A Systematic Review of MicroRNAs in Hemorrhagic Neurovascular Disease: Cerebral Cavernous Malformations as a Paradigm
Abstract
1. Introduction
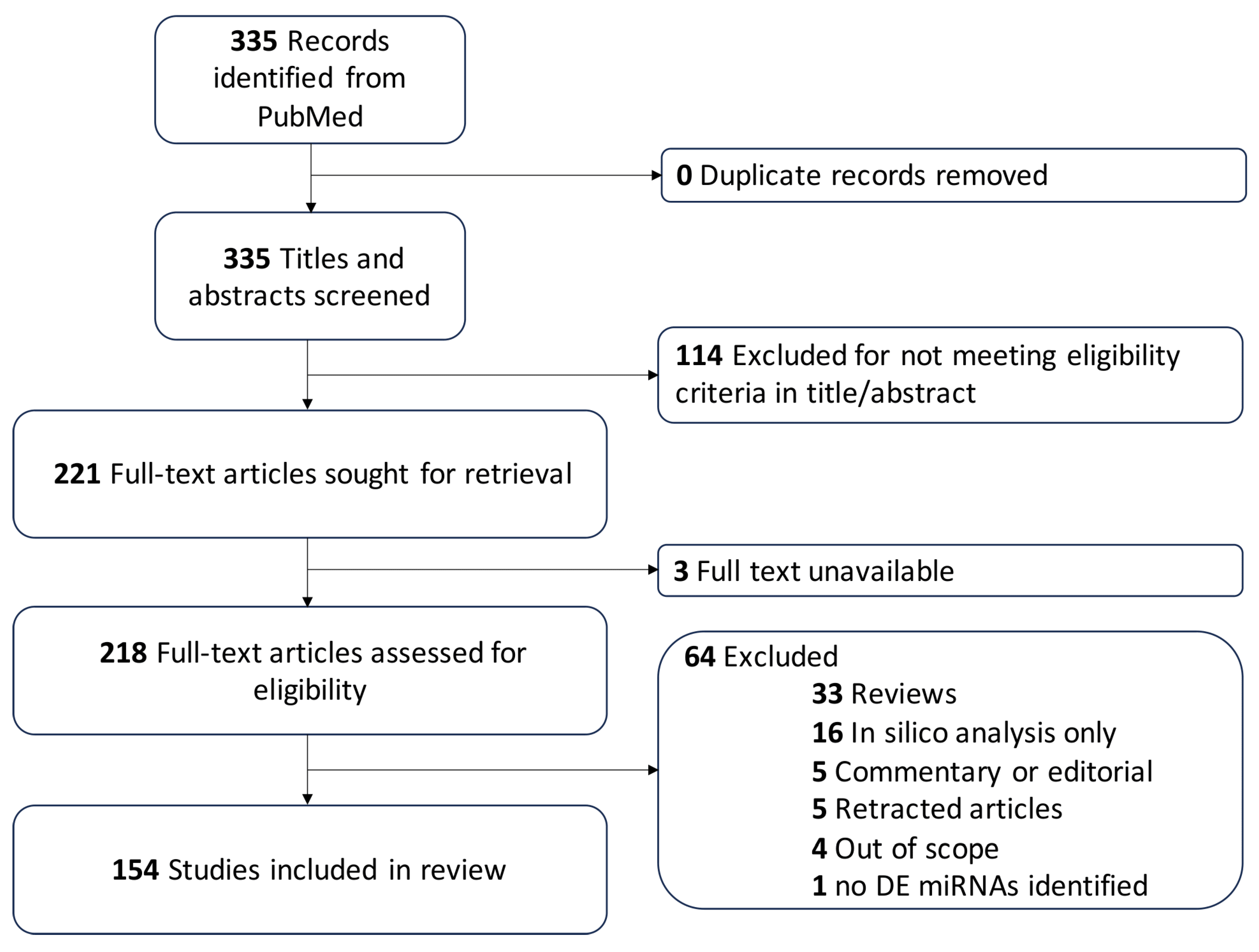
2. Methods
3. Results
3.1. Cerebral Cavernous Malformations
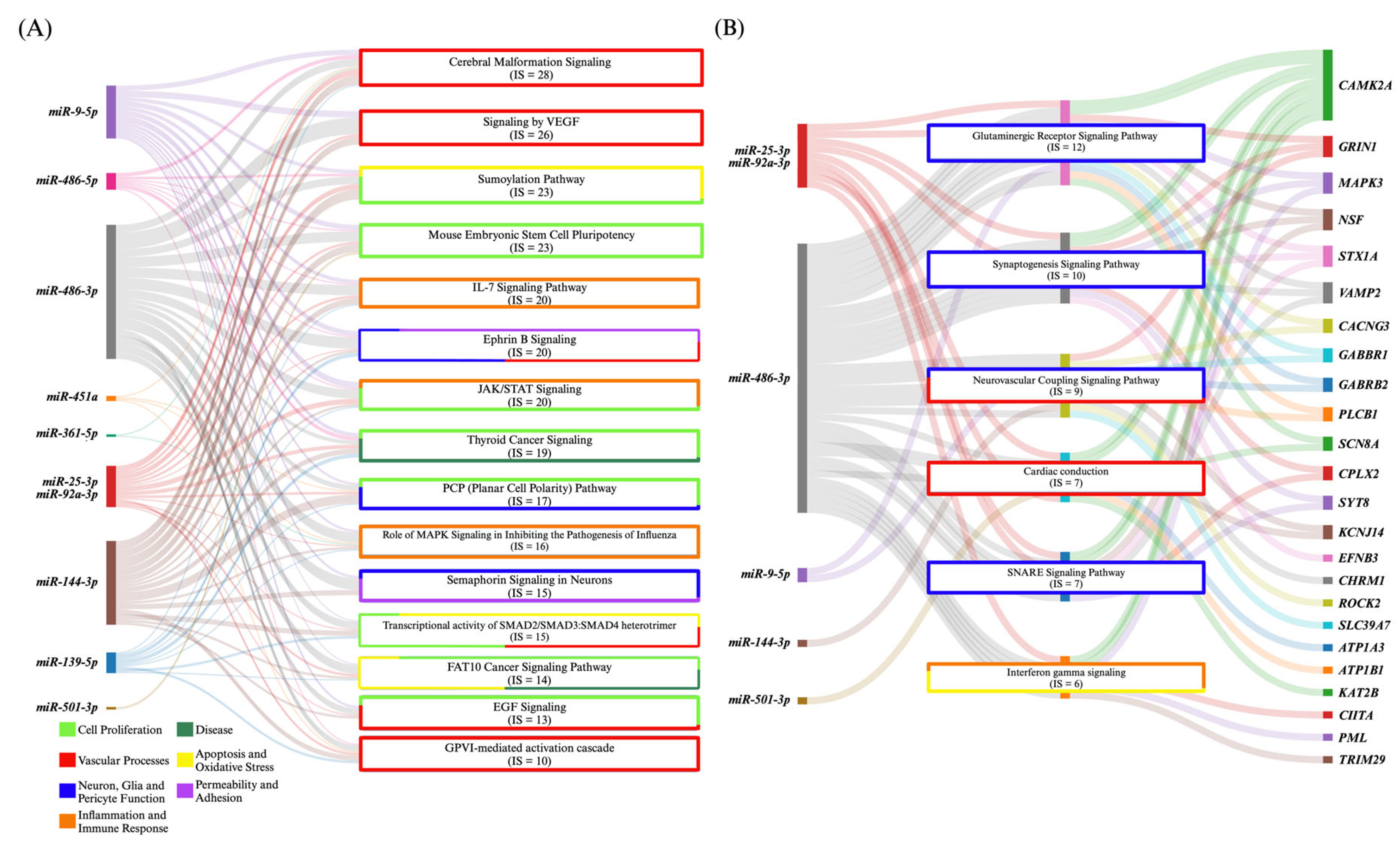
3.1.1. Dysregulated Intracellular miRNAs Are Mechanistically Tied to Vascular Pathobiology
3.1.2. CCM miRNAs Are Differentially Expressed in Mouse and Human Tissue
3.1.3. Circulating miRNAs as Clinical Markers of CCM and Symptomatic Hemorrhage
3.2. Arteriovenous Malformations
3.3. Moyamoya Disease
3.3.1. MiRNAs Are Mechanistically Associated with MMD
3.3.2. Circulating miRNAs Are Differentially Expressed in MMD Patients
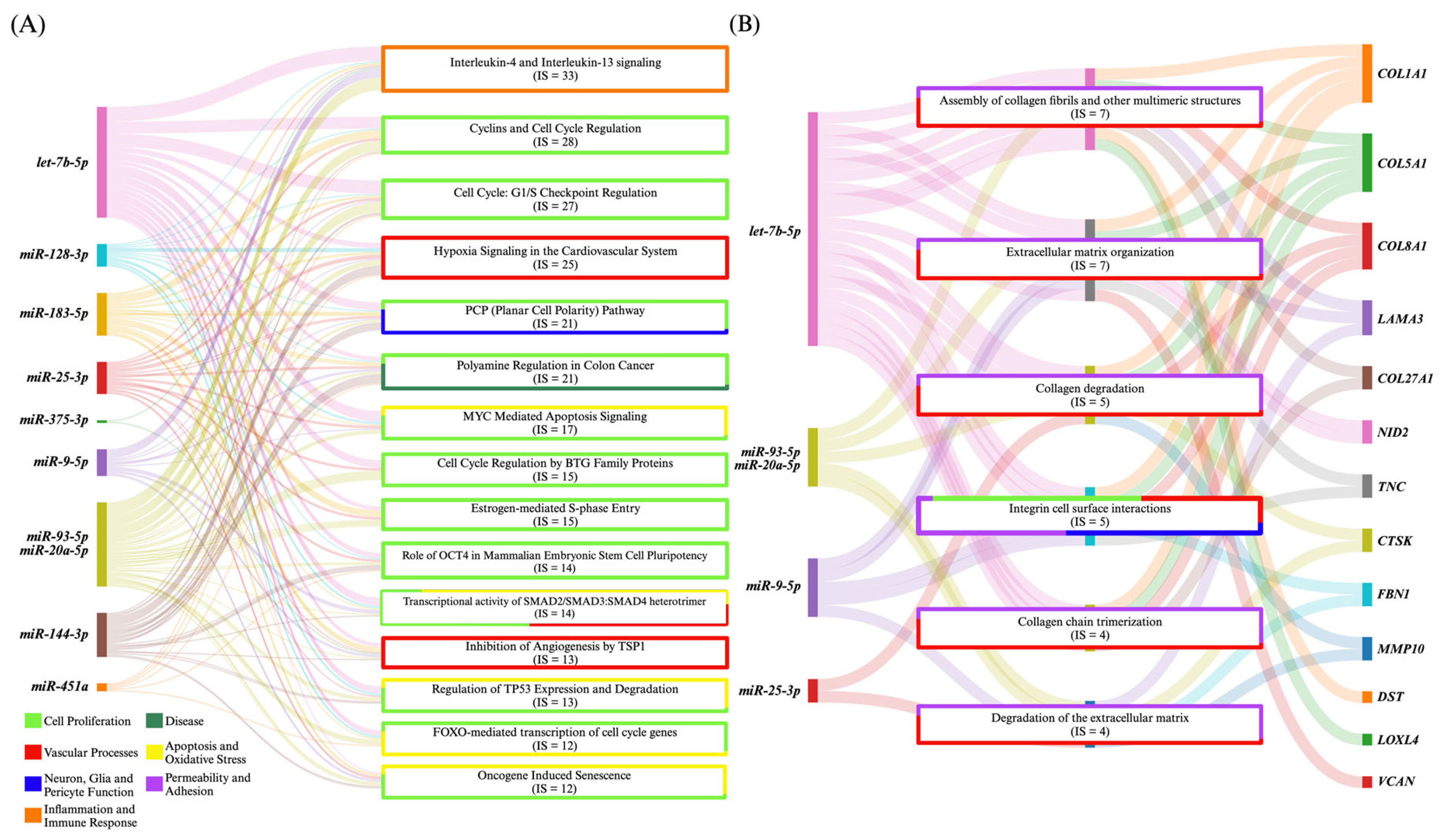
3.4. Intracerebral Hemorrhage
3.4.1. MiRNAs Are Shown to Modulate Brain Vascular Integrity and Adhesion
3.4.2. MiRNAs Are Shown to Modulate Apoptosis/Ferroptosis
3.4.3. MiRNAs Are Shown to Modulate Neuroinflammation After ICH in Microglia
3.4.4. MiRNAs Are Shown to Modulate Neuroinflammation After ICH in Neurons
3.4.5. MiRNAs Are Shown to Modulate Neuroinflammation After ICH in Immune Cells
3.4.6. Circulating miRNAs Are Dysregulated in ICH Patients
4. Discussion
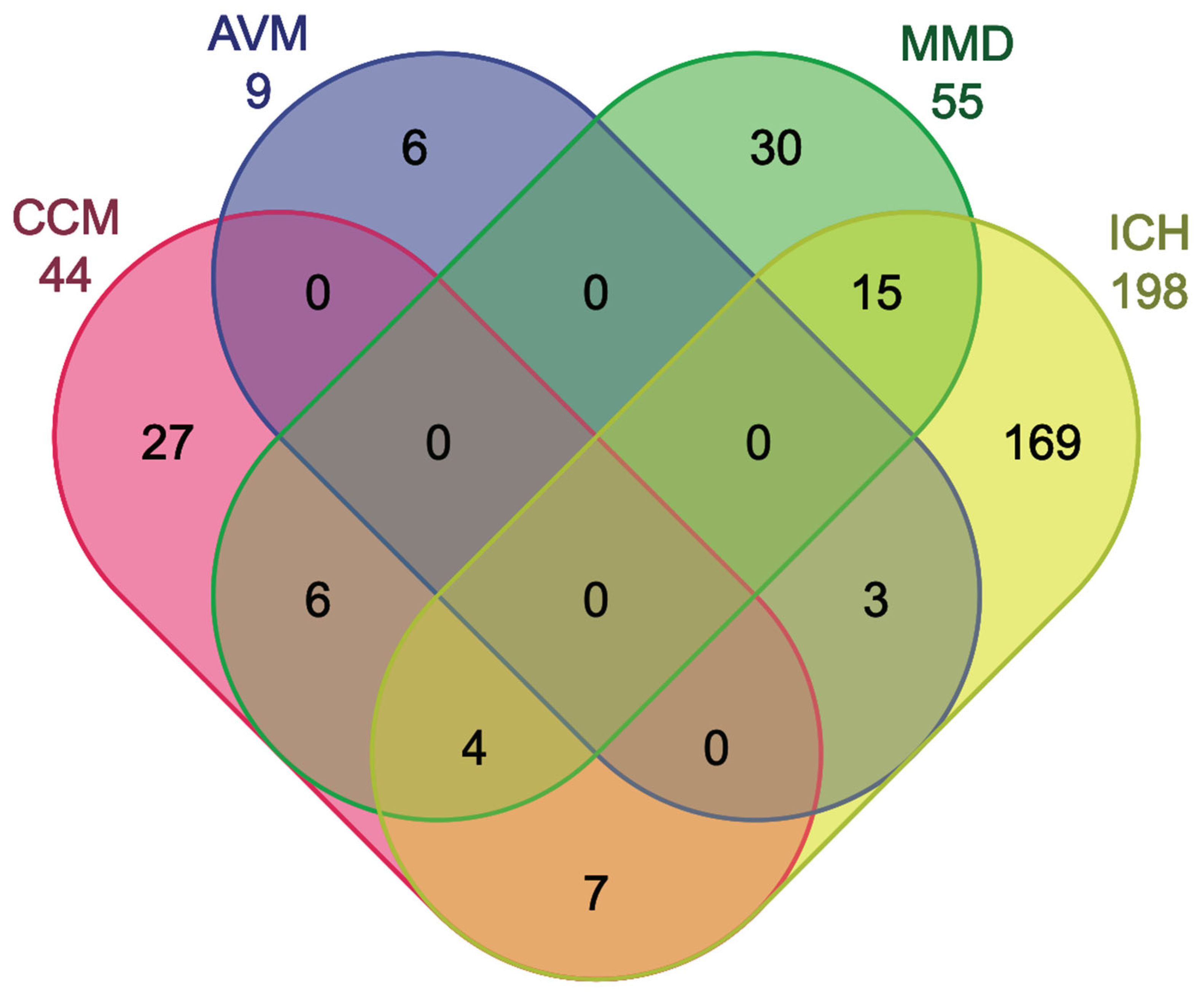
4.1. MiRNA Commonalities of CCM and AVM
4.2. MiRNA Commonalities Between CCM and MMD
4.3. MiRNA Commonalities of CCM and ICH
4.4. MiRNA Commonalities of MMD, ICH, and CCM
4.5. Distinct miRNAs in CCM, AVM, MMD, and ICH and Their Implications
5. Limitations
6. Conclusions and Future Directions
Supplementary Materials
Funding
Conflicts of Interest
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Owolabi, M.O. Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023, 22, 1160–1206. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Papi, G.; Insalata, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Comparison between Ischemic and Hemorrhagic Strokes in Functional Outcome at Discharge from an Intensive Rehabilitation Hospital. Diagnostics 2020, 11, 38. [Google Scholar] [CrossRef]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Takasaki, S. Roles of microRNAs in cancers and development. Methods Mol. Biol. 2015, 1218, 375–413. [Google Scholar] [CrossRef]
- Tsai, P.C.; Liao, Y.C.; Wang, Y.S.; Lin, H.F.; Lin, R.T.; Juo, S.H. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J. Vasc. Res. 2013, 50, 346–354. [Google Scholar] [CrossRef]
- Kala, R.; Peek, G.W.; Hardy, T.M.; Tollefsbol, T.O. MicroRNAs: An emerging science in cancer epigenetics. J. Clin. Bioinform. 2013, 3, 6. [Google Scholar] [CrossRef]
- Fellizar, A.; Refuerzo, V.; Ramos, J.D.; Albano, P.M. Expression of specific microRNAs in tissue and plasma in colorectal cancer. J. Pathol. Transl. Med. 2023, 57, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, M.; Romano, G.; Wernicke, D.; Croce, C.M. MicroRNA and cancer—A brief overview. Adv. Biol. Regul. 2015, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lyne, S.B.; Girard, R.; Koskimaki, J.; Zeineddine, H.A.; Zhang, D.; Cao, Y.; Li, Y.; Stadnik, A.; Moore, T.; Lightle, R.; et al. Biomarkers of cavernous angioma with symptomatic hemorrhage. JCI Insight 2019, 4, e128577. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Z.; Cao, F.; Teng, Z.; Huang, Z.; Sun, X. Rs41291957 polymorphism in the promoter region of microRNA-143 serves as a prognostic biomarker for patients with intracranial hemorrhage. Mol. Med. Rep. 2021, 23, 295. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- What will it take to get miRNA therapies to market? Nat. Biotechnol. 2024, 42, 1623–1624. [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Koskimaki, J.; Zhang, D.; Li, Y.; Saadat, L.; Moore, T.; Lightle, R.; Polster, S.P.; Carrion-Penagos, J.; Lyne, S.B.; Zeineddine, H.A.; et al. Transcriptome clarifies mechanisms of lesion genesis versus progression in models of Ccm3 cerebral cavernous malformations. Acta Neuropathol. Commun. 2019, 7, 132. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Li, Y.; Girard, R.; Srinath, A.; Cruz, D.V.; Ciszewski, C.; Chen, C.; Lightle, R.; Romanos, S.; Sone, J.Y.; Moore, T.; et al. Transcriptomic signatures of individual cell types in cerebral cavernous malformation. Cell Commun. Signal. 2024, 22, 23. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, Y.; Xing, X.; Zhao, Z.; Lu, Y.; Sun, Y.; Zhuang, Z.; Wang, M.; Ji, W.; He, Y. Arsenic-induced anti-angiogenesis via miR-425-5p-regulated CCM3. Toxicol. Lett. 2016, 254, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Choi, J.; Ting, K.K.; Coleman, P.; Chen, J.; Cogger, V.C.; Wan, L.; Shi, Z.; Moller, T.; et al. Targeting miR-27a/VE-cadherin interactions rescues cerebral cavernous malformations in mice. PLoS Biol. 2020, 18, e3000734. [Google Scholar] [CrossRef] [PubMed]
- Romanos, S.G.; Srinath, A.; Li, Y.; Xie, B.; Chen, C.; Li, Y.; Moore, T.; Bi, D.; Sone, J.Y.; Lightle, R.; et al. Circulating Plasma miRNA Homologs in Mice and Humans Reflect Familial Cerebral Cavernous Malformation Disease. Transl. Stroke Res. 2023, 14, 513–529. [Google Scholar] [CrossRef]
- Schwefel, K.; Spiegler, S.; Ameling, S.; Much, C.D.; Pilz, R.A.; Otto, O.; Volker, U.; Felbor, U.; Rath, M. Biallelic CCM3 mutations cause a clonogenic survival advantage and endothelial cell stiffening. J. Cell. Mol. Med. 2019, 23, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Bali, K.K.; Baisantry, A.; Geffers, R.; Samii, A.; Bertalanffy, H. Genome-Wide Sequencing Reveals MicroRNAs Downregulated in Cerebral Cavernous Malformations. J. Mol. Neurosci. 2017, 61, 178–188. [Google Scholar] [CrossRef]
- Srinath, A.; Xie, B.; Li, Y.; Sone, J.Y.; Romanos, S.; Chen, C.; Sharma, A.; Polster, S.; Dorrestein, P.C.; Weldon, K.C.; et al. Plasma metabolites with mechanistic and clinical links to the neurovascular disease cavernous angioma. Commun. Med. 2023, 3, 35. [Google Scholar] [CrossRef]
- Chen, B.; Tao, W.; Yan, L.; Zeng, M.; Song, L.; Huang, Z.; Chen, F. Molecular feature of arterial remodeling in the brain arteriovenous malformation revealed by arteriovenous shunt rat model and RNA sequencing. Int. Immunopharmacol. 2022, 107, 108653. [Google Scholar] [CrossRef]
- Huang, J.; Song, J.; Qu, M.; Wang, Y.; An, Q.; Song, Y.; Yan, W.; Wang, B.; Wang, X.; Zhang, S.; et al. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann. Neurol. 2017, 82, 371–384. [Google Scholar] [CrossRef]
