Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Homocysteine and Hypovitaminosis in Parkinson’s Disease
4. ADMA in Parkinson’s Disease
5. Homocysteine and Hypovitaminosis in Alzheimer’s Disease
6. ADMA in Alzheimer’s Disease
7. Homocysteine and Hypovitaminosis in Multiple Sclerosis
8. ADMA in Multiple Sclerosis
9. Strengths and Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| 27-OHC | 27-hydroxycholesterol |
| 5LO | 5-lipoxygenase |
| AA | Anthranilic acid |
| AD | Alzheimer’s disease |
| ADMA | Asymmetric dimethylarginine |
| ALA | Alpha-lipoic acid |
| ApoE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| Aβ | Amyloid β |
| BBB | Blood–brain barrier |
| BHMT | Betaine-homocysteine methyltransferase |
| CBF | Cerebral blood flow |
| cECs | Cerebral endothelial cells |
| CH2THF | 5,10-Methylenetetrahydrofolate |
| CH3THF | 5-methyltetrahydrofolate |
| CNS | Central nervous system |
| COMT | Catechol-O-methyltransferase |
| CSF | Cerebrospinal fluid |
| DDAH | Dimethylarginine dimethylaminohydodrolase |
| DMA | Dimethylamine |
| DMG | Dimethylglycine |
| DM-PP2Ac | De-methylated PP2A catalytic subunit |
| DR4/5 | Death receptor 4 and 5 |
| EAE | Autoimmune encephalomyelitis |
| EDSS | Expanded Disability Status Score |
| ET | Endothelin |
| FAD | Flavin adenine dinucleotide |
| HAA | 3-hydroxyanthranilic acid |
| HCs | Healthy controls |
| HCTL | Homocysteine thiolactone |
| Hcy | Homocysteine |
| HHcy | Hyperhomocysteinemia |
| HK | 3-hydroxykynurenine |
| iNOS | inducible Nitric Oxide Synthase |
| KA | Kynurenic acid |
| KAT | Kynurenine aminotransferase |
| Kyn | kynurenine |
| KYNU | Kynureninase |
| MBP | Myelin basic protein |
| MCI | Mild Cognitive Impairment |
| MeSe | Methionine synthase |
| MMA | Methylmalonic acid |
| MOG | Myelin oligodendrocyte glycoprotein |
| M-PP2Ac | Methylated PP2A catalytic subunit |
| MS | Multiple sclerosis |
| MSSS | Multiple Sclerosis Severity Score |
| MTHFR | Methylenetetrahydrofolate reductase |
| NMO | Neuromyelitis optica |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| ONDs | Other neurologic diseases |
| PD | Parkinson’s disease |
| PLP | Pyridoxal 5-phosphate |
| PP2A | Protein phosphatase 2A |
| PRMT-1 | Protein arginine methyltransferases-1 |
| PRMT-4 | Protein arginine methyltransferase-4 |
| PS1 | Presenilin 1 |
| pS262-tau | Ser262 hyperphosphorylated tau |
| PTMs | Post-translational modifications |
| ROS | Reactive oxygen species |
| RR | Relapsing–remitting |
| SAH | S-adenosyl homocysteine |
| SAM | S-adenosyl methionine |
| SDMA | Symmetric dimethylarginine |
| SP | Secondary progressive |
| TCA | Tricarboxylic acid |
| THF | Tetrahydrofolate |
| tHcy | Total homocysteine |
| VCID | Cognitive impairment and dementia |
| XA | Xanthurenic acid |
References
- Kirbas, S.; Kirbas, A.; Tufekci, A.; Cumhur Cure, M.; Cakmak, S.; Yazici, T.; Cure, E. Serum Levels of Homocysteine, Asymmetric Dimethylarginine and Nitric Oxide in Patients with Parkinson’s Disease. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2016, 71, 71–75. [Google Scholar] [CrossRef]
- Ientile, R.; Curro’, M.; Ferlazzo, N.; Condello, S.; Caccamo, D.; Pisani, F. Homocysteine, Vitamin Determinants and Neurological Diseases. Front. Biosci. 2010, 2, 359–372. [Google Scholar] [CrossRef]
- Dayal, S.; Rodionov, R.N.; Arning, E.; Bottiglieri, T.; Kimoto, M.; Murry, D.J.; Cooke, J.P.; Faraci, F.M.; Lentz, S.R. Tissue-Specific Downregulation of Dimethylarginine Dimethylaminohydrolase in Hyperhomocysteinemia. Am. J. Physiol.—Heart Circ. Physiol. 2008, 295, 816–825. [Google Scholar] [CrossRef]
