Rainbow Trout (Oncorhynchus mykiss) Pre-Smolts Treated with 11-Deoxycorticosterone Regulate Liver Carbohydrate Metabolism and Gill Osmoregulation
Abstract
1. Introduction
2. Results
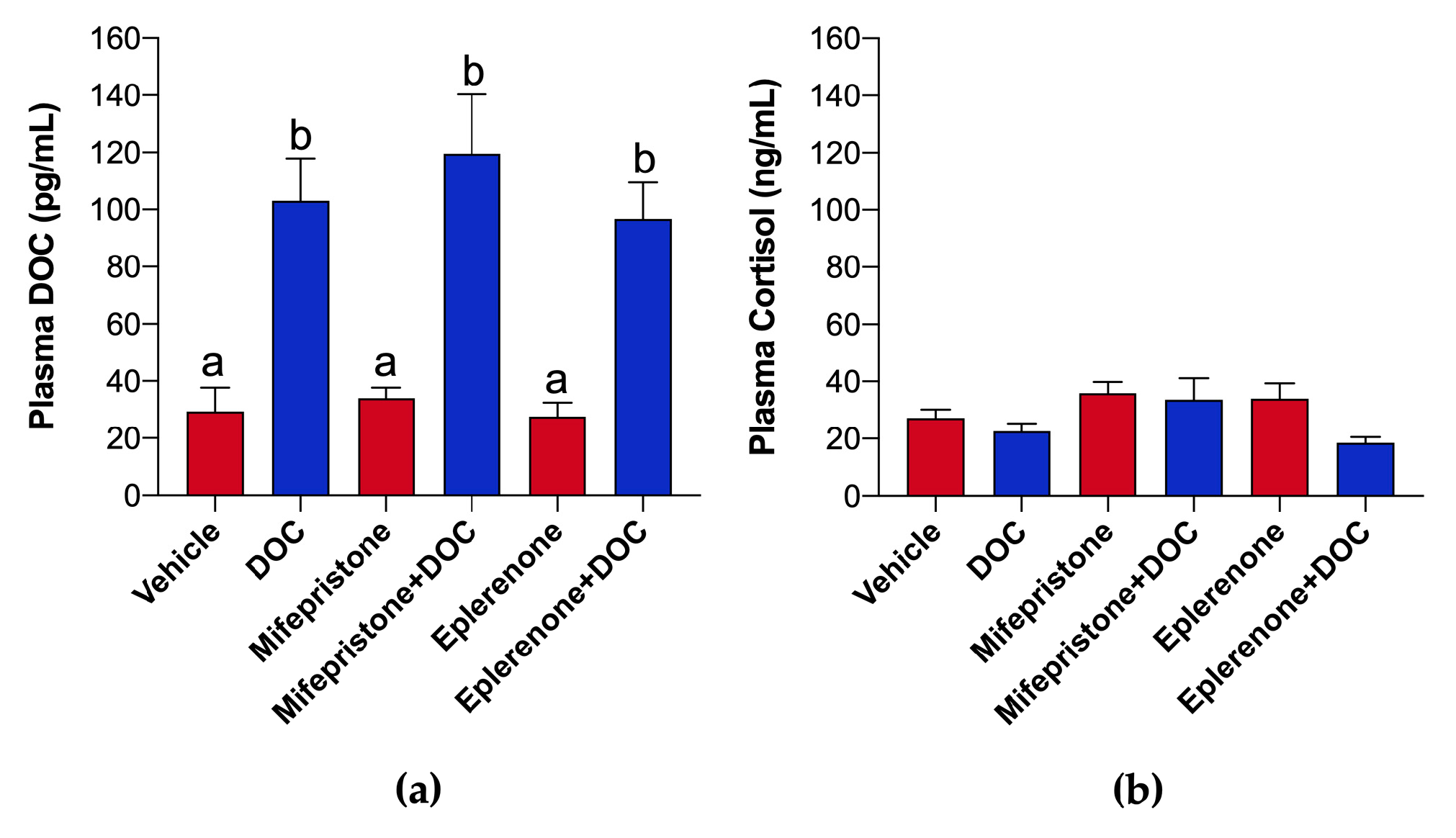
2.1. DOC and Cortisol in Plasma
2.2. Glucose, Lactzate, Pyruvate Plasma Levels, and Glycogen Content in Liver
2.3. Detection of Plasma Solutes and Muscle Water Content
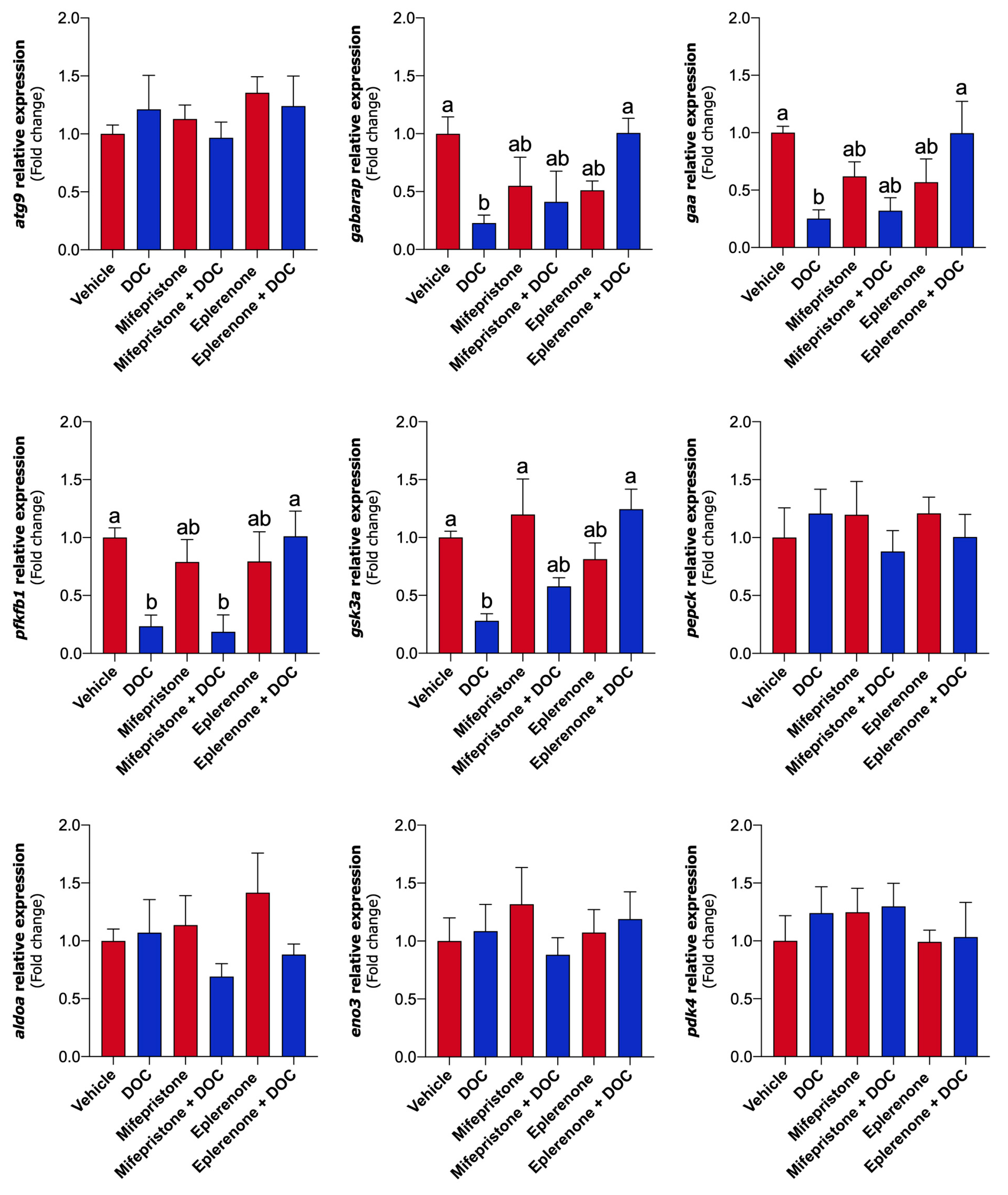
2.4. DOC-Induced Transcriptional Response Related to Carbohydrate Metabolism in the Liver
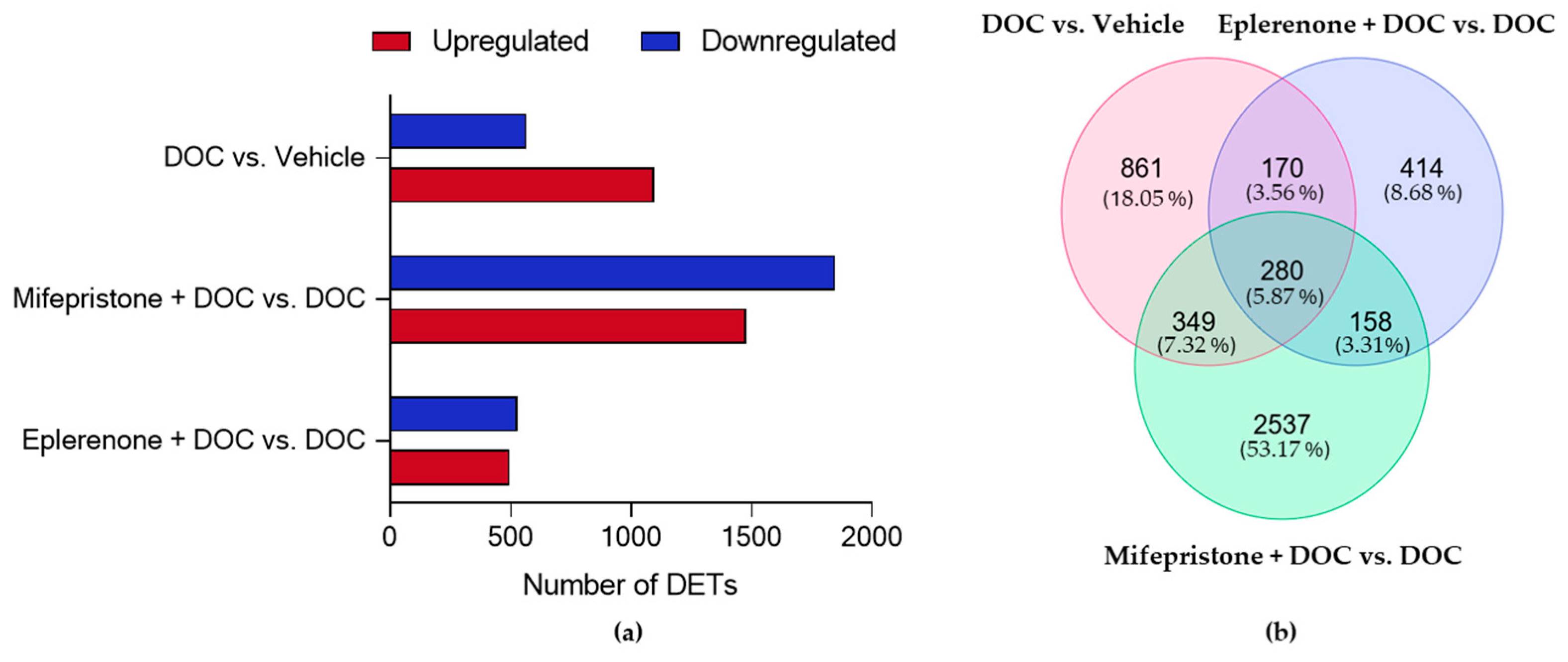
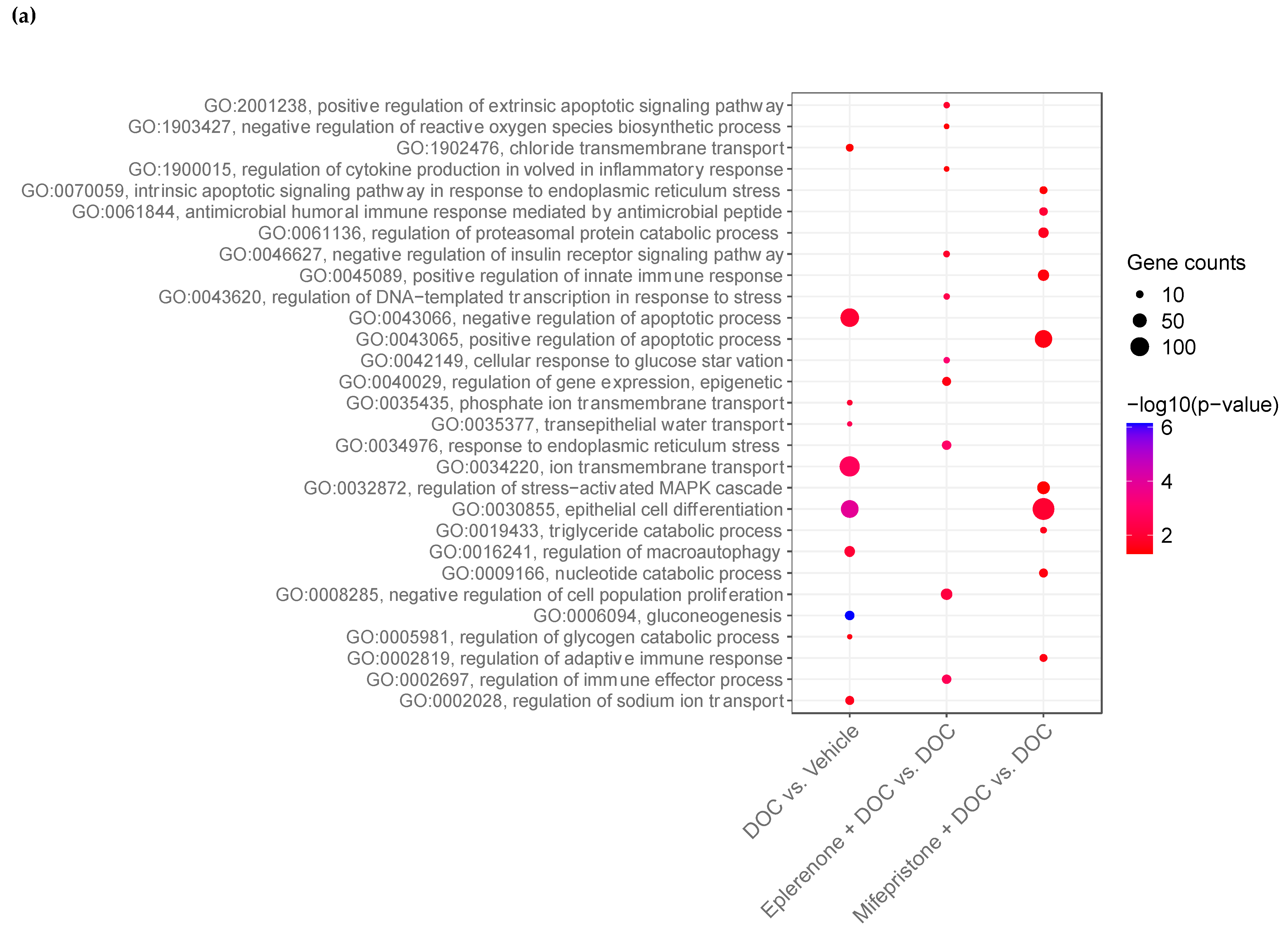
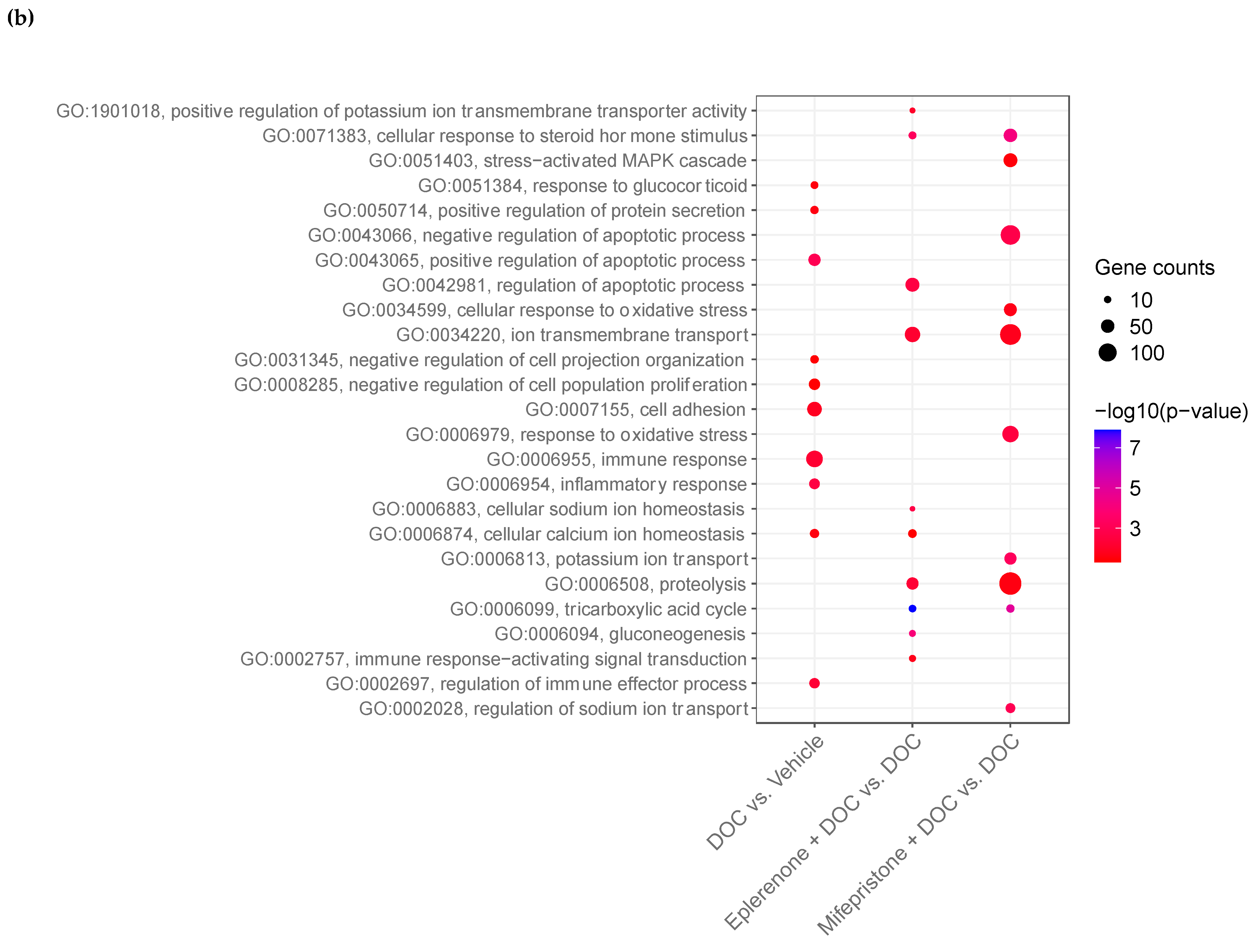
2.5. DOC Induces a Transcriptomic Response of the Rainbow Trout Gill Mediated by the Glucocorticoid Receptor (GR) and the Mineralocorticoid Receptor (MR)
3. Discussion
4. Materials and Methods
4.1. Experimental Protocol
4.2. Sampling Process
4.3. DOC and Cortisol Detection in Plasma
4.4. Measurement of Metabolites in Plasma
4.5. Quantification of Glycogen in Liver Tissue
4.6. Quantification of Solutes in Plasma
4.7. Detection of the Muscle Water Content
4.8. RNA Extraction
4.9. Real-Time PCR in Liver
4.10. Library Construction, RNA-Seq Analysis, and Functional Annotation Analysis in Gills
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOC | 11-deoxycorticosterone |
| MS | Mass spectrometry |
| ELISA | Enzyme-linked immunoadsorption assay |
| GR | Glucocorticoid receptor |
| MR | Mineralocorticoid receptor |
| DETs | Differentially expressed transcripts |
| SEM | Standard error of the mean |
| ANOVA | One-way analysis of variance |
| qPCR | Real-time PCR |
| GO | Gene ontology |
| RQN | RNA quality number |
| padj | p value adjust |
References
- Ceballos-Concha, A.; Asche, F.; Cárdenas-Retamal, R. Salmon Aquaculture in Chile: Production Growth and Socioeconomic Impacts. Rev. Aquac. 2025, 17, e12993. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar] [CrossRef]
- Myklatun, L.E.; Madaro, A.; Philip, A.J.P.; Pedersen, A.Ø.; Remø, S.; Hansen, T.J.; Fraser, T.W.K.; Sigholt, T.; Stefansson, S.; Fjelldal, P.G. Long term effects of smolt and post-smolt production strategy on mortality, growth, sexual maturation and melanized focal changes in farmed Atlantic salmon (Salmo salar L.). Aquac. 2025, 602, 742371. [Google Scholar] [CrossRef]
- Nisembaum, L.G.; Martin, P.; Lecomte, F.; Falcón, J. Melatonin and osmoregulation in fish: A focus on Atlantic salmon Salmo salar smoltification. J. Neuroendocrinol. 2021, 33, e12955. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Bordeleau, X.; Cooke, S.J.; Davidsen, J.G.; Eldøy, S.H.; Eliason, E.J.; Moore, A.; Aarestrup, K. Life-history strategies in salmonids: The role of physiology and its consequences. Biol. Rev. 2021, 96, 2304–2320. [Google Scholar] [CrossRef]
- Araujo, G.S.; Silva, J.W.A.; Cotas, J.; Pereira, L. Fish Farming Techniques: Current Situation and Trends. J. Mar. Sci. Eng. 2022, 10, 1598. [Google Scholar] [CrossRef]
- Valdivieso, A.; Caballero-Huertas, M.; Moraleda-Prados, J.; Piferrer, F.; Ribas, L. Exploring the Effects of Rearing Densities on Epigenetic Modifications in the Zebrafish Gonads. Int. J. Mol. Sci. 2023, 24, 16002. [Google Scholar] [CrossRef]
