Natriuretic Peptides and Soluble ST2 Improve Echocardiographic and Invasive Long-Term Survival Prediction in Patients Evaluated for Diastolic Dysfunction
Abstract
1. Introduction
2. Results
3. Discussion
Study Strengths and Limitations
4. Materials and Methods
4.1. Biomarker Quantification
4.2. Left Ventricular End-Diastolic Pressure Measurement
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; McKelvie, R.; Don-Wauchope, A.; Santaguida, P.L.; Ali, U.; Balion, C.; Hill, S.; Booth, R.; Brown, J.A.; Bustamam, A.; et al. A systematic review of BNP and NT-proBNP in the management of heart failure: Overview and methods. Heart Fail. Rev. 2014, 19, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Biomarkers in Heart Failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Januzzi, J.L. Soluble ST2 in Heart Failure. Heart Fail. Clin. 2018, 14, 41–48. [Google Scholar] [CrossRef]
- Shah, R.V.; Januzzi, J.L. ST2: A Novel Remodeling Biomarker in Acute and Chronic Heart Failure. Curr. Heart Fail. Rep. 2010, 7, 9–14. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Pál, K.; Mănescu, I.-B.; Lupu, S.; Dobreanu, M. Emerging Biomarkers for Predicting Clinical Outcomes in Patients with Heart Disease. Life 2023, 13, 230. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Pal, K.; Vacariu, V.; Drincal, R.-K.; Ion, A.A.; Adorján, I.; Oltean, T.; Hadadi, L. Addition of eptifibatide and manual thrombus aspiration to ticagrelor does not improve long-term survival after STEMI treated with primary PCI. Front. Pharmacol. 2024, 15, 1415025. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Lupu, S.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Stan, A.; Scurtu, A.-C.; Aniței, D.; Harpa, M.; Brînzaniuc, K. Natriuretic peptides and soluble ST2 improves echocardiographic diagnosis of elevated left ventricular filling pressures. Sci. Rep. 2024, 14, 22171. [Google Scholar] [CrossRef]