- Boisvert, F.M.; Côté, J.; Boulanger, M.C.; Richard, S. A Proteomic Analysis of Arginine-Methylated Protein Complexes. Mol. Cell. Proteom. 2003, 2, 1319–1330. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Z.Q.; Xia, L.; Feng, Q.; Erdjument-Bromage, H.; Strahl, B.D.; Briggs, S.D.; Allis, C.D.; Wong, J.; Tempst, P.; et al. Methylation of Histone H4 at Arginine 3 Facilitating Transcriptional Activation by Nuclear Hormone Receptor. Science 2001, 293, 853–857. [Google Scholar] [CrossRef]
- Böger, R.H. Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, Explains the “L-Arginine Paradox” and Acts as a Novel Cardiovascular Risk Factor. J. Nutr. 2004, 134, 2842–2847. [Google Scholar] [CrossRef]
- Devlin, A.M.; Arning, E.; Bottiglieri, T.; Faraci, F.M.; Rozen, R.; Lentz, S.R. Effect of Mthfr Genotype on Diet-Induced Hyperhomocysteinemia and Vascular Function in Mice. Blood 2004, 103, 2624–2629. [Google Scholar] [CrossRef]
- Homocysteine Studies Collaboration. Homocysteine and Risk of Ischemic Heart Disease and Stroke: A Meta-Analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef]
- Spoelstra-De Man, A.M.E.; Teerlink, T.; Brouwer, C.B.; Rauwerda, J.A.; Stehouwer, C.D.A.; Smulders, Y.M. No Effect of B Vitamins on ADMA Levels in Patients at Increased Cardiovascular Risk. Clin. Endocrinol. 2006, 64, 495–501. [Google Scholar] [CrossRef]
- Stühlinger, M.C.; Tsao, P.S.; Her, J.H.; Kimoto, M.; Balint, R.F.; Cooke, J.P. Homocysteine Impairs the Nitric Oxide Synthase Pathway Role of Asymmetric Dimethylarginine. Circulation 2001, 104, 2569–2575. [Google Scholar] [CrossRef]
- Gao, J.; Cahill, C.M.; Huang, X.; Roffman, J.L.; Lamon-Fava, S.; Fava, M.; Mischoulon, D.; Rogers, J.T. S-Adenosyl Methionine and Transmethylation Pathways in Neuropsychiatric Diseases Throughout Life. Neurotherapeutics 2018, 15, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Seminara, L.; Cavallaro, R.A.; D’Anselmi, F.; Scarpa, S. S-Adenosylmethionine/Homocysteine Cycle Alterations Modify DNA Methylation Status with Consequent Deregulation of PS1 and BACE and Beta-Amyloid Production. Mol. Cell. Neurosci. 2005, 28, 195–204. [Google Scholar] [CrossRef]
- Bertuccio, M.P.; Currò, M.; Caccamo, D.; Ientile, R. Dietary Intake and Genetic Background Influence Vitamin Needs during Pregnancy. Healthcare 2022, 10, 768. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Arginine, Transsulfuration, and Folic Acid Pathway Metabolomics in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 2180. [Google Scholar] [CrossRef] [PubMed]
- Reffo, A.; Gabelli, C. Hyperhomocysteinemia and Dementia Associated With Severe Cortical Atrophy, but No Amyloid Burden. J. Geriatr. Psychiatry Neurol. 2022, 35, 57–61. [Google Scholar] [CrossRef]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef]
- Tomic, S.; Pekic, V.; Popijac, Z.; Pucic, T.; Vinkovic, M.P.; Kuric, T.G.; Popovic, Z. Hyperhomocysteinemia Influenced Malnutrition in Parkinson’s Disease Patients. Neurol. Sci. 2018, 39, 1691–1695. [Google Scholar] [CrossRef]
- Bakeberg, M.C.; Jefferson, A.; Riley, M.; Byrnes, M.; Ghosh, S.; Mastaglia, F.L.; Horne, M.K.; McGregor, S.; Stell, R.; Kenna, J.; et al. Elevated Serum Homocysteine Levels Have Differential Gender-Specific Associations with Motor and Cognitive States in Parkinson’s Disease. Park. Dis. 2019, 2019, 3124295. [Google Scholar] [CrossRef]
- Licking, N.; Murchison, C.; Cholerton, B.; Zabetian, C.P.; Hu, S.C.; Montine, T.J.; Peterson-Hiller, A.L.; Chung, K.A.; Edwards, K.; Leverenz, J.B.; et al. Homocysteine and Cognitive Function in Parkinson’s Disease. Park. Relat. Disord. 2017, 44, 1–5. [Google Scholar] [CrossRef]
