Reanalysis of Published Histological Data Can Help to Characterize Neuronal Death After Spinal Cord Injury
Abstract
1. Introduction
- Creating an open data repository to store and share images, as well as the results from their analysis;
- Depositing and documenting 168 images of full transversal sections of mice spinal cords from the study by Reigada et al. [16] on the neuroprotective effects of ucf-101 in the damaged spinal cord;
- Comparing the precision and reproducibility of manual-, threshold- and neural network-based neuron identification methods;
- Exploring how neuronal death distributes within the spinal cord sections in Reigada’s mouse model of SCI and which neurons become protected by the treatment with the anti-apoptotic drug ucf-101.
2. Results
2.1. Open Access Repository
- A documented image repository of spinal cord sections stained with neuronal markers.
- Test benches for comparing methods to analyse naïve and injured spinal cords.
- Results from the analysis of the images in the neuroCLUEDO repository.
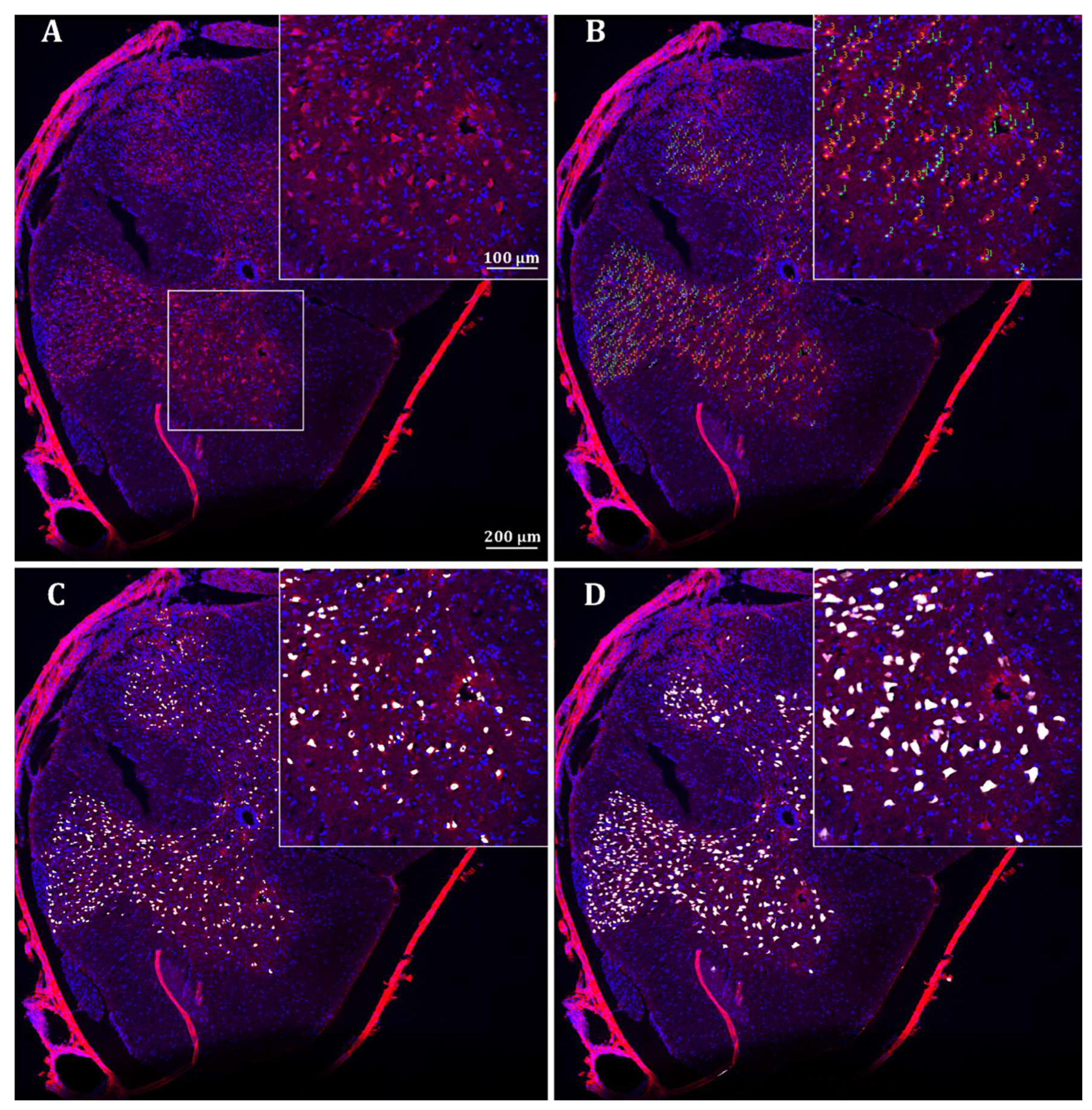
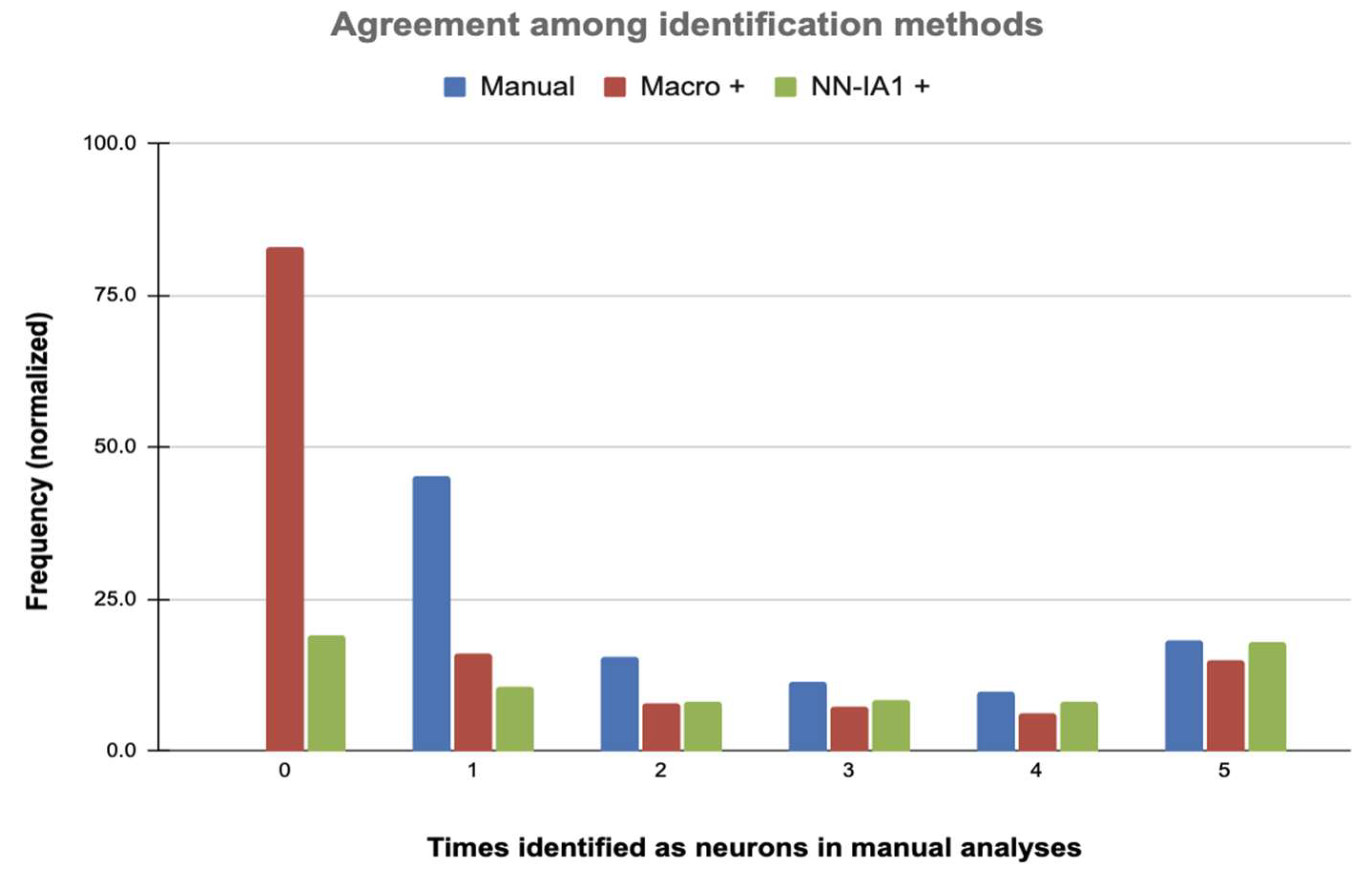
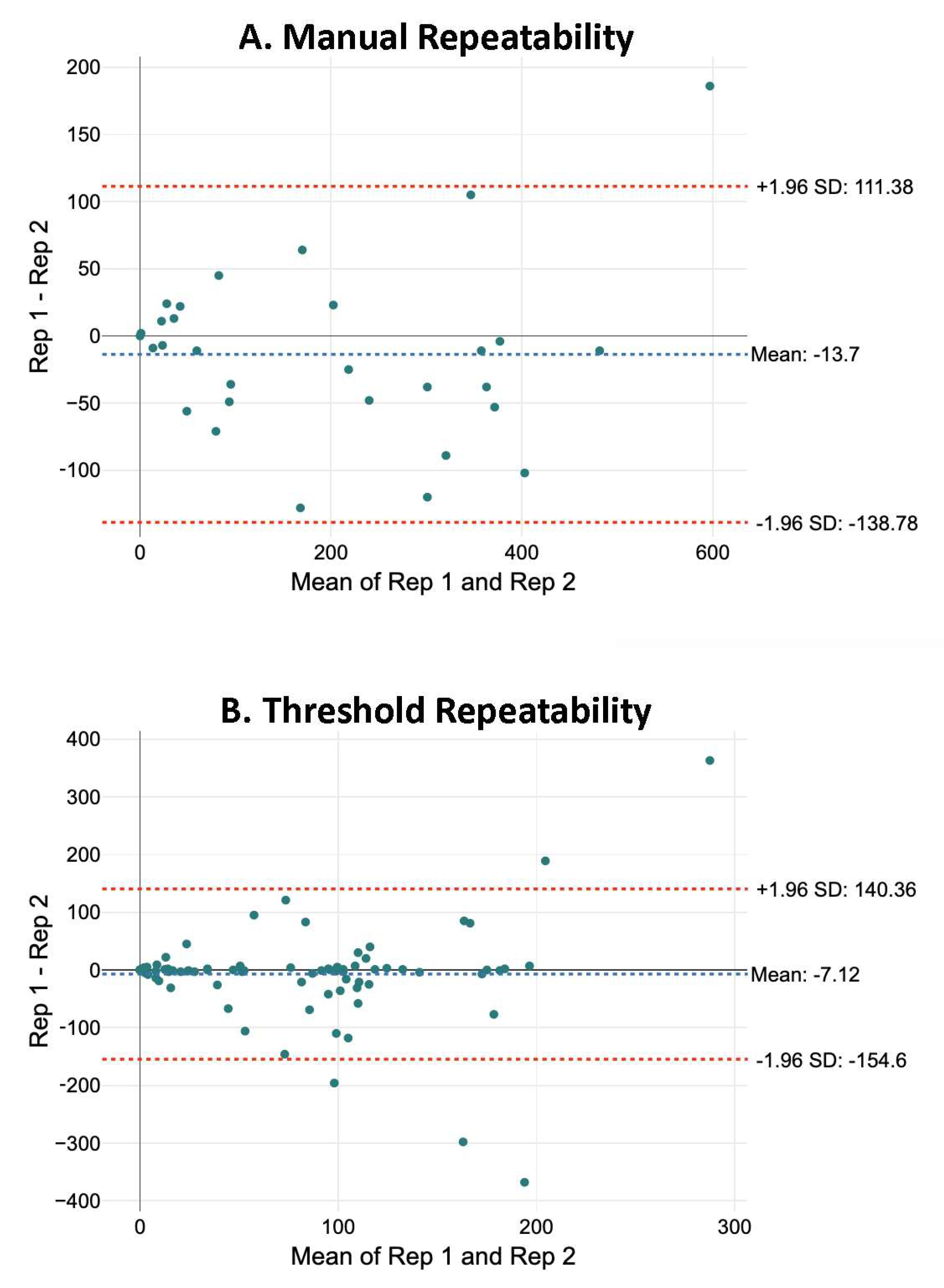
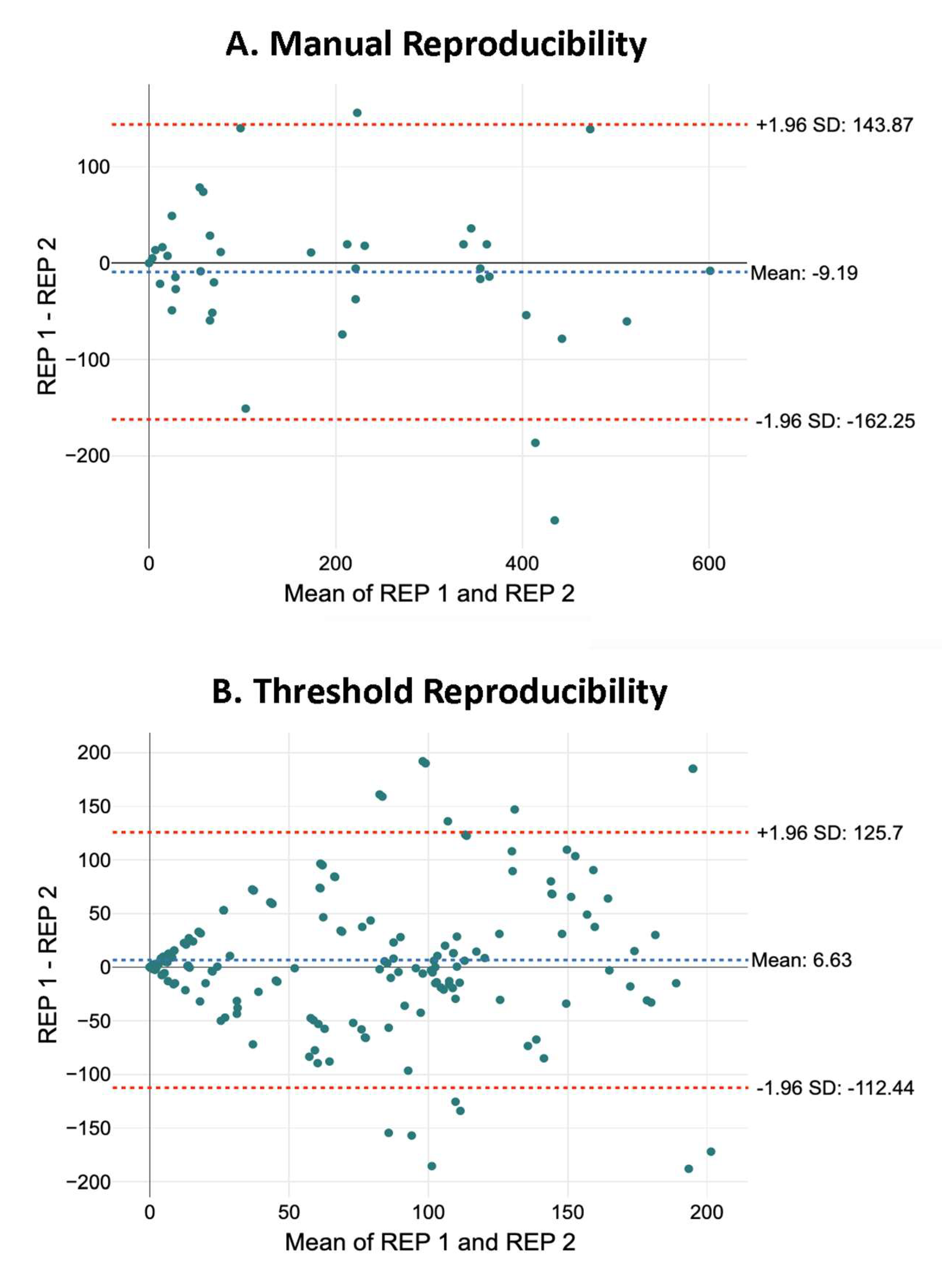