- Marin-Ramos, N.I.; Thein, T.Z.; Ghaghada, K.B.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. miR-18a Inhibits BMP4 and HIF-1alpha Normalizing Brain Arteriovenous Malformations. Circ. Res. 2020, 127, e210–e231. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Shi, Y.; Huang, G.; Chen, L.; Tan, H.; Wang, Z.; Yin, C.; Hu, J. Deep Sequencing of Small RNAs in Blood of Patients with Brain Arteriovenous Malformations. World Neurosurg. 2018, 115, e570–e579. [Google Scholar] [CrossRef]
- Ferreira, R.; Santos, T.; Amar, A.; Gong, A.; Chen, T.C.; Tahara, S.M.; Giannotta, S.L.; Hofman, F.M. Argonaute-2 promotes miR-18a entry in human brain endothelial cells. J. Am. Heart Assoc. 2014, 3, e000968. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Santos, T.; Amar, A.; Tahara, S.M.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke 2014, 45, 293–297. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Huo, R.; Wang, J.; Xu, H.; Zhao, S.; Zhang, J.; Sun, Y.; Jiao, Y.; Weng, J.; Zhao, J.; et al. Exosomal miR-3131 derived from endothelial cells with KRAS mutation promotes EndMT by targeting PICK1 in brain arteriovenous malformations. CNS Neurosci. Ther. 2023, 29, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; Qin, X.; Wang, J.; Zhang, B.; Jin, F. Plasma-derived exosomes contributes to endothelial-to-mesenchymal transition in Moyamoya disease. Heliyon 2024, 10, e26748. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Zhang, X.; Ma, X.; He, X.; Gan, C.; Zou, X.; Wang, S.; Shu, K.; Lei, T.; et al. CircZXDC Promotes Vascular Smooth Muscle Cell Transdifferentiation via Regulating miRNA-125a-3p/ABCC6 in Moyamoya Disease. Cells 2022, 11, 3792. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Y.; He, X.; Zhang, X.; Liu, Y.; Yang, Y.; Yue, P.; Liu, Y.; Gan, C.; Shu, K.; et al. Endothelial Cell-Derived Let-7c-Induced TLR7 Activation on Smooth Muscle Cell Mediate Vascular Wall Remodeling in Moyamoya Disease. Transl. Stroke Res. 2023, 14, 608–623. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, J.; Long, T.; Chen, F.; Wang, Z.; Chen, S.; Zhang, G.; Li, M.; Zhang, S.; Kang, H.; et al. miR-6760-5p suppresses neoangiogenesis by targeting Yes-associated protein 1 in patients with moyamoya disease undergoing indirect revascularization. Gene 2025, 937, 149152. [Google Scholar] [CrossRef]
- Dai, D.; Lu, Q.; Huang, Q.; Yang, P.; Hong, B.; Xu, Y.; Zhao, W.; Liu, J.; Li, Q. Serum miRNA signature in Moyamoya disease. PLoS ONE 2014, 9, e102382. [Google Scholar] [CrossRef]
- Gu, X.; Jiang, D.; Yang, Y.; Zhang, P.; Wan, G.; Gu, W.; Shi, J.; Jiang, L.; Chen, B.; Zheng, Y.; et al. Construction and Comprehensive Analysis of Dysregulated Long Noncoding RNA-Associated Competing Endogenous RNA Network in Moyamoya Disease. Comput. Math. Methods Med. 2020, 2020, 2018214. [Google Scholar] [CrossRef]
- Huang, D.; Qi, H.; Yang, H.; Chen, M. Plasma exosomal microRNAs are non-invasive biomarkers of moyamoya disease: A pilot study. Clinics 2023, 78, 100247. [Google Scholar] [CrossRef]
- Kang, K.; Shen, Y.; Zhang, Q.; Lu, J.; Ju, Y.; Ji, R.; Li, N.; Wu, J.; Yang, B.; Lin, J.; et al. MicroRNA Expression in Circulating Leukocytes and Bioinformatic Analysis of Patients With Moyamoya Disease. Front. Genet. 2022, 13, 816919. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Fallen, S.; Zhou, Y.; Baxter, D.; Scherler, K.; Kuo, M.F.; Wang, K. The Impact of Moyamoya Disease and RNF213 Mutations on the Spectrum of Plasma Protein and MicroRNA. J. Clin. Med. 2019, 8, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Yokoyama, K.; Kanamori, F.; Mamiya, T.; Uda, K.; Araki, Y.; Wakabayashi, T.; Yoshikawa, K.; Saito, R. Moyamoya disease-specific extracellular vesicle-derived microRNAs in the cerebrospinal fluid revealed by comprehensive expression analysis through microRNA sequencing. Acta Neurochir. 2023, 165, 2045–2055. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeon, Y.J.; Lee, B.E.; Kim, T.G.; Choi, J.U.; Kim, D.S.; Kim, N.K. Association of the miR-146aC>G, miR-196a2C>T, and miR-499A>G polymorphisms with moyamoya disease in the Korean population. Neurosci. Lett. 2012, 521, 71–75. [Google Scholar] [CrossRef]
- Uchino, H.; Ito, M.; Kazumata, K.; Hama, Y.; Hamauchi, S.; Terasaka, S.; Sasaki, H.; Houkin, K. Circulating miRNome profiling in Moyamoya disease-discordant monozygotic twins and endothelial microRNA expression analysis using iPS cell line. BMC Med. Genom. 2018, 11, 72. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Y.; Chen, S.; Zhang, G.; Li, M.; Zhang, S.; Qi, S.; Feng, W. Use of a panel of four microRNAs in CSF as a predicted biomarker for postoperative neoangiogenesis in moyamoya disease. CNS Neurosci. Ther. 2021, 27, 908–918. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Y.; Faleti, O.D.; Zhao, Q.; Liu, J.; Zhang, G.; Li, M.; Qi, S.; Feng, W.; Lyu, X. A Panel of Exosome-Derived miRNAs of Cerebrospinal Fluid for the Diagnosis of Moyamoya Disease. Front. Neurosci. 2020, 14, 548278. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Jin, F.; Li, G.; Cui, C.; Feng, S. Exosomal MicroRNAs: Biomarkers of moyamoya disease and involvement in vascular cytoskeleton reconstruction. Heliyon 2024, 10, e32022. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, Z.; Zhang, J.; Xu, X.; Liu, P.; Guan, W.; Jing, L.; Peng, T.; Teng, J.; Jia, Y. Elevated Serum MicroRNA Let-7c in Moyamoya Disease. J. Stroke Cerebrovasc. Dis. 2015, 24, 1709–1714. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Niu, J.Z. miR-222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar] [CrossRef]
- Cepparulo, P.; Cuomo, O.; Vinciguerra, A.; Torelli, M.; Annunziato, L.; Pignataro, G. Hemorrhagic Stroke Induces a Time-Dependent Upregulation of miR-150-5p and miR-181b-5p in the Bloodstream. Front. Neurol. 2021, 12, 736474. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, H.; Lv, C.; Mao, C.; Cui, Y. Long non-coding RNA H19 protects against intracerebral hemorrhage injuries via regulating microRNA-106b-5p/acyl-CoA synthetase long chain family member 4 axis. Bioengineered 2021, 12, 4004–4015. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, L.; Ma, W. Mechanism of SOX10 in ferroptosis of hippocampal neurons after intracerebral hemorrhage via the miR-29a-3p/ACSL4 axis. J. Neurophysiol. 2023, 129, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Wang, Y.P.; Zhang, X.; Li, G.X.; Zheng, K.; Duan, C.Z. lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after intracerebral hemorrhage by targeting miR-709 in mice. Brain Res. Bull. 2020, 162, 20–29. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, J.C.; Pan, M.X.; Chen, S.F.; Zhao, D.; Zhang, Y.; Liao, H.B.; Zhuang, Y.; Lei, R.X.; Wang, S.; et al. l-lysine confers neuroprotection by suppressing inflammatory response via microRNA-575/PTEN signaling after mouse intracerebral hemorrhage injury. Exp. Neurol. 2020, 327, 113214. [Google Scholar] [CrossRef]
