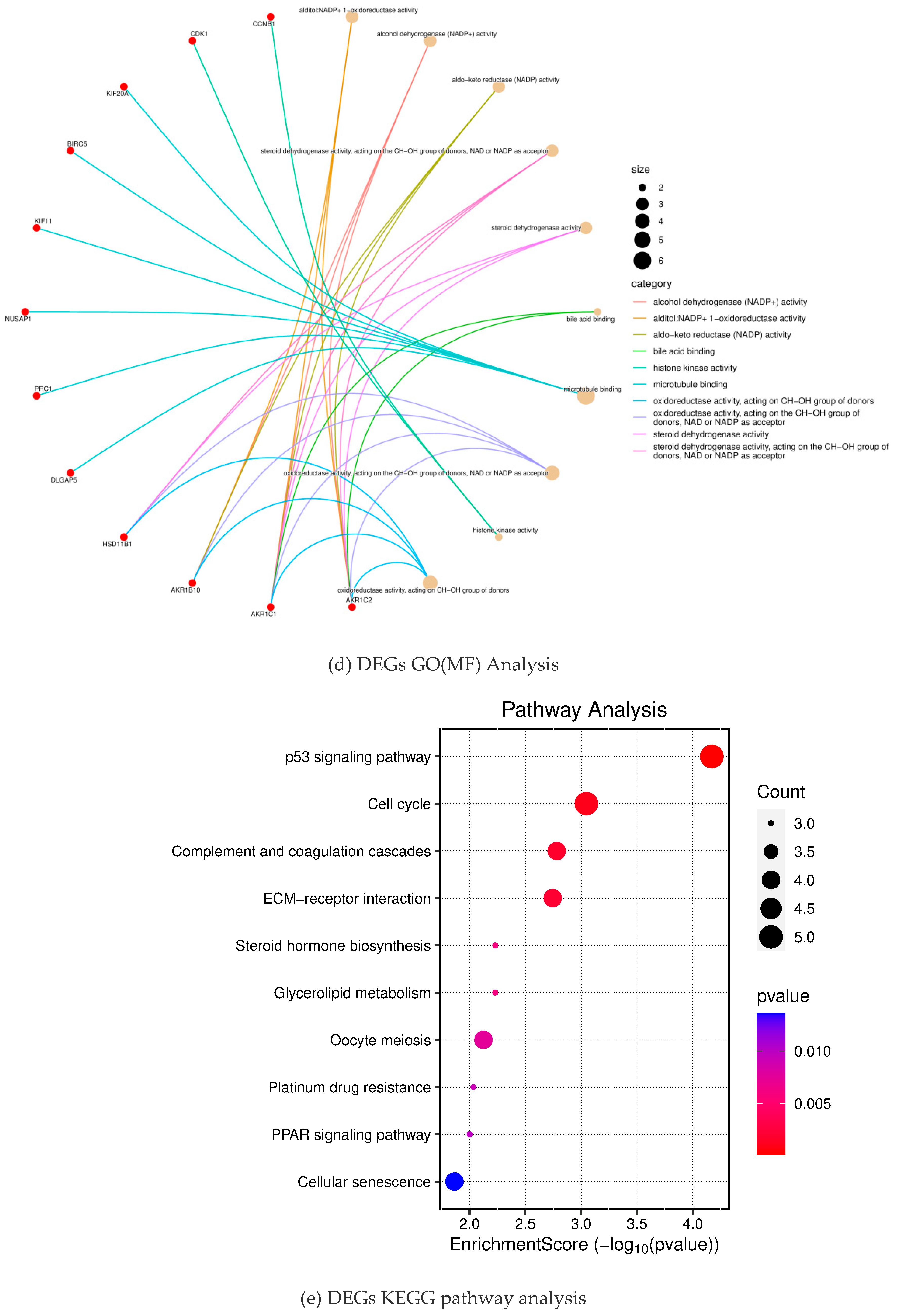
2.2. Results of the GO KEGG Enrichment Analysis
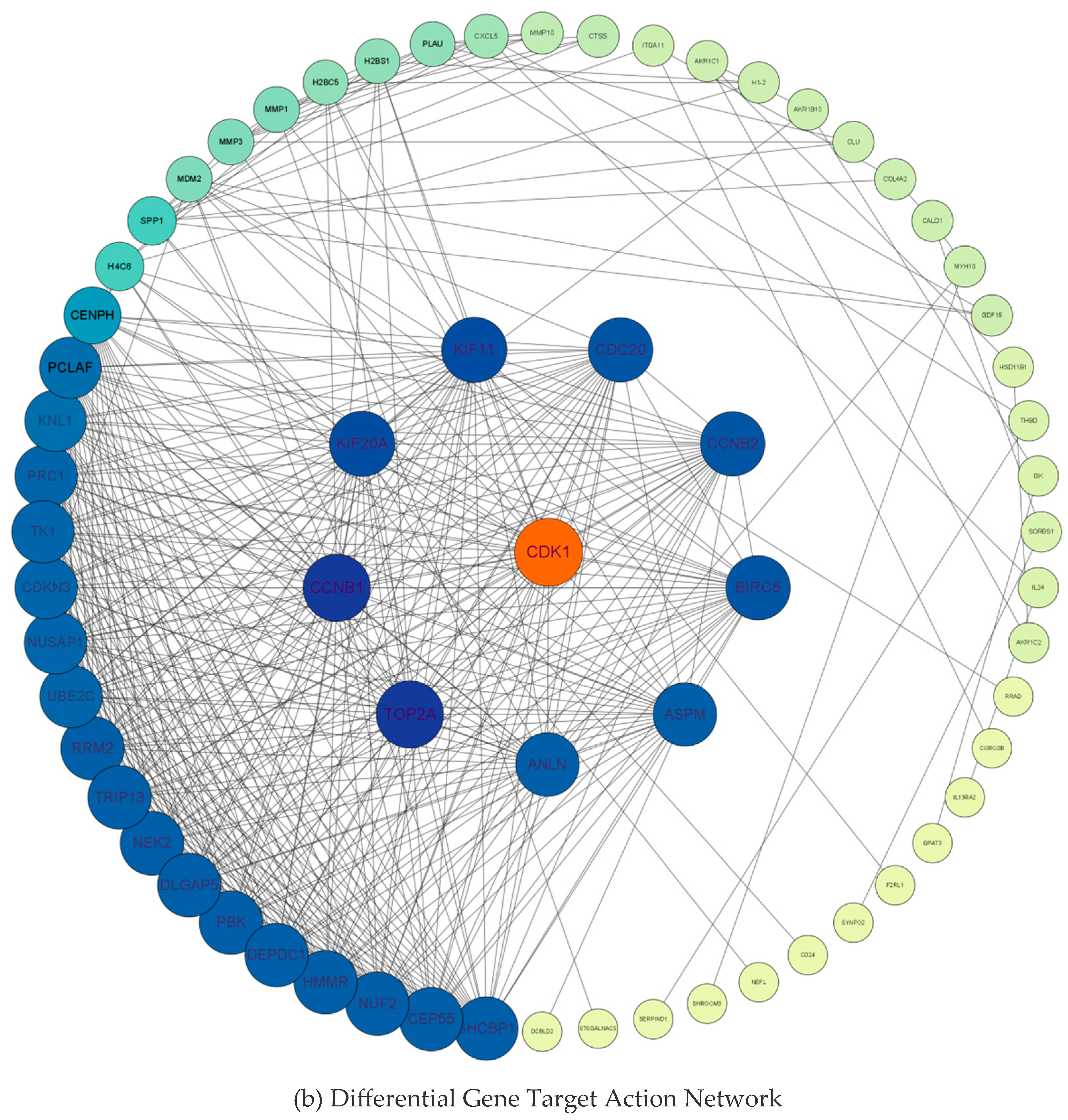
2.3. Construction of PPI Networks and Screening of Key Hub Genes
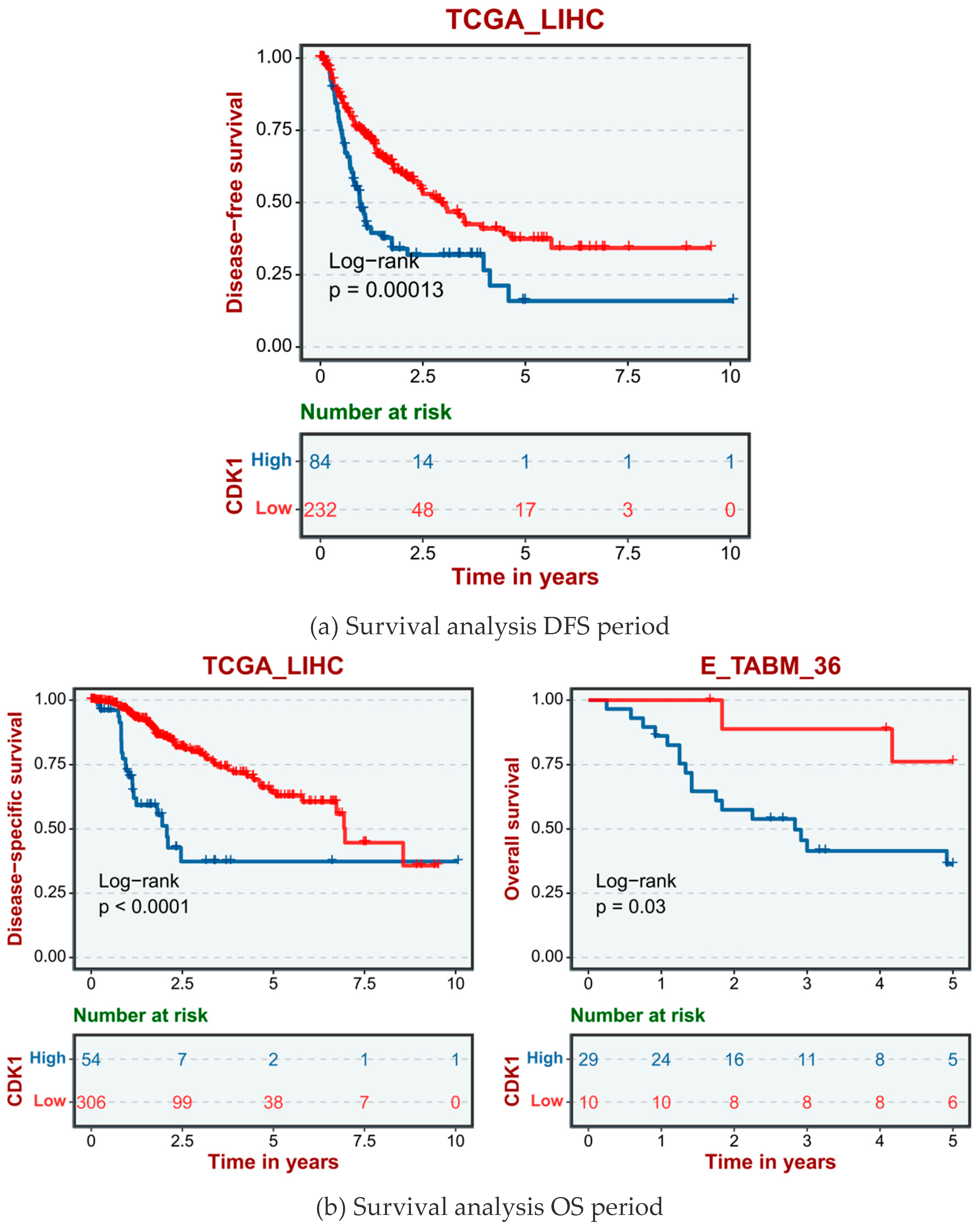
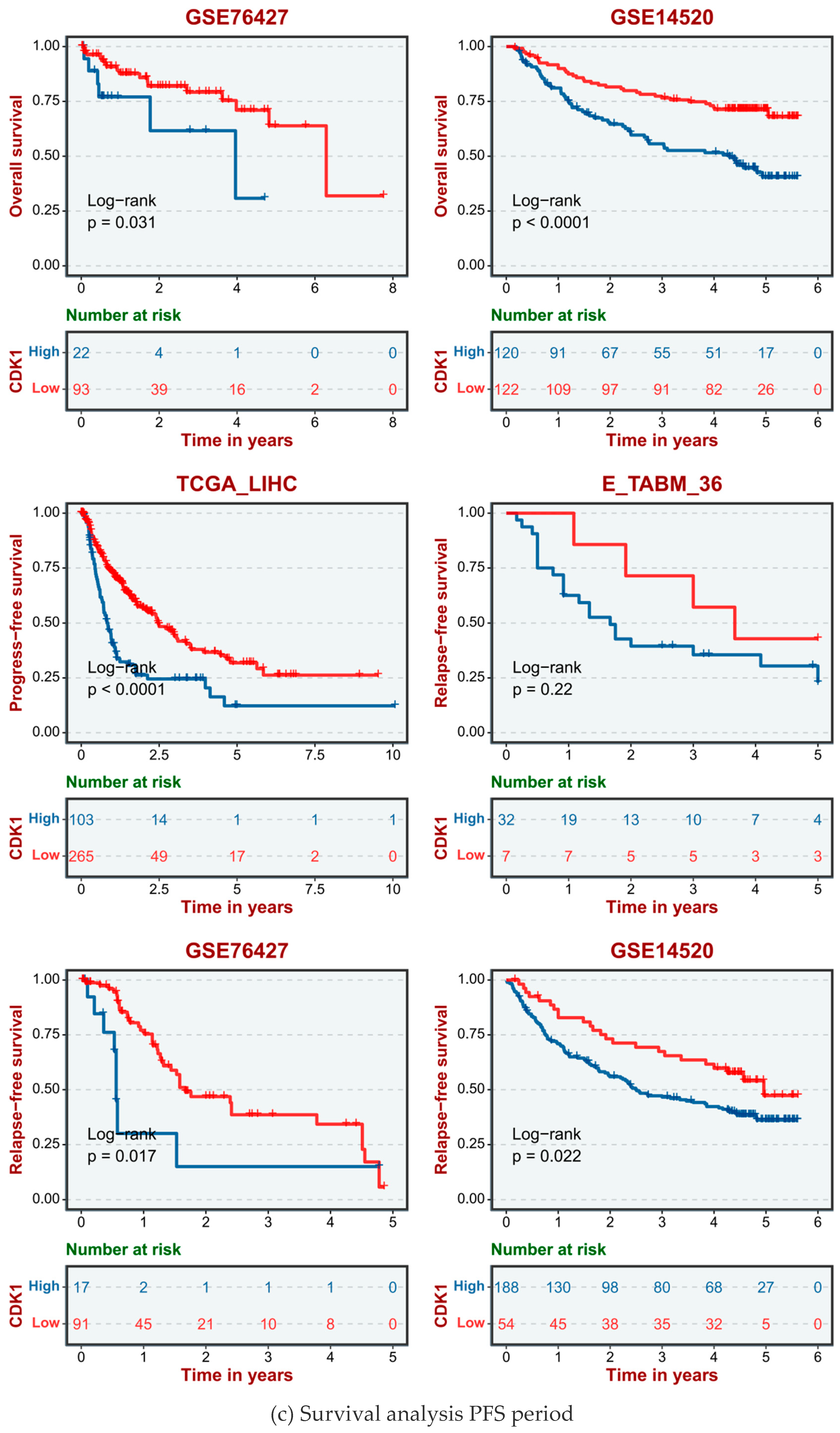
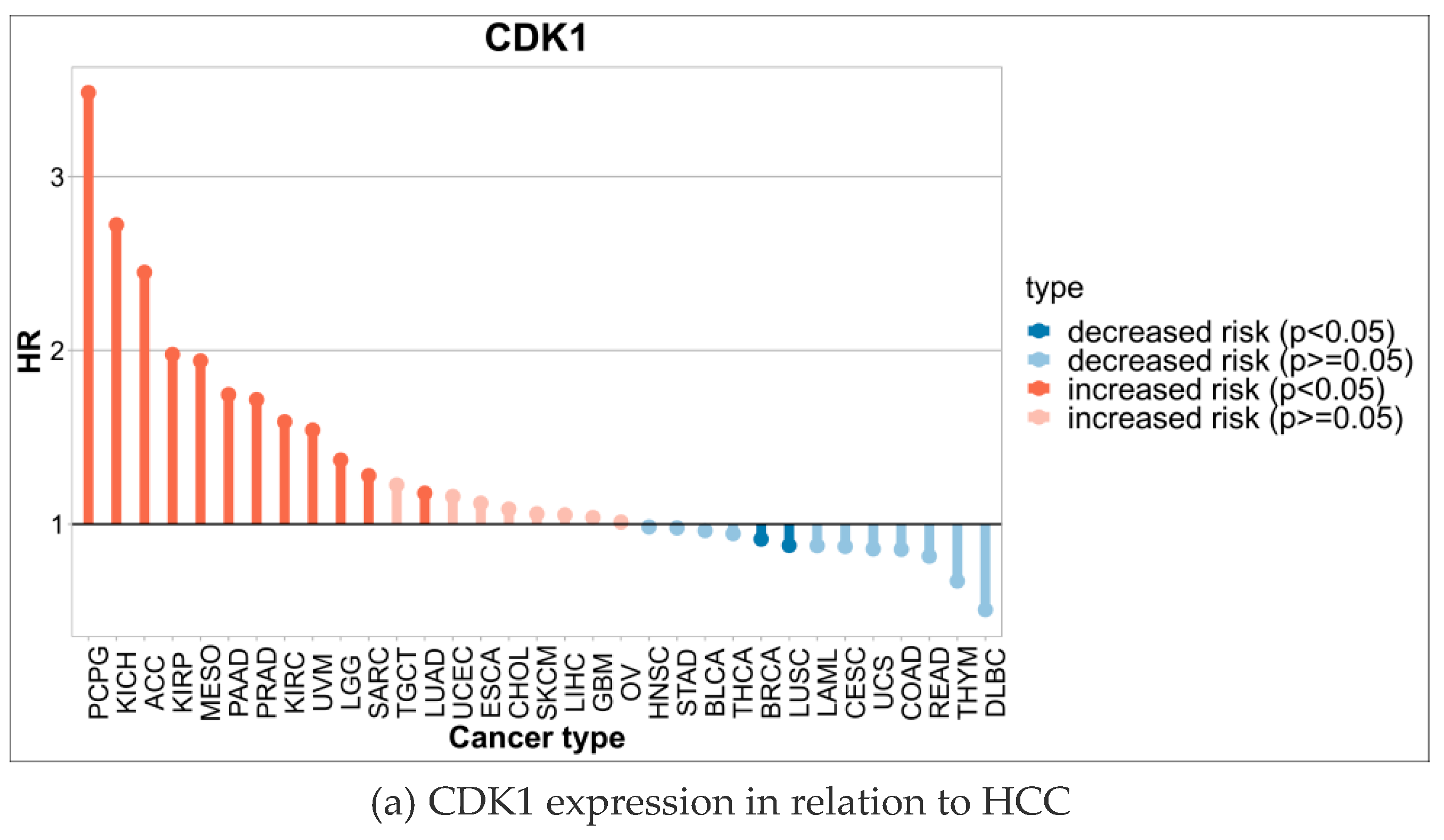
2.4. High Expression of CDK1 in HCC Cells Significantly Reduced the Survival Rate of Hepatocellular Carcinoma Patients
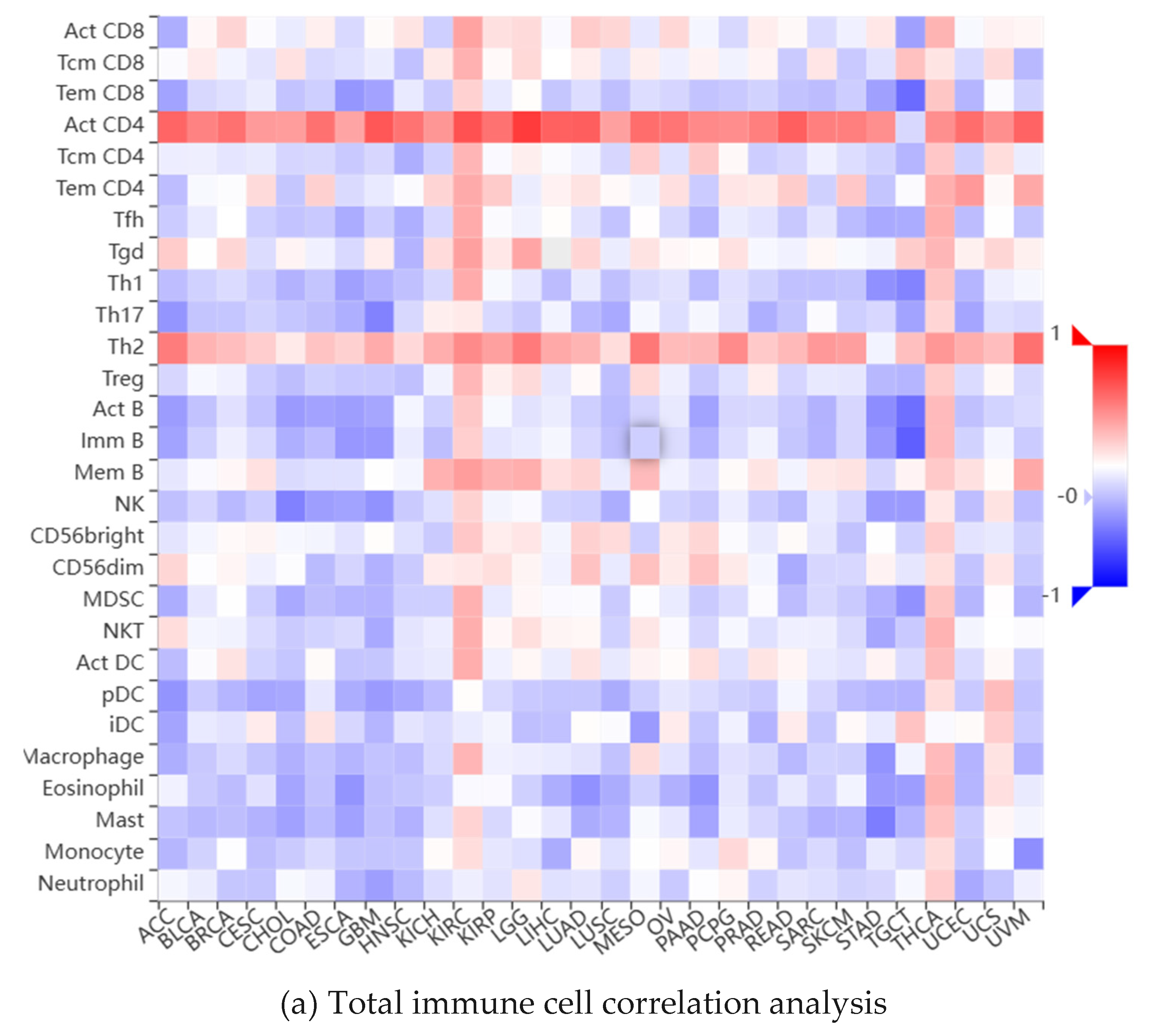
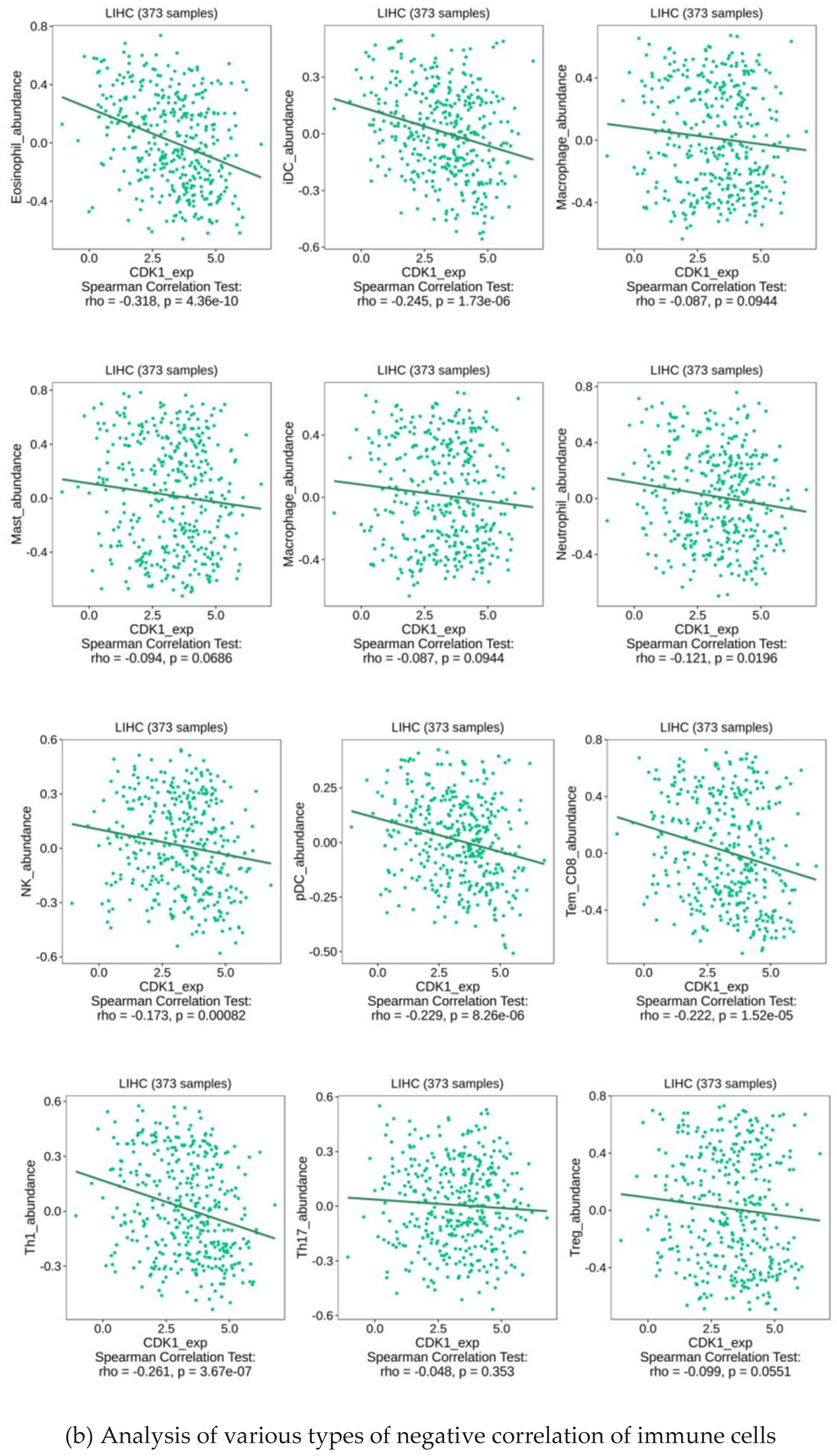
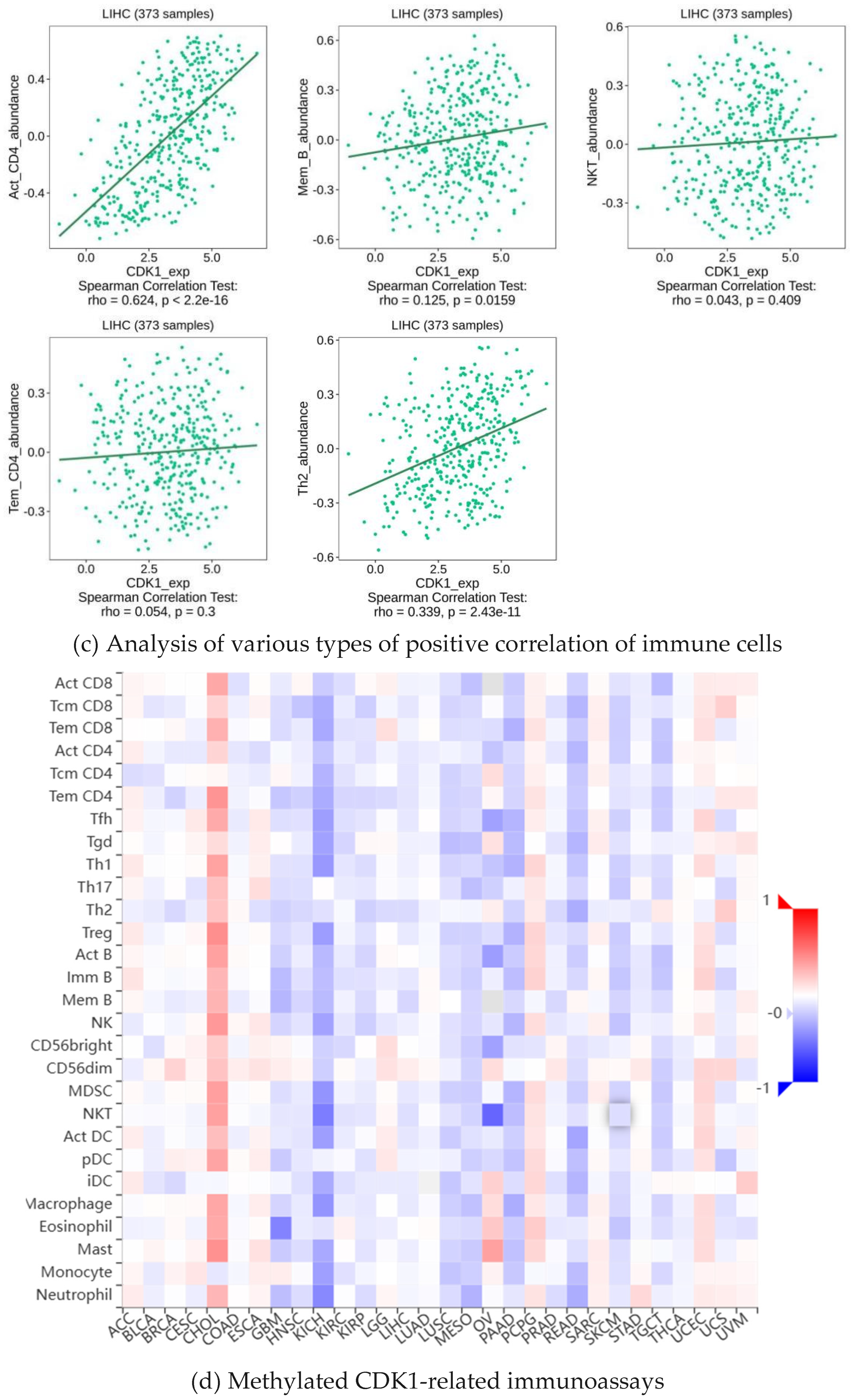
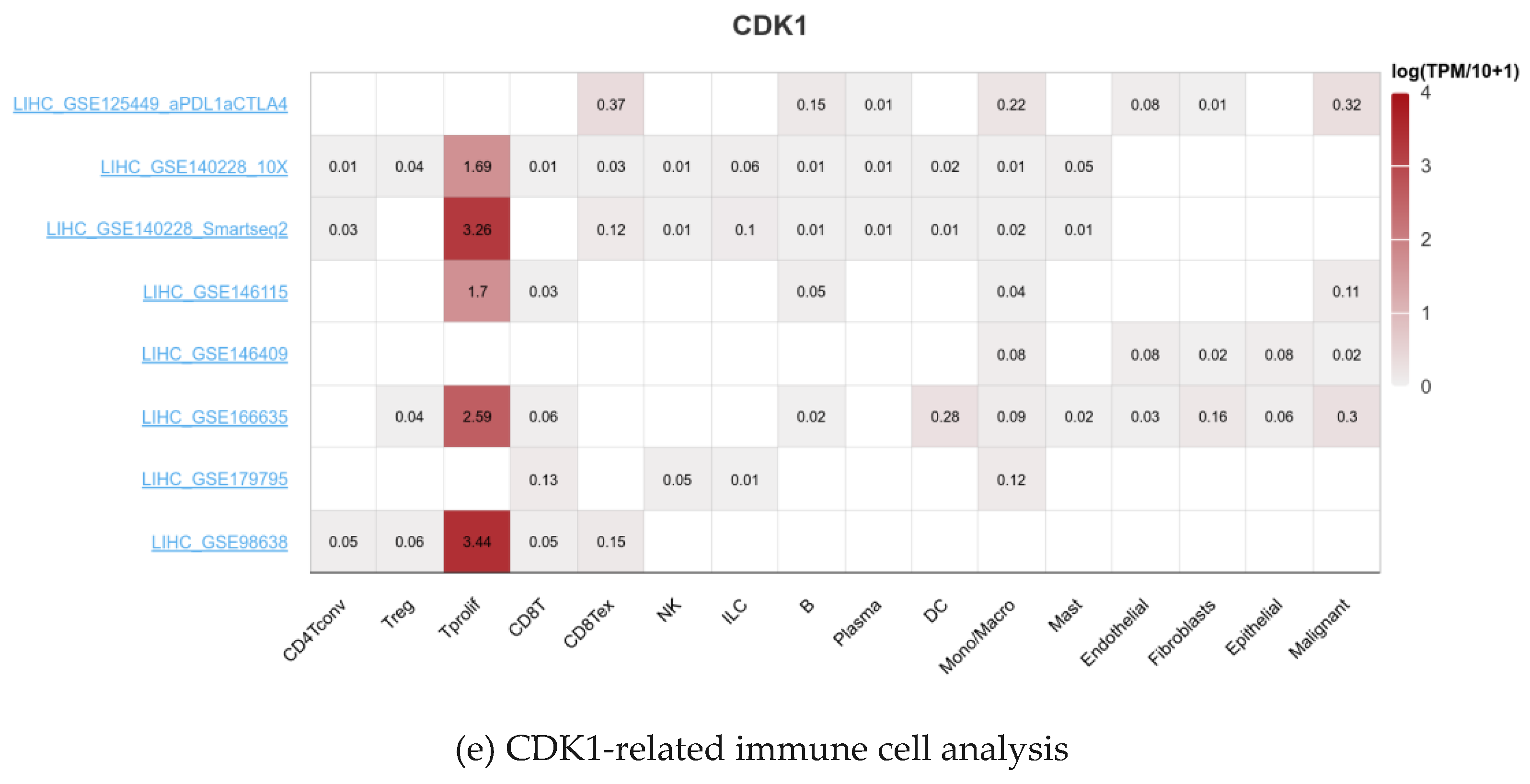
2.5. Analysis of Immune Infiltration of CDK1
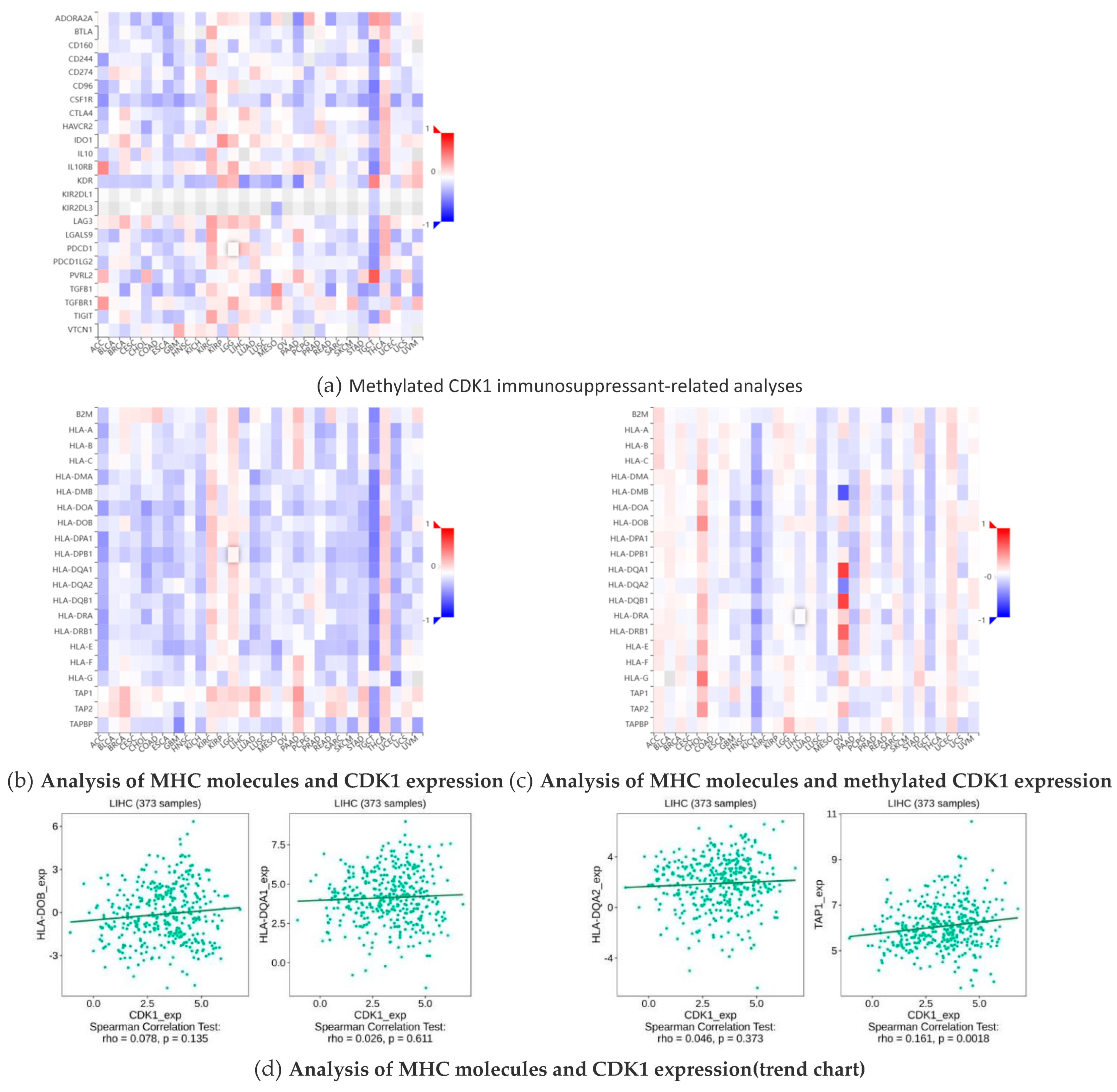
2.6. Methylation of CDK1 Disables Immunosuppressants in Hepatocellular Carcinoma
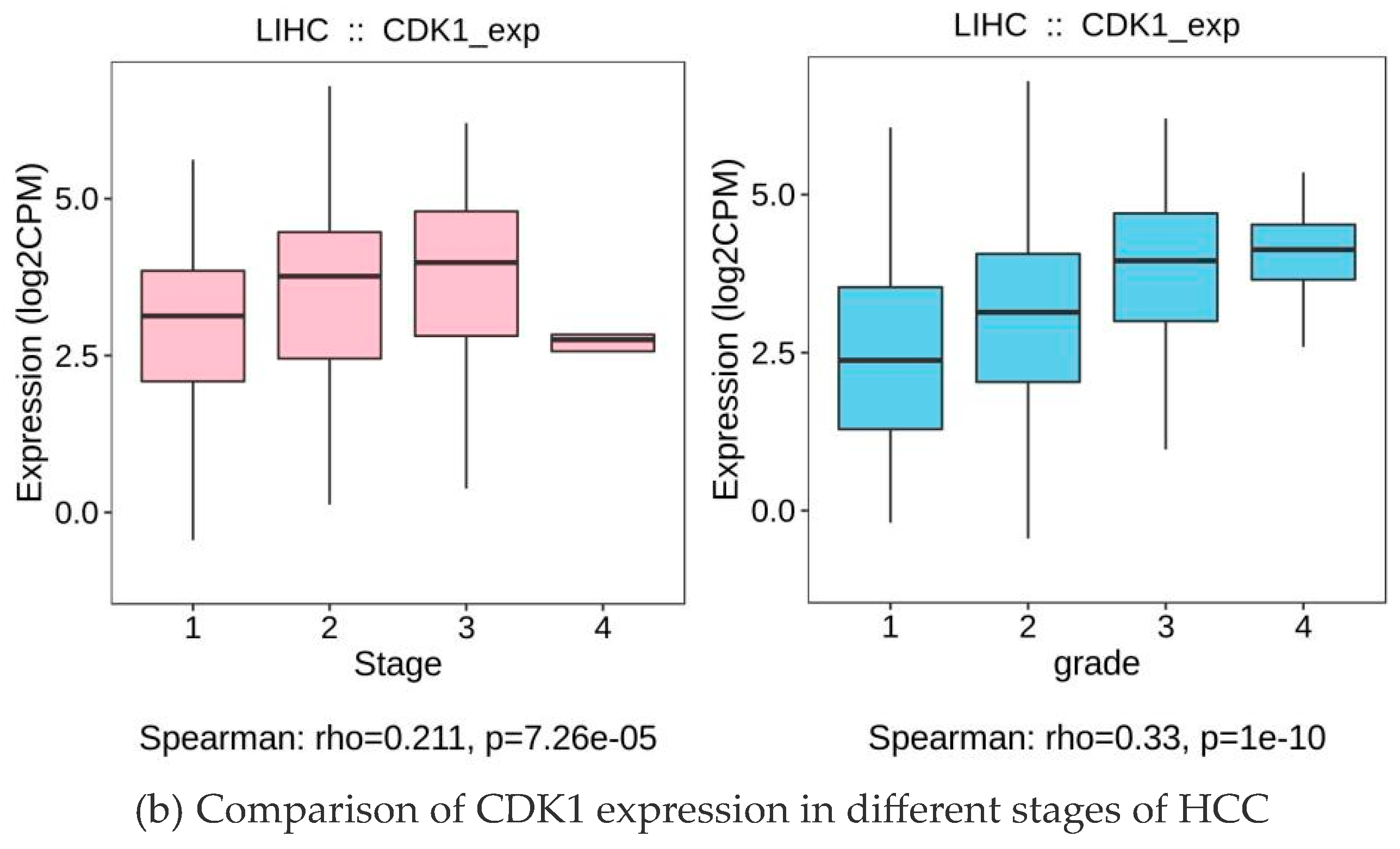
2.7. CDK1 Expression Was Positively Correlated with the Grade and Staging of the Tumors
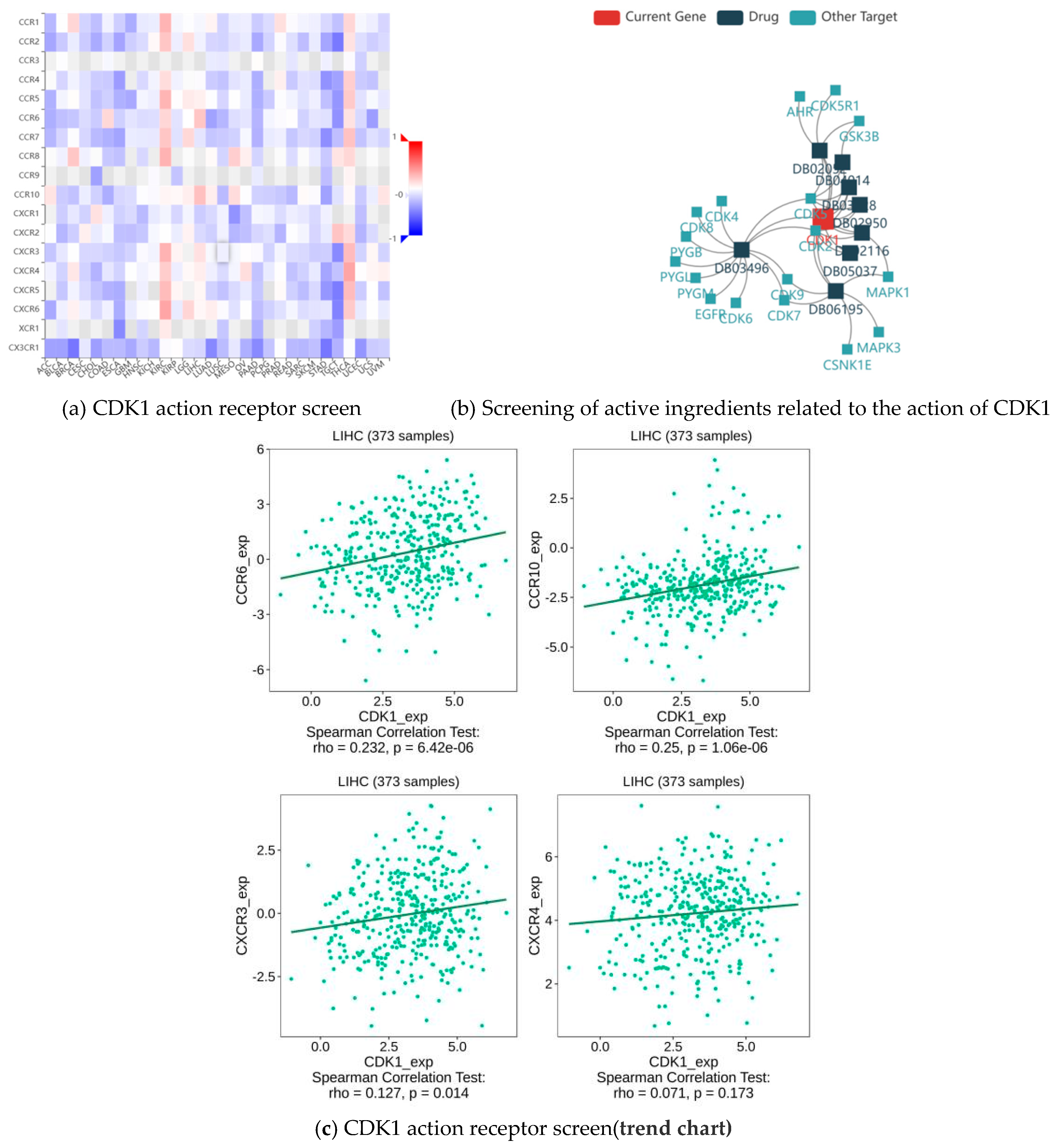
2.8. CDK1 Action Receptor and Active Component of HCC Treatment
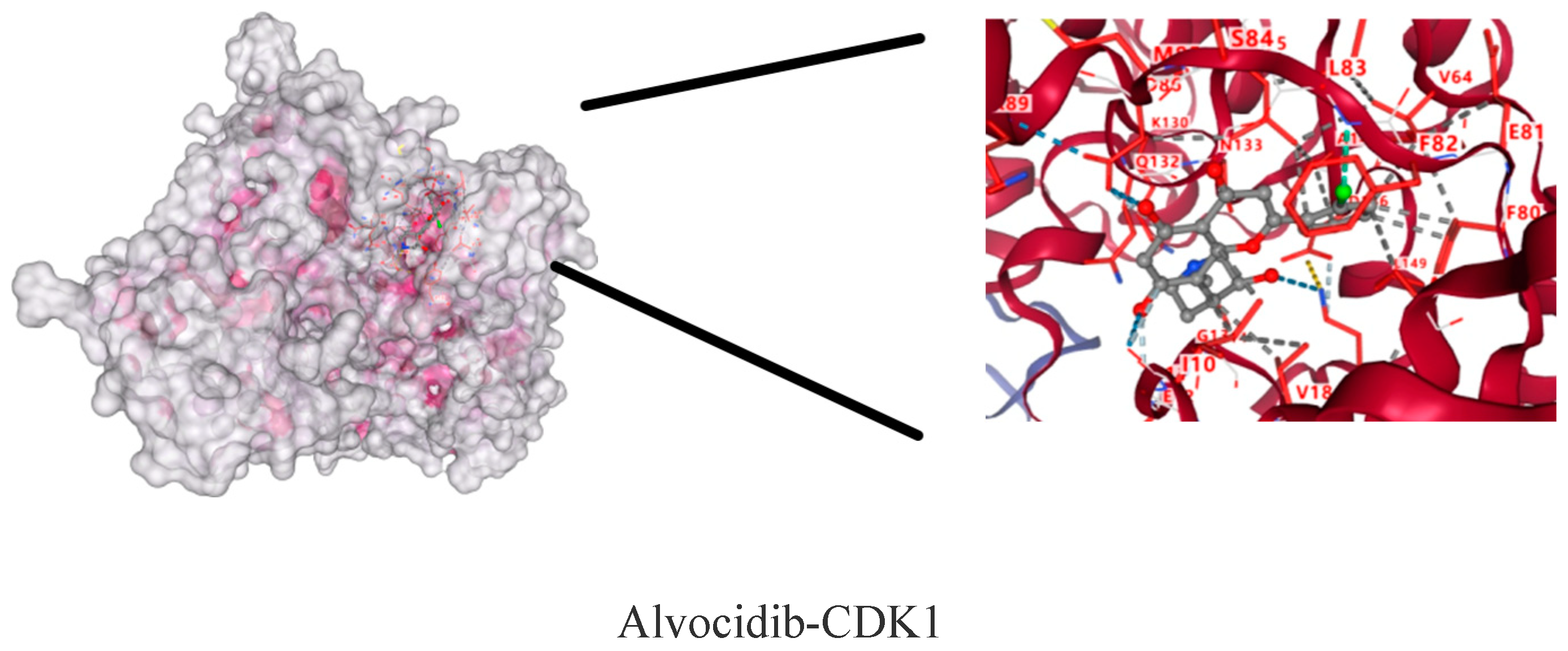
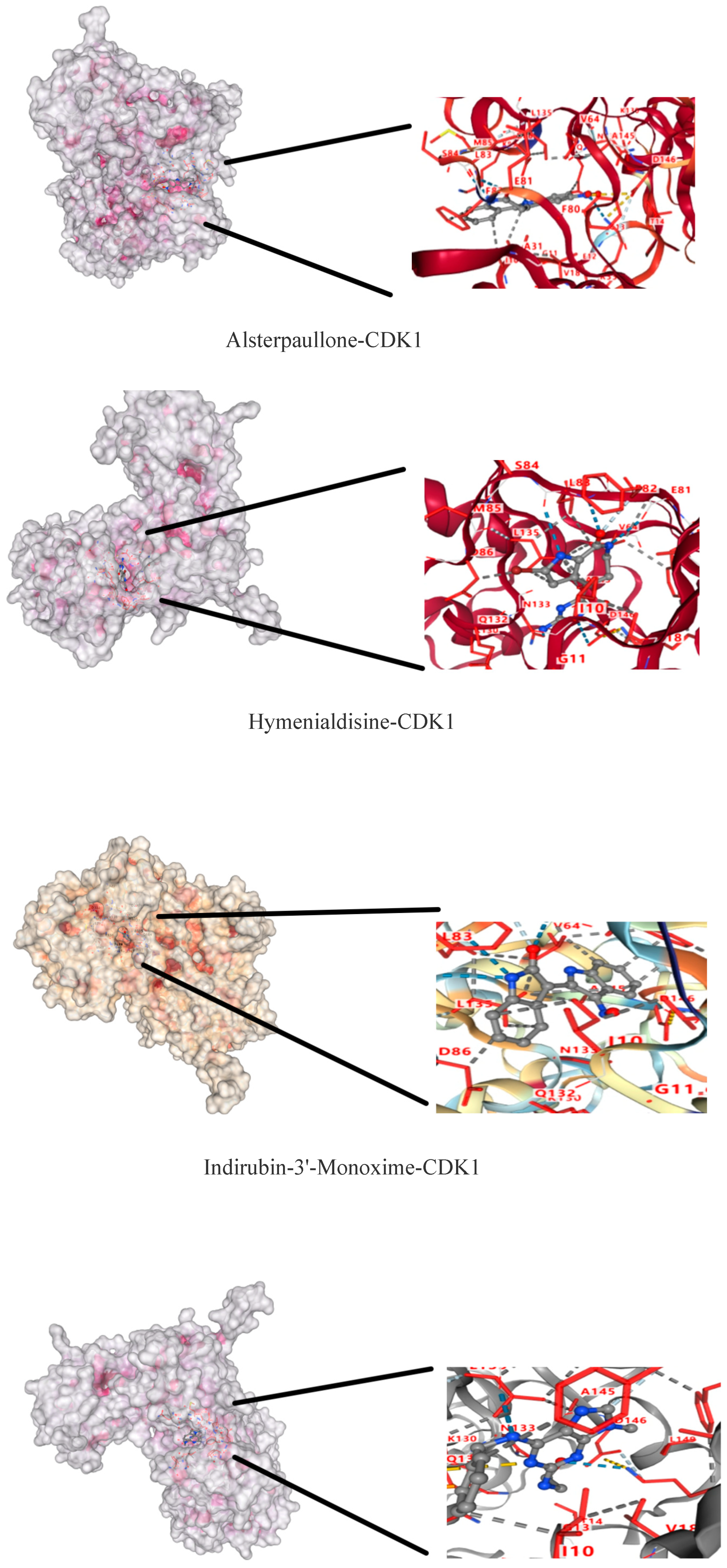
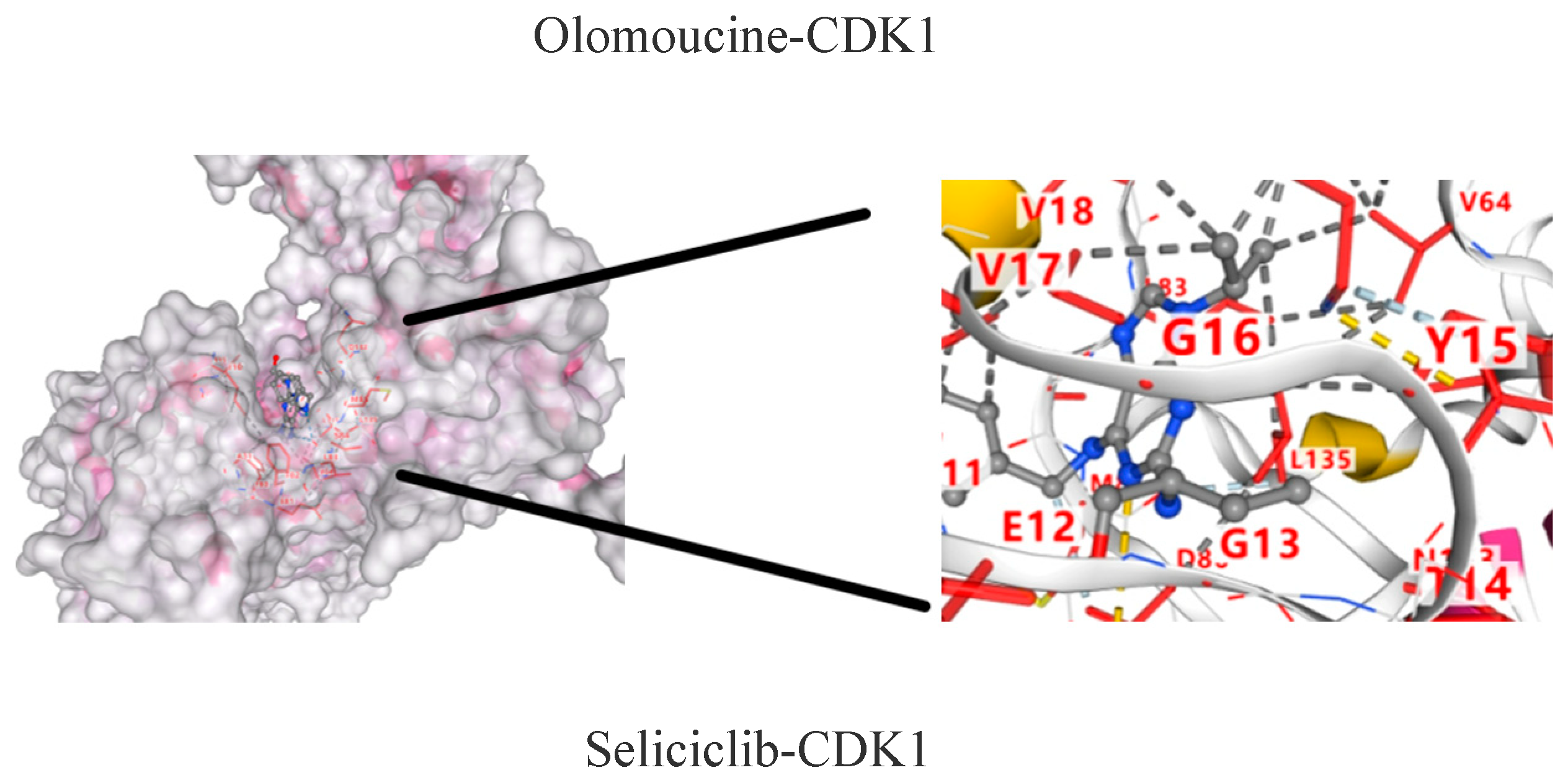
2.9. Molecular Docking Results of CDK1 and Active Components
3. Discussion
4. Materials and Method
4.1. Acquisition of RNA-Seq Data of Activated Hepatic Stellate Cells (HSCs)
4.2. The Differential Gene Expression Analysis of the Microarray Data
4.3. Protein Interaction (PPI) Networks of DEGs
4.4. Enrichment Analysis and Functional Annotation of the 2.4 DEGs
4.5. Analysis of Survival Prognosis Related to CDK1
4.6. Analysis of Immune Cell Infiltration
4.7. Analysis of the Interaction of CDK1 with Immunosuppressants and MHC Molecules
4.8. Association Between CDK1 Expression and Stage Grade of Hepatocellular Carcinoma in Human
4.9. Selection of Receptors and Drugs for CDK1 Action in Human Hepatocellular Carcinoma
4.10. Molecular Docking and Kinetic Simulation for the Validation of the Effective Components for the Treatment of Hepatocellular Carcinoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, E.; Viatour, P. Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Liu, Z.; Zhang, A.; Li, N. Identification of key genes and pathways in hepatocellular carcinoma: A preliminary bioinformatics analysis. Medicine 2019, 98, e14287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aydın, M.M.; Akçalı, K.C. Liver fibrosis. Turk. J. Gastroenterol. 