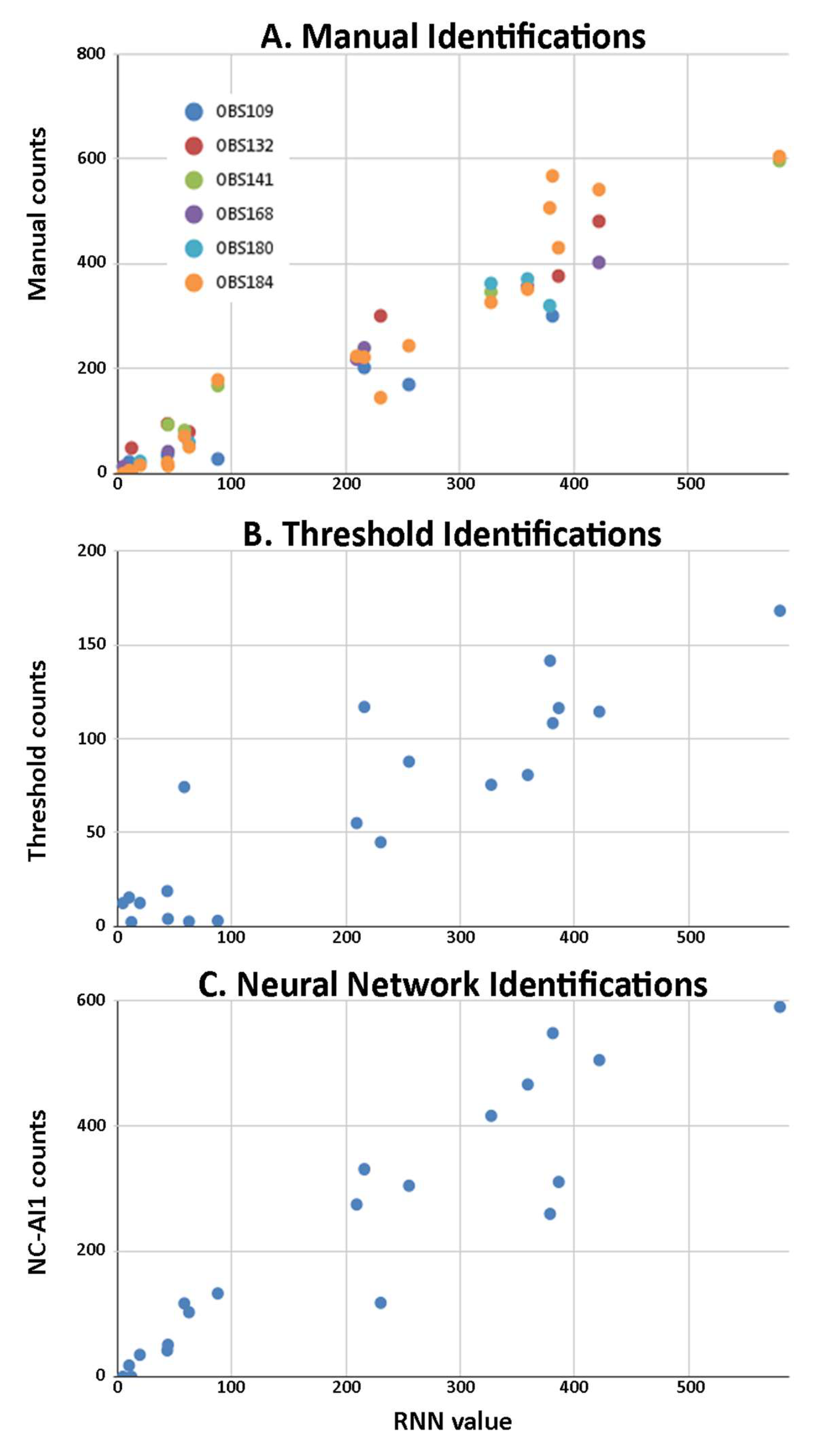
2.2. Comparison of Methods for Identifying Neurons
2.2.1. Neuronal Identifications
2.2.2. Number of Neurons
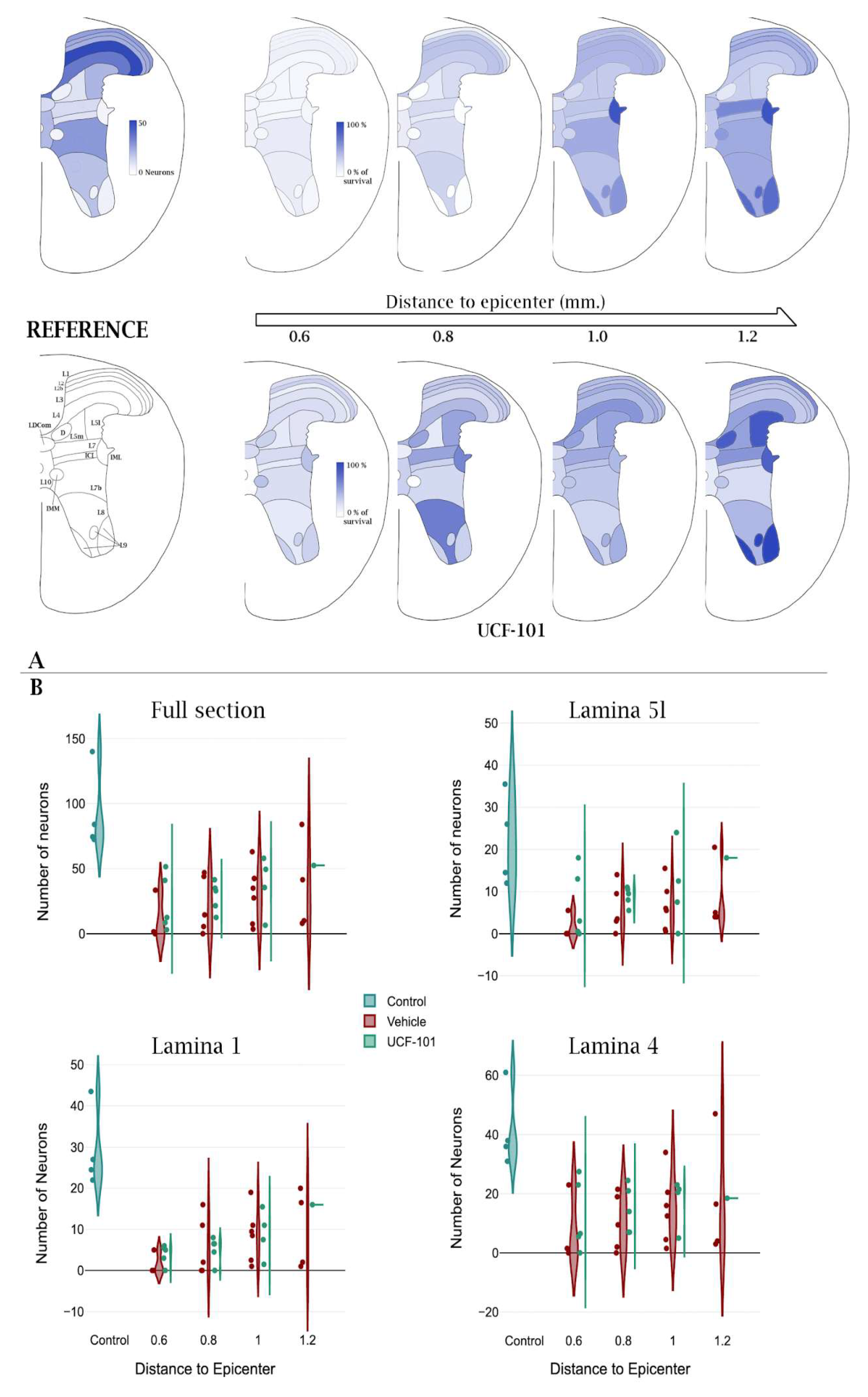
2.3. Effects of Injury and ucf-101 Treatment on Neuronal Death
2.3.1. General Description of the Obtained Data
2.3.2. Neuronal Loss After Spinal Cord Injury
2.3.3. Neurons Protected by ucf-101
3. Discussion
3.1. Repository on Spinal Cord Injury
3.2. Test Bench for Neuronal Identification Methods
3.3. Analysis of Neuronal Death and Neuroprotection
3.4. Perspectives
4. Materials and Methods
