Mechanisms of Different Motor Neurons in the Occurrence of Spasticity After Spinal Cord Injury: A Narrative Review
Abstract
1. Introduction
2. Hyperexcitability of Motor Neurons: The Core Mechanism Underlying Post-SCI Spasticity
2.1. H-Reflex and Spasticity Manifestations
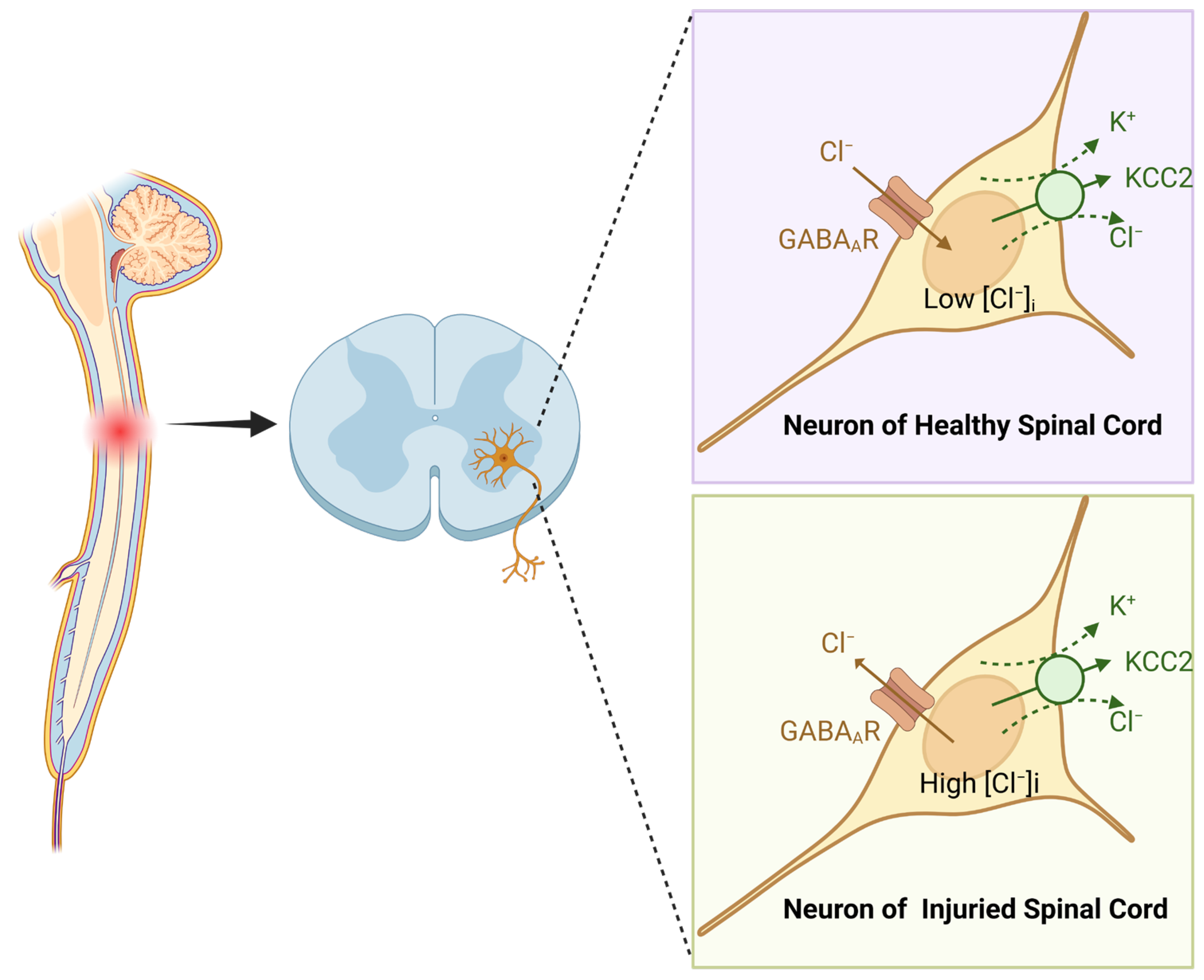
2.2. Voltage-Gated Conductances in MN Hyperexcitability Pathogenesis
| Ref. | Experimental Animals | Experimental Models | Treatment | The Changes in Motor Neurons Following Pharmacological Inhibition of Spasticity Post-SCI | ||
|---|---|---|---|---|---|---|
| [42] | Adult female Sprague-Dawley rats | Spinal Contusion Injury at T8/T9 | Intraspinal microstimulation | Following SCI, motor neurons exhibit hyperexcitability, resulting in spasticity. |
| |
| [43] | Eight-to-ten-week-old male and female C57/Bl6 mice expressing YFP under a Thy1 promoter | Spinal Contusion Injury at L1–L2 | Romidepsin |
|
| |
| [44] | Adult C57BL/6J mice of both sexes | Spinal Contusion Injury at T10; Spinal transection injury at T10 | STR, PTX |
|
| |
| [45] | Eight-to-ten-week-old male and female mice (c57/bl6) | Spinal Contusion Injury at L1–L2 |
| |||
| [46] | Selective Rac1KO in astrocytes using a cre-lox system (GFAP-cre/Rac1flox/flox) mouse | Spinal Contusion Injury at L1–L2 |
| |||
| [47] | Adult C57BL6/J female mice | Complete spinal cord transection at T10 | Strychnine, Bicuculline, α-5-HT, Citalopram, Nimodipine |
| Crucial ion channel receptors modulate the excitability of motor neurons |
|
| [48] | Adult female Sprague-Dawley rats | Spinal Contusion Injury at T9–T10 | NMD |
|
| |
| [49] | 1.5–2 months old and 180–200 g male Wistar rats | 7-Day Rat Hindlimb Suspension (The origin of this activity is somewhat akin to muscle spasticity after spinal cord injuries and is the result of KCC2 content decline in the spinal cord’s motor neurons) | CLP-290 |
|
| |
| [50] | Adult Wistar Han female rats | Spinal Cord transected at T8/T9 | AAV6-GFP-shRNA-CAPN1and AAV6-GFP-shRNA-scramble |
|
| |
| [51] | Adult female Sprague Dawley rats | Spinal transection injury at T12 | Bumetanide |
|
| |
3. Differential Remodeling of Motor Neurons in Hyperreflexia Development
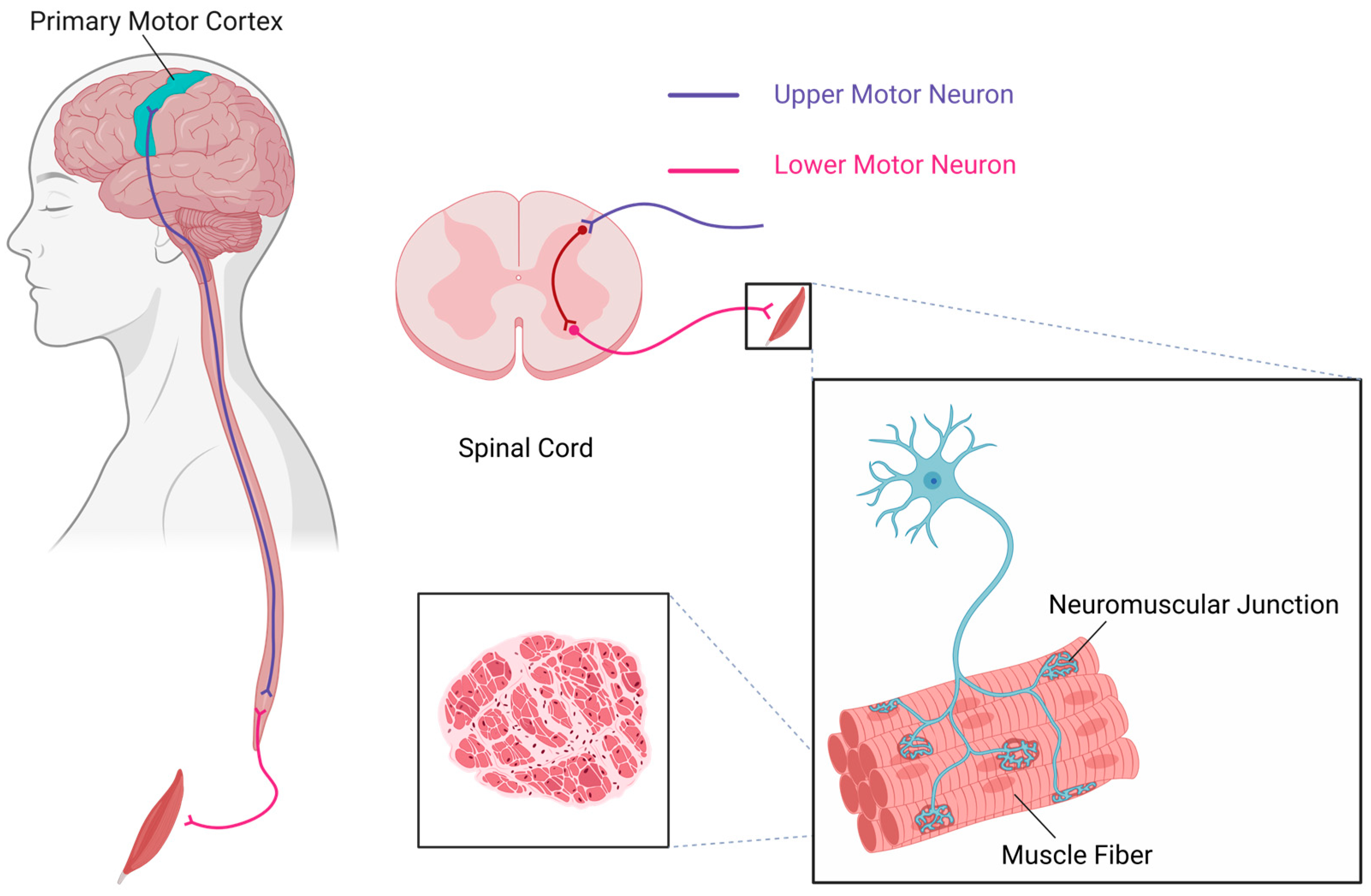
3.1. Upper Motor Neurons and Lower Motor Neurons
3.1.1. The Role of Upper Motor Neurons in Motor Control and Dysfunction Following Injury
3.1.2. Multiple Inputs to Lower Motor Neurons and Their Role in Motor Control
Spinal Motor Neurons and Their Pathophysiological Role in Spinal Cord Injury
Mechanisms of Elevated Fatigability and Enhanced Twitch Force in Spastic Muscles Post-SCI
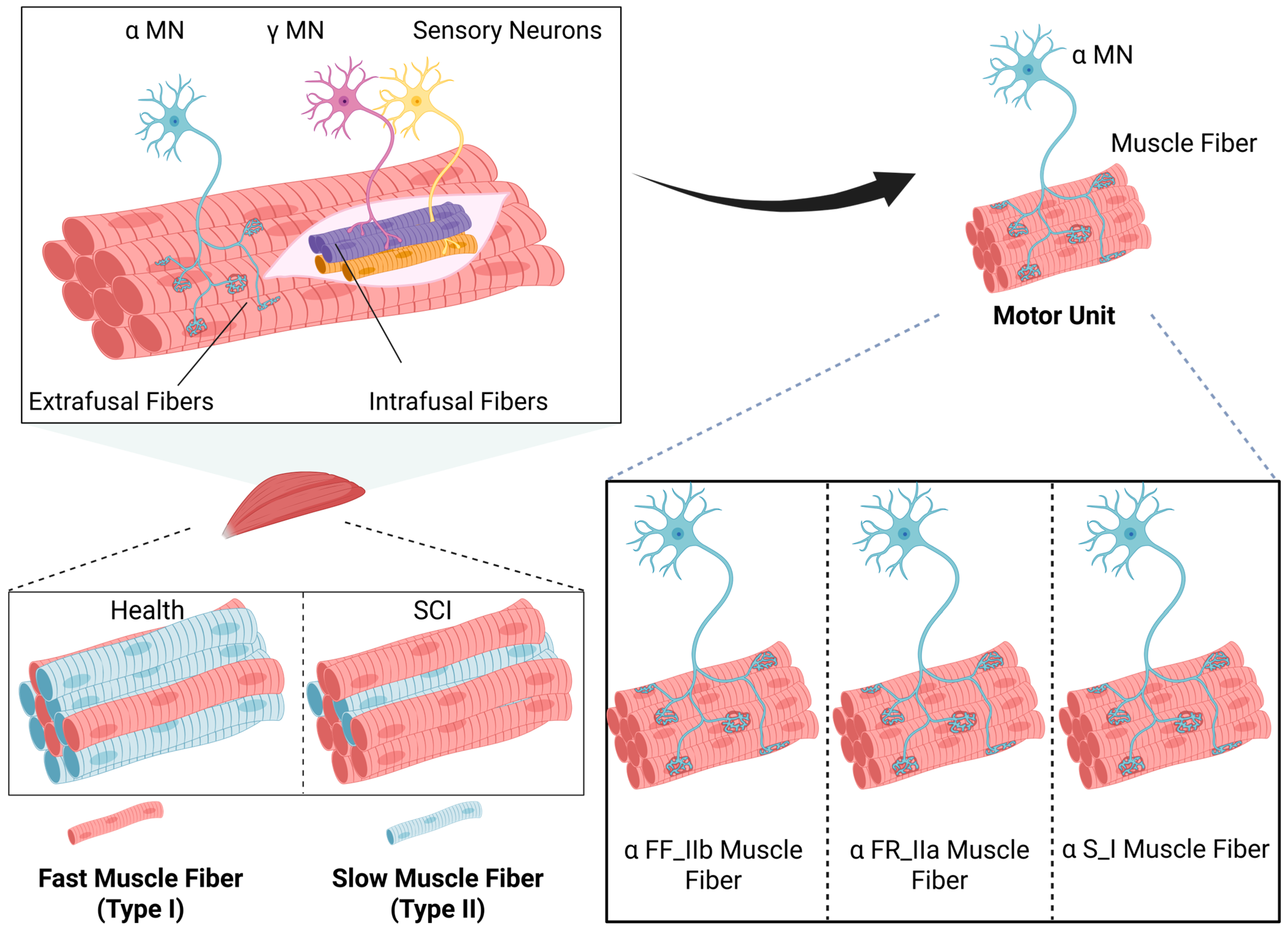
3.2. α, γ, and β Motor Neurons
3.2.1. Functional Changes in α-Motor Neurons Post-SCI and Their Association with Muscle Spasticity
3.2.2. Functional Changes in γ-Motor Neurons Post-SCI and Their Association with Muscle Spasticity
3.2.3. Functional Changes in β-Motor Neurons Post-SCI and Their Association with Muscle Spasticity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCI | spinal cord injury |