2018, 29, 14–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218, Erratum in: J. Clin. Investig. 2005, 115, 1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Zhou, X.; Wang, X.; Jin, Y.; Zhou, L.; Ye, J. Progress of mesenchymal stem cells (MSCs) & MSC-Exosomes combined with drugs intervention in liver fibrosis. Biomed. Pharmacother. 2024, 176, 116848. [Google Scholar] [CrossRef] [PubMed]
- Luangmonkong, T.; Parichatikanond, W.; Olinga, P. Targeting collagen homeostasis for the treatment of liver fibrosis: Opportunities and challenges. Biochem. Pharmacol. 2023, 215, 115740. [Google Scholar] [CrossRef] [PubMed]
- Poilil Surendran, S.; George Thomas, R.; Moon, M.J.; Jeong, Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997–7006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Zhou, J.; Han, J. Analysis of the clinicopathological characteristics of patients with autoimmune hepatitis and drug-induced liver injury. J. Pract. Liver Dis. 2023, 26, 218–221. [Google Scholar]
- Yang, Y.; Liu, Q.; Guo, X.; Yuan, Q.; Nian, S.; Kang, P.; Xu, Z.; Li, L.; Ye, Y. Systematic Pan-Cancer Analysis Identifies CDK1 as an Immunological and Prognostic Biomarker. J. Oncol. 2022, 2022, 8115474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, L.; Xu, Q.; Huang, J. Effect of Uine on proliferation and apoptosis of human thyroid cancer cells. Anhui Med. 2021, 12. [Google Scholar]
- He, Y.; Xie, J.; Zheng, L.; Guo, C.; Ma, Y. Expression and significance of CDK 1 and AURKA in the serum of patients with HBV-associated hepatocellular carcinoma. J. Clin. Hepatobiliary Dis. 2024, 40, 1390–1396. [Google Scholar]
- Ren, L.; Yang, Y.; Li, W.; Zheng, X.; Liu, J.; Li, S.; Yang, H.; Zhang, Y.; Ge, B.; Zhang, S.; et al. CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, G2/M phase transition, and PANoptosis. J. Transl. Med. 2022, 20, 444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Li, Y.; Hao, H.; Wang, Y.; Zhou, Z.; Wang, Z.; Chu, X. Screening Hub Genes as Prognostic Biomarkers of Hepatocellular Carcinoma by Bioinformatics Analysis. Cell Transplant. 2019, 28 (Suppl. 1), 76S–86S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Zhang, J. Prognosis of breast cancer based on cox proportional hazards regression model, lasso and survival tree. Stat. Appl. 2018, 7, 99–110. [Google Scholar]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, S.; Li, Y.; Wei, Z. Identification of HCC molecular biomarkers based on differential co-expression. Genom. Appl. Biol. 2021, 40, 429–434. [Google Scholar]