4.1. Images
4.2. Neuron Identifications
4.3. Reanalysis of ucf-101 Effects on Neuronal Survival
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| D | Dorsal Nucleus of Clarke |
| ICI | Intercalated Nucleus |
| IML | Intermediolateral Nucleus |
| IMM | Intermediomedial Nucleus |
| L1 to L10 | Lamina 1 to Lamina 10 |
| LDCom | Lumbar Dorsal Commissural Nucleus |
| MIASCI | Minimum Information about Spinal Cord Injury Experiments |
| NN | Neural Network |
| OSF | Open Science Framework |
| RCD | Regulated Cell Death |
| RNN | Reference Number of Neurons |
| SCI | Spinal Cord Injury |
| TUNEL | Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling |
| WT | Wild-Type |
| Xiap | X-Linked Inhibitor of Apoptosis |
Appendix A. Supplementary Methods
Appendix A.1. Neuron Identification Methods
Appendix A.2. Data Analysis
- For sections analysed five times:
- For sections analysed three times:
Appendix A.3. Reanalysis of ucf-101 Effects on Neuronal Survival
References
- Bickenbach, J.; Officer, A.; Shakespeare, T.; von Groote, P. International Perspectives on Spinal Cord Injury. 2013. Available online: https://apps.who.int/iris/handle/10665/94190 (accessed on 5 March 2025).
