Diagnostic Potential of CD44, CD133, and VDR in Epithelial Ovarian Tumors: Association with Histopathology Parameters
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. CD44, CD133, and VDR Expressions in Epithelial Ovarian Tumors
2.3. CD44, CD133, and VDR Expression Levels in Ovarian Cancers
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Histopathological Evaluation
4.3. Tissue Microarray Construction
4.4. Immunohistochemistry Analysis and Evaluation of Immunostaining Intensity Scoring Methods
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.C.; Smolenkov, A.; Batra, S.K.; Ratajczak, M.Z.; Kakar, S.S. Ovarian Cancer Stem Cells: Unraveling a Germline Connection. Stem Cells Dev. 2017, 26, 1781–1803. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.-Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721–8743. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Ahlawat, N.; Srivastava, A. Ovarian Cancer Stem Cells: Newer Horizons. J. Obstet. Gynaecol. India 2021, 71, 115–117. [Google Scholar] [CrossRef]
- Ng, A.; Barker, N. Ovary and fimbrial stem cells: Biology, niche and cancer origins. Nat. Rev. Mol. Cell Biol. 2015, 16, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef]
- Schwitalla, S. Tumor cell plasticity: The challenge to catch a moving target. J. Gastroenterol. 2014, 49, 618–627. [Google Scholar] [CrossRef]
- Ji, M.; Liu, L.; Hou, Y.; Li, B. 1α,25-Dihydroxyvitamin D3 restrains stem cell-like properties of ovarian cancer cells by enhancing vitamin D receptor and suppressing CD44. Oncol. Rep. 2019, 41, 3393–3403. [Google Scholar] [CrossRef]
- Boxberg, M.; Götz, C.; Haidari, S.; Dorfner, C.; Jesinghaus, M.; Drecoll, E.; Boskov, M.; Wolff, K.D.; Weichert, W.; Haller, B.; et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 2018, 73, 559–572. [Google Scholar] [CrossRef]