- Bansal, M.; Marwick, T.H. Natriuretic Peptides and Filling Pressure at Rest and Stress. Heart Fail. Clin. 2008, 4, 71–86. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Zhang, Y.; Ky, B. ST2 and patient prognosis in chronic heart failure. Am. J. Cardiol. 2015, 115, 64B–69B. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cui, X.; Xu, Y.; Wang, Y.; Dong, Y.; Liao, Y.; Li, W.; Li, X.; Yang, J.; Zhou, J.; et al. The effects of angiotensin receptor-neprilysin inhibitors on clinical outcomes in heart failure with mildly reduced or preserved ejection fraction: Real-world evidence from the CCA Database-HF Center. Eur. Heart J. 2024, 45, ehae666.1089. [Google Scholar] [CrossRef]
- Dong, G.; Chen, H.; Zhang, H.; Gu, Y. Long-Term and Short-Term Prognostic Value of Circulating Soluble Suppression of Tumorigenicity-2 Concentration in Chronic Heart Failure: A Systematic Review and Meta-Analysis. Cardiology 2021, 146, 433–440. [Google Scholar] [CrossRef]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.W.; Wu, A.H.B.; Fang, J.C.; et al. High-Sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure. Circ. Heart Fail. 2011, 4, 180–187. [Google Scholar] [CrossRef]
- Lupón, J.; de Antonio, M.; Galán, A.; Vila, J.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Combined Use of the Novel Biomarkers High-Sensitivity Troponin T and ST2 for Heart Failure Risk Stratification vs Conventional Assessment. Mayo Clin. Proc. 2013, 88, 234–243. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT−proBNP and High-Sensitivity Troponin T. JACC 2018, 72, 2309–2320. [Google Scholar] [CrossRef]