- Lan, L.; Li, J.; Ren, Q. Evaluation of the value of the novel markers AFP-L3 and PIVKAII in HCC diagnosis. J. Kunming Med. Univ. Kunming Yike Daxue Xuebao 2023, 44, 136–141. [Google Scholar]
- Zou, Y.; Ruan, S.; Jin, L.; Chen, Z.; Han, H.; Zhang, Y.; Jian, Z.; Lin, Y.; Shi, N.; Jin, H. CDK1, CCNB1, and CCNB2 are Prognostic Biomarkers and Correlated with Immune Infiltration in Hepatocellular Carcinoma. Med. Sci. Monit. 2020, 26, e925289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Bode, A.M.; Zhang, T. Targeting CDK1 in cancer: Mechanisms and implications. NPJ Precis. Oncol. 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sunada, S.; Saito, H.; Zhang, D.; Xu, Z.; Miki, Y. CDK1 inhibitor controls G2/M phase transition and reverses DNA damage sensitivity. Biochem. Biophys. Res. Commun. 2021, 550, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, W.; Liu, J. Screening and bioinformatics analysis of potential biological gene markers for virus-associated liver cancer based on GEO data. J. Mod. Lab. Med. 2021, 36, 106–110. [Google Scholar]
- Ginzinger, D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002, 30, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Zheng, W.; Ye, H.; Yang, L. CDK 1 participates in the development of hepatocellular carcinoma and its value of inhibitor application. Clin. Pharmacol. Ther. China 2021, 26, 1086–1094. [Google Scholar]
- Li, J. Kkilling of Paclitaxel and CDK 1 Inhibitor and Molecular Mechanism. Ph.D. Thesis, Guangzhou University of Traditional Chinese Medicine, Guangzhou, China, 2012. [Google Scholar]
- Zhou, X.; Gu, D.; Tong, C.; Zeng, J.; Wu, H.; Zhao, X. The therapeutic mechanism of the P on CCl 4 induced liver fibrosis in mice. Shaanxi Tradit. Chin. Med. 2022, 43, 151–156. [Google Scholar]
- Jones, M.C.; Askari, J.A.; Humphries, J.D.; Humphries, M.J. Cell adhesion is regulated by CDK1 during the cell cycle. J. Cell Biol. 2018, 217, 3203–3218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Li, J.; Chen, R. Progress in liver fibrosis and the immune microenvironment of liver cancer. J. Fudan Univ. Med. Ed. 2023, 50, 889–896. [Google Scholar]