- Guadagno, E.; Borrelli, G.; Califano, M.; Calì, G.; Solari, D.; Caro, M.D.B.D. Immunohistochemical expression of stem cell markers CD44 and nestin in glioblastomas: Evaluation of their prognostic significance. Pathol. Res. Pract. 2016, 212, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Sabunga, O.D.; Kaelan, C.; Zainuddin, A.A.; Sungowati, N.K.; Cangara, M.H.; Miskad, U. Expression of CD133 Cancer Stem Cell Marker in IDH-Mutant and IDH-wildtype (Isocitrate Dehydrogenase) Astrocytoma. Asian Pac. J. Cancer Prev. 2022, 23, 3051–3059. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Siegler, E.L.; Siriwon, N.; Wang, P. Therapeutic strategies for targeting cancer stem cells. J. Cancer Metastasis Treat. 2016, 2, 233–242. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, A.; Song, H.; Tao, J.; Yang, H.; Zuo, M. Prognostic value of cancer stem cell marker CD133 in ovarian cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3080–3088. [Google Scholar]
- Ikram, D.; Masadah, R.; Nelwan, B.J.; Zainuddin, A.A.; Ghaznawie, M.; Wahid, S. CD133 Act as an Essential Marker in Ovarian Carcinogenesis. Asian Pac. J. Cancer Prev. 2012, 22, 3525–3531. [Google Scholar] [CrossRef]
- Ma, H.; Tian, T.; Cui, Z. Targeting ovarian cancer stem cells: A new way out. Stem Cell Res. Ther. 2023, 14, 28. [Google Scholar] [CrossRef]
- Klemba, A.; Purzycka-Olewiecka, J.K.; Wcisło, G.; Czarnecka, A.M.; Lewicki, S.; Lesyng, B.; Szczylik, C.; Kieda, C. Surface markers of cancer stem-like cells of ovarian cancer and their clinical relevance. Contemp. Oncol. 2018, 22, 48–55. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer stem cells-origins and biomarkers: Perspectives for targeted personalized therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Lee, S.-M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef]
- Sheeley, M.P.; Andolino, C.; Kiesel, V.A.; Teegarden, D. Vitamin D regulation of energy metabolism in cancer. Br. J. Pharmacol. 2022, 179, 2890–2905. [Google Scholar] [CrossRef]
- Ong, J.-S.; Cuellar-Partida, G.; Lu, Y.; Fasching, P.A.; Hein, A.; Burghaus, S.; Beckmann, M.W.; Lambrechts, D.; Van Nieuwenhuysen, E.; Vergote, I.; et al. Australian Ovarian Cancer Study. Association of vitamin D levels and risk of ovarian cancer: A Mendelian randomization study. Int. J. Epidemiol. 2016, 45, 1619–1630. [Google Scholar] [CrossRef]