- Cui, H.; Yang, A.; Zhou, H.; Wang, Y.; Luo, J.; Zhou, J.; Liu, T.; Li, P.; Zhou, J.; Hu, E.; et al. Thrombin-induced miRNA-24-1-5p upregulation promotes angiogenesis by targeting prolyl hydroxylase domain 1 in intracerebral hemorrhagic rats. J. Neurosurg. 2020, 134, 1515–1526. [Google Scholar] [CrossRef]
- Di, Y.L.; Yu, Y.; Zhao, S.J.; Huang, N.; Fei, X.C.; Yao, D.D.; Ai, L.; Lyu, J.H.; He, R.Q.; Li, J.J.; et al. Formic acid induces hypertension-related hemorrhage in hSSAO(TG) in mice and human. Exp. Neurol. 2022, 358, 114208. [Google Scholar] [CrossRef]
- Ding, H.; Jia, Y.; Lv, H.; Chang, W.; Liu, F.; Wang, D. Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neuroinflammation after diabetic intracerebral hemorrhage via the miR-183-5p/PDCD4/NLRP3 pathway. J. Endocrinol. Investig. 2021, 44, 2685–2698. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, B.; Sun, Z.; Huang, S.; Han, L.; Nie, H.; Chen, G.; Liu, S.; Zhang, Y.; Bao, N.; et al. LncRNA-FENDRR mediates VEGFA to promote the apoptosis of brain microvascular endothelial cells via regulating miR-126 in mice with hypertensive intracerebral hemorrhage. Microcirculation 2018, 25, e12499. [Google Scholar] [CrossRef]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des. Dev. Ther. 2020, 14, 3143–3158. [Google Scholar] [CrossRef]
- Fan, W.; Li, X.; Zhang, D.; Li, H.; Shen, H.; Liu, Y.; Chen, G. Detrimental Role of miRNA-144-3p in Intracerebral Hemorrhage Induced Secondary Brain Injury is Mediated by Formyl Peptide Receptor 2 Downregulation Both In Vivo and In Vitro. Cell Transplant. 2019, 28, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wei, Y. LncRNA PVT1 promotes neuroinflammation after intracerebral hemorrhage by regulating the miR-128-3p/TXNIP axis. Int. J. Neurosci. 2024, 1–15, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, G.; Li, B.; Cao, C.; Cao, D.; Li, X.; Li, H.; Ye, M.; Shen, H.; Chen, G. BMAL1 attenuates intracerebral hemorrhage-induced secondary brain injury in rats by regulating the Nrf2 signaling pathway. Ann. Transl. Med. 2021, 9, 1617. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ge, X.; Wang, C.; Yin, Z.; Jia, Z.; Hu, T.; Li, M.; Wang, D.; Han, Z.; Wang, L.; et al. Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation. Brain Sci. 2023, 13, 639. [Google Scholar] [CrossRef]
- Guo, Q.; Su, H.; He, J.B.; Li, H.Q.; Sha, J.J. MiR-590-5p alleviates intracerebral hemorrhage-induced brain injury through targeting Peli1 gene expression. Biochem. Biophys. Res. Commun. 2018, 504, 61–67. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Yao, X.; Meng, M.; Wan, Y.; Cheng, Y. Mechanism of HDAC1 Regulating Iron Overload-Induced Neuronal Oxidative Damage After Cerebral Hemorrhage. Mol. Neurobiol. 2024, 61, 7549–7566. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, Y.; Liu, X.; Chen, Y.; Zhou, F.; Yang, B. Oxygen glucose deprivation-pretreated astrocyte-derived exosomes attenuates intracerebral hemorrhage (ICH)-induced BBB disruption through miR-27a-3p/ARHGAP25/Wnt/beta-catenin axis. Fluids Barriers CNS 2024, 21, 8. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; He, Z. MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 80, 106141. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; Shi, J.; He, Z. MicroRNA-23b alleviates neuroinflammation and brain injury in intracerebral hemorrhage by targeting inositol polyphosphate multikinase. Int. Immunopharmacol. 2019, 76, 105887. [Google Scholar] [CrossRef]
- Hu, L.T.; Wang, B.Y.; Fan, Y.H.; He, Z.Y.; Zheng, W.X. Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage. Neural Regen. Res. 2023, 18, 560–567. [Google Scholar] [CrossRef]
- Huan, S.; Jin, J.; Shi, C.X.; Li, T.; Dai, Z.; Fu, X.J. Overexpression of miR-146a inhibits the apoptosis of hippocampal neurons of rats with cerebral hemorrhage by regulating autophagy. Hum. Exp. Toxicol. 2020, 39, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, F.; Zhu, J.; Zeng, W.; Liu, Y. MiR-26a inhibits the inflammatory response of microglia by targeting HMGA2 in intracerebral hemorrhage. J. Int. Med. Res. 2020, 48, 300060520929615. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Meng, J.; Zhang, C.; Qi, J.; Wu, H. Consistency of mouse models with human intracerebral hemorrhage: Core targets and non-coding RNA regulatory axis. Aging 2024, 16, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.H.; Yu, J.S.; Kim, S.; Kim, S.H.; Park, D.K.; Moon, J.; et al. Inhibition of Let7c microRNA is neuroprotective in a rat intracerebral hemorrhage model. PLoS ONE 2014, 9, e97946. [Google Scholar] [CrossRef]
- Kong, F.; Zhou, J.; Zhou, W.; Guo, Y.; Li, G.; Yang, L. Protective role of microRNA-126 in intracerebral hemorrhage. Mol. Med. Rep. 2017, 15, 1419–1425. [Google Scholar] [CrossRef]
- Kong, Y.; Li, S.; Zhang, M.; Xu, W.; Chen, Q.; Zheng, L.; Liu, P.; Zou, W. Acupuncture Ameliorates Neuronal Cell Death, Inflammation, and Ferroptosis and Downregulated miR-23a-3p After Intracerebral Hemorrhage in Rats. J. Mol. Neurosci. 2021, 71, 1863–1875. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Shi, S.; Deng, X.; Zeng, X.; Li, Y.; Li, S.; Bai, P. Ubiquitin-like 4A alleviates the progression of intracerebral hemorrhage by regulating oxidative stress and mitochondrial damage. Exp. Anim. 2024, 73, 421–432. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Xia, H.; Wu, Y.; Wu, X.; Chen, S. Sevoflurane protects against intracerebral hemorrhage via microRNA-133b/FOXO4/BCL2 axis. Int. Immunopharmacol. 2023, 114, 109453. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, J.; Wang, Z.; Yang, Z.; Shu, Y.; Gan, S.; Wang, Z.; Lu, W. The phosphokinase activity of IRE1a prevents the oxidative stress injury through miR-25/Nox4 pathway after ICH. CNS Neurosci. Ther. 2024, 30, e14537. [Google Scholar] [CrossRef]