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 23, 309–331. [Google Scholar] [CrossRef]
- Traumatic Spinal Cord Injury: Facts and Figures at a Glance 2025. Available online: https://bpb-us-w2.wpmucdn.com/sites.uab.edu/dist/f/392/files/2025/02/2025-Facts-and-Figures.pdf (accessed on 7 April 2025).
- Dietz, V.A.; Roberts, N.; Knox, K.; Moore, S.; Pitonak, M.; Barr, C.; Centeno, J.; Leininger, S.; New, K.C.; Nowell, P.; et al. Fighting for recovery on multiple fronts: The past, present, and future of clinical trials for spinal cord injury. Front. Cell Neurosci. 2022, 16, 977679. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Kirshblum, S. Clinical Trials in Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 654–668. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Shakouri-Motlagh, A.; Mokhatab, M.; Rezvan, M.; Sharif-Alhoseini, M.; Shokraneh, F.; Moshayedi, P.; Rahimi-Movaghar, V. The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran. J. Basic Med. Sci. 2018, 21, 546. [Google Scholar] [CrossRef]
- Kuzhandaivel, A.; Nistri, A.; Mazzone, G.L.; Mladinic, M. Molecular mechanisms underlying cell death in spinal networks in relation to locomotor activity after acute injury in vitro. Front. Cell Neurosci. 2011, 5, 9. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Wu, Y.P.; Chen, W.S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287. [Google Scholar]
- Liu, M.; Wu, W.; Li, H.; Li, S.; Huang, L.T.; Yang, Y.Q.; Sun, Q.; Wang, C.X.; Yu, Z.; Hang, C.H. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord. Med. 2015, 38, 745–753. [Google Scholar] [CrossRef]
- Wu, J.; Lipinski, M.M. Autophagy in neurotrauma: Good, bad, or dysregulated. Cells 2019, 8, 693. [Google Scholar] [CrossRef]
- Beattie, M.S.; Hermann, G.E.; Rogers, R.C.; Bresnahan, J.C. Cell death in models of spinal cord injury. Prog. Brain Res. 2002, 137, 37–47. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, S.; Shi, L.; Li, J.; Ning, G.; Kong, X.; Feng, S. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021, 54, e12992. [Google Scholar] [CrossRef] [PubMed]
- Skinnider, M.A.; Gautier, M.; Teo, A.Y.Y.; Kathe, C.; Hutson, T.H.; Laskaratos, A.; de Coucy, A.; Regazzi, N.; Aureli, V.; James, N.D.; et al. Single-cell and spatial atlases of spinal cord injury in the Tabulae Paralytica. Nature 2024, 631, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Fiederling, F.; Hammond, L.A.; Ng, D.; Mason, C.; Dodd, J. Tools for efficient analysis of neurons in a 3D reference atlas of whole mouse spinal cord. Cell Rep. Methods 2021, 1, 100074. [Google Scholar] [CrossRef]
- Reigada, D.; Nieto-Díaz, M.; Navarro-Ruiz, R.; Caballero-Lopez, M.J.; Del Aguila, A.; Munoz-Galdeano, T.; Maza, R.M. Acute administration of ucf-101 ameliorates the locomotor impairments induced by a traumatic spinal cord injury. Neuroscience 2015, 300, 404–417. [Google Scholar] [CrossRef]
- Foster, E.D.; Deardorff, A. Open Science Framework (OSF). J. Med. Libr. Assoc. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Watson, C.; Paxinos, G.; Kayalioglu, G.; Heise, C. Atlas of the Mouse Spinal Cord. In The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas; Watson, C., Paxinos, G., Kayalioglu, G., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2009; pp. 308–379. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Luo, J.; Wu, M.; Gopukumar, D.; Zhao, Y. Big Data Application in Biomedical Research and Health Care: A Literature Review. Biomed. Inform. Insights 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Lemmon, V.P.; Ferguson, A.R.; Popovich, P.G.; Xu, X.M.; Snow, D.M.; Igarashi, M.; Beattie, C.E.; Bixby, J.L. MIASCI Consortium. Minimum Information About a Spinal Cord Injury Experiment: A Proposed Reporting Standard for Spinal Cord Injury Experiments. J. Neurotrauma 2014, 31, 1354–1361. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Guo, Y.; Hu, H.; Chen, K. Quantification of surviving neurons after contusion, dislocation, and distraction spinal cord injuries using automated methods. J. Exp. Neurosci. 2019, 13, 1179069519869617. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-convex Polygons. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), Granada, Spain, 16–20 September 2018. [Google Scholar] [CrossRef]