- Dai, J.; Jiang, J. New progress in the diagnosis of tumor markers in hepatocellular carcinoma. Diagn. Theory Pract. 2023, 22, 486–493. [Google Scholar]
- Zhang, Y. Hsa_circ_0006091 as a Molecular Marker in Hepatocellular Carcinoma. Ph.D. Thesis, Anhui Medical University, Hefei, China, 2021. [Google Scholar]
- Mi, N.; Bai, M.; Gao, L.; Ma, H.; Fu, W.; Lin, Y.; Meng, W. Screening and validation of HCC prognostic markers based on bioinformatics methods. Life Sci. Res. 2022, 26, 538–548. [Google Scholar]
- Liao, H.; Ji, F.; Ying, S. CDK1: Beyond cell cycle regulation. Aging 2017, 9, 2465–2466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Yu, S.; Chen, L.; Lai, Z.; Liu, H.; Liu, H.; Shen, C. Effect of mesenchymal stem cells combined with immunosuppressants on immune rejection in a liver transplant rat model. J. Clin. Hepatol. Linchuang Gandanbing Zazhi 2024, 40, 1209–1214. [Google Scholar]
- Jia, Y.; Zhao, F.; Zhang, C.; Jiang, M.; Kong, L.; Li, D.; Li, C. Ininhibitory effect of Chinese medicine liver rehabilitation on TNF-α-mediated inflammatory liver injury and liver fibrosis in rats. Chin. J. Microecol. 2016, 28, 146–150. [Google Scholar] [CrossRef]
- Du, M.; Jiang, Z.; Zhang, L. Progress in liver injury caused by commonly used immunosuppressive agents. Drug Eval. Study 2022, 45, 982–988. [Google Scholar]
- Li, W.; Ding, Z.; Wang, D.; Pan, Y.; Liu, Y.; Zhang, S.; Li, J.; Yan, M. Bioinformatics analysis based on osteosarcoma core driver gene screening and construction of a predicted gene model for patient survival. J. Jilin Univ. Med. Ed. 2021, 47, 1570–1580. [Google Scholar]
- Huang, Y.; Xia, L.; Lei, Q.; Zhang, C.; Liu, H.; Li, L. The protective effect and mechanism of buside D on immune liver fibrosis in rats. J. Army Med. Univ. 2022, 44, 1410–1420. [Google Scholar]
- Huang, W.; Feng, C. Association study between LBH and progression of liver fibrosis and distribution of immune cells in the liver. Chin. J. General. Surg. 2023, 32, 1053–1060. [Google Scholar]
- Du, Y.; Zhu, S.; Zeng, H.; Wang, Z.; Huang, Y.; Zhou, Y.; Zhang, W.; Zhu, J.; Yang, C. Research Progress on the Effect of Autophagy and Exosomes on Liver Fibrosis. Curr. Stem Cell Res. Ther. 2024, 19, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, W.; Li, X. A new perspective on liver fibrosis therapy: Targeting macrophage metabolism. J. Clin. Hepatol. Linchuang Gandanbing Zazhi 2023, 39, 922–928. [Google Scholar]