- van Muilekom, D.R.; Mueller, J.; Lindemeyer, J.; Schultheiß, T.; Maser, E.; Seibel, H.; Rebl, A.; Schulz, C.; Goldammer, T. Salinity change evokes stress and immune responses in Atlantic salmon with microalgae showing limited potential for dietary mitigation. Front. Physiol. 2024, 15, 1338858. [Google Scholar] [CrossRef]
- Murugananthkumar, R.; Sudhakumari, C.C. Understanding the impact of stress on teleostean reproduction. Aquac. Fish. 2022, 7, 553–561. [Google Scholar] [CrossRef]
- Culbert, B.M.; Regish, A.M.; Hall, D.J.; McCormick, S.D.; Bernier, N.J. Neuroendocrine Regulation of Plasma Cortisol Levels During Smoltification and Seawater Acclimation of Atlantic Salmon. Front. Endocrinol. 2022, 13, 859817. [Google Scholar] [CrossRef]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef]
- Trubitt, R.T.; Rabeneck, D.B.; Bujak, J.K.; Bossus, M.C.; Madsen, S.S.; Tipsmark, C.K. Transepithelial resistance and claudin expression in trout RTgill-W1 cell line: Effects of osmoregulatory hormones. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 182, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Hepatic Glucose Metabolism and Its Disorders in Fish. In Recent Advances in Animal Nutrition and Metabolism; Wu, G., Ed.; Springer: Cham, Switzerland, 2022; Volume 1354, pp. 207–236. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Chen, W.Z.; Hu, Y.C.; Gamage, L.; Lee, T.H.; Ho, C.W. Regulation of PGC-1α of the Mitochondrial Energy Metabolism Pathway in the Gills of Indian Medaka (Oryzias dancena) under Hypothermal Stress. Int. J. Mol. Sci. 2023, 24, 16187. [Google Scholar] [CrossRef] [PubMed]
- Arterbery, A.S.; Fergus, D.J.; Fogarty, E.A.; Mayberry, J.; Deitcher, D.L.; Kraus, W.L.; Bass, A.H. Evolution of ligand specificity in vertebrate corticosteroid receptors. BMC Evol. Biol. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Schaaf, M.J. Molecular mechanisms of the stress-induced regulation of the inflammatory response in fish. Gen. Comp. Endocr. 2024, 345, 114387. [Google Scholar] [CrossRef]
- Toso, A.; Garoche, C.; Balaguer, P. Human and fish differences in steroid receptors activation: A review. Sci. Total Environ. 2024, 948, 174889. [Google Scholar] [CrossRef]
- Sturm, A.; Bury, N.; Dengreville, L.; Fagart, J.; Flouriot, G.; Rafestin-Oblin, M.E.; Prunet, P. 11-deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 2005, 146, 47–55. [Google Scholar] [CrossRef]
- Schoen, A.N.; Weinrauch, A.M.; Bouyoucos, I.A.; Treberg, J.R.; Anderson, W.G. Hormonal effects on glucose and ketone metabolism in a perfused liver of an elasmobranch, the North Pacific spiny dogfish, Squalus suckleyi. Gen. Comp. Endocrinol. 2024, 352, 114514. [Google Scholar] [CrossRef]
- Baker, M.E.; Katsu, Y. Progesterone: An enigmatic ligand for the mineralocorticoid receptor. Biochem. Pharmacol. 2020, 177, 113976. [Google Scholar] [CrossRef]
- Milla, S.; Wang, N.; Mandiki, S.; Kestemont, P. Corticosteroids: Friends or Foes of Teleost Fish Reproduction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef]