- Zhang, X.; Nicosia, S.V.; Bai, W. Vitamin D receptor is a novel drug target for ovarian cancer treatment. Curr. Cancer Drug Targets 2006, 6, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef]
- Czogalla, B.; Deuster, E.; Liao, Y.; Mayr, D.; Schmoeckel, E.; Sattler, C.; Kolben, T.; Hester, A.; Fürst, S.; Burges, A.; et al. Cytoplasmic VDR expression as an independent risk factor for ovarian cancer. Histochem. Cell Biol. 2020, 154, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Rafi, L.; Mitschele, T.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2003; Volume 164, pp. 239–246. [Google Scholar]
- Lurie, G.; Wilkens, L.R.; Thompson, P.J.; McDuffie, K.E.; Carney, M.E.; Terada, K.Y.; Goodman, M.T. Vitamin D receptor gene polymorphisms and epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Mostowska, A.; Sajdak, S.; Pawlik, P.; Lianeri, M.; Jagodzinski, P.P. Vitamin D receptor gene BsmI and FokI polymorphisms in relation to ovarian cancer risk in the Polish population. Genet. Test. Mol. Biomarkers 2013, 17, 183–187. [Google Scholar] [CrossRef]
- Muñoz-Galván, S.; Verdugo-Sivianes, E.M.; Santos-Pereira, J.M.; Estevez-García, P.; Carnero, A. Essential role of PLD2 in hypoxia-induced stemness and therapy resistance in ovarian tumors. J. Exp. Clin. Cancer Res. 2024, 43, 57. [Google Scholar] [CrossRef]
- Davenport, C.; Rai, N.; Sharma, P.; Deeks, J.J.; Berhane, S.; Mallett, S.; Saha, P.; Champaneria, R.; Bayliss, S.E.; Snell, K.I.; et al. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. Cochrane Database Syst. Rev. 2022, 7, CD011964. [Google Scholar] [PubMed]
- Fujimori, M.; Takahashi, T.; Furukawa, Y.; Takanashi, A.; Iizawa, Y.; Jimbo, M.; Soeda, S.; Fujimori, K.; Takeichi, K. Synchronous bilateral primary ovarian cancer with right endometroid carcinoma and left high-grade serous carcinoma: A case report and literature review. BMC Womens Health 2022, 22, 103. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615, Erratum in Nature 2012, 490, 298. [Google Scholar]