- Thanikachalam, P.V.; Ramamurthy, S.; Mallapu, P.; Varma, S.R.; Narayanan, J.; Abourehab, M.A.; Kesharwani, P. Modulation of IL-33/ST2 signaling as a potential new therapeutic target for cardiovascular diseases. Cytokine Growth Factor Rev. 2023, 71–72, 94–104. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Ramadan, A.M.; Daguindau, E.; Rech, J.C.; Chinnaswamy, K.; Zhang, J.; Hura, G.L.; Griesenauer, B.; Bolten, Z.; Robida, A.; Larsen, M.; et al. From proteomics to discovery of first-in-class ST2 inhibitors active in vivo. JCI Insight 2018, 3, e99208. [Google Scholar] [CrossRef]
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.V.; Shi, V.; Lefkowitz, M.; et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients with HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Barallat, J.; Zamora, E.; Urrutia, A.; Lupón, J. Head-to-Head Comparison of 2 Myocardial Fibrosis Biomarkers for Long-Term Heart Failure Risk Stratification. J. Am. Coll. Cardiol. 2014, 63, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.B.; Wians, F.; Jaffe, A. Biological variation of galectin-3 and soluble ST2 for chronic heart failure: Implication on interpretation of test results. Am. Heart J. 2013, 165, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Călburean, P.-A.; Pannone, L.; Sorgente, A.; Gauthey, A.; Monaco, C.; Strazdas, A.; Almorad, A.; Bisignani, A.; Bala, G.; Ramak, R.; et al. Heart rate variability and microvolt T wave alternans changes during ajmaline test may predict prognosis in Brugada syndrome. Clin. Auton. Res. 2023, 33, 51–62. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E. Prediction models need appropriate internal, internal-external, and external validation. J. Clin. Epidemiol. 