- Kiilerich, P.; Servili, A.; Péron, S.; Valotaire, C.; Goardon, L.; Leguen, I.; Prunet, P. Regulation of the Corticosteroid Signalling System in Rainbow Trout HPI Axis during Confinement Stress. Gen. Comp. Endocrinol. 2018, 258, 184–193. [Google Scholar] [CrossRef]
- Zuloaga, R.; Ahumada-Langer, L.; Aedo, J.E.; Molina, A.; Valdés, J.A. 11-Deoxycorticosterone (DOC)’s Action on the Gill Osmoregulation of Juvenile Rainbow Trout (Oncorhynchus mykiss). Biology 2024, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Kiilerich, P.; Pedersen, S.H.; Kristiansen, K.; Madsen, S.S. Corticosteroid regulation of Na+, K+-ATPase α1-isoform expression in Atlantic salmon gill during smolt development. Gen. Comp. Endocrinol. 2011, 170, 283–289. [Google Scholar] [CrossRef]
- Milla, S.; Massart, S.; Mathieu, C.; Wang, N.; Douny, C.; Douxfils, J.; Scippo, M.L.; De Pauw, E.; Dieu, M.; Silvestre, F.; et al. Physiological and proteomic responses to corticosteroid treatments in Eurasian perch, Perca fluviatilis: Investigation of immune-related parameters. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, R.; Ahumada-Langer, L.; Aedo, J.E.; Molina, A.; Valdés, J.A. Early metabolic and transcriptomic regulation in rainbow trout (Oncorhynchus mykiss) liver by 11-deoxycorticosterone through two corticosteroid receptors pathways. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 298, 111746. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, R.; Aravena-Canales, D.; Aedo, J.E.; Osorio-Fuentealba, C.; Molina, A.; Valdés, J.A. Effect of 11-Deoxycorticosterone in the Transcriptomic Response to Stress in Rainbow Trout Skeletal Muscle. Genes 2023, 14, 512. [Google Scholar] [CrossRef]
- Kiilerich, P.; Geffroy, B.; Valotaire, C.; Prunet, P. Endogenous Regulation of 11-Deoxycorticosterone (DOC) and Corticosteroid Receptors (CRs) during Rainbow Trout Early Development and the Effects of Corticosteroids on Hatching. Gen. Comp. Endocrinol. 2018, 265, 22–30. [Google Scholar] [CrossRef]
- McCormick, S.D.; Regish, A.; O’dea, M.F.; Shrimpton, J.M. Are we missing a mineralocorticoid in teleost fish? Effects of cortisol, deoxycorticosterone and aldosterone on osmoregulation, gill Na+, K+-ATPase activity and isoform mRNA levels in Atlantic salmon. Gen. Comp. Endocrinol. 2008, 157, 35–40. [Google Scholar] [CrossRef]
- Kiilerich, P.; Tipsmark, C.K.; Borski, R.J.; Madsen, S.S. Differential effects of cortisol and 11-deoxycorticosterone on ion transport protein mRNA levels in gills of two euryhaline teleosts, Mozambique tilapia (Oreochromis mossambicus) and striped bass (Morone saxatilis). J. Endocrinol. 2011, 209, 115–126. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; McCormick, S.D. 11-Deoxycortisol is a stress responsive and gluconeogenic hormone in a jawless vertebrate, the sea lamprey (Petromyzon marinus). J. Exp. Biol. 2021, 224, jeb241943. [Google Scholar] [CrossRef]
- El Mohajer, L.; Chevalier, C.; Chardard, D.; Schaerlinger, B.; Fontaine, P.; Milla, S. Corticosteroid plasma kinetics and gonadal receptor gene expression during the reproductive cycle in female Eurasian Perch: Investigation of the roles of corticosteroids in vitellogenesis. Theriogenology 2023, 202, 61–73. [Google Scholar] [CrossRef]