- Ke, S.; Wang, W.; Liao, H.; Peng, Z.; Qiu, X.; Tang, H.; Li, Q.; Xia, X. Bioinformatics methods to screen the core genes in glioblastoma. Bioinformatics 2020, 18, 56–64. [Google Scholar]
- Halligan, S.; Mallett, S. The current state of imaging biomarker development and evaluation. Br. J. Radiol. 2025, tqaf027, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Song, Y.; Jin, L.; Li, H.; Song, Y.; Liu, Z. Progress in safety of CRISPR/Cas 9 gene editing technology. Chin. J. Pathog. Biol. 2024, 19, 976–979. [Google Scholar] [CrossRef]
- Rose, S.A.; Wroblewska, A.; Dhainaut, M.; Yoshida, H.; Shaffer, J.M.; Bektesevic, A.; Ben-Zvi, B.; Rhoads, A.; Kim, E.Y.; Yu, B.; et al. A microRNA expression and regulatory element activity atlas of the mouse immune system. Nat. Immunol. 2021, 22, 914–927. [Google Scholar] [CrossRef]
- Hu, J.; Wei, L.; Teng, X.; Du, Y. The value of digital PCR technique in the differential diagnosis of benign and malignant thyroid nodules. Lab. Med. Clin. 2024, 17, 2537–2541. [Google Scholar]


| Gene Symbol | adj.p.Val | p Value | t | B | logFC |
|---|---|---|---|---|---|
| TOP2A | 0.12789 | 0.00535 | 7.3 | −1.89819 | 6.1906911 |
| CALD1 | 0.05537 | 0.000559 | 15.8 | 0.85216 | 6.10536365 |
| RRM2 | 0.38828 | 0.0432 | 3.38 | −4.55825 | 5.6877799 |
| CDK1 | 0.06921 | 0.00108 | 12.7 | 0.08781 | 5.681935 |
| ANLN | 0.04192 | 0.000229 | 21.3 | 1.81079 | 5.6470256 |
| DLGAP5 | 0.1093 | 0.00366 | 8.33 | −1.4181 | 5.58844865 |
| NEK2 | 0.03218 | 0.000111 | 27.2 | 2.50046 | 5.4713496 |
| CDKN3 | 0.06542 | 0.000872 | 13.6 | 0.33851 | 5.4477173 |
| PRC1 | 0.00433 | 7.92 × 10−8 | 307 | 4.6163 | 5.438704 |
| HMMR | 0.11977 | 0.00466 | 7.66 | −1.72192 | 5.4364495 |
| TOP2A | 0.10102 | 0.00287 | 9.06 | −1.11079 | 5.34630875 |
| APOBEC3B | 0.1972 | 0.0135 | 5.26 | −3.07882 | 5.2771654 |
| ASPM | 0.02438 | 0.0000279 | 43.2 | 3.50872 | 5.25215545 |
| SHROOM3 | 0.07134 | 0.00123 | 12.1 | −0.07062 | 5.1339738 |
| CDK1 | 0.0469 | 0.000364 | 18.3 | 1.32791 | 5.11074285 |
| MIR503///MIR424///MIR503HG | 0.26429 | 0.0231 | 4.31 | −3.76446 | 4.9491692 |
| CDKN3 | 0.02826 | 0.0000529 | 34.9 | 3.0913 | 4.9007493 |
| CDK1 | 0.04122 | 0.000222 | 21.6 | 1.84474 | 4.88621195 |
| MYH10 | 0.10263 | 0.003 | 8.92 | −1.16686 | 4.8265022 |