- Körber, N. MIA is an open-source standalone deep learning application for microscopic image analysis. Cell Rep. Meth. 2023, 3, 100517. [Google Scholar] [CrossRef]
- Franco-Barranco, D.; Andrés-San Román, J.A.; Gómez-Gálvez, P.; Escudero, L.M.; Muñoz-Barrutia, A.; Arganda-Carreras, I. BiaPy: A ready-to-use library for bioimage analysis pipelines. In Proceedings of the 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena de Indias, Colombia, 18–21 April 2023. [Google Scholar] [CrossRef]
- Reigada, D.; Maza, R.M.; Muñoz-Galdeano, T.; Barreda-Manso, M.A.; Soto, A.; Lindholm, D.; Navarro-Ruíz, R.; Nieto-Díaz, M. Overexpression of the X-Linked Inhibitor of Apoptosis Protein (XIAP) in Neurons Improves Cell Survival and the Functional Outcome after Traumatic Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2791. [Google Scholar] [CrossRef] [PubMed]
- Sauerbeck, A.D.; Goldstein, E.Z.; Alfredo, A.N.; Norenberg, M.; Marcillo, A.; McTigue, D.M. Alpha-synuclein increases in rodent and human spinal cord injury and promotes inflammation and tissue loss. Sci. Rep. 2021, 11, 11720. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Wu, Y.; Qiu, J.; Lang-Lazdunski, L.; Hirt, L.; Waeber, C.; Hyman, B.T.; Yuan, J.; Moskowitz, M.A. Fas receptor and neuronal cell death after spinal cord ischemia. J. Neurosci. 2000, 20, 6879–6887. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.; Siegelin, M.D.; Dehghani, F.; Cilenti, L.; Zervos, A.S.; Rami, A. The serine protease Omi/HtrA2 is involved in XIAP cleavage and in neuronal cell death following focal cerebral ischemia/reperfusion. Neurochem. Int. 2007, 50, 172–180. [Google Scholar] [CrossRef]
- Goffredo, D.; Rigamonti, D.; Zuccato, C.; Tartari, M.; Valenza, M.; Cattaneo, E. Prevention of cytosolic IAPs degradation: A potential pharmacological target in Huntington’s Disease. Pharmacol. Res. 2005, 52, 140–150. [Google Scholar] [CrossRef]
- Ding, X.; Patel, M.; Shen, D.; Herzlich, A.A.; Cao, X.; Villasmil, R.; Klupsch, K.; Tuo, J.; Downward, J.; Chan, C.C. Enhanced HtrA2/Omi expression in oxidative injury to retinal pigment epithelial cells and murine models of neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4957–4966. [Google Scholar] [CrossRef]
- Lu, J.; Ashwell, K.W.S.; Waite, P. Advances in secondary spinal cord injury. Spine 2000, 23, 1859–1866. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 96, 813–880. [Google Scholar] [CrossRef]
- Muñoz-Galdeano, T.; Reigada, D.; Del Águila, Á.; Velez, I.; Caballero-López, M.J.; Maza, R.M.; Nieto-Díaz, M. Cell Specific Changes of Autophagy in a Mouse Model of Contusive Spinal Cord Injury. Front. Cell Neurosci. 2018, 12, 164. [Google Scholar] [CrossRef]
- Bogovic, J.A.; Hanslovsky, P.; Wong, A.; Saalfeld, S. Robust registration of calcium images by learned contrast synthesis. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016. [Google Scholar] [CrossRef]
- Wang, C.W.; Ka, S.M.; Chen, A. Robust image registration of biological microscopic images. Sci. Rep. 2014, 4, 6050. [Google Scholar] [CrossRef]
- Thevenaz, P.; Ruttimann, U.E.; Unser, M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Trans. Image Process 1997, 7, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Marstal, K.; Berendsen, F.; Staring, M.; Klein, S. SimpleElastix: A User-Friendly, Multi-lingual Library for Medical Image Registration. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Las Vegas, NV, USA, 26–30 June 2016. [Google Scholar] [CrossRef]
- Borovec, J.; Kybic, J.; Arganda-Carreras, I.; Sorokin, D.V.; Bueno, G.; Khvostikov, A.V.; Bakas, S.; Chang, E.I.; Heldmann, S.; Kartasalo, K.; et al. ANHIR: Automatic non-rigid histological image registration challenge. IEEE Trans. Med. Imaging 2020, 39, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Lein, E.; Hawrylycz, M.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide Atlas of Gene Expression in the Adult Mouse Brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Erö, C.; Gewaltig, M.-O.; Keller, D.; Markram, H. A Cell Atlas for the Mouse Brain. Front. Neuroinform. 2018, 12, 84. [Google Scholar] [CrossRef]
- Liu, X.Z.; Xu, X.M.; Hu, R.; Du, C.; Zhang, S.X.; McDonald, J.W.; Dong, H.X.; Wu, Y.J.; Fan, G.S.; Jacquin, M.F.; et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997, 17, 5395–5406. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 5 March 2025).