- Martignoni, E.; Tassorelli, C.; Nappi, G.; Zangaglia, R.; Pacchetti, C.; Blandini, F. Homocysteine and Parkinson’s Disease: A Dangerous Liaison? J. Neurol. Sci. 2007, 257, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zoccolella, S.; dell’Aquila, C.; Specchio, L.M.; Logroscino, G.; Lamberti, P. Elevated Homocysteine Levels in Parkinson’s Disease: Is There Anything besides L-Dopa Treatment? Curr. Med. Chem. 2010, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Anamnart, C.; Kitjarak, R. Effects of Vitamin B12, Folate, and Entacapone on Homocysteine Levels in Levodopa-Treated Parkinson’s Disease Patients: A Randomized Controlled Study. J. Clin. Neurosci. 2021, 88, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Xu, S.; Cao, L.D.; Wu, Q.Y. The Effect of Levodopa Benserazide Hydrochloride on Homocysteinemia Levels in Patients with Parkinson’s Disease and Treatment of Hyperhomocysteinemia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2409–2412. [Google Scholar]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic Analysis of Gut Microbiome Reveals the Role of Bacterial Folate and Homocysteine Metabolism in Parkinson’s Disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Brito, A.; Grapov, D.; Fahrmann, J.; Harvey, D.; Green, R.; Miller, J.W.; Fedosov, S.N.; Shahab-Ferdows, S.; Hampel, D.; Pedersen, T.L.; et al. The Human Serum Metabolome of Vitamin B-12 Deficiency and Repletion, and Associations with Neurological Function in Elderly Adults. J. Nutr. 2017, 147, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Brites, P.; Waterham, H.R.; Wanders, R.J.A. Functions and Biosynthesis of Plasmalogens in Health and Disease. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2004, 1636, 219–231. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and Functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Sezen, S.; Karadayi, M.; Yesilyurt, F.; Burul, F.; Gulsahin, Y.; Ozkaraca, M.; Okkay, U.; Gulluce, M. Acyclovir Provides Protection against 6-OHDA-Induced Neurotoxicity in SH-SY5Y Cells through the Kynurenine Pathway. Neurotoxicology 2025, 106, 1–9. [Google Scholar] [CrossRef]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef]
- Havelund, J.F.; Andersen, A.D.; Binzer, M.; Blaabjerg, M.; Heegaard, N.H.H.; Stenager, E.; Faergeman, N.J.; Gramsbergen, J.B. Changes in Kynurenine Pathway Metabolism in Parkinson Patients with L-DOPA-Induced Dyskinesia. J. Neurochem. 2017, 142, 756–766. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Ono, K. Metabolomics in Parkinson’s Disease and Correlation with Disease State. Metabolites 2025, 15, 208. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.-L.; Bjørk, M.-H.; Storstein, A.; Ueland, P.M.; Tysnes, O.-B. Severe Hyperhomocysteinemia in a Patient with Parkinson Disease. Clin. Chem. 2022, 68, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Gupta, E. Das Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism: Epidemiology, Metabolism and the Associated Diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Dowey, L.R.C.; Strain, J.J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin Lowers Homocysteine in Individuals Homozygous for the MTHFR 677C→T Polymorphism. Circulation 2006, 113, 74–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.X.; He, Z.Y.; Liu, X.; Liu, H.N. Association of MTHFR C677T with Total Homocysteine Plasma Levels and Susceptibility to Parkinson’s Disease: A Meta-Analysis. Neurol. Sci. 2015, 36, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher Cerebrospinal Fluid to Plasma Ratio of P-Cresol Sulfate and Indoxyl Sulfate in Patients with Parkinson’s Disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef]
