TMEM16A Maintains Acrosomal Integrity Through ERK1/2, RhoA, and Actin Cytoskeleton During Capacitation
Abstract
1. Introduction
2. Results
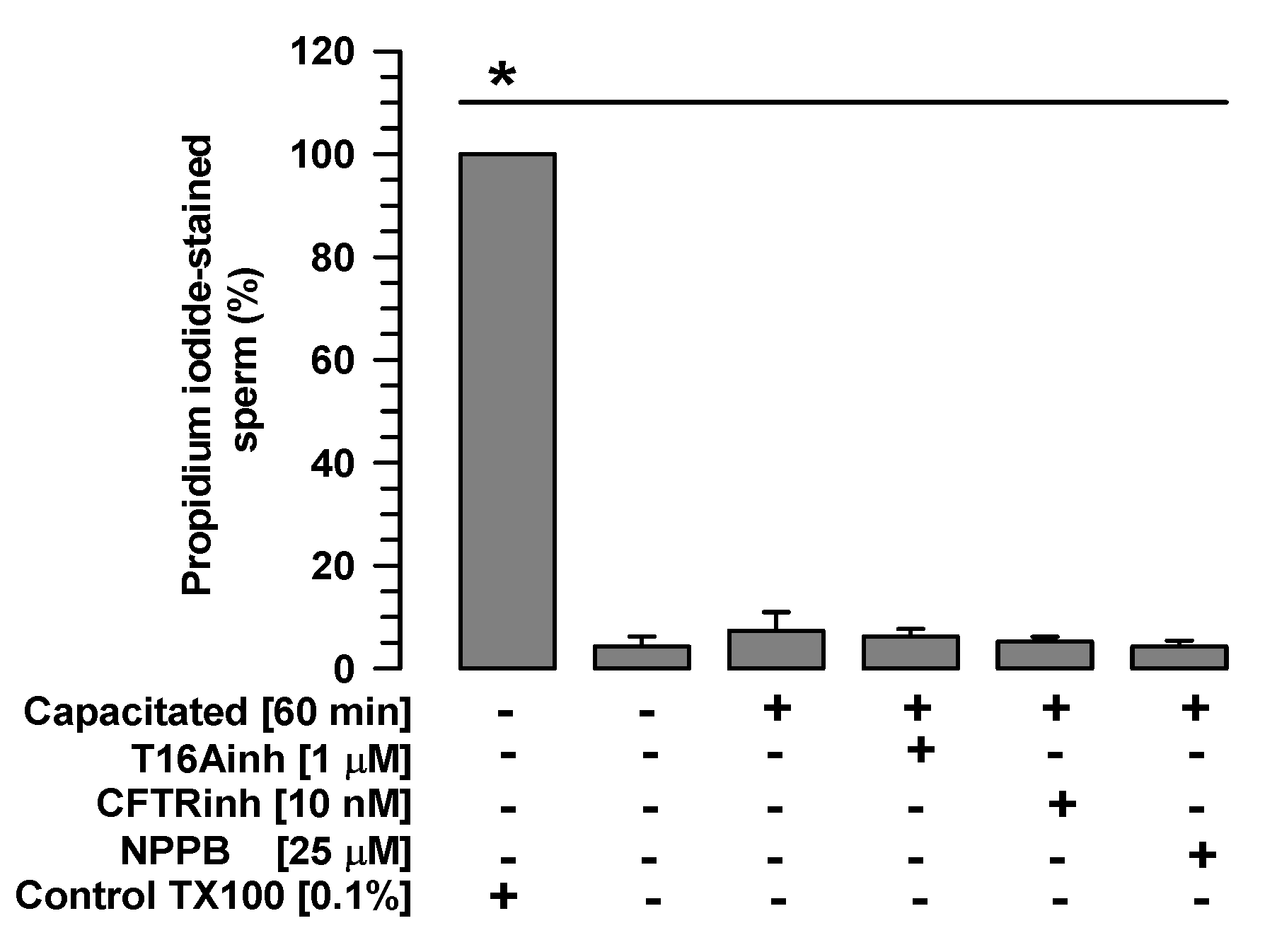
2.1. The Tested Inhibitors Do Not Alter Sperm Viability
2.2. TMEM16A Inhibition Disrupts the Acrosome Structure
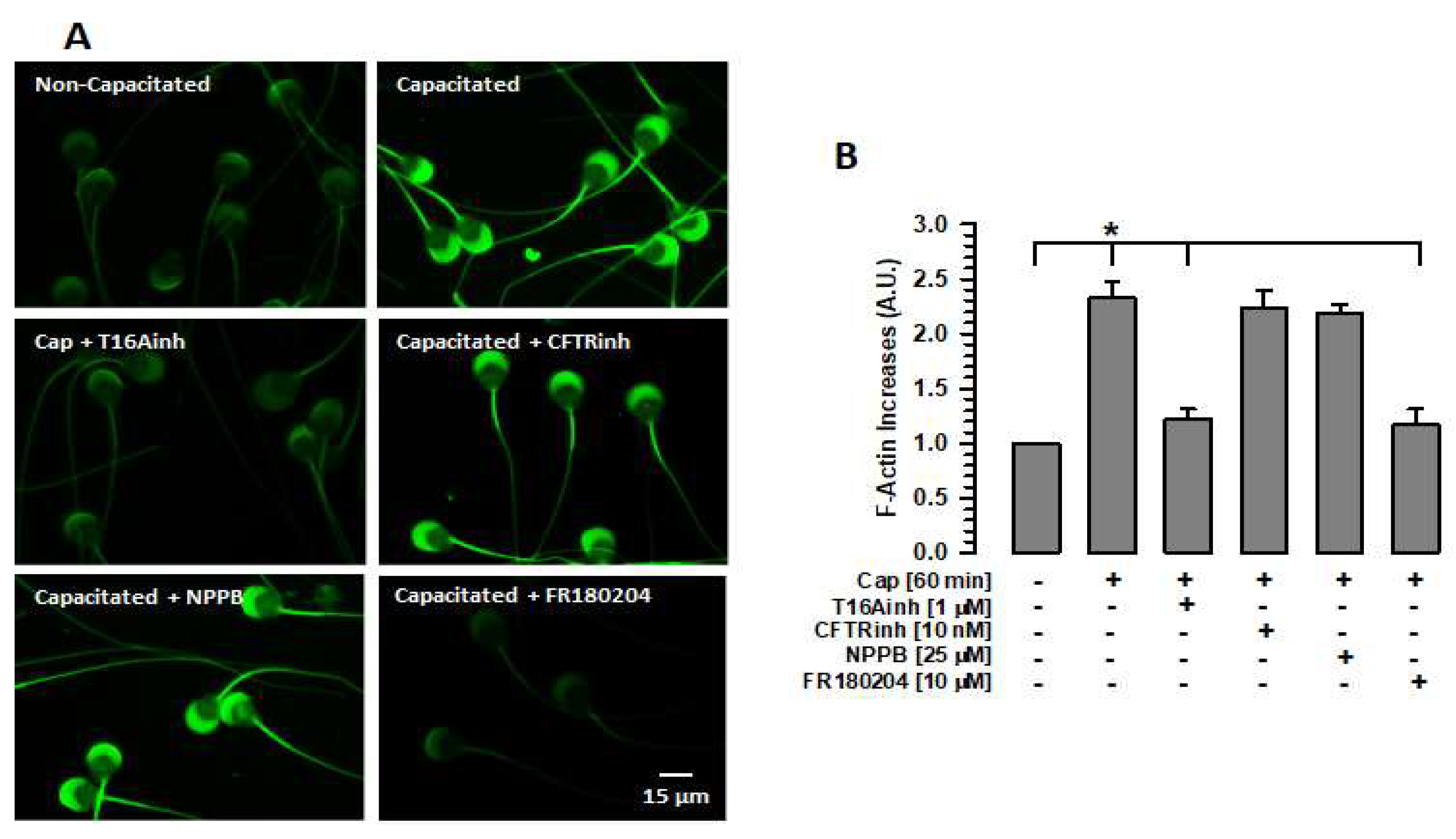
2.3. Inhibition of TMEM16A Prevents Actin Polymerization
2.4. TMEM16A, CFTR, and ClC3 Inhibition Alters Intracellular Cl− Homeostasis and the Intracellular pH
2.4.1. Intracellular Concentration of Cl−
2.4.2. Intracellular pH
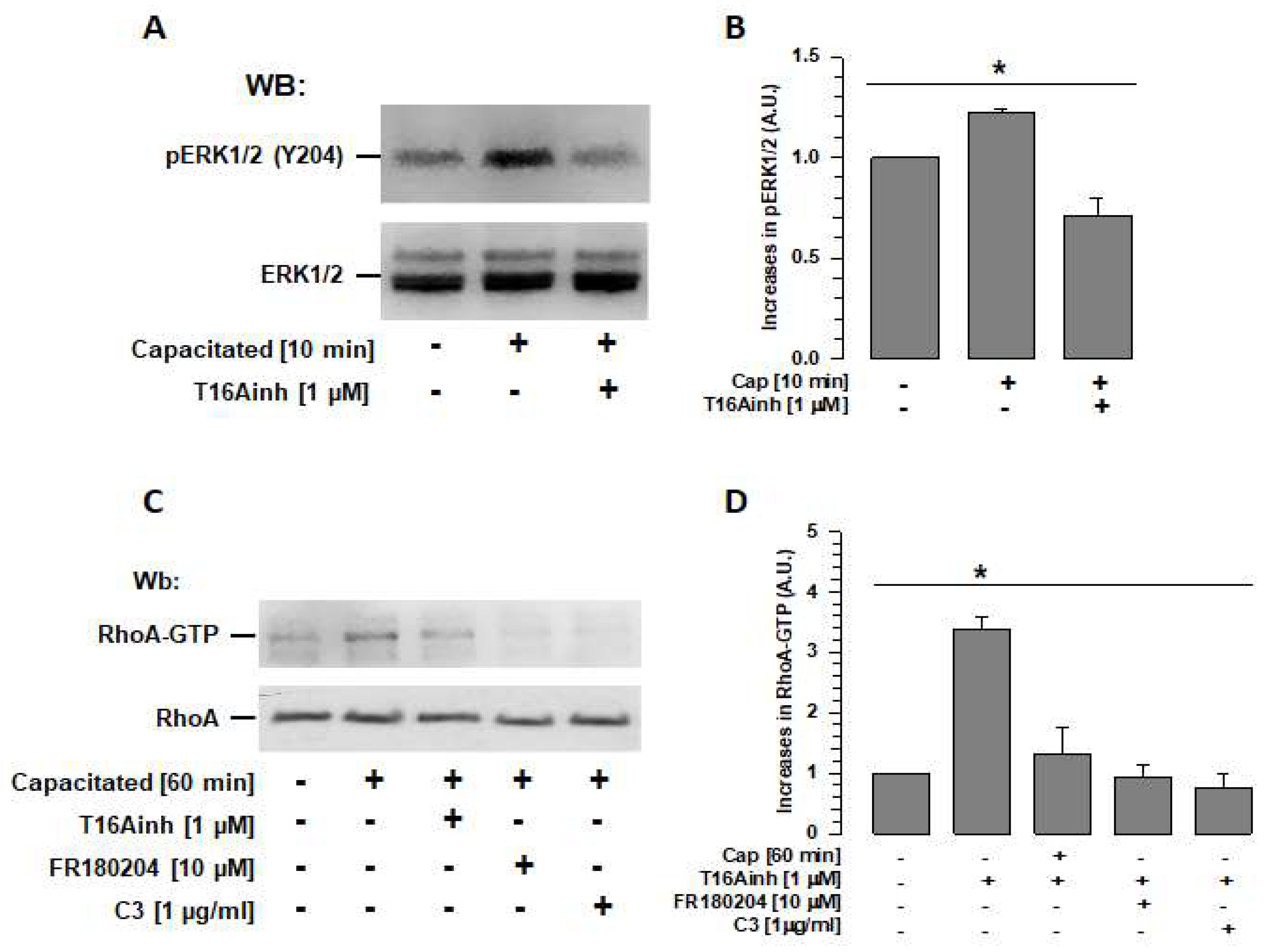
2.5. TMEM16A Inhibition Prevents ERK1/2 and RhoA Activation
2.6. TMEM16A, CFTR, and ClC3 Inhibition Inhibit the Normal Course of Capacitation and Spontaneous Acrosomal Reaction
3. Discussion
4. Materials and Methods
4.1. Reagents
4.1.1. Antibodies
4.1.2. Inhibitors
4.2. Experimental Animals
4.3. Capacitation Assay
4.4. Pharmacologic Inhibitors
4.5. Assessment of Sperm Viability
4.6. Assessment of Sperm Head Area
4.7. Immunofluorescence Assays
4.8. Intracellular Measurement of Cl− in the Sperm Population
4.9. Intracellular pH Measurement in the Sperm Population
4.10. Assessment of F-Actin
4.11. Rhotekin–Rho Binding Domain Pull-Down Assay
4.12. Immunoblotting
4.13. Evaluation of Capacitation Status by Staining with Chlortetracycline (CTC)
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolli, A.R.; Cesari, A. The Other Side of Capacitation: Role of Mouse Male Molecules in the Regulation of Time and Place of Capacitation. Reproduction 2023, 166, R73–R85. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.J.; Bastias, C.; Coulson, R.L.; Russell, L.D. Cytochalasin D Inhibits Penetration of Hamster Eggs by Guinea Pig and Human Spermatozoa. J. Androl. 1989, 10, 275–282. [Google Scholar] [CrossRef]