- Liu, D.Z.; Tian, Y.; Ander, B.P.; Xu, H.; Stamova, B.S.; Zhan, X.; Turner, R.J.; Jickling, G.; Sharp, F.R. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 2010, 30, 92–101. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Ge, L.; Xiao, H.; Huang, Y.; Zeng, L.; Jiang, Z.; Lu, M.; Hu, Z. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res. Ther. 2021, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mo, C.; Mao, X.; Lu, M.; Xu, L. Increasing miR-126 Can Prevent Brain Injury after Intracerebral Hemorrhage in Rats by Regulating ZEB1. Contrast Media Mol. Imaging 2022, 2022, 2698773. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Huang, K. Protective effect of silencing lncRNA HCP5 against brain injury after intracerebral hemorrhage by targeting miR-195-5p. BMC Neurosci. 2025, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, L.; Song, X.D.; Chen, H.X.; Yun, D.B.; Wang, L.; Zhang, Y. MicroRNA-7 attenuates secondary brain injury following experimental intracerebral hemorrhage via inhibition of NLRP3. J. Stroke Cerebrovasc. Dis. 2024, 33, 107670. [Google Scholar] [CrossRef]
- Matsuoka, H.; Tamura, A.; Kinehara, M.; Shima, A.; Uda, A.; Tahara, H.; Michihara, A. Levels of tight junction protein CLDND1 are regulated by microRNA-124 in the cerebellum of stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2018, 498, 817–823. [Google Scholar] [CrossRef]
- Nie, H.; Hu, Y.; Guo, W.; Wang, W.; Yang, Q.; Dong, Q.; Tang, Y.; Li, Q.; Tang, Z. miR-331-3p Inhibits Inflammatory Response after Intracerebral Hemorrhage by Directly Targeting NLRP6. Biomed. Res. Int. 2020, 2020, 6182464. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, D.; Wang, H.; Wan, Z.; Luo, Q.; Zhong, Y.; Yin, M.; Qing, Z.; Li, Z.; Bao, B.; et al. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell 2019, 18, e13022. [Google Scholar] [CrossRef]
- Qi, J.; Meng, C.; Mo, J.; Shou, T.; Ding, L.; Zhi, T. CircAFF2 Promotes Neuronal Cell Injury in Intracerebral Hemorrhage by Regulating the miR-488/CLSTN3 Axis. Neuroscience 2023, 535, 75–87. [Google Scholar] [CrossRef]
- Qu, X.; Wang, N.; Cheng, W.; Xue, Y.; Chen, W.; Qi, M. MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp. Ther. Med. 2019, 18, 3920–3928. [Google Scholar] [CrossRef]
- Ren, S.; Wu, G.; Huang, Y.; Wang, L.; Li, Y.; Zhang, Y. MiR-18a Aggravates Intracranial Hemorrhage by Regulating RUNX1-Occludin/ZO-1 Axis to Increase BBB Permeability. J. Stroke Cerebrovasc. Dis. 2021, 30, 105878. [Google Scholar] [CrossRef]
- Robles, D.; Guo, D.H.; Watson, N.; Asante, D.; Sukumari-Ramesh, S. Dysregulation of Serum MicroRNA after Intracerebral Hemorrhage in Aged Mice. Biomedicines 2023, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Zhou, C.; Ma, K.; Zhao, W.; Xiong, Q.; Yang, L.; Huang, Z.; Yang, Z. MiRNA-494 enhances M1 macrophage polarization via Nrdp1 in ICH mice model. J. Inflamm. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xu, X.; Yu, Z.; Li, H.; Shen, H.; Li, X.; Shen, M.; Chen, G. Rbfox-1 contributes to CaMKIIalpha expression and intracerebral hemorrhage-induced secondary brain injury via blocking micro-RNA-124. J. Cereb. Blood Flow Metab. 2021, 41, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yao, X.; Li, H.; Li, X.; Zhang, T.; Sun, Q.; Ji, C.; Chen, G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J. Mol. Neurosci. 2018, 64, 421–430. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Cui, H.F.; Qin, B.J. Monomethyl fumarate protects cerebral hemorrhage injury in rats via activating microRNA-139/Nrf2 axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5012–5019. [Google Scholar] [CrossRef]
- Song, H.; Xu, N.; Jin, S. miR-30e-5p attenuates neuronal deficit and inflammation of rats with intracerebral hemorrhage by regulating TLR4. Exp. Ther. Med. 2022, 24, 492. [Google Scholar] [CrossRef]
- Sun, J.; Xu, G. Mesenchymal Stem Cell-Derived Exosomal miR-150-3p Affects Intracerebral Hemorrhage By Regulating TRAF6/NF-kappaB Axis, Gut Microbiota and Metabolism. Stem Cell Rev. Rep. 2023, 19, 1907–1921. [Google Scholar] [CrossRef]
- Tang, J.; Yan, B.; Tang, Y.; Zhou, X.; Ji, Z.; Xu, F. Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int. J. Neurosci. 2023, 133, 1380–1393. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chang, C.H.; Chong, Y.B.; Wu, C.H.; Tsai, H.P.; Cheng, T.L.; Lin, C.L. MicroRNA-195-5p Inhibits Intracerebral Hemorrhage-Induced Inflammatory Response and Neuron Cell Apoptosis. Int. J. Mol. Sci. 2024, 25, 10321. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chang, C.H.; Chong, Y.B.; Wu, C.H.; Tsai, H.P.; Cheng, T.L.; Lin, C.L. MicroRNA-195-5p Attenuates Intracerebral-Hemorrhage-Induced Brain Damage by Inhibiting MMP-9/MMP-2 Expression. Biomedicines 2024, 12, 1373. [Google Scholar] [CrossRef]
- Walsh, K.B.; Zimmerman, K.D.; Zhang, X.; Demel, S.L.; Luo, Y.; Langefeld, C.D.; Wohleb, E.; Schulert, G.; Woo, D.; Adeoye, O. miR-181a Mediates Inflammatory Gene Expression After Intracerebral Hemorrhage: An Integrated Analysis of miRNA-seq and mRNA-seq in a Swine ICH Model. J. Mol. Neurosci. 2021, 71, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Y.; Li, G.S.; Tu, C.; Chen, W.L.; Wang, X.W.; Wang, Y.N.; Peng, L.B.; Tan, F. MicroNAR-194-5p hinders the activation of NLRP3 inflammasomes and alleviates neuroinflammation during intracerebral hemorrhage by blocking the interaction between TRAF6 and NLRP3. Brain Res. 2021, 1752, 147228. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tian, L.; Zhang, Z.; Liu, Z.; Li, K.; Zhang, Q.; Song, Y.; Qi, J. CircTrim37 Ameliorates Intracerebral Hemorrhage Outcomes by Modulating Microglial Polarization via the miR-30c-5p/SOCS3 Axis. Mol. Neurobiol. 2024, 61, 4038–4054. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, X.; Xiao, L.; Chen, Y. FoxO1 Silencing Facilitates Neurological Function Recovery in Intracerebral Hemorrhage Mice via the lncRNA GAS5/miR-378a-5p/Hspa5 Axis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106443. [Google Scholar] [CrossRef]
- Wang, B.Q.; He, M.; Wang, Y.; Liu, S.; Guo, Z.W.; Liu, Z.L. Hyperbaric oxygen ameliorates neuronal injury and neurological function recovery in rats with intracerebral hemorrhage by silencing microRNA-204-5p-targeted chloride channel protein 3. J. Physiol. Pharmacol. 2023, 74, 347–354. [Google Scholar] [CrossRef]
- Wang, C.; Cao, J.; Duan, S.; Xu, R.; Yu, H.; Huo, X.; Qian, Y. Effect of MicroRNA-126a-3p on Bone Marrow Mesenchymal Stem Cells Repairing Blood-brain Barrier and Nerve Injury after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 104748. [Google Scholar] [CrossRef]