- Schmitt, B.; Wolters, M.; Kressel, G.; Hülsmann, O.; Ströhle, A.; Kühn-Velten, W.N.; Lichtinghagen, R.; Bub, A.; Barth, S.W.; Stichtenoth, D.O.; et al. Effects of Combined Supplementation with B Vitamins and Antioxidants on Plasma Levels of Asymmetric Dimethylarginine (ADMA) in Subjects with Elevated Risk for Cardiovascular Disease. Atherosclerosis 2007, 193, 168–176. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A Randomized Placebo-Controlled Trial of Using B Vitamins to Prevent Cognitive Decline in Older Mild Cognitive Impairment Patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Narayan, S.K.; Saxby, B.K.; Firbank, M.J.; O’Brien, J.T.; Harrington, F.; McKeith, I.G.; Hansrani, M.; Stansby, G.; Ford, G.A. Plasma Homocysteine and Cognitive Decline in Older Hypertensive Subjects. Int. Psychogeriatr. 2011, 23, 1607–1615. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Gorelick, P.B. Risk Factors for Vascular Dementia and Alzheimer Disease. Stroke 2004, 35, 2620–2622. [Google Scholar] [CrossRef]
- Tucker, K.L.; Qiao, N.; Scott, T.; Rosenberg, I.; Spiro, A. High Homocysteine and Low B Vitamins Predict Cognitive Decline in Aging Men: The Veterans Affairs Normative Aging Study. Am. J. Clin. Nutr. 2005, 82, 627–635. [Google Scholar] [CrossRef]
- Singh, M.; Prakash, A. Possible Role of Endothelin Receptor against Hyperhomocysteinemia and β-Amyloid Induced AD Type of Vascular Dementia in Rats. Brain Res. Bull. 2017, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, T.; Riemenschneider, M.; Förstl, H.; Henriksen, G.; Klunk, W.E.; Mathis, C.A.; Shiga, T.; Wester, H.J.; Kurz, A.; Drzezga, A. Beta Amyloid in Alzheimer’s Disease: Increased Deposition in Brain Is Reflected in Reduced Concentration in Cerebrospinal Fluid. Biol. Psychiatry 2009, 65, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of Homocysteine Neurotoxicity in Neurodegenerative Diseases with Special Reference to Dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef]
- Polito, L.; Poloni, T.E.; Vaccaro, R.; Abbondanza, S.; Mangieri, M.; Davin, A.; Villani, S.; Guaita, A. High Homocysteine and Epistasis between MTHFR and APOE: Association with Cognitive Performance in the Elderly. Exp. Gerontol. 2016, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Jasbi, P.; Pascual, A.S.; North, S.; Kwatra, N.; Weissig, V.; Gu, H.; Bottiglieri, T.; Jadavji, N.M. Ischemic Stroke and Dietary Vitamin B12 Deficiency in Old-Aged Females: Impaired Motor Function, Increased Ischemic Damage Size, and Changed Metabolite Profiles in Brain and Cecum Tissue. Nutrients 2022, 14, 2960. [Google Scholar] [CrossRef]
- Sah, R.P.; Vidya, C.S.; Pereira, P.; Jayaram, S.; Yadav, A.K.; Sujatha, P. Elevated Homocysteine Level and Brain Atrophy Changes as Markers to Screen the Alzheimer Disease: Case Series. Ann. Geriatr. Med. Res. 2024, 28, 116–120. [Google Scholar] [CrossRef]
- Ueno, A.; Hamano, T.; Enomoto, S.; Shirafuji, N.; Nagata, M.; Kimura, H.; Ikawa, M.; Yamamura, O.; Yamanaka, D.; Ito, T.; et al. Influences of Vitamin B(12) Supplementation on Cognition and Homocysteine in Patients with Vitamin B(12) Deficiency and Cognitive Impairment. Nutrients 2022, 14, 1494. [Google Scholar] [CrossRef]
- Baroni, L.; Bonetto, C.; Rizzo, G.; Bertola, C.; Caberlotto, L.; Bazzerla, G. Association Between Cognitive Impairment and Vitamin B12, Folate, and Homocysteine Status in Elderly Adults: A Retrospective Study. J. Alzheimers Dis. 2019, 70, 443–453. [Google Scholar] [CrossRef]