- Castellani-Ceresa, L.; Mattioli, M.; Radaelli, G.; Barboni, B.; Brivio, M.F. Actin Polymerization in Boar Spermatozoa: Fertilization Is Reduced with Use of Cytochalasin D. Mol. Reprod. Dev. 1993, 36, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gutierrez, M.; Contreras, R.G.; Mujica, A. Cytochalasin-D Retards Sperm Incorporation Deep into the Egg Cytoplasm but Not Membrane Fusion with the Egg Plasma Membrane. Mol. Reprod. Dev. 2002, 63, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the Actin Cytoskeleton During Mammalian Sperm Capacitation and Acrosome Reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- Delgado-Buenrostro, N.L.; Hernandez-Gonzalez, E.O.; Segura-Nieto, M.; Mujica, A. Actin Polymerization in the Equatorial and Postacrosomal Regions of Guinea Pig Spermatozoa During the Acrosome Reaction Is Regulated by G Proteins. Mol. Reprod. Dev. 2005, 70, 198–210. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, D.; Salgado-Lucio, M.L.; Roa-Espitia, A.L.; Fierro, R.; Gonzalez-Marquez, H.; Cordero-Martinez, J.; Hernandez-Gonzalez, E.O. Rac1 Is Necessary for Capacitation and Acrosome Reaction in Guinea Pig Spermatozoa. J. Cell. Biochem. 2019, 121, 2864–2876. [Google Scholar] [CrossRef]
- Breitbart, H.; Cohen, G.; Rubinstein, S. Role of Actin Cytoskeleton in Mammalian Sperm Capacitation and the Acrosome Reaction. Reproduction 2005, 129, 263–268. [Google Scholar] [CrossRef]
- Reyes-Miguel, T.; Roa-Espitia, A.L.; Baltierrez-Hoyos, R.; Hernandez-Gonzalez, E.O. Cdc42 Drives Rhoa Activity and Actin Polymerization During Capacitation. Reproduction 2020, 160, 393–404. [Google Scholar] [CrossRef]
- Salgado-Lucio, M.L.; Ramirez-Ramirez, D.; Jorge-Cruz, C.Y.; Roa-Espitia, A.L.; Hernandez-Gonzalez, E.O. Fak Regulates Actin Polymerization During Sperm Capacitation Via the Erk2/Gef-H1/Rhoa Signaling Pathway. J. Cell. Sci. 2020, 133, jcs239186. [Google Scholar] [CrossRef]