| PICs | persistent inward currents |
| ADLs | activities of daily living |
| QOL | quality of life |
| LLR | long-lasting reflex |
| EPSP | excitatory postsynaptic potential |
| KCC2 | K+-Cl− cotransporter 2 |
| GABA | γ-aminobutyric acid |
| IPSP | inhibitory postsynaptic potential |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| NMDAR | N-methyl-D-aspartate receptor |
| NMD | nimodipine |
| PA | proprioceptive afferent |
| GABApre | GABAergic inhibitory presynaptic regulation of 1A terminals |
| RDD | rate-dependent depression |
| STR | Strychnine |
| PTX | picrotoxin |
| GLT-1 | glutamate transporter-1 |
| PSD-95 | postsynaptic density protein 95 |
| SCT | spinal cord transection |
| GL | Gastrocnemius lateralis |
| TA | Tibialis anterior |
| aSCI | acute spinal cord transections |
| CNS | central nervous system |
| UMN | upper motor neuron |
| LMN | lower motor neuron |
| HSP | hereditary spastic paraplegia |
| PLS | primary lateral sclerosis |
| ALS | amyotrophic lateral sclerosis |
| SMA | spinal muscular atrophy |
| dHMNs | distal hereditary motor neuropathies |
| GBS | Guillain–Barré syndrome |
| MMN | multifocal motor neuropathy |
| CIDP | chronic inflammatory demyelinating polyneuropathy |
| MRRF | movement-related receptive field |
| FFR | force–frequency relationship |
| αMN | α-motor neuron |
| βMN | β-motor neuron |
| γMN | γ-motor neuron |
| αFF | fast-twitch fatigable |
| αFR | fast-twitch fatigue-resistant |
| αS | slow-twitch fatigue-resistant |
| AHP | afterhyperpolarization |
References
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Hicks, A.L. Spasticity after spinal cord injury. Spinal Cord 2005, 43, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sangari, S.; Perez, M.A. Prevalence of spasticity in humans with spinal cord injury with different injury severity. J. Neurophysiol. 2022, 128, 470–479. [Google Scholar] [CrossRef]
- Stifani, N. Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci. 2014, 8, 293. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabilit. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Decq, P. Pathophysiology of spasticity. Neurochirurgie 2003, 49, 163–184. [Google Scholar]
- Gorassini, M.A.; Knash, M.E.; Harvey, P.J.; Bennett, D.J.; Yang, J.F. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 2004, 127, 2247–2258. [Google Scholar] [CrossRef]
- Hiersemenzel, L.P.; Curt, A.; Dietz, V. From spinal shock to spasticity: Neuronal adaptations to a spinal cord injury. Neurology 2000, 54, 1574–1582. [Google Scholar] [CrossRef]
- Therkildsen, E.R.; Kaster, P.; Nielsen, J.B. A scoping review on muscle cramps and spasms in upper motor neuron disorder-two sides of the same coin? Front. Neurol. 2024, 15, 1360521. [Google Scholar] [CrossRef]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Finnerup, N.B. Neuropathic pain and spasticity: Intricate consequences of spinal cord injury. Spinal Cord 2017, 55, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Esteban, E.; Taylor, J.; Abián-Vicén, J.; Albu, S.; Simón-Martínez, C.; Torricelli, D.; Gómez-Soriano, J. Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation 2013, 33, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Emos, M.C.; Agarwal, S. Neuroanatomy, Upper Motor Neuron Lesion. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Marsden, J.; Stevenson, V.; Jarrett, L. Treatment of spasticity. Handb. Clin. Neurol. 2023, 196, 497–521. [Google Scholar] [CrossRef]