| DEPDC1 | 0.02348 | 0.0000137 | 54.8 | 3.86617 | 4.82202725 |
| NUF2 | 0.06714 | 0.00096 | 13.2 | 0.22503 | 4.80625185 |
| CDC20 | 0.02141 | 0.00000649 | 70.4 | 4.13784 | 4.7577602 |
| SHCBP1 | 0.41379 | 0.048 | 3.24 | −4.69101 | 4.746537 |
| NUSAP1 | 0.04944 | 0.000412 | 17.5 | 1.19152 | 4.6542621 |
| MEGF6 | 0.14361 | 0.00702 | 6.64 | −2.24408 | 4.6460274 |
| KIAA0101 | 0.19579 | 0.0133 | 5.29 | −3.05921 | 4.59005025 |
| CCNB2 | 0.06324 | 0.000747 | 14.3 | 0.51913 | 4.54231145 |
| TK1 | 0.07043 | 0.00116 | 12.4 | 0.00481 | 4.53425635 |
| ST6GALNAC5 | 0.04696 | 0.000367 | 18.2 | 1.31903 | 4.4934988 |
| TRIP13 | 0.21995 | 0.0166 | 4.87 | −3.34373 | 4.42963645 |
| KIF11 | 0.04077 | 0.000217 | 21.7 | 1.86588 | 4.3848553 |
| ANLN | 0.07284 | 0.00134 | 11.8 | −0.17191 | 4.37412475 |
| UBE2C | 0.09333 | 0.0024 | 9.63 | −0.88967 | 4.129496 |
| BIRC5 | 0.02343 | 0.00000957 | 61.8 | 4.00897 | 4.04899235 |
| GALNT15 | 0.107 | 0.00348 | 8.48 | −1.35462 | 4.0395688 |
| PBK | 0.10906 | 0.00364 | 8.35 | −1.40896 | 4.0233828 |
| CCNB1 | 0.18706 | 0.0121 | 5.46 | −2.94424 | 3.98987825 |
| SYNPO2 | 0.17546 | 0.0107 | 5.71 | −2.78445 | 3.9871827 |
| TK1 | 0.08096 | 0.00175 | 10.7 | −0.49598 | 3.942686 |
| ID4 | 0.19244 | 0.0128 | 5.36 | −3.01187 | 3.8204956 |
| CEP55 | 0.02348 | 0.0000113 | 58.5 | 3.94689 | 3.8085747 |
| CENPH | 0.03812 | 0.000187 | 22.8 | 2.01352 | 3.78016685 |
| KNL1 | 0.02538 | 0.0000349 | 40.1 | 3.37228 | 3.74212255 |
| COL4A2 | 0.13932 | 0.00662 | 6.78 | −2.16919 | 3.7290946 |
| ITGA11 | 0.20015 | 0.014 | 5.19 | −3.12318 | 3.72201025 |
| PTN | 0.08079 | 0.00173 | 10.8 | −0.4872 | 3.6569635 |
| SORBS1 | 0.17335 | 0.0105 | 5.76 | −2.75316 | 3.60330895 |
| OXTR | 0.03626 | 0.000157 | 24.2 | 2.18018 | 3.58367625 |
| KIF20A | 0.12046 | 0.00473 | 7.62 | −1.74108 | 3.54996585 |
| NREP | 0.186 | 0.012 | 5.48 | −2.92871 | 3.5390412 |
| GPAT3 | 0.14374 | 0.00703 | −6.63 | −2.24599 | −4.0068785 |
| LRRN3 | 0.02391 | 0.0000265 | −43.9 | 3.53655 | −4.0428889 |
| GDF15 | 0.03792 | 0.000179 | −23.2 | 2.05611 | −4.1874975 |
| LOC101930400///AKR1C2///AKR1C1 | 0.26356 | 0.023 | −4.32 | −3.75854 | −4.2147407 |
| H2BFS | 0.23672 | 0.0191 | −4.63 | −3.52323 | −4.2734285 |
| HIST1H4H | 0.0728 | 0.00132 | −11.8 | −0.1598 | −4.2883313 |
| KCNJ2 | 0.12826 | 0.00549 | −7.23 | −1.93174 | −4.3042694 |
| F2RL1 | 0.04937 | 0.000407 | −17.6 | 1.20609 | −4.3446080 |