- Bednarska-Makaruk, M.; Graban, A.; Sobczyńska-Malefora, A.; Harrington, D.J.; Mitchell, M.; Voong, K.; Dai, L.; Łojkowska, W.; Bochyńska, A.; Ryglewicz, D.; et al. Homocysteine Metabolism and the Associations of Global DNA Methylation with Selected Gene Polymorphisms and Nutritional Factors in Patients with Dementia. Exp. Gerontol. 2016, 81, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Jernerén, F.; Turner, C.; Hart, K.; Tabet, N. Homocysteine Concentrations in the Cognitive Progression of Alzheimer’s Disease. Exp. Gerontol. 2017, 99, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Anusree, V.R.; Satheesh, G.; Vijayakumar, G.; Chandran, M.; Simon, L.; Lakshmi, S.; Pillai, M.R.; Jaleel, A. Hyperhomocysteinemia-Related Serum Metabolome Alterations Not Normalized by Short-Term Folic Acid Treatment. Metabolomics 2021, 17, 47. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Yu, H.; Hao, L.; Ju, M.; Feng, W.; Guo, Z.; Sun, X.; Fan, Q.; Xiao, R. Vitamin D, Folic Acid and Vitamin B(12) Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients 2022, 15, 132. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Yu, M.; Zhang, X.; Zhang, M.; Wilson, J.X.; Huang, G. Folic Acid Administration Inhibits Amyloid β-Peptide Accumulation in APP/PS1 Transgenic Mice. J. Nutr. Biochem. 2015, 26, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lu, W.; Zhang, C.; Wang, G.; Hou, Y.; Zhou, J.; Wang, Y. Folic Acid and S-Adenosylmethionine Reverse Homocysteine-Induced Alzheimer’s Disease-like Pathological Changes in Rat Hippocampus by Modulating PS1 and PP2A Methylation Levels. Brain Res. 2024, 1841, 149095. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Chinnathambi, S. Prion-Like Propagation of Post-Translationally Modified Tau in Alzheimer’s Disease: A Hypothesis. J. Mol. Neurosci. 2018, 65, 480–490. [Google Scholar] [CrossRef]
- Zhang, C.-E.; Wei, W.; Liu, Y.-H.; Peng, J.-H.; Tian, Q.; Liu, G.-P.; Zhang, Y.; Wang, J.-Z. Hyperhomocysteinemia Increases Beta-Amyloid by Enhancing Expression of Gamma-Secretase and Phosphorylation of Amyloid Precursor Protein in Rat Brain. Am. J. Pathol. 2009, 174, 1481–1491. [Google Scholar] [CrossRef]
- Zhang, C.-E.; Tian, Q.; Wei, W.; Peng, J.-H.; Liu, G.-P.; Zhou, X.-W.; Wang, Q.; Wang, D.-W.; Wang, J.-Z. Homocysteine Induces Tau Phosphorylation by Inactivating Protein Phosphatase 2A in Rat Hippocampus. Neurobiol. Aging 2008, 29, 1654–1665. [Google Scholar] [CrossRef]
- Guo, J.; Ni, S.; Li, Q.; Wang, J.Z.; Yang, Y. Folate/Vitamin B Alleviates Hyperhomocysteinemia-Induced Alzheimer-Like Pathologies in Rat Retina. Neurosci. Bull. 2019, 35, 325–335. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Ni, S.; Zhang, S.; Li, Q.; Zeng, P.; Pi, G.; Liu, E.; Sun, D.-S.; Liu, Y.; et al. Elevation of PS262-Tau and Demethylated PP2A in Retina Occurs Earlier than in Hippocampus During Hyperhomocysteinemia. J. Alzheimers Dis. 2019, 68, 367–381. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kruyer, A.; Yao, Y.; Feierman, E.; Richards, A.; Strickland, S.; Norris, E.H. Hyperhomocysteinemia Exacerbates Alzheimer’s Disease Pathology by Way of the β-Amyloid Fibrinogen Interaction. J. Thromb. Haemost. 2016, 14, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.; Parodi-Rullan, R.; Vazquez-Torres, R.; Canepa, E.; Fossati, S. Homocysteine Potentiates Amyloid β-Induced Death Receptor 4- and 5-Mediated Cerebral Endothelial Cell Apoptosis, Blood Brain Barrier Dysfunction and Angiogenic Impairment. Aging Cell 2024, 23, e14106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Barrero, C.; Gupta, S.; Kruger, W.D.; Merali, S.; Praticò, D. Homocysteine Modulates 5-Lipoxygenase Expression Level via DNA Methylation. Aging Cell 2017, 16, 273–280. [Google Scholar] [CrossRef]
- Li, J.-G.; Barrero, C.; Merali, S.; Praticò, D. Five Lipoxygenase Hypomethylation Mediates the Homocysteine Effect on Alzheimer’s Phenotype. Sci. Rep. 2017, 7, 46002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, X.; Liu, L.; Chen, R. Serum Metabolome Alterations in Hyperhomocysteinemia Based on Targeted and Non-Targeted MS-Platforms. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2024, 1247, 124336. [Google Scholar] [CrossRef]