- Bandaru, S.P.; Liu, S.; Waxman, S.G.; Tan, A.M. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J. Neurophysiol. 2015, 113, 1598–1615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, D.L.; Toda, T.; Narushima, M.; Eto, K.; Takayama, C.; Ooba, T.; Wake, H.; Moorhouse, A.J.; Nabekura, J. KCC2 downregulation after sciatic nerve injury enhances motor function recovery. Sci. Rep. 2023, 13, 7871. [Google Scholar] [CrossRef]
- Huang, J.; Pan, X.; Yan, N. Structural biology and molecular pharmacology of voltage-gated ion channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 904–925. [Google Scholar] [CrossRef]
- Buen, E.P.; Salgado-Ceballos, H.; González-Tapia, D.; Leal-Cortés, C.; Mondragón-Lozano, R.; Sánchez-Torres, S.; Álvarez-Mejía, L.; Fabela-Sánchez, O.; Martínez-Torres, N.I.; González-Ramírez, M.M.; et al. Spinogenesis and Plastic Changes in the Dendritic Spines of Spinal Cord Motoneurons After Traumatic Injury in Rats. Arch. Med. Res. 2017, 48, 609–615. [Google Scholar] [CrossRef]
- Bannon, N.M.; Chistiakova, M.; Volgushev, M. Synaptic Plasticity in Cortical Inhibitory Neurons: What Mechanisms May Help to Balance Synaptic Weight Changes? Front. Cell. Neurosci. 2020, 14, 204. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ott, K.; Porter, R.W.; Stuart, D. Low frequency depression of the H wave in normal and spinal man. Exp. Neurol. 1966, 15, 140–156. [Google Scholar] [CrossRef]
- León, F.; Manzo, L.; Kababie, R.; Figueroa, J.; Cuellar, C.; Herrero, P. Effects of Dry Needling on Spasticity in Multiple Sclerosis Evaluated Through the Rate-Dependent Depression of the H Reflex: A Case Report. Int. Med. Case Rep. J. 2023, 16, 293–302. [Google Scholar] [CrossRef]
- Wieters, F.; Gruhn, M.; Büschges, A.; Fink, G.R.; Aswendt, M. Terminal H-reflex Measurements in Mice. J. Vis. Exp. 2022, e63304. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E. Electrophysiological assessment of the spinal mechanisms underlying spasticity. Electroencephalogr. Clin. Neurophysiol. Suppl. 1990, 41, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Calcutt, N.A.; Zhou, X. Rate-Dependent Depression of the Hoffmann Reflex: Practical Applications in Painful Diabetic Neuropathy. Diabetes Metab. J. 2024, 48, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Kronenberg, M.F.; Stĕtkárová, I.; Paske, W.C.; Stokić, D.S. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology 1996, 47, 231–237. [Google Scholar] [CrossRef]
- Pelletier, C.A.; Hicks, A.L. The length-tension relationship of human dorsiflexor and plantarflexor muscles after spinal cord injury. Spinal Cord 2010, 48, 202–206. [Google Scholar] [CrossRef]