- Santi, C.M.; Orta, G.; Salkoff, L.; Visconti, P.E.; Darszon, A.; Trevino, C.L. K+ and Cl− Channels and Transporters in Sperm Function. Curr. Top. Dev. Biol. 2013, 102, 385–421. [Google Scholar] [CrossRef] [PubMed]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation Mechanisms and Implications of Sperm Membrane Hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Orta, G.; Ferreira, G.; Jose, O.; Trevino, C.L.; Beltran, C.; Darszon, A. Human Spermatozoa Possess a Calcium-Dependent Chloride Channel That May Participate in the Acrosomal Reaction. J. Physiol. 2012, 590, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Martinez, J.; Reyes-Miguel, T.; Rodriguez-Paez, L.; Garduno-Siciliano, L.; Maldonado-Garcia, D.; Roa-Espitia, A.L.; Hernandez-Gonzalez, E.O. Tmem16a Inhibition Impedes Capacitation and Acquisition of Hyperactivated Motility in Guinea Pig Sperm. J. Cell. Biochem. 2018, 119, 5944–5959. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, E.O.; Trevino, C.L.; Castellano, L.E.; de la Vega-Beltran, J.L.; Ocampo, A.Y.; Wertheimer, E.; Visconti, P.E.; Darszon, A. Involvement of Cystic Fibrosis Transmembrane Conductance Regulator in Mouse Sperm Capacitation. J. Biol. Chem. 2007, 282, 24397–24406. [Google Scholar] [CrossRef]
- Xu, W.M.; Shi, Q.X.; Chen, W.Y.; Zhou, C.X.; Ni, Y.; Rowlands, D.K.; Yi Liu, G.; Zhu, H.; Ma, Z.G.; Wang, X.F.; et al. Cystic Fibrosis Transmembrane Conductance Regulator Is Vital to Sperm Fertilizing Capacity and Male Fertility. Proc. Natl. Acad. Sci. USA 2007, 104, 9816–9821. [Google Scholar] [CrossRef]
- Yeung, C.H.; Barfield, J.P.; Cooper, T.G. Chloride Channels in Physiological Volume Regulation of Human Spermatozoa. Biol. Reprod. 2005, 73, 1057–1063. [Google Scholar] [CrossRef]
- Liu, S.W.; Li, Y.; Zou, L.L.; Guan, Y.T.; Peng, S.; Zheng, L.X.; Deng, S.M.; Zhu, L.Y.; Wang, L.W.; Chen, L.X. Chloride Channels Are Involved in Sperm Motility and Are Downregulated in Spermatozoa from Patients with Asthenozoospermia. Asian J. Androl. 2017, 19, 418–424. [Google Scholar] [CrossRef]