- Wang, J.; Teng, F.; Liu, S.; Pan, X.; Yang, B.; Wu, W. lncRNA SND1-IT1 delivered via intracerebral hemorrhage-derived exosomes affect the growth of human microglia by regulating the miR-124-3p/MTF1 axis. J. Cell. Physiol. 2023, 238, 366–378. [Google Scholar] [CrossRef]
- Wang, M.; Mungur, R.; Lan, P.; Wang, P.; Wan, S. MicroRNA-21 and microRNA-146a negatively regulate the secondary inflammatory response of microglia after intracerebral hemorrhage. Int. J. Clin. Exp. Pathol. 2018, 11, 3348–3356. [Google Scholar]
- Wang, M.D.; Wang, Y.; Xia, Y.P.; Dai, J.W.; Gao, L.; Wang, S.Q.; Wang, H.J.; Mao, L.; Li, M.; Yu, S.M.; et al. High Serum MiR-130a Levels Are Associated with Severe Perihematomal Edema and Predict Adverse Outcome in Acute ICH. Mol. Neurobiol. 2016, 53, 1310–1321. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Y.; Xu, J.; Gao, H. miR-140-5p Attenuates Neuroinflammation and Brain Injury in Rats Following Intracerebral Hemorrhage by Targeting TLR4. Inflammation 2019, 42, 1869–1877. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Wu, L.; Duan, X.; Hu, Y.; Sun, Y.; Wei, Y.; Dong, Z.; Wu, C.; Yu, D.; et al. Deletion of MicroRNA-144/451 Cluster Aggravated Brain Injury in Intracerebral Hemorrhage Mice by Targeting 14-3-3zeta. Front. Neurol. 2020, 11, 551411. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Pang, Y.; Yu, Z.; Hua, W.; Gu, Y.; Qi, J.; Wu, H. miR-183-5p alleviates early injury after intracerebral hemorrhage by inhibiting heme oxygenase-1 expression. Aging 2020, 12, 12869–12895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Cheng, M.; Hu, E.; Yan, Q.; Zheng, F.; Guo, X.; Zhang, W.; Li, H.; Li, Z.; et al. Buyang huanwu decoction promotes remyelination via miR-760-3p/GPR17 axis after intracerebral hemorrhage. J. Ethnopharmacol. 2024, 328, 118126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Hao, Y.; Jin, F.; Tang, L.; Xu, X.; He, Z.; Wang, Y. Expression profile of circular RNAs in blood samples of Northern Chinese males with intracerebral hemorrhage shows downregulation of hsa-circ-0090829. Heliyon 2024, 10, e35864. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, K.; Wang, J.J.; Hua, W.; Liu, Q.; Sun, Y.L.; Qi, J.P.; Song, Y.J. Bone marrow-derived mesenchymal stem cell-derived exosome-loaded miR-129-5p targets high-mobility group box 1 attenuates neurological-impairment after diabetic cerebral hemorrhage. World J. Diabetes 2024, 15, 1979–2001. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, L.; Shi, H.; Yang, Z. miR-181b regulates ER stress induced neuron death through targeting Heat Shock Protein A5 following intracerebral haemorrhage. Immunol. Lett. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, B.; Fu, F.; Huang, S.; Yang, Z. Hemoglobin enhances miRNA-144 expression and autophagic activation mediated inflammation of microglia via mTOR pathway. Sci. Rep. 2017, 7, 11861. [Google Scholar] [CrossRef]
- Wei, M.; Li, C.; Yan, Z.; Hu, Z.; Dong, L.; Zhang, J.; Wang, X.; Li, Y.; Zhang, H. Activated Microglia Exosomes Mediated miR-383-3p Promotes Neuronal Necroptosis Through Inhibiting ATF4 Expression in Intracerebral Hemorrhage. Neurochem. Res. 2021, 46, 1337–1349. [Google Scholar] [CrossRef]
- Wu, T.S.; Lin, Y.T.; Huang, Y.T.; Yu, F.Y.; Liu, B.H. Ochratoxin A triggered intracerebral hemorrhage in embryonic zebrafish: Involvement of microRNA-731 and prolactin receptor. Chemosphere 2020, 242, 125143. [Google Scholar] [CrossRef]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; He, Z. MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem. Biophys. Res. Commun. 2017, 494, 144–151. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, Z.; Wan, W.; Pan, W.; Xu, J. miR-145-5p targets MMP2 to protect brain injury in hypertensive intracerebral hemorrhage via inactivation of the Wnt/beta-catenin signaling pathway. Ann. Transl. Med. 2022, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Qiao, M.; Xuan, J. lncRNA MEG3 Downregulation Relieves Intracerebral Hemorrhage by Inhibiting Oxidative Stress and Inflammation in an miR-181b-Dependent Manner. Med. Sci. Monit. 2021, 27, e929435. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.F.; Fang, X.Y.; Zhu, S.H.; Xu, X.H.; Zhang, Z.X.; Wang, Z.F.; Zhao, Z.Q.; Ding, Y.J.; Tao, L.Y. Glucocorticoid treatment inhibits intracerebral hemorrhage-induced inflammation by targeting the microRNA-155/SOCS-1 signaling pathway. Mol. Med. Rep. 2016, 14, 3798–3804. [Google Scholar] [CrossRef]
- Xu, W.; Li, F.; Liu, Z.; Xu, Z.; Sun, B.; Cao, J.; Liu, Y. MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury. Oncotarget 2017, 8, 70669–70684. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, B.; Mao, J.; Sun, Z. Knockdown of long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1 protects against intracerebral hemorrhage through microRNA-146a-mediated inhibition of inflammation and oxidative stress. Bioengineered 2022, 13, 3969–3980. [Google Scholar] [CrossRef]
- Yang, W.; Ding, N.; Luo, R.; Zhang, Q.; Li, Z.; Zhao, F.; Zhang, S.; Zhang, X.; Zhou, T.; Wang, H.; et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact. Mater. 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Yang, W.S.; Shen, Y.Q.; Yang, X.; Li, X.H.; Xu, S.H.; Zhao, L.B.; Li, R.; Xiong, X.; Bai, S.J.; Wu, Q.Y.; et al. MicroRNA Transcriptomics Analysis Identifies Dysregulated Hedgehog Signaling Pathway in a Mouse Model of Acute Intracerebral Hemorrhage Exposed to Hyperglycemia. J. Stroke Cerebrovasc. Dis. 2022, 31, 106281. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, L.; Xi, J.; Liu, X.; Yang, H.; Luo, Q.; Xie, F.; Niu, J.; Meng, P.; Tian, X.; et al. Mesenchymal stem cell-derived extracellular vesicles mitigate neuronal damage from intracerebral hemorrhage by modulating ferroptosis. Stem Cell Res. Ther. 2024, 15, 255. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, J.; Liao, Y.; Gan, S.; Zhu, S.; Xu, S.; Shu, Y.; Lu, W. ER Stress is Involved in Mast Cells Degranulation via IRE1alpha/miR-125/Lyn Pathway in an Experimental Intracerebral Hemorrhage Mouse Model. Neurochem. Res. 2022, 47, 1598–1609. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, X.; Zhang, J.; Huang, X.; Zhang, X.; Wang, J.; Shi, H.; Yu, A. Let-7a promotes microglia M2 polarization by targeting CKIP-1 following ICH. Immunol. Lett. 2018, 202, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, L.; Xian, R.; Yuan, B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol. Immunol. 2015, 65, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chen, Z.; Ouyang, Y.; Zhang, H.; Wan, Z.; Wang, H.; Wu, W.; Yin, X. Thrombin-induced, TNFR-dependent miR-181c downregulation promotes MLL1 and NF-kappaB target gene expression in human microglia. J. Neuroinflamm. 2017, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhang, T.; Duan, H.; Pan, Y.; Zhang, X.; Yang, G.; Wang, J.; Deng, Y.; Yang, Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-alpha pathway in intracerebral hemorrhage. Immunol. Lett. 2017, 182, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhang, T.; Zhong, W.; Duan, H.; Wang, S.; Ye, P.; Wang, J.; Zhong, S.; Yang, Z. miRNA-144 induces microglial autophagy and inflammation following intracerebral hemorrhage. Immunol. Lett. 2017, 182, 18–23. [Google Scholar] [CrossRef]