- Biering-Sørensen, B.; Kristensen, I.B.; Kjaer, M.; Biering-Sørensen, F. Muscle after spinal cord injury. Muscle Nerve 2009, 40, 499–519. [Google Scholar] [CrossRef]
- Rank, M.M.; Murray, K.C.; Stephens, M.J.; D’Amico, J.; Gorassini, M.A.; Bennett, D.J. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J. Neurophysiol. 2011, 105, 410–422. [Google Scholar] [CrossRef]
- Li, Y.; Bennett, D.J. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J. Neurophysiol. 2003, 90, 857–869. [Google Scholar] [CrossRef]
- Deuschl, G.; Lücking, C.H. Physiology and clinical applications of hand muscle reflexes. Electroencephalogr. Clin. Neurophysiol. Suppl. 1990, 41, 84–101. [Google Scholar] [CrossRef]
- Li, Y.; Gorassini, M.A.; Bennett, D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J. Neurophysiol. 2004, 91, 767–783. [Google Scholar] [CrossRef]
- Revill, A.L.; Chu, N.Y.; Ma, L.; LeBlancq, M.J.; Dickson, C.T.; Funk, G.D. Postnatal development of persistent inward currents in rat XII motoneurons and their modulation by serotonin, muscarine and noradrenaline. J. Physiol. 2019, 597, 3183–3201. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ge, X.; Dai, Y. Cholinergic modulation of persistent inward currents is mediated by activating muscarinic receptors of serotonergic neurons in the brainstem of ePet-EYFP mice. Exp. Brain Res. 2022, 240, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- ElBasiouny, S.M.; Schuster, J.E.; Heckman, C.J. Persistent inward currents in spinal motoneurons: Important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2010, 121, 1669–1679. [Google Scholar] [CrossRef]
- Gracies, J.M. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef]
- Wang, J.X.; Yang, X.; Zhang, J.J.; Zhou, T.T.; Zhu, Y.L.; Wang, L.Y. Effects of Shaoyao Gancao decoction on contents of amino acids and expressions of receptors in brains of spastic paralysis rats. Zhongguo Zhong Yao Za Zhi 2016, 41, 1100–1106. [Google Scholar] [CrossRef]
- Faist, M.; Mazevet, D.; Dietz, V.; Pierrot-Deseilligny, E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain 1994, 117, 1449–1455. [Google Scholar] [CrossRef]
- Fogarty, M.J. Inhibitory Synaptic Influences on Developmental Motor Disorders. Int. J. Mol. Sci. 2023, 24, 6962. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.E.; Grau, J.W. Ionic Plasticity: Common Mechanistic Underpinnings of Pathology in Spinal Cord Injury and the Brain. Cells 2022, 11, 2910. [Google Scholar] [CrossRef]
- Talifu, Z.; Pan, Y.; Gong, H.; Xu, X.; Zhang, C.; Yang, D.; Gao, F.; Yu, Y.; Du, L.; Li, J. The role of KCC2 and NKCC1 in spinal cord injury: From physiology to pathology. Front. Physiol. 2022, 13, 1045520. [Google Scholar] [CrossRef]
- Bennett, D.J.; Li, Y.; Siu, M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J. Neurophysiol. 2001, 86, 1955–1971. [Google Scholar] [CrossRef]
- Bandres, M.F.; Gomes, J.L.; McPherson, J.G. Intraspinal microstimulation of the ventral horn has therapeutically relevant cross-modal effects on nociception. Brain Commun. 2024, 6, fcae280. [Google Scholar] [CrossRef] [PubMed]