2016, 69, 245–247. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr. Evaluating Discrimination of Risk Prediction Models: The C Statistic. JAMA 2015, 314, 1063–1064. [Google Scholar] [CrossRef]


| Parameter | 1-Year Survival | Long-Term Survival | |||
|---|---|---|---|---|---|
| Alive (n = 100) | Deceased (n = 10) | p * | HR | p ** | |
| Male sex | 75 (75%) | 8 (80%) | 1.00 | 1.01 | 0.98 |
| Age (years) | 65.9 (56.0–71.7) | 59.4 ± 18.3 | 0.52 | 0.99 | 0.68 |
| BMI (kg/m2) | 29.05 ± 4.51 | 27.36 ± 3.61 | 0.25 | 0.92 | 0.17 |
| Arterial hypertension | 70 (70%) | 3 (30%) | 0.01 | 0.43 | 0.09 |
| Hyperlipidemia | 76 (76%) | 5 (50%) | 0.13 | 0.56 | 0.26 |
| Diabetes mellitus | 31 (31%) | 4 (40%) | 0.71 | 2.10 | 0.14 |
| Peripheral artery disease | 19 (19%) | 1 (10%) | 0.67 | 0.59 | 0.49 |
| Stroke | 9 (9%) | 0 (0%) | 0.60 | 0.63 | 0.66 |
| COPD | 6 (6%) | 1 (10%) | 1.00 | 0.9 | 0.92 |
| Chronic kidney disease | 5 (5%) | 3 (30%) | 0.02 | 3.74 | 0.03 |
| Atrial fibrillation | 18 (18%) | 5 (50%) | 0.03 | 2.45 | 0.08 |
| Coronary artery disease | 68 (68%) | 5 (50%) | 0.31 | 0.44 | 0.10 |
| History of PCI/CABG | 39 (39%) | 4 (40%) | 1.00 | 0.91 | 0.86 |
| History of MI | 27 (27%) | 2 (20%) | 0.72 | 1.30 | 0.62 |
| Valvular heart disease | 12 (12%) | 8 (80%) | 0.0005 | 8.25 | 0.0001 |
| Dilated cardiomyopathy | 21 (21%) | 5 (50%) | 0.04 | 2.21 | 0.12 |
| Concentric hypertrophy | 35 (35%) | 3 (30%) | 1.00 | 0.86 | 0.78 |
| QRS duration (ms) | 85 (80–100) | 121.4 ± 41.12 | 0.03 | 1.02 | 0.01 |
| Creatinine clearance | 105.19 ± 30.77 | 91.47 ± 38.6 | 0.19 | 0.99 | 0.19 |
| LVEF ≤ 40 | 22 (22%) | 5 (50%) | 0.06 | 2.04 | 0.17 |