- Perez-Cornejo, P.; Gokhale, A.; Duran, C.; Cui, Y.; Xiao, Q.; Hartzell, H.C.; Faundez, V. Anoctamin 1 (Tmem16a) Ca2+-Activated Chloride Channel Stoichiometrically Interacts with an Ezrin-Radixin-Moesin Network. Proc. Natl. Acad. Sci. USA 2012, 109, 10376–10381. [Google Scholar] [CrossRef]
- Danielsson, J.; Vink, J.; Hyuga, S.; Fu, X.W.; Funayama, H.; Wapner, R.; Blanks, A.M.; Gallos, G. Anoctamin Channels in Human Myometrium: A Novel Target for Tocolysis. Reprod. Sci. 2018, 25, 1589–1600. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.; Zeng, X.; Huang, C.; Ma, M.; Lv, X.; Zhang, Y.; Sun, L.; Wang, G.; Du, Y.; et al. Tmem16a Inhibits Angiotensin Ii-Induced Basilar Artery Smooth Muscle Cell Migration in a Wnk1-Dependent Manner. Acta Pharm. Sin. B 2021, 11, 3994–4007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, L.; Ma, K.; Yu, J.; Wu, H.; Wei, M.; Xiao, Q. Cell-Specific Mechanisms of Tmem16a Ca2+-Activated Chloride Channel in Cancer. Mol. Cancer 2017, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Monterisi, S.; Favia, M.; Guerra, L.; Cardone, R.A.; Marzulli, D.; Reshkin, S.J.; Casavola, V.; Zaccolo, M. Cftr Regulation in Human Airway Epithelial Cells Requires Integrity of the Actin Cytoskeleton and Compartmentalized Camp and Pka Activity. J. Cell. Sci. 2012, 125 Pt 5, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Favia, M.; Guerra, L.; Fanelli, T.; Cardone, R.A.; Monterisi, S.; Di Sole, F.; Castellani, S.; Chen, M.; Seidler, U.; Reshkin, S.J.; et al. Na+/H+ Exchanger Regulatory Factor 1 Overexpression-Dependent Increase of Cytoskeleton Organization Is Fundamental in the Rescue of F508del Cystic Fibrosis Transmembrane Conductance Regulator in Human Airway Cfbe41o- Cells. Mol. Biol. Cell. 2010, 21, 73–86. [Google Scholar] [CrossRef]