- Kauer, S.D.; Benson, C.A.; Carrara, J.M.; Tarafder, A.A.; Ibrahim, Y.H.; Estacion, M.A.; Waxman, S.G.; Tan, A.M. PAK1 inhibition with Romidepsin attenuates H-reflex hyperexcitability after spinal cord injury. J. Physiol. 2024, 602, 5061–5081. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, A.; Birch, D.; Heckman, C.J.; Tysseling, V. Muscle Spasms after Spinal Cord Injury Stem from Changes in Motoneuron Excitability and Synaptic Inhibition, Not Synaptic Excitation. J. Neurosci. 2024, 44, e1695232023. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.A.; King, J.F.; Kauer, S.D.; Waxman, S.G.; Tan, A.M. Increased astrocytic GLT-1 expression in tripartite synapses is associated with SCI-induced hyperreflexia. J. Neurophysiol. 2023, 130, 1358–1366. [Google Scholar] [CrossRef]
- Benson, C.A.; Olson, K.L.; Patwa, S.; Kauer, S.D.; King, J.F.; Waxman, S.G.; Tan, A.M. Conditional Astrocyte Rac1KO Attenuates Hyperreflexia after Spinal Cord Injury. J. Neurosci. 2024, 44, e1670222023. [Google Scholar] [CrossRef]
- Jiang, M.C.; Birch, D.V.; Heckman, C.J.; Tysseling, V.M. The Involvement of Ca(V)1.3 Channels in Prolonged Root Reflexes and Its Potential as a Therapeutic Target in Spinal Cord Injury. Front. Neural Circuits 2021, 15, 642111. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, X.; He, Z.; Zhang, R.; Zhang, S.; Wang, M.; Chen, H.; Wang, W. Nimodipine Promotes Functional Recovery After Spinal Cord Injury in Rats. Front. Pharmacol. 2021, 12, 733420. [Google Scholar] [CrossRef]
- Sergeeva, X.V.; Sharlo, K.A.; Tyganov, S.A.; Kalashnikov, V.E.; Shenkman, B.S. Molecular Signaling Effects behind the Spontaneous Soleus Muscle Activity Induced by 7-Day Rat Hindlimb Suspension. Int. J. Mol. Sci. 2024, 25, 8316. [Google Scholar] [CrossRef]
- Kerzonkuf, M.; Verneuil, J.; Brocard, C.; Dingu, N.; Trouplin, V.; Ramirez Franco, J.J.; Bartoli, M.; Brocard, F.; Bras, H. Knockdown of calpain1 in lumbar motoneurons reduces spasticity after spinal cord injury in adult rats. Mol. Ther. 2024, 32, 1096–1109. [Google Scholar] [CrossRef]
- Caron, G.; Bilchak, J.; Côté, M.P. Bumetanide increases postsynaptic inhibition after chronic SCI and decreases presynaptic inhibition with step-training. J. Physiol. 2023, 601, 1425–1447. [Google Scholar] [CrossRef]
- Binder, M.D.; Heckman, C.J.; Powers, R.K. Relative strengths and distributions of different sources of synaptic input to the motoneurone pool: Implications for motor unit recruitment. Adv. Exp. Med. Biol. 2002, 508, 207–212. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Morita, H.; Wenzelburger, R.; Deuschl, G.; Gossard, J.P.; Hultborn, H. Recruitment gain of spinal motor neuron pools in cat and human. Exp. Brain Res. 2019, 237, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulos-Stournaras, S.; Iles, J.F. Motor neuron columns in the lumbar spinal cord of the rat. J. Comp. Neurol. 1983, 217, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Hinckley, C.A.; Hilde, K.L.; Driscoll, S.P.; Poon, T.H.; Montgomery, J.M.; Pfaff, S.L. Identification of a cellular node for motor control pathways. Nat. Neurosci. 2014, 17, 586–593. [Google Scholar] [CrossRef]