| MMP10 | 0.1383 | 0.00651 | −6.82 | −2.1471 | −4.3788025 |
| CD24 | 0.16218 | 0.00915 | −6.05 | −2.58109 | −4.4126261 |
| DUSP6 | 0.06921 | 0.00107 | −12.7 | 0.09775 | −4.4189292 |
| MDM2 | 0.03616 | 0.000155 | −24.3 | 2.19471 | −4.4415299 |
| CNIH3 | 0.17998 | 0.0113 | −5.61 | −2.84847 | −4.4545942 |
| HIST1H2BC///HIST1H2BI///HIST1H2BE///HIST1H2BF///HIST1H2BG | 0.03057 | 0.0000729 | −31.3 | 2.84933 | −4.5961153 |
| HIST1H1C | 0.30462 | 0.0292 | −3.94 | −4.06465 | −4.7696097 |
| CORO2B | 0.19916 | 0.0138 | −5.21 | −3.11184 | −4.7864721 |
| HIST1H2BC | 0.06714 | 0.000945 | −13.2 | 0.24434 | −4.7936413 |
| HIST1H4H | 0.17579 | 0.0108 | −5.7 | −2.79027 | −4.8343656 |
| MIR146A | 0.03218 | 0.000101 | −28.1 | 2.58075 | −4.8751408 |
| PDK4 | 0.07133 | 0.00121 | −12.2 | −0.05298 | −4.8824006 |
| CTSS | 0.23602 | 0.019 | −4.64 | −3.51716 | −4.8909467 |
| PLAU | 0.08098 | 0.00175 | −10.7 | −0.49883 | −4.9086587 |
| RRAD | 0.06324 | 0.000748 | −14.3 | 0.51759 | −4.9342201 |
| GK | 0.04656 | 0.00035 | −18.5 | 1.37074 | −4.9782953 |
| BTBD11 | 0.11079 | 0.0039 | −8.15 | −1.49722 | −5.0217412 |
| CLU | 0.04927 | 0.000402 | −17.7 | 1.22063 | −5.1144443 |
| AKR1B10 | 0.05446 | 0.00053 | −16.1 | 0.91266 | −5.1255345 |
| CD24 | 0.09974 | 0.0028 | −9.14 | −1.08137 | −5.2352129 |
| DCBLD2 | 0.06186 | 0.000702 | −14.6 | 0.59178 | −5.3608140 |
| LINC00973 | 0.02391 | 0.0000267 | −43.9 | 3.53382 | −5.4841241 |
| MMP1 | 0.13772 | 0.00646 | −6.84 | −2.13708 | −5.5256527 |
| AADAC | 0.02391 | 0.0000221 | −46.7 | 3.63589 | −5.5888679 |
| SERPIND1 | 0.00966 | 0.00000035 | −186 | 4.56539 | −5.6156946 |
| KYNU | 0.06773 | 0.001 | −13 | 0.1743 | −5.6242355 |
| ST3GAL6 | 0.03218 | 0.000107 | −27.6 | 2.5333 | −5.7433365 |
| PLAU | 0.31948 | 0.0315 | −3.83 | −4.16154 | −5.7927380 |
| SLC16A6 | 0.03812 | 0.000186 | −22.9 | 2.01797 | −5.8349129 |
| CD24 | 0.07227 | 0.00127 | −12 | −0.11069 | −5.9268551 |
| THBD | 0.12169 | 0.00481 | −7.58 | −1.76274 | −5.9403651 |
| CD24 | 0.07242 | 0.00128 | −11.9 | −0.12211 | −6.0572334 |
| IL13RA2 | 0.04214 | 0.000237 | −21.1 | 1.77762 | −6.3166654 |
| CXCL5 | 0.03057 | 0.0000727 | −31.3 | 2.85071 | −6.428763 |
| SPP1 | 0.09665 | 0.0026 | −9.37 | −0.99003 | −6.4387306 |
| HSD11B1 | 0.04578 | 0.000335 | −18.8 | 1.41684 | −6.5452208 |
| NEFL | 0.02141 | 0.00000638 | −70.8 | 4.14284 | −6.9522091 |