- McCloskey, D.T.; Doherty, L.; Dai, Y.P.; Miller, L.; Hume, J.R.; Yamboliev, I.A. Hypotonic Activation of Short ClC3 Isoform Is Modulated by Direct Interaction Between Its Cytosolic C-Terminal Tail and Subcortical Actin Filaments. J. Biol. Chem. 2007, 282, 16871–16877. [Google Scholar] [CrossRef]
- Cooper, T.G.; Yeung, C.H. Involvement of Potassium and Chloride Channels and Other Transporters in Volume Regulation by Spermatozoa. Curr. Pharm. Des. 2007, 13, 3222–3230. [Google Scholar] [CrossRef]
- Delgado-Bermudez, A.; Yeste, M.; Bonet, S.; Pinart, E. A Review on the Role of Bicarbonate and Proton Transporters During Sperm Capacitation in Mammals. Int. J. Mol. Sci. 2022, 23, 6333. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, T.; Sato-Numata, K.; Islam, M.R.; Ando-Akatsuka, Y.; Numata, T.; Kubo, M.; Shimizu, T.; Kurbannazarova, R.S.; Marunaka, Y.; et al. Cell Volume-Activated and Volume-Correlated Anion Channels in Mammalian Cells: Their Biophysical, Molecular, and Pharmacological Properties. Pharmacol. Rev. 2019, 71, 49–88. [Google Scholar] [CrossRef]
- Nakamura, M.; Moriya, M.; Baba, T.; Michikawa, Y.; Yamanobe, T.; Arai, K.; Okinaga, S.; Kobayashi, T. An Endoplasmic Reticulum Protein, Calreticulin, Is Transported into the Acrosome of Rat Sperm. Exp. Cell. Res. 1993, 205, 101–110. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Wolkowicz, M.J.; Klotz, K.; Bush, L.A.; Westbrook, V.A.; Shibahara, H.; Shetty, J.; Coonrod, S.A.; Reddi, P.P.; Shannon, J.; et al. Co-Localization of the Inositol 1,4,5-Trisphosphate Receptor and Calreticulin in the Equatorial Segment and in Membrane Bounded Vesicles in the Cytoplasmic Droplet of Human Spermatozoa. Mol. Hum. Reprod. 2001, 7, 923–933. [Google Scholar] [CrossRef]