| LVEF = 41–49% | 15 (15%) | 1 (10%) | 1.00 | 0.85 | 0.82 |
| LVEF ≥ 50% | 63 (63%) | 4 (40%) | 0.20 | 0.59 | 0.29 |
| Beta-blocker | 78 (78%) | 8 (80%) | 1.00 | 1.13 | 0.84 |
| ACEI/ARB | 73 (73%) | 3 (30%) | 0.009 | 0.44 | 0.11 |
| MRA | 33 (33%) | 8 (80%) | 0.007 | 3.37 | 0.01 |
| ARNI | 16 (16%) | 3 (30%) | 0.38 | 1.26 | 0.71 |
| SGLT2 inhibitors | 20 (20%) | 5 (50%) | 0.04 | 2.53 | 0.07 |
| Loop diuretics | 31 (31%) | 9 (90%) | 0.001 | 6.44 | 0.001 |
| Parameter | 1-Year Survival | Long-Term Survival | |||
|---|---|---|---|---|---|
| Alive (n = 100) | Deceased (n = 10) | p * | HR | p ** | |
| Serum biomarkers | |||||
| MR-proANP (pg/mL) | 138 (98–193) | 229.2 ± 159.4 | 0.09 | 1.00 | 0.13 |
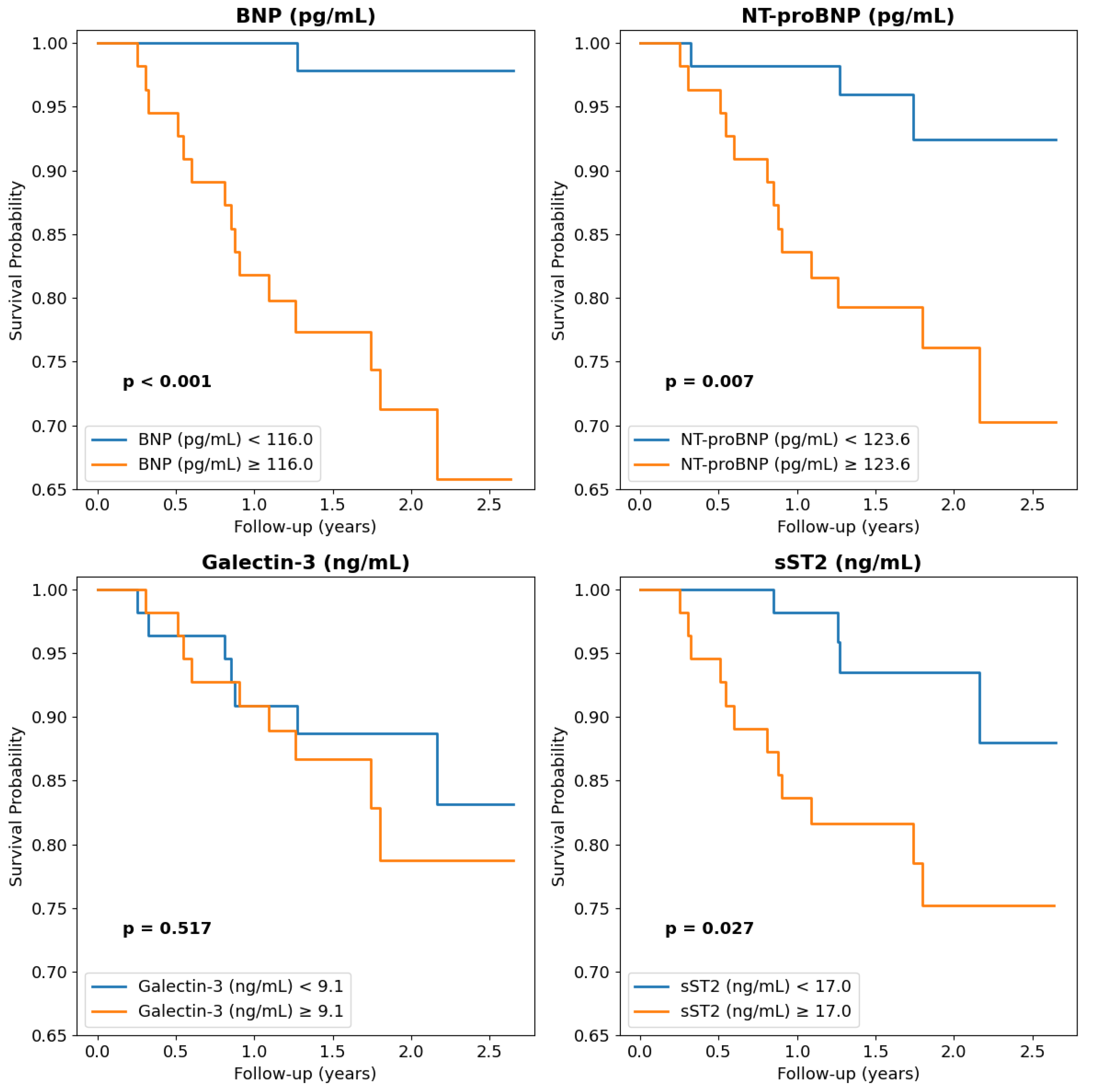
| BNP (pg/mL) | 106 (55–240) | 464 (448–531) | 0.0001 | 1.02 | 0.0001 |
| NT-proBNP (pg/mL) | 104 (29–341) | 2230 ± 1510 | 0.00002 | 1.02 | 0.0001 |
| sST2 (ng/mL) | 16.3 (12.7–21.4) | 42.3 ± 27.3 | 0.0007 | 1.06 | 0.0001 |
| Galectin-3 (ng/mL) | 9.0 (7.1–11.1) | 12.8 ± 8.7 | 0.25 | 1.13 | 0.009 |
| MR-proADM (pg/mL) | 216 (126–575) | 188 (121–234) | 0.33 | 1.00 | 0.51 |
| Echocardiographic parameters | |||||
| LVEF (%) | 50 (41–55) | 35 ± 16 | 0.02 | 0.97 | 0.09 |
| Average GLS (%) | −16.4 (−17.9–−11.3) | −9.0 ± 4.6 | 0.002 | 1.14 | 0.007 |
| LVEDV (mL/m2) | 53.9 (46.6–66.9) | 80.0 ± 34.7 | 0.07 | 1.01 | 0.36 |
| LVESV (mL/m2) | 26.2 (20.9–42.4) | 63.4(25.5–91.8) | 0.02 | 1.01 | 0.004 |
| Stroke volume (mL/m2) | 26.6 (22.7–31.7) | 31.2 ± 10.3 | 0.33 | 1.04 | 0.27 |
| LA volume (mL/m2) | 35.6 (29.7–43.7) | 59.6 ± 17.2 | 0.0004 | 1.05 | 0.0001 |
| LARS (%) | 14.9 (10.6–18.3) | 7.9 ± 4.0 | 0.0004 | 0.89 | 0.008 |
| RA volume (mL/m2) | 25.5 (21.5–31.6) | 40.4 ± 13.2 | 0.01 | 1.04 | 0.002 |