| MMP3 | 0.27605 | 0.025 | −4.18 | −3.86739 | −7.1158428 |
| THBD | 0.02666 | 0.0000419 | −37.7 | 3.25337 | −7.5474292 |
| IL24 | 0.05373 | 0.000515 | −16.2 | 0.94497 | −7.646795 |
| RAB27B | 0.03057 | 0.0000737 | −31.2 | 2.84013 | −7.6470385 |
| EHF | 0.02343 | 0.00000969 | −61.5 | 4.00437 | −7.7647490 |
| Parameter | Numerical Value |
|---|---|
| number of nodes: | 85 |
| number of edges: | 447 |
| average node degree: | 10.5 |
| expected number of edges: | 82 |
| PPI enrichment p-value: | <1.0 × 10−16 |
| ID | Name | Drug Type | Targets |
|---|---|---|---|
| DB02052 | Indirubin-3′-Monoxime | Small Molecule | AHR, CDK1, CDK2, CDK5, CDK5R1, GSK3B |
| DB02116 | Olomoucine | Small Molecule | CDK1, CDK2, CDK5, MAPK1 |
| DB02950 | Hymenialdisine | Small Molecule | CDK1, CDK2, CDK5 |
| DB03496 | Alvocidib | Small Molecule | CDK1, CDK2, CDK4, CDK5, CDK6, CDK7, CDK8, CDK9, EGFR, PYGB, PYGL,… |
| DB04014 | Alsterpaullone | Small Molecule | CDK1, CDK5, GSK3B |
| DB06195 | Seliciclib | Small Molecule | CDK1, CDK2, CDK7, CDK9, CSNK1E, MAPK1, MAPK3 |
| Active Ingredients | Binding Afinity (eV) |
|---|---|
| CDK1 | |
| Indirubin-3′-Monoxime | −2.6 |
| Olomoucine | −6.7 |
| Hymenialdisine | −6.3 |
| Alvocidib | −8.7 |
| Alsterpaullone | −7.8 |
| Seliciclib | −8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Li, Z. Identification of CDK1 as a Biomarker for the Treatment of Liver Fibrosis and Hepatocellular Carcinoma Through Bioinformatics Analysis. Int. J. Mol. Sci. 2025, 26, 3816. https://doi.org/10.3390/ijms26083816
Qin J, Li Z. Identification of CDK1 as a Biomarker for the Treatment of Liver Fibrosis and Hepatocellular Carcinoma Through Bioinformatics Analysis. International Journal of Molecular Sciences. 2025; 26(8):3816. https://doi.org/10.3390/ijms26083816
Chicago/Turabian StyleQin, Jiayi, and Zhuan Li. 2025. "Identification of CDK1 as a Biomarker for the Treatment of Liver Fibrosis and Hepatocellular Carcinoma Through Bioinformatics Analysis" International Journal of Molecular Sciences 26, no. 8: 3816. https://doi.org/10.3390/ijms26083816
APA StyleQin, J., & Li, Z. (2025). Identification of CDK1 as a Biomarker for the Treatment of Liver Fibrosis and Hepatocellular Carcinoma Through Bioinformatics Analysis. International Journal of Molecular Sciences, 26(8), 3816. https://doi.org/10.3390/ijms26083816