- Petru, E.; Huber, C.; Sampl, E.; Haas, J. Comparison of Primary Tumor Size in Stage I and III Epithelial Ovarian Cancer. Anticancer Res. 2018, 38, 6507–6511. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Rizvi, A.; Cui, T.; Han, C.; Banerjee, A.; Naseem, I.; Zheng, Y.; Wani, A.A.; Wang, Q.-E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget 2018, 9, 14481–14491. [Google Scholar] [CrossRef]
- Królewska-Daszczyńska, P.; Wendlocha, D.; Smycz-Kubańska, M.; Stępień, S.; Mielczarek-Palacz, A. Cancer stem cells markers in ovarian cancer: Clinical and therapeutic significance (Review). Oncol. Lett. 2022, 24, 465. [Google Scholar] [CrossRef]
- Muñoz-Galván, S.; Carnero, A. Targeting Cancer Stem Cells to Overcome Therapy Resistance in Ovarian Cancer. Cells 2020, 9, 1402. [Google Scholar] [CrossRef]
- Wan Kamarul Zaman, W.S.; Nurul, A.A.; Nordin, F. Stem cells and cancer stem cells: The Jekyll and Hyde scenario and their implications in stem cell therapy. Biomedicines 2021, 9, 1245. [Google Scholar] [CrossRef]
- Keyvani, V.; Farshchian, M.; Esmaeili, S.-A.; Yari, H.; Moghbeli, M.; Nezhad, S.-R.K.; Abbaszadegan, M.R. Ovarian cancer stem cells and targeted therapy. J. Ovarian Res. 2019, 12, 120. [Google Scholar] [CrossRef]
- Zhou, J.; Du, Y.; Lu, Y.; Luan, B.; Xu, C.; Yu, Y.; Zhao, H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients Through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, K.; Zheng, R.; Derwahl, M. 1,25 dihydroxyvitamin D3 inhibits the proliferation of thyroid cancer stem-like cells via cell cycle arrest. Endocr. Res. 2016, 41, 71–80. [Google Scholar] [CrossRef] [PubMed]
- So, J.Y.; Lee, H.J.; Smolarek, A.K.; Paul, S.; Wang, C.-X.; Maehr, H.; Uskokovic, M.; Zheng, X.; Conney, A.H.; Cai, L.; et al. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol. Pharmacol. 2011, 79, 360–367. [Google Scholar] [CrossRef]
- Silvagno, F.; De Vivo, E.; Attanasio, A.; Gallo, V.; Mazzucco, G.; Pescarmona, G. Mitochondrial Localization of Vitamin D Receptor in Human Platelets and Differentiated Megakaryocytes. PLoS ONE 2010, 5, e8670. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barral, A.; Bustamante-Madrid, P.; Ferrer-Mayorga, G.; Barbáchano, A.; Larriba, M.J.; Muñoz, A. Vitamin D Effects on Cell Differentiation and Stemness in Cancer. Cancers 2020, 12, 2413. [Google Scholar] [CrossRef]