- McHanwell, S.; Biscoe, T.J. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1981, 293, 477–508. [Google Scholar] [CrossRef]
- Gunay, A.; Shin, H.H.; Gozutok, O.; Gautam, M.; Ozdinler, P.H. Importance of lipids for upper motor neuron health and disease. Semin. Cell Dev. Biol. 2021, 112, 92–104. [Google Scholar] [CrossRef]
- Ivanhoe, C.B.; Reistetter, T.A. Spasticity: The misunderstood part of the upper motor neuron syndrome. Am. J. Phys. Med. Rehabil. 2004, 83, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.M.; Ghasemi, M.; Brown, R.H., Jr. Emerging mechanisms of molecular pathology in ALS. J. Clin. Investig. 2015, 125, 2548. [Google Scholar] [CrossRef]
- Jo, H.J.; Perez, M.A. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 2020, 143, 1368–1382. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Danner, S.M.; Mayr, W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artif. Organs 2015, 39, 823–833. [Google Scholar] [CrossRef]
- Burke, R.E.; Levine, D.N.; Salcman, M.; Tsairis, P. Motor units in cat soleus muscle: Physiological, histochemical and morphological characteristics. J. Physiol. 1974, 238, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T.; Kanouchi, T.; Shibuya, K.; Noto, Y.; Yagi, Y.; Inaba, A.; Abe, K.; Misawa, S.; Orimo, S.; Kobayashi, T.; et al. Spreading of amyotrophic lateral sclerosis lesions--multifocal hits and local propagation? J. Neurol. Neurosurg. Psychiatry 2014, 85, 85–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verschueren, A. Motor neuropathies and lower motor neuron syndromes. Rev. Neurol. 2017, 173, 320–325. [Google Scholar] [CrossRef]
- Garg, N.; Park, S.B.; Vucic, S.; Yiannikas, C.; Spies, J.; Howells, J.; Huynh, W.; Matamala, J.M.; Krishnan, A.V.; Pollard, J.D.; et al. Differentiating lower motor neuron syndromes. J. Neurol. Neurosurg. Psychiatry 2017, 88, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Alaburda, A.; Perrier, J.F.; Hounsgaard, J. Mechanisms causing plateau potentials in spinal motoneurones. Adv. Exp. Med. Biol. 2002, 508, 219–226. [Google Scholar] [CrossRef]
- Heckmann, C.J.; Gorassini, M.A.; Bennett, D.J. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 2005, 31, 135–156. [Google Scholar] [CrossRef]
- Kennedy, D.S.; McNeil, C.J.; Gandevia, S.C.; Taylor, J.L. Effects of fatigue on corticospinal excitability of the human knee extensors. Exp. Physiol. 2016, 101, 1552–1564. [Google Scholar] [CrossRef]
- Taylor, J.L.; Todd, G.; Gandevia, S.C. Evidence for a supraspinal contribution to human muscle fatigue. Clin. Exp. Pharmacol. Physiol. 2006, 33, 400–405. [Google Scholar] [CrossRef]
- Binder-Macleod, S.A.; McDermond, L.R. Changes in the force-frequency relationship of the human quadriceps femoris muscle following electrically and voluntarily induced fatigue. Phys. Ther. 1992, 72, 95–104. [Google Scholar] [CrossRef]