| Component | Files | Description | |
|---|---|---|---|
| Image Repository: ucf-101 files (https://osf.io/qe9ys/; accessed on 7 April 2025) | .lif files (13) | Each .lif file comprises all confocal images (stacks) of the spinal cord sections from one individual (in .lif format). | |
| 2015 Reigada.pdf | The original article where images were published (.pdf format). | ||
| metadata images.xlsx | Spreadsheet with data on each section (individual, treatment, number of Z, rostrocaudal position, etc.; in .xlsx format). | ||
| MIASCI data.xlsx | Minimum information about spinal cord injury experiments of the study (in .xlsx format). | ||
| Test Benches/ Neuron Detection/ TestBench1. ucf-101 images (https://osf.io/7agyq/; accessed on 7 April 2025) | tif images folder | A folder comprising two zip files that contain the images (in .tif format) of the comparison (20 images) and training sets (87 images). | |
| Manual Identifications Folder | |||
| Manual ID_Spreadsheet for data collection.xlsx | Excel spreadsheet employed by the observers for data collection (in .xlsx format). Includes instructions. | ||
| Manual ID_identifications xml.zip | A file containing folders for each image with the image, identifications of each observer, and the overlap of all of them. Identification data are presented in .xml format exported from the count tool of Fiji ver. 2.16.0/1.54p. | ||
| Threshold Macro Nukleator Folder | |||
| Threshold ID_macro.txt | ImageJ/Fiji macro for neuron thresholding developed and employed in the Reigada et al. [16] study (in .txt format). | ||
| Threshold ID_idetifications.zip | zip file containing 20 files with the neuronal identifications derived obtained with the threshold macro (in .tif format). | ||
| Neural Network NC-AI1 Folder | |||
| Neural Network_NC_AI1.nn | Neural network developed in this study. In .nn format of CellSens Dimension (Olympus) ver. 3.1 software. To be used with CellSens Dimension software. | ||
| NC-AI1 Training.zip | A zip-compressed folder comprising the different types of files employed to train the neural network (in .ets format). | ||
| NC-AI1 Identifications.zip | A zip-compressed folder containing the files of the comparison set with the neural network identifications (in .tif format). | ||
| NC-AI1 ImageJ macro.ijm | Macro to identify as neurons those particles above 25 µm2 composed of pixels above 50% probability according to the neural network (in ImageJ’s .ijm format). | ||
| Ibañez 2021_Degree Project_in spanish.pdf | Final degree project of Nadia Ibañez-Barranco describing the original results of this test bench (in .pdf format, in Spanish). | ||
| Analysis | |||
| Overlapping.zip | A file containing folders for five images with the image identifications of all observers and the overlap with threshold and neural network identifications. Identification data are presented in xml format exported from the count tool of ImageJ or Fiji. | ||
| Data and Analyses.xlsx | Excel file with all data and analyses from the comparisons of the manual, Nukleator, and NC-AI1 methods | ||
| Neuronal death reconstructions: Which neurons protect ucf-101? (https://osf.io/fhxqs/; accessed on 7 April 2025) | Processed images.zip | A folder containing the images with layers detailing neuronal identifications and annotations of Rexed laminae from Watson et al. [18]’s Atlas (in .tif format). This includes files (in .xml format) containing the landmarks employed for image registration. | |
| Software_bigdataviewer_fiji-6.2.1.jar | Fiji vs 2.16.0/1.54p plugins for image registration using BigWarp, a tool for landmark-based deformable image alignment (in .jar format). | ||
| Software_bigwarp_fiji-7.0.2.jar | |||
| data &analyses.xlsx | Metadata, raw and processed data on the numbers of neurons per Rexed laminae and results of their analysis (in .xlsx format). | ||
| R Analysis Neuronal Death.txt | Statistical analysis of the data in R ver 4.4.0 (in .txt format) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Amezcua, P.; Ibáñez-Barranco, N.; Reigada, D.; Novillo, I.; Soto, A.; Barreda-Manso, M.A.; Muñoz-Galdeano, T.; Maza, R.M.; Esteban, F.J.; Nieto-Díaz, M. Reanalysis of Published Histological Data Can Help to Characterize Neuronal Death After Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 3749. https://doi.org/10.3390/ijms26083749
Ruiz-Amezcua P, Ibáñez-Barranco N, Reigada D, Novillo I, Soto A, Barreda-Manso MA, Muñoz-Galdeano T, Maza RM, Esteban FJ, Nieto-Díaz M. Reanalysis of Published Histological Data Can Help to Characterize Neuronal Death After Spinal Cord Injury. International Journal of Molecular Sciences. 2025; 26(8):3749. https://doi.org/10.3390/ijms26083749
Chicago/Turabian StyleRuiz-Amezcua, Pablo, Nadia Ibáñez-Barranco, David Reigada, Irene Novillo, Altea Soto, María Asunción Barreda-Manso, Teresa Muñoz-Galdeano, Rodrigo M. Maza, Francisco J. Esteban, and Manuel Nieto-Díaz. 2025. "Reanalysis of Published Histological Data Can Help to Characterize Neuronal Death After Spinal Cord Injury" International Journal of Molecular Sciences 26, no. 8: 3749. https://doi.org/10.3390/ijms26083749
APA StyleRuiz-Amezcua, P., Ibáñez-Barranco, N., Reigada, D., Novillo, I., Soto, A., Barreda-Manso, M. A., Muñoz-Galdeano, T., Maza, R. M., Esteban, F. J., & Nieto-Díaz, M. (2025). Reanalysis of Published Histological Data Can Help to Characterize Neuronal Death After Spinal Cord Injury. International Journal of Molecular Sciences, 26(8), 3749. https://doi.org/10.3390/ijms26083749