- Yu, M.; Tian, T.; Zhang, J.; Hu, T. miR-141-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting ZEB2. J. Clin. Neurosci. 2022, 99, 253–260. [Google Scholar] [CrossRef]
- Yu, N.; Tian, W.; Liu, C.; Zhang, P.; Zhao, Y.; Nan, C.; Jin, Q.; Li, X.; Liu, Y. miR-122-5p Promotes Peripheral and Central Nervous System Inflammation in a Mouse Model of Intracerebral Hemorrhage via Disruption of the MLLT1/PI3K/AKT Signaling. Neurochem. Res. 2023, 48, 3665–3682. [Google Scholar] [CrossRef]
- Yuan, B.; Shen, H.; Lin, L.; Su, T.; Zhong, L.; Yang, Z. MicroRNA367 negatively regulates the inflammatory response of microglia by targeting IRAK4 in intracerebral hemorrhage. J. Neuroinflamm. 2015, 12, 206. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ren, X.M.; Li, H.B.; Wei, W.; Wang, K.X.; Li, Y.M.; Hu, J.L.; Li, X. Effect of miR-130a on neuronal injury in rats with intracranial hemorrhage through PTEN/PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4890–4897. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; Hao, Y.; Tang, L.; He, Z. MicroRNA-26a-5p alleviates neuronal apoptosis and brain injury in intracerebral hemorrhage by targeting RAN binding protein 9. Acta Histochem. 2020, 122, 151571. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lian, L.; Zhang, C.; He, Z. Glycine-Histidine-Lysine (GHK) Alleviates Astrocytes Injury of Intracerebral Hemorrhage via the Akt/miR-146a-3p/AQP4 Pathway. Front. Neurosci. 2020, 14, 576389. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lv, Q.; Gao, J.; Hu, L.; He, Z. MicroRNA-21 Overexpression Promotes the Neuroprotective Efficacy of Mesenchymal Stem Cells for Treatment of Intracerebral Hemorrhage. Front. Neurol. 2018, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Wang, R.; Duan, Z.; Wang, H. A blockade of microRNA-155 signal pathway has a beneficial effect on neural injury after intracerebral haemorrhage via reduction in neuroinflammation and oxidative stress. Arch. Physiol. Biochem. 2022, 128, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Fan, Q.Y.; Qiu, Z.; Chen, S. MiR-7 alleviates secondary inflammatory response of microglia caused by cerebral hemorrhage through inhibiting TLR4 expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5597–5604. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; He, Y.; Li, D.; Ma, X.; Liu, Q.; Hao, J. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem. Int. 2017, 107, 182–190. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, J.; Zhang, Y.; Jiang, X.; Tian, Y.; Zheng, X.; Wang, K.; Cui, J. Elevated miR-29a Contributes to Axonal Outgrowth and Neurological Recovery After Intracerebral Hemorrhage via Targeting PTEN/PI3K/Akt Pathway. Cell. Mol. Neurobiol. 2021, 41, 1759–1772. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Yuan, G.Q.; Zhang, G.G.; Chen, Y.T.; Nie, Q.Q.; Wang, Z. Identification of CCL20 as a Key Biomarker of Inflammatory Responses in the Pathogenesis of Intracerebral Hemorrhage. Inflammation 2023, 46, 1290–1304. [Google Scholar] [CrossRef]
- Zhu, Z.; Mo, S.; Wang, X.; Meng, M.; Qiao, L. Circ-AGTPBP1 promotes white matter injury through miR-140-3p/Pcdh17 axis role of Circ-AGTPBP1 in white matter injury. J. Bioenerg. Biomembr. 2024, 56, 1–14. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, G.; Chen, S.; Wu, X.; Li, J.; Wang, J.; Chen, D.; Liu, X.; Wang, J.; Li, Y.; et al. MicroRNA-451 Regulates Angiogenesis in Intracerebral Hemorrhage by Targeting Macrophage Migration Inhibitory Factor. Mol. Neurobiol. 2024, 61, 10481–10499. [Google Scholar] [CrossRef]
- Bao, W.D.; Zhou, X.T.; Zhou, L.T.; Wang, F.; Yin, X.; Lu, Y.; Zhu, L.Q.; Liu, D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell 2020, 19, e13235. [Google Scholar] [CrossRef]
- Fang, Y.; Hong, X. miR-124-3p Inhibits Microglial Secondary Inflammation After Basal Ganglia Hemorrhage by Targeting TRAF6 and Repressing the Activation of NLRP3 Inflammasome. Front. Neurol. 2021, 12, 653321. [Google Scholar] [CrossRef]
- Fu, X.; Niu, T.; Li, X. MicroRNA-126-3p Attenuates Intracerebral Hemorrhage-Induced Blood-Brain Barrier Disruption by Regulating VCAM-1 Expression. Front. Neurosci. 2019, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Wang, H.; Huang, Q.; Wang, G.; Zhang, H.B. MicroRNA-23a-3p promotes the perihematomal edema formation after intracerebral hemorrhage via ZO-1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liu, X.; Wang, X.; Hu, J.; Chang, S.; Cui, X. LncRNA FGD5-AS1 accelerates intracerebral hemorrhage injury in mice by adsorbing miR-6838-5p to target VEGFA. Brain Res. 2022, 1776, 147751. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, L.; Wang, P.; Yu, J.; Zhong, J.; Tang, Q.; Zhu, T.; Chen, K.; Li, F.; Hong, P.; et al. Extracellular vesicles from neural stem cells safeguard neurons in intracerebral hemorrhage by suppressing reactive astrocyte neurotoxicity. Cell Rep. 2024, 43, 114854. [Google Scholar] [CrossRef]
- Liang, T.; Liu, R.; Liu, J.; Hong, J.; Gong, F.; Yang, X. miRNA506 Activates Sphk1 Binding with Sirt1 to Inhibit Brain Injury After Intracerebral Hemorrhage via PI3K/AKT Signaling Pathway. Mol. Neurobiol. 2025, 62, 4093–4114. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.Y.; He, Z.Y. MicroRNA-181c provides neuroprotection in an intracerebral hemorrhage model. Neural Regen. Res. 2020, 15, 1274–1282. [Google Scholar] [CrossRef]