- Jan, M.; Cueto, R.; Jiang, X.; Lu, L.; Sardy, J.; Xiong, X.; Yu, J.E.; Pham, H.; Khan, M.; Qin, X.; et al. Molecular Processes Mediating Hyperhomocysteinemia-Induced Metabolic Reprogramming, Redox Regulation and Growth Inhibition in Endothelial Cells. Redox Biol. 2021, 45, 102018. [Google Scholar] [CrossRef]
- Seaks, C.E.; Weekman, E.M.; Sudduth, T.L.; Xie, K.; Wasek, B.; Fardo, D.W.; Johnson, L.A.; Bottiglieri, T.; Wilcock, D.M. Apolipoprotein E Ε4/4 Genotype Limits Response to Dietary Induction of Hyperhomocysteinemia and Resulting Inflammatory Signaling. J. Cereb. Blood Flow Metab. 2022, 42, 771–787. [Google Scholar] [CrossRef]
- Weekman, E.M.; Woolums, A.E.; Sudduth, T.L.; Wilcock, D.M. Hyperhomocysteinemia-Induced Gene Expression Changes in the Cell Types of the Brain. ASN Neuro 2017, 9, 1759091417742296. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; Ramirez, S.H.; Nenov, M.N. Asymmetric Dimethylarginine Induces Maladaptive Function of the Blood-Brain Barrier. Front. Cell Dev. Biol. 2024, 12, 1476386. [Google Scholar] [CrossRef]
- Luo, Y.; Yue, W.; Quan, X.; Wang, Y.; Zhao, B.; Lu, Z. Asymmetric Dimethylarginine Exacerbates Aβ-Induced Toxicity and Oxidative Stress in Human Cell and Caenorhabditis Elegans Models of Alzheimer Disease. Free Radic. Biol. Med. 2015, 79, 117–126. [Google Scholar] [CrossRef]
- Tarkowski, E.; Ringqvist, Å.; Blennow, K.; Wallin, A.; Wennmalm, Å. Intrathecal Release of Nitric Oxide in Alzheimer’s Disease and Vascular Dementia. Dement. Geriatr. Cogn. Disord. 2000, 11, 322–326. [Google Scholar] [CrossRef]
- Clemons, G.A.; Silva, A.C.E.; Acosta, C.H.; Udo, M.S.B.; Tesic, V.; Rodgers, K.M.; Wu, C.Y.-C.; Citadin, C.T.; Lee, R.H.-C.; Neumann, J.T.; et al. Protein Arginine Methyltransferase 4 Modulates Nitric Oxide Synthase Uncoupling and Cerebral Blood Flow in Alzheimer’s Disease. J. Cell. Physiol. 2024, 239, e30858. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Singh, I.; Singh, A.K.; Khan, M.; Won, J. Asymmetric Dimethylarginine Exacerbates Cognitive Dysfunction Associated with Cerebrovascular Pathology. FASEB J. 2020, 34, 6808–6823. [Google Scholar] [CrossRef] [PubMed]
- Fleszar, M.G.; Wiśniewski, J.; Zboch, M.; Diakowska, D.; Gamian, A.; Krzystek-Korpacka, M. Targeted Metabolomic Analysis of Nitric Oxide/L-Arginine Pathway Metabolites in Dementia: Association with Pathology, Severity, and Structural Brain Changes. Sci. Rep. 2019, 9, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.G.; Pirko, I.; Lucchinetti, C.F. Pathology of Multiple Sclerosis: Where Do We Stand? Contin. Lifelong Learn. Neurol. 2013, 19, 901–921. [Google Scholar] [CrossRef]
- Review, A.S.; Martínez-tomé, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Nutrition Facts in Multiple Sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, Demyelination, Remyelination and Repair in Multiple Sclerosis. J. Neurol. Sci. 2005, 233, 93–97. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Frick, B.; Wirleitner, B.C.; Winkler, B.S.P.; Schennach, H.D.; Fuchs, B.S.P. Moderate Hyperhomocysteinemia and Immune Activation. Curr. Pharm. Biotechnol. 2005, 5, 107–118. [Google Scholar] [CrossRef]