- Strehle, D.; Schnauss, J.; Heussinger, C.; Alvarado, J.; Bathe, M.; Kas, J.; Gentry, B. Transiently Crosslinked F-Actin Bundles. Eur. Biophys. J. 2011, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zoca, G.B.; Celeghini, E.C.C.; Pugliesi, G.; de Carvalho, C.P.T.; Assumpcao, M.; Siqueira, A.F.P.; Oliveira, L.Z.; Lanconi, R.; de Arruda, R.P. Influence of Seminal Plasma During Different Stages of Bovine Sperm Cryopreservation. Reprod. Domest. Anim. 2021, 56, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.V.; Salicioni, A.M.; Liu, W.; Trevino, C.L.; Chavez, J.; Hernandez-Gonzalez, E.O.; Darszon, A.; Visconti, P.E. Chloride Is Essential for Capacitation and for the Capacitation-Associated Increase in Tyrosine Phosphorylation. J. Biol. Chem. 2008, 283, 35539–35550. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Xu, W.M.; Chen, Z.H.; Ni, Y.; Yuan, Y.Y.; Zhou, S.C.; Zhou, W.W.; Tsang, L.L.; Chung, Y.W.; Hoglund, P.; et al. Cl− Is Required for HCO3− Entry Necessary for Sperm Capacitation in Guinea Pig: Involvement of a Cl−/HCO3− Exchanger (Slc26a3) and Cftr. Biol. Reprod. 2009, 80, 115–123. [Google Scholar] [CrossRef]
- Chavez, J.C.; Hernandez-Gonzalez, E.O.; Wertheimer, E.; Visconti, P.E.; Darszon, A.; Trevino, C.L. Participation of the Cl−/HCO3− Exchangers Slc26a3 and Slc26a6, the Cl− Channel Cftr, and the Regulatory Factor Slc9a3r1 in Mouse Sperm Capacitation. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef]
- Roa-Espitia, A.L.; Hernandez-Rendon, E.R.; Baltierrez-Hoyos, R.; Munoz-Gotera, R.J.; Cote-Velez, A.; Jimenez, I.; Gonzalez-Marquez, H.; Hernandez-Gonzalez, E.O. Focal Adhesion Kinase Is Required for Actin Polymerization and Remodeling of the Cytoskeleton During Sperm Capacitation. Biol. Open 2016, 5, 1189–1199. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Hebel, M.; Waberski, D.; Weitze, K.F.; Topfer-Petersen, E. Requirement for an Intact Cytoskeleton for Volume Regulation in Boar Spermatozoa. Reproduction 2004, 127, 105–115. [Google Scholar] [CrossRef]
- Bastian, Y.; Roa-Espitia, A.L.; Mujica, A.; Hernandez-Gonzalez, E.O. Calpain Modulates Capacitation and Acrosome Reaction through Cleavage of the Spectrin Cytoskeleton. Reproduction 2010, 140, 673–684. [Google Scholar] [CrossRef]