| MV E/A ratio | 8.65 (7.49–10.5) | 14.77 ± 5.15 | 0.0004 | 1.59 | 0.005 |
| MV DT (msec) | 213.88 ± 52.36 | 201.61 ± 48.34 | 0.47 | 0.99 | 0.27 |
| Mitral average E/e’ | 0.85 (0.74–1.45) | 1.94 ± 0.84 | 0.007 | 1.30 | 0.0001 |
| Diastolic dysfunction | 20 (20%) | 8 (80%) | 0.0005 | 5.57 | 0.0009 |
| Diastolic dysfunction grade | 1 (0–1) | 2 (2–3) | 0.0003 | 2.21 | 0.0008 |
| Left ventricular catheterization parameters | |||||
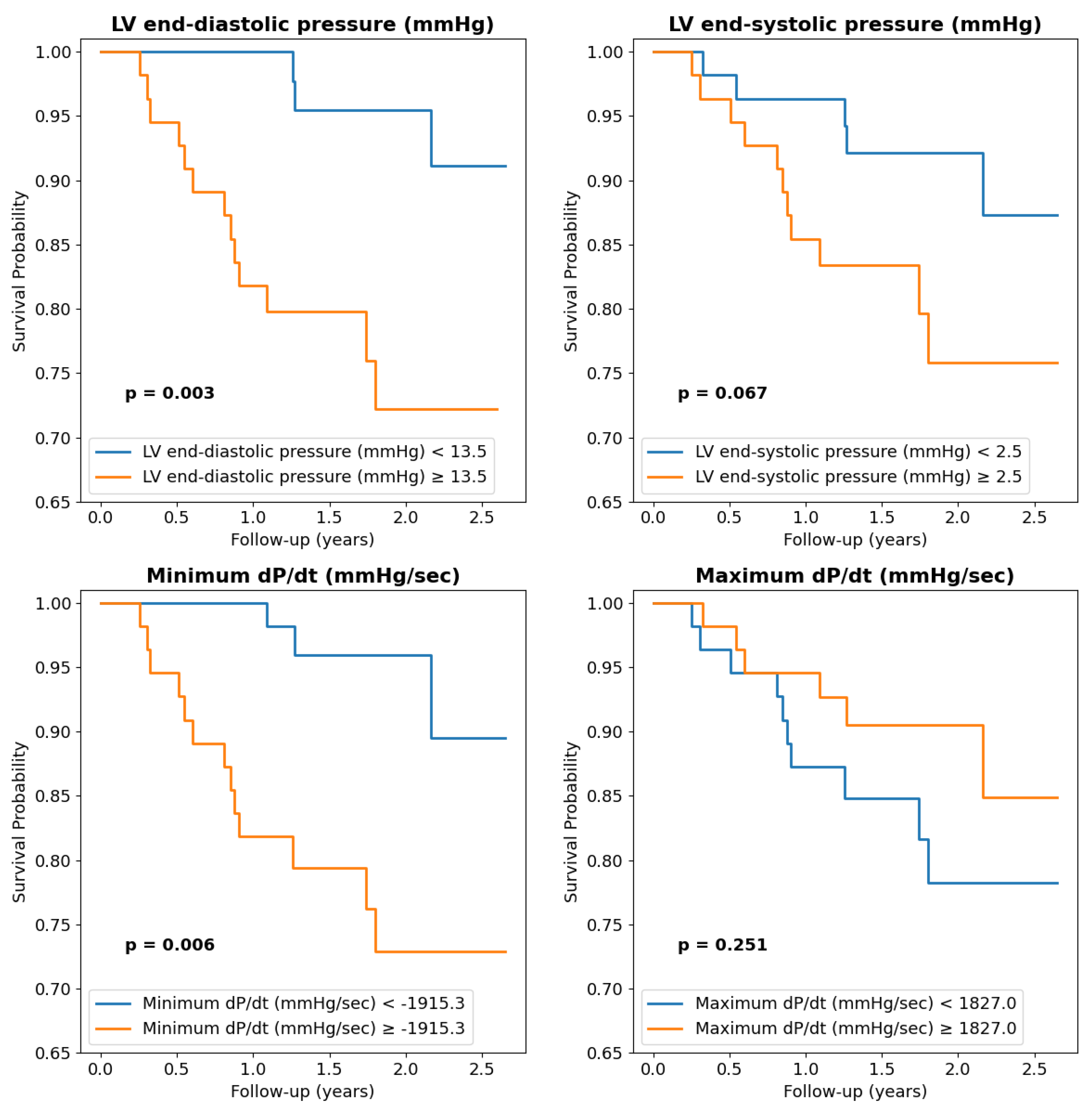
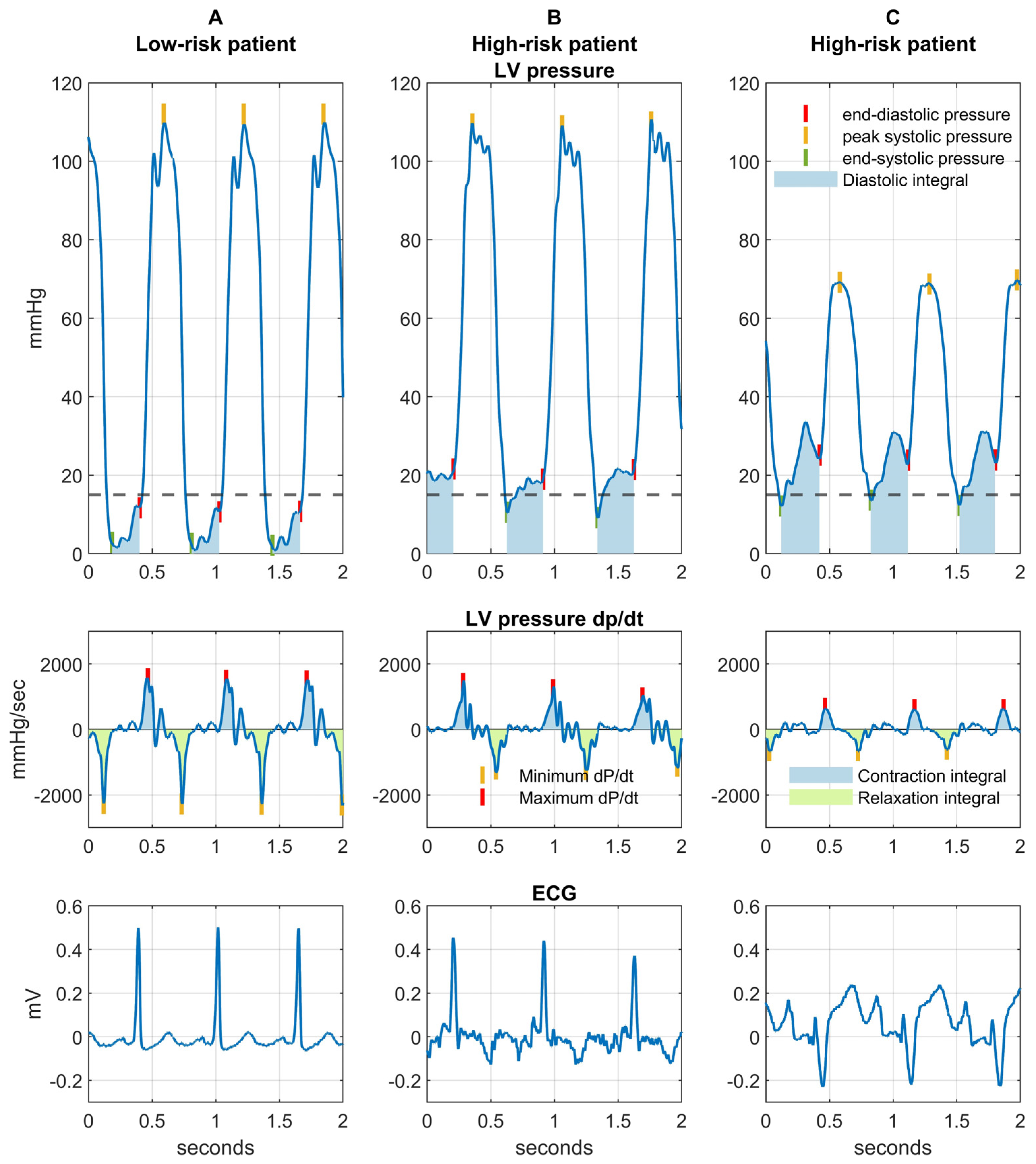
| LVEDP (mmHg) | 12 (8–19) | 20 ± 5 | 0.01 | 1.05 | 0.04 |
| LV end-systolic pressure (mmHg) | 2 (−1–6) | 9 (7–10) | 0.01 | 1.08 | 0.06 |
| Minimum dP/dt (mmHg/sec) | −1979 (−2260–−1492) | −1161 ± 389 | 0.0003 | 1.02 | 0.001 |
| Maximum dP/dt (mmHg/sec) | 1841 (1373–2179) | 1415 ± 737 | 0.04 | 1.00 | 0.06 |
| LV contraction integral (mmHg) | 158.0 ± 33.9 | 120.6 ± 51.5 | 0.04 | 0.98 | 0.03 |
| LV relaxation integral (mmHg) | 140.1 ± 27.8 | 109.4 ± 34.2 | 0.001 | 0.98 | 0.009 |
| LV diastolic integral (mmHg·sec) | 22 (16–32.4) | 26.1 (23.5–32.7) | 0.15 | 1.01 | 0.73 |
| Parameter * | HR (95% CI) | p | C-Statistic |
|---|---|---|---|
| Non-invasive setting (clinical and echocardiographic parameters) | |||
| E/E’ ratio > 9.8 | 2.02 (1.05–4.10) | 0.02 | 0.786 |
| NT-proBNP > 123 pg/mL | 2.25 (1.09–3.41) | 0.005 | |
| sST2 > 17 ng/mL | 1.58 (0.47–2.69) | 0.01 | |
| LVESV > 41 mL/m2 | 1.15 (1.01–2.31) | 0.04 | |
| Invasive setting (clinical, echocardiographic and catheterization parameters) | |||
| Minimum dP/dt > −1915 mmHg/s | 2.13 (1.12–4.21) | 0.03 | 0.823 |
| NT-proBNP > 123 pg/mL | 2.54 (1.37–3.72) | 0.005 | |
| sST2 > 17 ng/mL | 1.50 (0.34–2.60) | 0.01 | |