- Dardiotis, E.; Arseniou, S.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.F.A.; Michalopoulou, A.; Andravizou, A.; Dastamani, M.; Paterakis, K.; et al. Vitamin B12, Folate, and Homocysteine Levels and Multiple Sclerosis: A Meta-Analysis. Mult. Scler. Relat. Disord. 2017, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Largani, M.; Pourvali-Talatappeh, P.; Rousta, A.M.; Karimi-Kivi, M.; Noroozi, E.; Mahjoob, A.; Asaadi, Y.; Shahmohammadi, A.; Sadeghi, S.; Shakeri, S.; et al. A Review on Potential Roles of Vitamins in Incidence, Progression, and Improvement of Multiple Sclerosis. eNeurologicalSci 2018, 10, 37–44. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Han, J.; Hu, W. Serum Levels of Homocysteine, Vitamin B12 and Folate in Patients with Multiple Sclerosis: An Updated Meta-Analysis. Int. J. Med. Sci. 2020, 17, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, M.; Mamarabadi, M.; Mohebi, N.; Razjouyan, H.; Aghaei, M. Homocysteine, Vitamin B12 and Folate Levels in Iranian Patients with Multiple Sclerosis: A Case Control Study. Clin. Neurol. Neurosurg. 2013, 115, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple Aspects of Homocysteine Neurotoxicity: Glutamate Excitotoxicity, Kinase Hyperactivation and DNA Damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Bełtowski, J.; Bartosik-Psujek, H.; Wójcicka, G.; Rejdak, K. Processes of Plasma Protein N-Homocysteinylation in Multiple Sclerosis. Int. J. Neurosci. 2017, 127, 709–715. [Google Scholar] [CrossRef]
- Lioudyno, V.I.; Tsymbalova, E.A.; Chernyavskaya, E.A.; Scripchenko, E.Y.; Bisaga, G.N.; Dmitriev, A.V.; Abdurasulova, I.N. Association of Increased Homocysteine Levels with Impaired Folate Metabolism and Vitamin B Deficiency in Early-Onset Multiple Sclerosis. Biochemistry (Moscow) 2024, 89, 562–573. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Flauzino, T.; Sabino, B.S.; Kallaur, A.P.; Alfieri, D.F.; Kaimen-Maciel, D.R.; Morimoto, H.K.; de Almeida, E.R.D.; Lozovoy, M.A.B.; Reiche, E.M.V.; et al. Elevated Plasma Homocysteine Levels Are Associated with Disability Progression in Patients with Multiple Sclerosis. Metab. Brain Dis. 2018, 33, 1393–1399. [Google Scholar] [CrossRef]
- Pan, L.; Yin, Y.; Chen, J.; Ma, Z.; Chen, Y.; Deng, X.; Zhang, H.T.; Leng, H.; Wu, K. Homocysteine, Vitamin B12, and Folate Levels in Patients with Multiple Sclerosis in Chinese Population: A Case-Control Study and Meta-Analysis. Mult. Scler. Relat. Disord. 2019, 36, 101395. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Bełtowski, J.; Wójcicka, G.; Bartosik-Psujek, H.; Rejdak, K. Cladribine Treatment Improved Homocysteine Metabolism and Increased Total Serum Antioxidant Activity in Secondary Progressive Multiple Sclerosis Patients. Oxid. Med. Cell. Longev. 2020, 2020, 1654754. [Google Scholar] [CrossRef]
- Bhargava, P.; Gocke, A.; Calabresi, P.A. 1,25-Dihydroxyvitamin D3 Impairs the Differentiation of Effector Memory T Cells in Vitro in Multiple Sclerosis Patients and Healthy Controls. J. Neuroimmunol. 2015, 279, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Schwarz, A.; Korporal-Kuhnke, M.; Faller, S.; Jarius, S.; Wildemann, B. Hypovitaminosis D Upscales B-Cell Immunoreactivity in Multiple Sclerosis. J. Neuroimmunol. 2016, 294, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Miele, G.; Abbadessa, G.; Cavalla, P.; Valentino, P.; Marfia, G.A.; Landi, D.; Bosa, C.; Vercellino, M.; De Martino, A.; Ponzano, M.; et al. Association of Vitamin D Serum Levels and Vitamin D Supplementation with B Cell Kinetics and Disease Activity in Multiple Sclerosis Patients Treated with Ocrelizumab: An Italian Multi-Center Study. Mult. Scler. Relat. Disord. 2022, 68, 104395. [Google Scholar] [CrossRef]
- Bäcker-Koduah, P.; Bellmann-Strobl, J.; Scheel, M.; Wuerfel, J.; Wernecke, K.D.; Dörr, J.; Brandt, A.U.; Paul, F. Vitamin D and Disease Severity in Multiple Sclerosis—Baseline Data From the Randomized Controlled Trial (EVIDIMS). Front. Neurol. 2020, 11, 129. [Google Scholar] [CrossRef]