- Hermansen, S.K.; Christensen, K.G.; Jensen, S.S.; Kristensen, B.W. Inconsistent immunohistochemical expression patterns of four different CD133 antibody clones in glioblastoma. J. Histochem. Cytochem. 2011, 59, 391–407. [Google Scholar] [CrossRef]
- Kazama, S.; Kishikawa, J.; Kiyomatsu, T.; Kawai, K.; Nozawa, H.; Ishihara, S.; Watanabe, T. Expression of the stem cell marker CD133 is related to tumor development in colorectal carcinogenesis. Asian J. Surg. 2018, 41, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar]
- Wu, S.; Tan, Y.; Li, F.; Han, Y.; Zhang, S.; Lin, X. CD44: A cancer stem cell marker and therapeutic target in leukemia treatment. Front. Immunol. 2024, 15, 1354992. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, K.H.; Song, J.Y.; Choi, S.J.; Kim, L.; Park, I.S.; Han, J.Y.; Kim, J.M.; Chu, Y.C. Construction of high-density tissue microarrays at low cost by using self-made manual microarray kits and recipient paraffin blocks. Korean J. Pathol. 2012, 46, 562–568. [Google Scholar] [CrossRef]
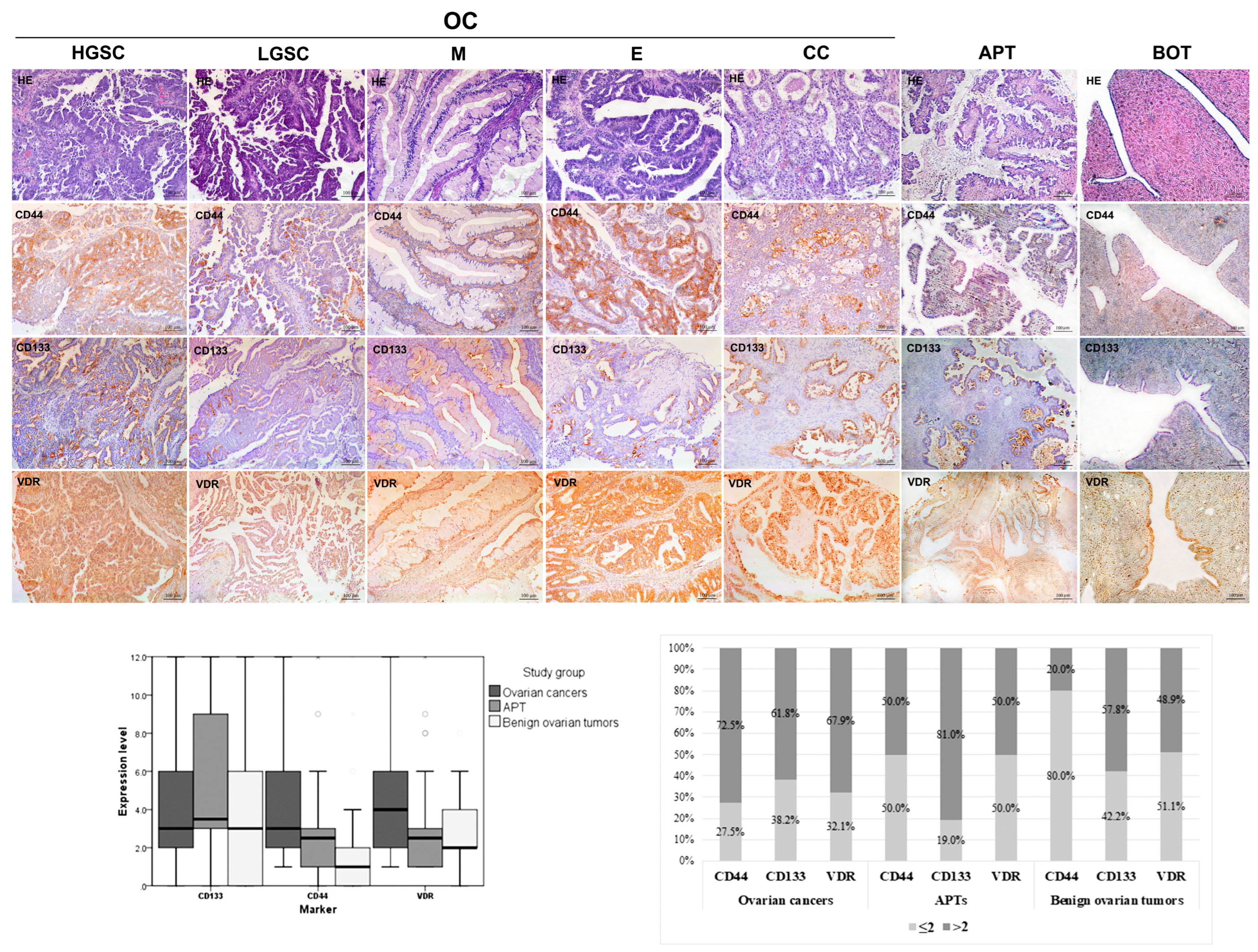

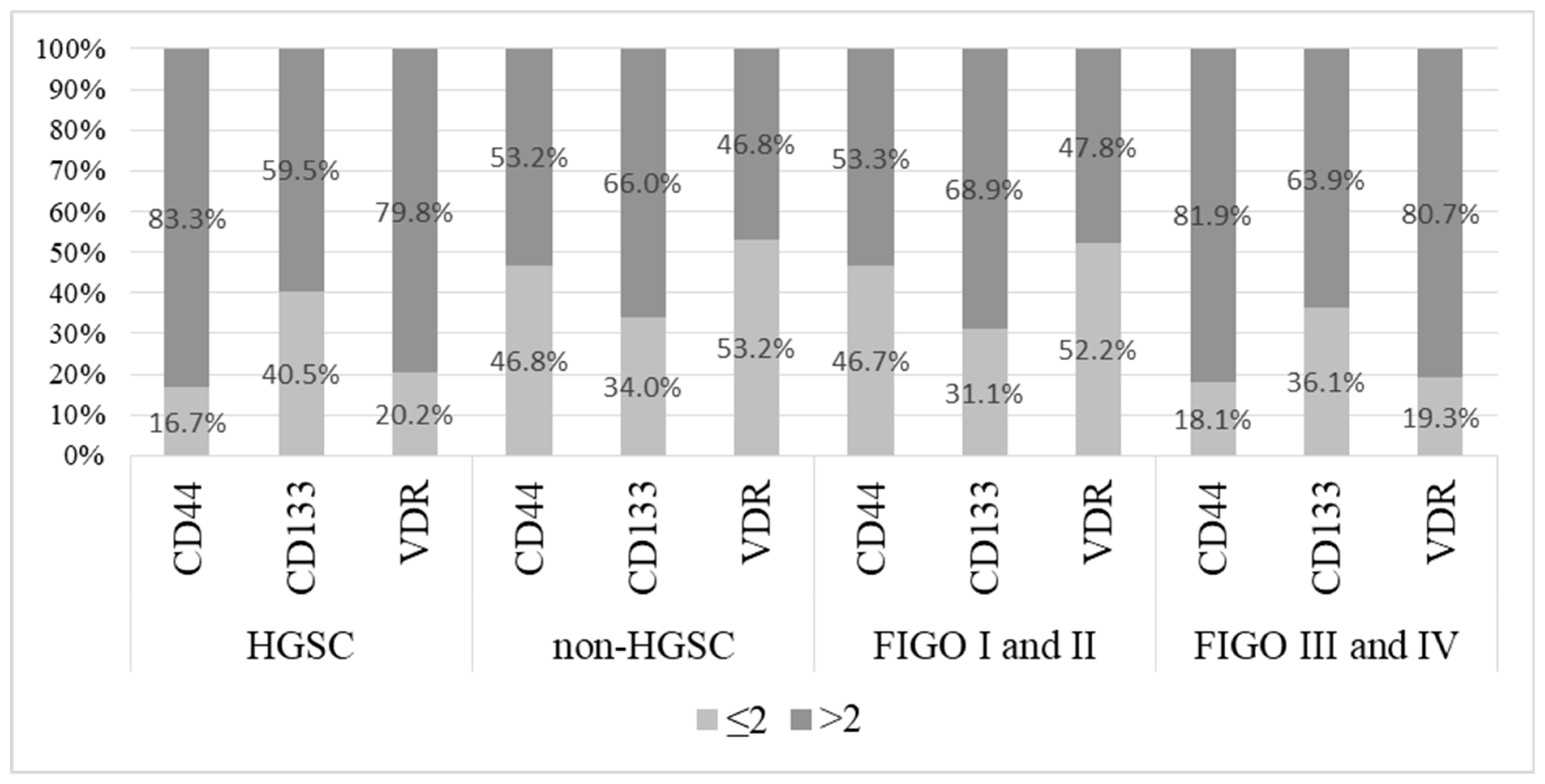
| Characteristics | OC n = 131 | APT n = 42 | BOT n = 45 | OC vs. APT | OC vs. BOT | APT vs. BOT |
|---|---|---|---|---|---|---|
| Woman | ||||||
| Age (years), Mean ± SD | 60.79 ± 10.14 | 45.76 ± 17.25 | 48.69 ± 15.52 | <0.001 | <0.001 | 0.545 |
| Menopause, n (%) | 110 (84.0) | 20 (47.6) | 23 (51.1) | <0.001 | <0.001 | 0.745 |
| Tumor | ||||||
| Laterality | ||||||
| Unilateral | 49 (37.4) | 36 (85.7) | 40 (88.9) | <0.001 | <0.001 | 0.656 |
| Bilateral | 82 (62.6) | 6 (14.3) | 5 (11.1) | |||
| Median diameter (mm), (min–max) | 80 (18–245) | 100 (30–310) | 65 (3–190) | 0.015 | 0.125 | 0.003 |
| Characteristics | Description n = 131 (%) |
|---|---|
| Histological type | |
| HGSC | 83 (63.4) |
| LGSC | 22 (16.8) |
| Mucinous | 7 (5.3) |
| Endometrioid | 8 (6.1) |
| Clear cell | 11 (8.4) |
| FIGO stage | |
| I | 59 (45.0) |
| II | 9 (6.9) |
| III | 62 (47.3) |
| IV | 1 (0.8) |
| Differentiation (Grade) | |
| I | 43 (32.8) |
| II | 9 (6.9) |
| III | 79 (60.3) |