| E/E’ ratio > 9.8 | 1.76 (1.62–2.92) | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, H.; Călburean, P.-A.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Scurtu, A.-C.; Stan, A.; Aniței, D.; Brînzaniuc, K.; Hadadi, L.; et al. Natriuretic Peptides and Soluble ST2 Improve Echocardiographic and Invasive Long-Term Survival Prediction in Patients Evaluated for Diastolic Dysfunction. Int. J. Mol. Sci. 2025, 26, 3713. https://doi.org/10.3390/ijms26083713
Suciu H, Călburean P-A, Huțanu A, Oprica M, Opriș DR, Scurtu A-C, Stan A, Aniței D, Brînzaniuc K, Hadadi L, et al. Natriuretic Peptides and Soluble ST2 Improve Echocardiographic and Invasive Long-Term Survival Prediction in Patients Evaluated for Diastolic Dysfunction. International Journal of Molecular Sciences. 2025; 26(8):3713. https://doi.org/10.3390/ijms26083713
Chicago/Turabian StyleSuciu, Horațiu, Paul-Adrian Călburean, Adina Huțanu, Mădălina Oprica, Diana Roxana Opriș, Anda-Cristina Scurtu, Alexandru Stan, David Aniței, Klara Brînzaniuc, László Hadadi, and et al. 2025. "Natriuretic Peptides and Soluble ST2 Improve Echocardiographic and Invasive Long-Term Survival Prediction in Patients Evaluated for Diastolic Dysfunction" International Journal of Molecular Sciences 26, no. 8: 3713. https://doi.org/10.3390/ijms26083713
APA StyleSuciu, H., Călburean, P.-A., Huțanu, A., Oprica, M., Opriș, D. R., Scurtu, A.-C., Stan, A., Aniței, D., Brînzaniuc, K., Hadadi, L., & Harpa, M. (2025). Natriuretic Peptides and Soluble ST2 Improve Echocardiographic and Invasive Long-Term Survival Prediction in Patients Evaluated for Diastolic Dysfunction. International Journal of Molecular Sciences, 26(8), 3713. https://doi.org/10.3390/ijms26083713