- Mujica, A.; Navarro-Garcia, F.; Hernandez-Gonzalez, E.O.; De Lourdes Juarez-Mosqueda, M. Perinuclear Theca During Spermatozoa Maturation Leading to Fertilization. Microsc. Res. Tech. 2003, 61, 76–87. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Lesich, K.A. Functional Anatomy of the Mammalian Sperm Flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhao, C.; Huo, R.; Guo, X.J.; Lin, M.; Huang, X.Y.; Mao, Y.D.; Zhou, Z.M.; Sha, J.H. The Role of Ezrin-Associated Protein Network in Human Sperm Capacitation. Asian J. Androl. 2010, 12, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, E.O.; Lecona-Valera, A.N.; Escobar-Herrera, J.; Mujica, A. Involvement of an F-Actin Skeleton on the Acrosome Reaction in Guinea Pig Spermatozoa. Cell Motil. Cytoskelet. 2000, 46, 43–58. [Google Scholar] [CrossRef]
- Schreiber, R.; Ousingsawat, J.; Kunzelmann, K. Epithelial Anoctamins. Cell Calcium 2024, 120, 102885. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Urminska, D.; Duracka, M.; Tvrda, E. Signaling Roleplay between Ion Channels During Mammalian Sperm Capacitation. Biomedicines 2023, 11, 2519. [Google Scholar] [CrossRef]
- Navarro, B.; Kirichok, Y.; Chung, J.J.; Clapham, D.E. Ion Channels that Control Fertility in Mammalian Spermatozoa. Int. J. Dev. Biol. 2008, 52, 607–613. [Google Scholar] [CrossRef]
- Dona, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (Ae1). Int. J. Mol. Sci. 2020, 21, 4063. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Pinto, N.A.; Torres Rodriguez, P.; Romarowski, A.; Vicens Sanchez, A.; Visconti, P.E.; Darszon, A.; Trevino, C.L.; Buffone, M.G. Essential Role of Cftr in Pka-Dependent Phosphorylation, Alkalinization, and Hyperpolarization During Human Sperm Capacitation. J. Cell. Physiol. 2017, 232, 1404–1414. [Google Scholar] [CrossRef]