- Pivonello, R.; Ferrigno, R.; De Martino, M.C.; Simeoli, C.; Di Paola, N.; Pivonello, C.; Barba, L.; Negri, M.; De Angelis, C.; Colao, A. Medical treatment of Cushing’s disease: An overview of the current and recent clinical trials. Front. Endocrinol. 2020, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lee, T.H.; Tseng, D.Y. Glucocorticoid Receptor Mediates Cortisol Regulation of Glycogen Metabolism in Gills of the Euryhaline Tilapia (Oreochromis mossambicus). Fishes 2023, 8, 267. [Google Scholar] [CrossRef]
- Aedo, J.; Aravena-Canales, D.; Ruiz-Jarabo, I.; Oyarzún, R.; Molina, A.; Martínez-Rodríguez, G.; Valdés, J.A.; Mancera, J.M. Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA. Animals 2021, 11, 3310. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, J.; Peña-Herrejón, G.A.; Aguirre-Becerra, H. Fish Responses to Alternative Feeding Ingredients under Abiotic Chronic Stress. Animals 2024, 14, 765. [Google Scholar] [CrossRef]
- Heden, T.D.; Chow, L.S.; Hughey, C.C.; Mashek, D.G. Regulation and role of glycophagy in skeletal muscle energy metabolism. Autophagy 2022, 18, 1078–1089. [Google Scholar] [CrossRef]
- Koutsifeli, P.; Varma, U.; Daniels, L.J.; Annandale, M.; Li, X.; Neale, J.P.; Hayes, S.; Weeks, K.; James, S.; Delbridge, L.M.; et al. Glycogen-autophagy: Molecular machinery and cellular mechanisms of glycophagy. J. Biol. Chem. 2022, 298, 102093. [Google Scholar] [CrossRef]
- Wu, L.X.; Xu, Y.C.; Pantopoulos, K.; Tan, X.Y.; Wei, X.L.; Zheng, H.; Luo, Z. Glycophagy mediated glucose-induced changes of hepatic glycogen metabolism via OGT1-AKT1-FOXO1Ser238 pathway. J. Nutr. Biochem. 2023, 117, 109337. [Google Scholar] [CrossRef]
- Mancini, M.C.; Noland, R.C.; Collier, J.J.; Burke, S.J.; Stadler, K.; Heden, T.D. Lysosomal glucose sensing and glycophagy in metabolism. Trends. Endocrinol. Metab. 2023, 34, 764–777. [Google Scholar] [CrossRef]
- Raposo de Magalhães, C.; Farinha, A.P.; Blackburn, G.; Whitfield, P.D.; Carrilho, R.; Schrama, D.; Cerqueira, M.; Rodrigues, P.M. Gilthead Seabream Liver Integrative Proteomics and Metabolomics Analysis Reveals Regulation by Different Prosurvival Pathways in the Metabolic Adaptation to Stress. Int. J. Mol. Sci. 2022, 23, 15395. [Google Scholar] [CrossRef]
- Zhou, Z.; He, Y.; Wang, S.; Wang, Y.; Shan, P.; Li, P. Autophagy regulation in teleost fish: A double-edged sword. Aquaculture 2022, 558, 738369. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Z.; Wang, D.; Xiao, J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta Biochim. Biophys. Sinica 2020, 52, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Zhang, X.; Ma, J.; Liu, Y.; Cui, L.; Wang, F. Glycolysis in Innate Immune Cells Contributes to Autoimmunity. Front. Immunol. 2022, 13, 920029. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. The mineralocorticoid receptor functions as a key glucose regulator in the skeletal muscle of zebrafish. Endocrinol. 2022, 163, bqac149. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, A.; Licciardello, G.; Fontana, C.M.; Tiso, N.; Argenton, F.; Dalla Valle, L. Glucocorticoid receptor activities in the zebrafish model: A review. J. Endocrinol. 2020, 247, R63–R82. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Jiang, X.; Hu, J.; Li, R.; Liu, Y.; Zhu, G.; Rong, X. Glucocorticoids induce osteoporosis mediated by glucocorticoid receptor-dependent and-independent pathways. Biomed. Pharmacother. 2020, 125, 109979. [Google Scholar] [CrossRef]
- Selvam, C.; Philip, A.J.P.; Lutfi, E.; Sigholt, T.; Norberg, B.; Bæverfjord, G.; Rosenlund, G.; Ruyter, B.; Sissener, N.H. Long-term feeding of Atlantic salmon with varying levels of dietary EPA + DHA alters the mineral status but does not affect the stress responses after mechanical delousing stress. Br. J. Nutr. 2022, 128, 2291–2307. [Google Scholar] [CrossRef]
- Hou, Z.S.; Wen, H.S.; Li, J.F.; He, F.; Li, Y.; Qi, X. Environmental hypoxia causes growth retardation, osteoclast differentiation and calcium dyshomeostasis in juvenile rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2020, 705, 135272. [Google Scholar] [CrossRef]
- Das, C.; Faught, E.; Vijayan, M.M. Cortisol rapidly stimulates calcium waves in the developing trunk muscle of zebrafish. Mol. Cell. Endocrinol. 2021, 520, 111067. [Google Scholar] [CrossRef]
- Das, C.; Rout, M.K.; Wildering, W.C.; Vijayan, M.M. Cortisol modulates calcium release-activated calcium channel gating in fish hepatocytes. Sci. Rep. 2021, 11, 9621. [Google Scholar] [CrossRef]
- Michea, L.; Delpiano, A.M.; Hitschfeld, C.; Lobos, L.; Lavandero, S.; Marusic, E.T. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinol. 2005, 146, 973–980. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kozaka, T.; Takahashi, A.; Kawauchi, H.; Ando, M. Medaka (Oryzias latipes) as a model for hypoosmoregulation of euryhaline fishes. Aquaculture 2001, 193, 347–354. [Google Scholar] [CrossRef]
- McCormick, S.D.; Taylor, M.L.; Regish, A.M. Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J. Exp. Biol. 2020, 223, jeb220251. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Milla, S.; Mandiki, S.N.M.; Douxfils, J.; Douny, C.; Scippo, M.L.; De Pauw, E.; Kestemont, P. First evidence of the possible implication of the 11- deoxycorticosterone (DOC) in immune activity of Eurasian perch (Perca fluviatilis, L.): Comparison with cortisol. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Milla, S.; Mandiki, S.N.M.; Douxfils, J.; Kestemont, P. In vivo response of some immune and endocrine variables to LPS in Eurasian perch (Perca fluviatilis, L.) and modulation of this response by two corticosteroids, cortisol and 11-deoxycorticosterone. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 25–34. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yoshiki, M.; Takahashi, H.; Yoshida, M.; Ogino, Y.; Ikeuchi, T.; Nakamachi, T.; Konno, N.; Matsuda, K.; Sakamoto, H. Principal function of mineralocorticoid signaling suggested by constitutive knockout of the mineralocorticoid receptor in medaka fish. Sci. Rep. 2016, 29, 37991. [Google Scholar] [CrossRef]