- MacIntosh, B.R.; Willis, J.C. Force-frequency relationship and potentiation in mammalian skeletal muscle. J. Appl. Physiol. 2000, 88, 2088–2096. [Google Scholar] [CrossRef]
- Carroll, T.J.; Taylor, J.L.; Gandevia, S.C. Recovery of central and peripheral neuromuscular fatigue after exercise. J. Appl. Physiol. 2017, 122, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Castro, M.J.; Apple, D.F., Jr.; Staron, R.S.; Campos, G.E.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J. Appl. Physiol. 1999, 86, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Justicia, R.; Van der Stede, T.; Stocks, B.; Laitila, J.; Seaborne, R.A.; Van de Loock, A.; Lievens, E.; Samodova, D.; Marín-Arraiza, L.; Dmytriyeva, O.; et al. Human skeletal muscle fiber heterogeneity beyond myosin heavy chains. Nat. Commun. 2025, 16, 1764. [Google Scholar] [CrossRef]
- Olsson, M.C.; Krüger, M.; Meyer, L.H.; Ahnlund, L.; Gransberg, L.; Linke, W.A.; Larsson, L. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J. Physiol. 2006, 577, 339–352. [Google Scholar] [CrossRef]
- Kanning, K.C.; Kaplan, A.; Henderson, C.E. Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 2010, 33, 409–440. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal muscular atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E., 3rd. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Chalif, J.I.; Mentis, G.Z. Normal Development and Pathology of Motoneurons: Anatomy, Electrophysiological Properties, Firing Patterns and Circuit Connectivity. Adv. Neurobiol. 2022, 28, 63–85. [Google Scholar] [CrossRef]
- Luo, J.; Lei, K.Y.; Shi, S.; Xu, X.L.; Sun, X.L.; Song, M.J.; Zhang, H.P.; Li, L. The role of cis-regulatory elements in the determination and transformation of muscle fiber type in animal skeletal muscles. Yi Chuan 2025, 47, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Lilliu, E.; Choi, R.; Hilber, K.; Launikonis, B.; Koenig, X. Comparison of Phasic Store-Operated Calcium Entry in Rat Slow- and Fast-Twitch Muscle Fibers. Acta Physiol. 2025, 241, e70059. [Google Scholar] [CrossRef]
- Kaplan, A.; Spiller, K.J.; Towne, C.; Kanning, K.C.; Choe, G.T.; Geber, A.; Akay, T.; Aebischer, P.; Henderson, C.E. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 2014, 81, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, M.; Cassar-Malek, I.; Meunier, B.; Picard, B. A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur. J. Histochem. 2014, 58, 2254. [Google Scholar] [CrossRef]
- Thomas, C.K.; Butler, J.E.; Zijdewind, I. Patterns of pathological firing in human motor units. Adv. Exp. Med. Biol. 2002, 508, 237–244. [Google Scholar] [CrossRef]
- Piotrkiewicz, M. Possible changes in motor neuron discharge characteristics in presymptomatic amyotrophic lateral sclerosis. J. Physiol. 2024, 602, 6631–6635. [Google Scholar] [CrossRef]