- Cordero-Martinez, J.; Flores-Alonso, J.C.; Aguirre-Alvarado, C.; Oviedo, N.; Alcantara-Farfan, V.; Garcia-Perez, C.A.; Bermudez-Ruiz, K.F.; Jimenez-Gutierrez, G.E.; Rodriguez-Paez, L. Influence of Echeveria Gibbiflora Dc Aqueous Crude Extract on Mouse Sperm Energy Metabolism and Calcium-Dependent Channels. J. Ethnopharmacol. 2020, 248, 112321. [Google Scholar] [CrossRef]
- Ward, C.R.; Storey, B.T. Determination of the Time Course of Capacitation in Mouse Spermatozoa Using a Chlortetracycline Fluorescence Assay. Dev. Biol. 1984, 104, 287–296. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roa-Espitia, A.L.; Reyes-Miguel, T.; Salgado-Lucio, M.L.; Cordero-Martínez, J.; Tafoya-Domínguez, D.; Hernández-González, E.O. TMEM16A Maintains Acrosomal Integrity Through ERK1/2, RhoA, and Actin Cytoskeleton During Capacitation. Int. J. Mol. Sci. 2025, 26, 3750. https://doi.org/10.3390/ijms26083750
Roa-Espitia AL, Reyes-Miguel T, Salgado-Lucio ML, Cordero-Martínez J, Tafoya-Domínguez D, Hernández-González EO. TMEM16A Maintains Acrosomal Integrity Through ERK1/2, RhoA, and Actin Cytoskeleton During Capacitation. International Journal of Molecular Sciences. 2025; 26(8):3750. https://doi.org/10.3390/ijms26083750
Chicago/Turabian StyleRoa-Espitia, Ana L., Tania Reyes-Miguel, Monica L. Salgado-Lucio, Joaquín Cordero-Martínez, Dennis Tafoya-Domínguez, and Enrique O. Hernández-González. 2025. "TMEM16A Maintains Acrosomal Integrity Through ERK1/2, RhoA, and Actin Cytoskeleton During Capacitation" International Journal of Molecular Sciences 26, no. 8: 3750. https://doi.org/10.3390/ijms26083750
APA StyleRoa-Espitia, A. L., Reyes-Miguel, T., Salgado-Lucio, M. L., Cordero-Martínez, J., Tafoya-Domínguez, D., & Hernández-González, E. O. (2025). TMEM16A Maintains Acrosomal Integrity Through ERK1/2, RhoA, and Actin Cytoskeleton During Capacitation. International Journal of Molecular Sciences, 26(8), 3750. https://doi.org/10.3390/ijms26083750