- Bury, N.R.; Sturm, A.; Le Rouzic, P.; Lethimonier, C.; Ducouret, B.; Guiguen, Y.; Robinson-Rechavi, M.; Laudet, V.; Rafestin-Oblin, M.E.; Prunet, P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003, 31, 141–156. [Google Scholar] [CrossRef]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of hormone-receptor complexity by molecular exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hyodo, S.; Takagi, W.A. A possible principal function of corticosteroid signaling that is conserved in vertebrate evolution: Lessons from receptor-knockout small fish. J. Steroid Biochem. Mol. Biol. 2018, 184, 57–61. [Google Scholar] [CrossRef]
- Pan, J.; Chen, L.; Ji, Y.; Huang, Y.; Bu, X.; Zhu, J.; Li, E.; Qin, J.; Wang, X. A crucial role in osmoregulation against hyperosmotic stress: Carbohydrate and inositol metabolism in Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2023, 28, 101433. [Google Scholar] [CrossRef]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Seale, A.P.; Breves, J.P. Endocrine and osmoregulatory responses to tidally-changing salinities in fishes. Gen. Comp. Endocrinol. 2022, 326, 114071. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.P.; Cao, K.; Chang, R.J.; Goodearly, T.R.; Malintha, G.H.T.; Merlo, R.S.; Peterson, T.L.; Reighard, J.R. Salinity tolerance of fishes: Experimental approaches and implications for aquaculture production. Rev. Aquac. 2024, 16, 1351–1373. [Google Scholar] [CrossRef]
- Nemova, N.N.; Kaivarainen, E.I.; Rendakov, N.L.; Nikerova, K.M.; Efremov, D.A. Cortisol content and Na+/K+-ATPase activity under adaptation of juvenile pink salmon Oncorhynchus gorbuscha (Salmonidae) to salinity changes. J. Ichthyol. 2021, 61, 771–778. [Google Scholar] [CrossRef]
- Fridman, S. Ontogeny of the Osmoregulatory Capacity of Teleosts and the Role of Ionocytes. Front. Mar. Sci. 2020, 7, 709. [Google Scholar] [CrossRef]
- Bonzi, L.C.; Monroe, A.A.; Lehmann, R.; Berumen, M.L.; Ravasi, T.; Schunter, C. The time course of molecular acclimation to seawater in a euryhaline fish. Sci. Rep. 2021, 11, 18127. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; Breves, J.P. Molecular mechanisms of Cl− transport in fishes: New insights and their evolutionary context. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 207–216. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Váldes, J.A.; Gallardo-Escárate, C. Transcriptome Profiling of Long Non-coding RNAs During the Atlantic Salmon Smoltification Process. Mar. Biotechnol. 2021, 23, 308–320. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Yan, J.J.; Furukawa, F.; Hwang, P.P. Did acidic stress resistance in vertebrates evolve as Na+/H+ exchanger-mediated ammonia excretion in fish? BioEssays 2020, 42, 1900161. [Google Scholar] [CrossRef]
- Shih, S.W.; Yan, J.J.; Chou, M.Y.; Hwang, P.P. Recent progress and debates in molecular physiology of Na+ uptake in teleosts. Front. Mar. Sci. 2023, 10, 1066929. [Google Scholar] [CrossRef]
- Palmgren, M. Evolution of the sodium pump. Biochim. Biophys. Acta. Mol. Cell. Res. 2023, 1870, 119511. [Google Scholar] [CrossRef] [PubMed]