- Zijdewind, I.; Thomas, C.K. Firing patterns of spontaneously active motor units in spinal cord-injured subjects. J. Physiol. 2012, 590, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Zijdewind, I.; Gant, K.; Bakels, R.; Thomas, C.K. Do additional inputs change maximal voluntary motor unit firing rates after spinal cord injury? Neurorehabilit. Neural Repair 2012, 26, 58–67. [Google Scholar] [CrossRef]
- Wilkinson, K.A. Methodological advances for studying gamma motor neurons. Curr. Opin. Physiol. 2021, 19, 135–140. [Google Scholar] [CrossRef]
- D’Amico, J.M.; Condliffe, E.G.; Martins, K.J.; Bennett, D.J.; Gorassini, M.A. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front. Integr. Neurosci. 2014, 8, 36. [Google Scholar] [CrossRef]
- Enjin, A.; Leão, K.E.; Mikulovic, S.; Le Merre, P.; Tourtellotte, W.G.; Kullander, K. Sensorimotor function is modulated by the serotonin receptor 1d, a novel marker for gamma motor neurons. Mol. Cell. Neurosci. 2012, 49, 322–332. [Google Scholar] [CrossRef]
- Nishimura, K.; Ohta, M.; Saito, M.; Morita-Isogai, Y.; Sato, H.; Kuramoto, E.; Yin, D.X.; Maeda, Y.; Kaneko, T.; Yamashiro, T.; et al. Electrophysiological and Morphological Properties of α and γ Motoneurons in the Rat Trigeminal Motor Nucleus. Front. Cell. Neurosci. 2018, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; He, X.; Xiao, Q.; Alstermark, B.; Lan, N. Corticomuscular transmission of tremor signals by propriospinal neurons in Parkinson’s disease. PLoS ONE 2013, 8, e79829. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Knellwolf, T.P. Functional properties of human muscle spindles. J. Neurophysiol. 2018, 120, 452–467. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, C.D. Sensorimotor anatomy of gait, balance, and falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar] [CrossRef]
- Bessou, P.; Emonet-Dénand, F.; Laporte, Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J. Physiol. 1965, 180, 649–672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Zhang, Z.-Y.; Duan, Z.-X.; Mao, X.-A.; Wu, Y.-Y.; Rao, J.-S.; Du, X.-X. Mechanisms of Different Motor Neurons in the Occurrence of Spasticity After Spinal Cord Injury: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5162. https://doi.org/10.3390/ijms26115162
Gong H, Zhang Z-Y, Duan Z-X, Mao X-A, Wu Y-Y, Rao J-S, Du X-X. Mechanisms of Different Motor Neurons in the Occurrence of Spasticity After Spinal Cord Injury: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(11):5162. https://doi.org/10.3390/ijms26115162
Chicago/Turabian StyleGong, Han, Ze-Yan Zhang, Zhi-Xuan Duan, Xin-Ao Mao, Yuan-Yuan Wu, Jia-Sheng Rao, and Xiao-Xia Du. 2025. "Mechanisms of Different Motor Neurons in the Occurrence of Spasticity After Spinal Cord Injury: A Narrative Review" International Journal of Molecular Sciences 26, no. 11: 5162. https://doi.org/10.3390/ijms26115162
APA StyleGong, H., Zhang, Z.-Y., Duan, Z.-X., Mao, X.-A., Wu, Y.-Y., Rao, J.-S., & Du, X.-X. (2025). Mechanisms of Different Motor Neurons in the Occurrence of Spasticity After Spinal Cord Injury: A Narrative Review. International Journal of Molecular Sciences, 26(11), 5162. https://doi.org/10.3390/ijms26115162