- Pei, H.; Peng, Q.; Guo, S.; Gu, Y.; Sun, T.; Xu, D.; Jiang, Y.; Xie, J.; Zhang, L.; Zhu, Z. MiR-367 alleviates inflammatory injury of microglia by promoting M2 polarization via targeting CEBPA. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 878–887. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Wen, X.; Li, D.; Ouyang, Y.; Bao, B.; Zhong, Y.; Qin, Z.; Yin, M.; Chen, Z.; et al. Transforming growth factor-beta1 functions as a competitive endogenous RNA that ameliorates intracranial hemorrhage injury by sponging microRNA-93-5p. Mol. Med. Rep. 2021, 24, 499. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Sze, J.; Liu, Y.; Lin, S.; Yao, H.; Zhang, J.; Xie, D.; Liu, Q.; Kung, H.F.; et al. Plasma miR-124 Is a Promising Candidate Biomarker for Human Intracerebral Hemorrhage Stroke. Mol. Neurobiol. 2018, 55, 5879–5888. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Hu, Q.; Wang, J.; Zhang, S.; Cui, W.; Shi, Y.; Bai, H.; Zhou, J.; Han, L.; et al. Astrocyte-Derived Extracellular Vesicular miR-143-3p Dampens Autophagic Degradation of Endothelial Adhesion Molecules and Promotes Neutrophil Transendothelial Migration after Acute Brain Injury. Adv. Sci. 2024, 11, e2305339. [Google Scholar] [CrossRef]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; Scalzo, F.; He, Z. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J. Biol. Chem. 2018, 293, 20041–20050. [Google Scholar] [CrossRef]
- Xu, L.; Mo, C.; Lu, M.; Wang, P.; Liu, Y. MiR-20a-5p targets RBM24 and alleviates hypertensive intracerebral hemorrhage. Cell. Mol. Biol. 2023, 69, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, G.; Ye, J.; Jiang, J.; Xu, Q. Protective Effect of miR-340-5p against Brain Injury after Intracerebral Hemorrhage by Targeting PDCD4. Cerebrovasc. Dis. 2020, 49, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ander, B.P.; Jickling, G.C.; Zhan, X.; Hull, H.; Sharp, F.R.; Stamova, B. MicroRNA and their target mRNAs change expression in whole blood of patients after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2020, 40, 775–786. [Google Scholar] [CrossRef]
- Gareev, I.; Yang, G.; Sun, J.; Beylerli, O.; Chen, X.; Zhang, D.; Zhao, B.; Zhang, R.; Sun, Z.; Yang, Q.; et al. Circulating MicroRNAs as Potential Noninvasive Biomarkers of Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2020, 133, e369–e375. [Google Scholar] [CrossRef]
- Giordano, M.; Trotta, M.C.; Ciarambino, T.; D’Amico, M.; Schettini, F.; Sisto, A.D.; D’Auria, V.; Voza, A.; Malatino, L.S.; Biolo, G.; et al. Circulating miRNA-195-5p and -451a in Patients with Acute Hemorrhagic Stroke in Emergency Department. Life 2022, 12, 763. [Google Scholar] [CrossRef]
- Guo, D.; Liu, J.; Wang, W.; Hao, F.; Sun, X.; Wu, X.; Bu, P.; Zhang, Y.; Liu, Y.; Liu, F.; et al. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke 2013, 44, 1739–1742. [Google Scholar] [CrossRef]
- Kalani, M.Y.S.; Alsop, E.; Meechoovet, B.; Beecroft, T.; Agrawal, K.; Whitsett, T.G.; Huentelman, M.J.; Spetzler, R.F.; Nakaji, P.; Kim, S.; et al. Extracellular microRNAs in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J. Extracell. Vesicles 2020, 9, 1713540. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Shi, Q. Changes in Serum LncRNA MEG3/miR-181b and UCH-L1 Levels in Patients with Moderate and Severe Intracerebral Hemorrhage. Turk. Neurosurg. 2024, 34, 20–27. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Jin, F.; Tang, L.; He, Z.; He, Z. Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage. J. Int. Med. Res. 2016, 44, 419–432. [Google Scholar] [CrossRef]
- Zheng, H.W.; Wang, Y.L.; Lin, J.X.; Li, N.; Zhao, X.Q.; Liu, G.F.; Liu, L.P.; Jiao, Y.; Gu, W.K.; Wang, D.Z.; et al. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci. Ther. 2012, 18, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, J.L.; He, Z.Y.; Jin, F.; Tang, L. Association of Altered Serum MicroRNAs with Perihematomal Edema after Acute Intracerebral Hemorrhage. PLoS ONE 2015, 10, e0133783. [Google Scholar] [CrossRef] [PubMed]
- Kurata, A.; Miyasaka, Y.; Kitahara, T.; Kan, S.; Takagi, H. Subcortical cerebral hemorrhage with reference to vascular malformations and hypertension as causes of hemorrhage. Neurosurgery 1993, 32, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Margolis, G.; Odom, G.L.; Woodhall, B.; Bloor, B.M. The role of small angiomatous malformations in the production of intracerebral hematomas. J. Neurosurg. 1951, 8, 564–575. [Google Scholar] [CrossRef]
- Jellinger, K. Vascular malformations of the central nervous system: A morphological overview. Neurosurg. Rev. 1986, 9, 177–216. [Google Scholar] [CrossRef]
- Fischer, A.; Zalvide, J.; Faurobert, E.; Albiges-Rizo, C.; Tournier-Lasserve, E. Cerebral cavernous malformations: From CCM genes to endothelial cell homeostasis. Trends Mol. Med. 2013, 19, 302–308. [Google Scholar] [CrossRef]
- Glading, A.; Han, J.; Stockton, R.A.; Ginsberg, M.H. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell–cell junctions. J. Cell Biol. 2007, 179, 247–254. [Google Scholar] [CrossRef]
- Lai, C.C.; Nelsen, B.; Frias-Anaya, E.; Gallego-Gutierrez, H.; Orecchioni, M.; Herrera, V.; Ortiz, E.; Sun, H.; Mesarwi, O.A.; Ley, K.; et al. Neuroinflammation Plays a Critical Role in Cerebral Cavernous Malformation Disease. Circ. Res. 2022, 131, 909–925. [Google Scholar] [CrossRef]
- Maddaluno, L.; Rudini, N.; Cuttano, R.; Bravi, L.; Giampietro, C.; Corada, M.; Ferrarini, L.; Orsenigo, F.; Papa, E.; Boulday, G.; et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 2013, 498, 492–496. [Google Scholar] [CrossRef]
- Rath, M.; Schwefel, K.; Malinverno, M.; Skowronek, D.; Leopoldi, A.; Pilz, R.A.; Biedenweg, D.; Bekeschus, S.; Penninger, J.M.; Dejana, E.; et al. Contact-dependent signaling triggers tumor-like proliferation of CCM3 knockout endothelial cells in co-culture with wild-type cells. Cell. Mol. Life Sci. 2022, 79, 340. [Google Scholar] [CrossRef]
- Valentino, M.; Dejana, E.; Malinverno, M. The multifaceted PDCD10/CCM3 gene. Genes Dis. 2021, 8, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; An, R.; Wang, H.; Chen, L.; Shen, Y.; Cai, W.; Zhu, W. Oxidative stress-related circulating miRNA-27a is a potential biomarker for diagnosis and prognosis in patients with sepsis. BMC Immunol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Ferraris, C.; Berni, E.; Glading, A.J.; Retta, S.F. KRIT1: A Traffic Warden at the Busy Crossroads Between Redox Signaling and the Pathogenesis of Cerebral Cavernous Malformation Disease. Antioxid. Redox Signal 2023, 38, 496–528. [Google Scholar] [CrossRef]
- Retta, S.F.; Glading, A.J. Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: Two sides of the same coin. Int. J. Biochem. Cell Biol. 2016, 81, 254–270. [Google Scholar] [CrossRef]