- Barlow, I.L.; Mackay, E.; Wheater, E.; Goel, A.; Lim, S.; Zimmerman, S.; Woods, I.; Prober, D.A.; Rihel, J. The zebrafish mutant dreammist implicates sodium homeostasis in sleep regulation. Elife 2023, 12, RP87521. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yu, S.E.; Sun, Y.X.; Zhang, L.; Tan, Y.; Zhang, Y.Y.; Wang, S.; Zhou, Y.G.; Hu, L.S.; Dong, Y.W. Genome-wide identification and quantification of salinity-responsive Na+/K+-ATPase α-subunits in three salmonids. Aquaculture 2024, 582, 740514. [Google Scholar] [CrossRef]
- Kiilerich, P.; Milla, S.; Sturm, A.; Valotaire, C.; Chevolleau, S.; Giton, F.; Terrien, X.; Fiet, J.; Fostier, A.; Debrauwer, L.; et al. Implication of the mineralocorticoid axis in rainbow trout osmoregulation during salinity acclimation. J. Endocrinol. 2011, 209, 221–235. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakamoto, T. The role of “mineralocorticoids” in teleost fish: Relative importance of glucocorticoid signaling in the osmoregulation and “central” actions of mineralocorticoid receptor. Gen. Comp. Endocrinol. 2013, 181, 223–228. [Google Scholar] [CrossRef]
- Aedo, J.E.; Zuloaga, R.; Aravena-Canales, D.; Molina, A.; Valdés, J.A. Role of glucocorticoid and mineralocorticoid receptors in rainbow trout (Oncorhynchus mykiss) skeletal muscle: A transcriptomic perspective of cortisol action. Front. Physiol. 2023, 13, 1048008. [Google Scholar] [CrossRef]
- Faught, E.; Schaaf, M.J.M. The mineralocorticoid receptor plays a crucial role in macrophage development and function. Endocrinol. 2023, 164, bqad127. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lee, T.H.; Tseng, D.Y. Mineralocorticoid receptor mediates cortisol regulation of ionocyte development in tilapia (Oreochromis mossambicus). Fishes 2023, 8, 283. [Google Scholar] [CrossRef]
- Reyes-Contreras, M.; Glauser, G.; Rennison, D.J.; Taborsky, B. Early-life manipulation of cortisol and its receptor alters stress axis programming and social competence. Philos. Trans. R. Soc. B 2019, 374, 20180119. [Google Scholar] [CrossRef]
- Gao, R.; Yan, H.; Zhou, H.; Hu, M.; Ding, Y.; Shen, X.; Wang, J.; Wang, C.; Wang, L.; Jiang, C.; et al. Administration of mifepristone can induce masculinization and alter the expression of sex-related genes in Takifugu rubripes. Aquac. Rep. 2024, 36, 102172. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Ponce, C.; Zuloaga, R.; González, P.; Avendaño-Herrera, R.; Valdés, J.A.; Molina, A. Effects of crowding on the three main proteolytic mechanisms of skeletal muscle in rainbow trout (Oncorhynchus mykiss). BMC Vet. Res. 2020, 16, 294. [Google Scholar] [CrossRef] [PubMed]
- Dettleff, P.; Fuentes, M.; González, P.; Aedo, J.; Zuloaga, R.; Estrada, J.M.; Molina, A.; Valdes, J.A. Generating transcriptomic resources in the teleost fish black cusk-eel (Genypterus maculatus) to evaluate thermal stress in the liver under a climate change scenario. Fish Physiol. Biochem. 2025, 51, 75. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Navarro, C.; Elorriaga-Verplancken, F.R.; Galván-Magaña, F.; Valdivia-Anda, G.; Peña, R.; Ketchum, J.T.; Hoyos-Padilla, E.M. Stress biomarkers in the Giant Manta Mobula birostris associated to tourism in the Revillagigedo National Park, Mexico. Open J. Vet. Med. 2023, 13, 136–146. [Google Scholar] [CrossRef]