| Other parameters | |
| Lymphovascular invasion (yes) | 84 (64.1) |
| Necrosis (yes) | 80 (61.1) |
| Intratumor lymphocytic infiltration (yes) | 85 (64.9) |
| Peritumor lymphocytic infiltration (yes) | 96 (73.3) |
| Variables | CD44 | CD133 | VDR | Grade | FIGO Stage |
|---|---|---|---|---|---|
| CD44 | 1 | 0.2978 *** | 0.6496 *** | 0.3930 *** | 0.4028 *** |
| CD133 | 0.2978 *** | 1 | 0.4127 *** | −0.08114 | −0.07031 |
| VDR | 0.6496 *** | 0.4127 *** | 1 | 0.4170 *** | 0.4331 *** |
| Grade | −0.08114 | 0.4170 *** | 1 | 0.3875 *** | |
| FIGO stage | 0.4028 *** | −0.07031 | 0.4331 *** | 0.3875 *** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, L.; Šošić-Jurjević, B.; Ćirković, A.; Dragičević, S.; Filipović, B.; Milenković, S.; Dugalić, S.; Gojnić-Dugalić, M.; Nikolić, A. Diagnostic Potential of CD44, CD133, and VDR in Epithelial Ovarian Tumors: Association with Histopathology Parameters. Int. J. Mol. Sci. 2025, 26, 3729. https://doi.org/10.3390/ijms26083729
Jovanović L, Šošić-Jurjević B, Ćirković A, Dragičević S, Filipović B, Milenković S, Dugalić S, Gojnić-Dugalić M, Nikolić A. Diagnostic Potential of CD44, CD133, and VDR in Epithelial Ovarian Tumors: Association with Histopathology Parameters. International Journal of Molecular Sciences. 2025; 26(8):3729. https://doi.org/10.3390/ijms26083729
Chicago/Turabian StyleJovanović, Ljubiša, Branka Šošić-Jurjević, Anđa Ćirković, Sandra Dragičević, Branko Filipović, Svetlana Milenković, Stefan Dugalić, Miroslava Gojnić-Dugalić, and Aleksandra Nikolić. 2025. "Diagnostic Potential of CD44, CD133, and VDR in Epithelial Ovarian Tumors: Association with Histopathology Parameters" International Journal of Molecular Sciences 26, no. 8: 3729. https://doi.org/10.3390/ijms26083729
APA StyleJovanović, L., Šošić-Jurjević, B., Ćirković, A., Dragičević, S., Filipović, B., Milenković, S., Dugalić, S., Gojnić-Dugalić, M., & Nikolić, A. (2025). Diagnostic Potential of CD44, CD133, and VDR in Epithelial Ovarian Tumors: Association with Histopathology Parameters. International Journal of Molecular Sciences, 26(8), 3729. https://doi.org/10.3390/ijms26083729