- Ruiz, G.P.; Camara, H.; Fazolini, N.P.B.; Mori, M.A. Extracellular miRNAs in redox signaling: Health, disease and potential therapies. Free Radic. Biol. Med. 2021, 173, 170–187. [Google Scholar] [CrossRef]
- Wang, L.; Bayanbold, K.; Zhao, L.; Wang, Y.; Adamcakova-Dodd, A.; Thorne, P.S.; Yang, H.; Jiang, B.H.; Liu, L.Z. Redox sensitive miR-27a/b/Nrf2 signaling in Cr(VI)-induced carcinogenesis. Sci. Total Environ. 2022, 809, 151118. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, D.; Reece, E.A.; Wang, A.R.; Yang, P. Oxidative stress-induced miR-27a targets the redox gene nuclear factor erythroid 2-related factor 2 in diabetic embryopathy. Am. J. Obstet. Gynecol. 2018, 218, 136e1–136e10. [Google Scholar] [CrossRef]
- Perrelli, A.; Retta, S.F. Polymorphisms in genes related to oxidative stress and inflammation: Emerging links with the pathogenesis and severity of Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2021, 172, 403–417. [Google Scholar] [CrossRef]
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Elijovich, L.; Patel, P.V.; Hemphill, J.C., 3rd. Intracerebral hemorrhage. Semin. Neurol. 2008, 28, 657–667. [Google Scholar] [CrossRef]
- Haseeb, A.; Shafique, M.A.; Mustafa, M.S.; Singh, A.; Iftikhar, S.; Rangwala, B.S.; Waggan, A.I.; Fadlalla Ahmad, T.K.; Raja, S.; Raja, A. Neuroendoscopic versus Craniotomy Approach in Supratentorial Hypertensive Intracerebral Hemorrhage: An Updated Meta-Analysis. World Neurosurg. 2024, 190, e721–e747. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, D.; Liang, J.W. Intracerebral Hemorrhage. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zolboot, N.; Du, J.X.; Zampa, F.; Lippi, G. MicroRNAs Instruct and Maintain Cell Type Diversity in the Nervous System. Front. Mol. Neurosci. 2021, 14, 646072. [Google Scholar] [CrossRef] [PubMed]
- Flower, O.; Smith, M. The acute management of intracerebral hemorrhage. Curr. Opin. Crit. Care 2011, 17, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Walsh, K.; Woo, J.G.; Haverbusch, M.; Moomaw, C.J.; Broderick, J.P.; Kissela, B.M.; Kleindorfer, D.; Flaherty, M.L.; Woo, D. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2014, 23, e107–e111. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, G.; Lee, J.H.; Ryu, J.Y.; Oh, E.J.; Kim, H.M.; Kwak, S.; Hur, K.; Chung, H.Y. MicroRNA-135b-5p Is a Pathologic Biomarker in the Endothelial Cells of Arteriovenous Malformations. Int. J. Mol. Sci. 2024, 25, 4888. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Toyama, K.; Igase, M.; Spin, J.M.; Abe, Y.; Javkhlant, A.; Okada, Y.; Wagenhauser, M.U.; Schelzig, H.; Tsao, P.S.; Mogi, M. Exosome miR-501-3p Elevation Contributes to Progression of Vascular Stiffness. Circ. Rep. 2021, 3, 170–177. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, M.; Li, H.; Liu, H.; Wang, J.; Huang, J. TGFbeta1 as a Predictive Biomarker for Collateral Formation Within Ischemic Moyamoya Disease. Front. Neurol. 2022, 13, 899470. [Google Scholar] [CrossRef]
- Girard, R.; Zeineddine, H.A.; Koskimaki, J.; Fam, M.D.; Cao, Y.; Shi, C.; Moore, T.; Lightle, R.; Stadnik, A.; Chaudagar, K.; et al. Plasma Biomarkers of Inflammation and Angiogenesis Predict Cerebral Cavernous Malformation Symptomatic Hemorrhage or Lesional Growth. Circ. Res. 2018, 122, 1716–1721. [Google Scholar] [CrossRef]
- Li, Y.; Srinath, A.; Alcazar-Felix, R.J.; Hage, S.; Bindal, A.; Lightle, R.; Shenkar, R.; Shi, C.; Girard, R.; Awad, I.A. Inflammatory Mechanisms in a Neurovascular Disease: Cerebral Cavernous Malformation. Brain Sci. 2023, 13, 1336. [Google Scholar] [CrossRef]
- Kuosmanen, S.M.; Kansanen, E.; Kaikkonen, M.U.; Sihvola, V.; Pulkkinen, K.; Jyrkkanen, H.K.; Tuoresmaki, P.; Hartikainen, J.; Hippelainen, M.; Kokki, H.; et al. NRF2 regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res. 2018, 46, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassoni, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Padarti, A.; Zhang, J. Recent advances in cerebral cavernous malformation research. Vessel Plus 2018, 2, 21. [Google Scholar] [CrossRef]
- Hong, C.C.; Tang, A.T.; Detter, M.R.; Choi, J.P.; Wang, R.; Yang, X.; Guerrero, A.A.; Wittig, C.F.; Hobson, N.; Girard, R.; et al. Cerebral cavernous malformations are driven by ADAMTS5 proteolysis of versican. J. Exp. Med. 2020, 217, e20200140. [Google Scholar] [CrossRef]
- Du, S.; Shen, S.; Ding, S.; Wang, L. Suppression of microRNA-323-3p restrains vascular endothelial cell apoptosis via promoting sirtuin-1 expression in coronary heart disease. Life Sci. 2021, 270, 119065. [Google Scholar] [CrossRef]
- Nan, S.; Wang, Y.; Xu, C.; Wang, H. Interfering microRNA-410 attenuates atherosclerosis via the HDAC1/KLF5/IKBalpha/NF-kappaB axis. Mol. Ther. Nucleic Acids 2021, 24, 646–657. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcazar-Felix, R.J.; Jhaveri, A.; Iqbal, J.; Srinath, A.; Bennett, C.; Bindal, A.; Vera Cruz, D.; Romanos, S.; Hage, S.; Stadnik, A.; et al. A Systematic Review of MicroRNAs in Hemorrhagic Neurovascular Disease: Cerebral Cavernous Malformations as a Paradigm. Int. J. Mol. Sci. 2025, 26, 3794. https://doi.org/10.3390/ijms26083794
Alcazar-Felix RJ, Jhaveri A, Iqbal J, Srinath A, Bennett C, Bindal A, Vera Cruz D, Romanos S, Hage S, Stadnik A, et al. A Systematic Review of MicroRNAs in Hemorrhagic Neurovascular Disease: Cerebral Cavernous Malformations as a Paradigm. International Journal of Molecular Sciences. 2025; 26(8):3794. https://doi.org/10.3390/ijms26083794
Chicago/Turabian StyleAlcazar-Felix, Roberto J., Aditya Jhaveri, Javed Iqbal, Abhinav Srinath, Carolyn Bennett, Akash Bindal, Diana Vera Cruz, Sharbel Romanos, Stephanie Hage, Agnieszka Stadnik, and et al. 2025. "A Systematic Review of MicroRNAs in Hemorrhagic Neurovascular Disease: Cerebral Cavernous Malformations as a Paradigm" International Journal of Molecular Sciences 26, no. 8: 3794. https://doi.org/10.3390/ijms26083794
APA StyleAlcazar-Felix, R. J., Jhaveri, A., Iqbal, J., Srinath, A., Bennett, C., Bindal, A., Vera Cruz, D., Romanos, S., Hage, S., Stadnik, A., Lee, J., Lightle, R., Shenkar, R., Koskimäki, J., Polster, S. P., Girard, R., & Awad, I. A. (2025). A Systematic Review of MicroRNAs in Hemorrhagic Neurovascular Disease: Cerebral Cavernous Malformations as a Paradigm. International Journal of Molecular Sciences, 26(8), 3794. https://doi.org/10.3390/ijms26083794