- Farag, M.R.; Abo-Al-Ela, H.G.; Alagawany, M.; Azzam, M.M.; El-Saadony, M.T.; Rea, S.; Di Cerbo, A.; Nouh, D.S. Effect of quercetin nanoparticles on hepatic and intestinal enzymes and stress-related genes in Nile tilapia fish exposed to silver nanoparticles. Biomed. 2023, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, F.D.; Kültz, D. Direct ionic regulation of the activity of myo-inositol biosynthesis enzymes in Mozambique tilapia. PLoS ONE 2015, 10, e0123212. [Google Scholar] [CrossRef]
- Srivastav, S.; Mishra, D.; Srivastav, S.K.; Suzuki, N.; Srivastav, A.K. Estradiol Affects Prolactin Producing Cells and Calcium levels in a Teleost, Heteropneustesfossilis, Kept in Different Calcium Concentrations. Iran. J. Toxicol. 2017, 11, 45–51. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Moraes, F.R.D.; Imoto, M.E. Hematological parameters in two neotropical freshwater teleost, Leporinus macrocephalus (Anostomidae) and Prochilodus lineatus (Prochilodontidae). Biosci. J. Uberlândia 2008, 24, 96–101. [Google Scholar]
- Al-Jandal, N.J.; Wilson, R.W. A comparison of osmoregulatory responses in plasma and tissues of rainbow trout (Oncorhynchus mykiss) following acute salinity challenges. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 175–181. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J. Gene set enrichment analysis with topGO. Bioconduct. Improv. 2009, 27, 776. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; p. 260. [Google Scholar]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuloaga, R.; Ahumada-Langer, L.; Aedo, J.E.; Llanos-Azócar, K.; Molina, A.; Valdés, J.A. Rainbow Trout (Oncorhynchus mykiss) Pre-Smolts Treated with 11-Deoxycorticosterone Regulate Liver Carbohydrate Metabolism and Gill Osmoregulation. Int. J. Mol. Sci. 2025, 26, 3725. https://doi.org/10.3390/ijms26083725
Zuloaga R, Ahumada-Langer L, Aedo JE, Llanos-Azócar K, Molina A, Valdés JA. Rainbow Trout (Oncorhynchus mykiss) Pre-Smolts Treated with 11-Deoxycorticosterone Regulate Liver Carbohydrate Metabolism and Gill Osmoregulation. International Journal of Molecular Sciences. 2025; 26(8):3725. https://doi.org/10.3390/ijms26083725
Chicago/Turabian StyleZuloaga, Rodrigo, Luciano Ahumada-Langer, Jorge Eduardo Aedo, Katalina Llanos-Azócar, Alfredo Molina, and Juan Antonio Valdés. 2025. "Rainbow Trout (Oncorhynchus mykiss) Pre-Smolts Treated with 11-Deoxycorticosterone Regulate Liver Carbohydrate Metabolism and Gill Osmoregulation" International Journal of Molecular Sciences 26, no. 8: 3725. https://doi.org/10.3390/ijms26083725
APA StyleZuloaga, R., Ahumada-Langer, L., Aedo, J. E., Llanos-Azócar, K., Molina, A., & Valdés, J. A. (2025). Rainbow Trout (Oncorhynchus mykiss) Pre-Smolts Treated with 11-Deoxycorticosterone Regulate Liver Carbohydrate Metabolism and Gill Osmoregulation. International Journal of Molecular Sciences, 26(8), 3725. https://doi.org/10.3390/ijms26083725