- Balasooriya, N.N.; Elliott, T.M.; Neale, R.E.; Vasquez, P.; Comans, T.; Gordon, L.G. The Association between Vitamin D Deficiency and Multiple Sclerosis: An Updated Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2024, 90, 105804. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Callegaro, D.; Lana-Peixoto, M.A.; Talim, N.; Vidaletti, T.; De Paula Correâ, M.; Gomes, I. Hypovitaminosis D Association with Disease Activity in Relapsing Remitting Multiple Sclerosis in Brazil. J. Neurol. Sci. 2016, 363, 236–239. [Google Scholar] [CrossRef]
- Niedziela, N.; Nowak-Kiczmer, M.; Malciene, L.; Stasiołek, M.; Niedziela, J.T.; Czuba, Z.P.; Lis, M.; Sowa, A.; Adamczyk-Sowa, M. Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 10502. [Google Scholar] [CrossRef]
- Bucova, M.; Durmanova, V.; Cudrakova, D.; Blazickova, S.; Gmitterova, K.; Klimova, E.; Lisa, I.; Kluckova, K.; Majernikova, B. Decreased Plasma Levels of 25(OH)D in Multiple Sclerosis Patients. Correlation with Disease Severity Expressed by EDSS, MSSS, Progression Index and Herbert’s Scale Severity Grade. Bratisl. Lek. Listy 2019, 120, 723–729. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Barbero, P.; Caloni, B.; Naldi, P.; Cantello, R.; Dianzani, U.; Comi, C. Serum Vitamin D as a Marker of Impaired Information Processing Speed and Early Disability in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1521. [Google Scholar] [CrossRef]
- Agnello, L.; Scazzone, C.; Sasso, B.L.; Vidali, M.; Giglio, R.V.; Ciaccio, A.M.; Ragonese, P.; Salemi, G.; Ciaccio, M. Role of Multiple Vitamin D-Related Polymorphisms in Multiple Sclerosis Severity: Preliminary Findings. Genes 2022, 13, 1307. [Google Scholar] [CrossRef]
- Scazzone, C.; Agnello, L.; Sasso, B.L.; Ragonese, P.; Bivona, G.; Realmuto, S.; Iacolino, G.; Gambino, C.M.; Bellia, C.; Salemi, G.; et al. Klotho and Vitamin D in Multiple Sclerosis: An Italian Study. Arch. Med. Sci. 2020, 16, 842–847. [Google Scholar] [CrossRef]
- Elkama, A.; Karahalil, B. Role of Gene Polymorphisms in Vitamin D Metabolism and in Multiple Sclerosis. Arh. Hig. Rada Toksikol. 2018, 69, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2021, 59, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Reza Dorosty-Motlagh, A.; Mohammadzadeh Honarvar, N.; Sedighiyan, M.; Abdolahi, M. The Molecular Mechanisms of Vitamin A Deficiency in Multiple Sclerosis. J. Mol. Neurosci. 2016, 60, 82–90. [Google Scholar] [CrossRef]
- Saboor-Yaraghi, A.A.; Harirchian, M.H.; Mohammadzadeh Honarvar, N.; Bitarafan, S.; Abdolahi, M.; Siassi, F.; Salehi, E.; Sahraian, M.A.; Eshraghian, M.R.; Roostaei, T.; et al. The Effect of Vitamin A Supplementation on FoxP3 and TGF-β Gene Expression in Avonex-Treated Multiple Sclerosis Patients. J. Mol. Neurosci. 2015, 56, 608–612. [Google Scholar] [CrossRef]
- Lillico, R.; Zhou, T.; Khorshid Ahmad, T.; Stesco, N.; Gozda, K.; Truong, J.; Kong, J.; Lakowski, T.M.; Namaka, M. Increased Post-Translational Lysine Acetylation of Myelin Basic Protein Is Associated with Peak Neurological Disability in a Mouse Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. J. Proteome Res. 2018, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef]
- Smith, K.J.; Lassmann, H. The Role of Nitric Oxide in Multiple Sclerosis. Lancet Neurol. 2002, 1, 232–241. [Google Scholar] [CrossRef]
- Niedziela, N.; Adamczyk-Sowa, M.; Niedziela, J.T.; Mazur, B.; Kluczewska, E.; Sowa, P.; Gąsior, M. Assessment of Serum Nitrogen Species and Inflammatory Parameters in Relapsing-Remitting Multiple Sclerosis Patients Treated with Different Therapeutic Approaches. BioMed Res. Int. 2016, 2016, 4570351. [Google Scholar] [CrossRef]
- Ghasemi, M.; Fatemi, A. Pathologic Role of Glial Nitric Oxide in Adult and Pediatric Neuroinflammatory Diseases. Neurosci. Biobehav. Rev. 2014, 45, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; Abujrais, S.; Herman, S.; Khoonsari, P.E.; Åkerfeldt, T.; Svenningsson, A.; Burman, J.; Kultima, K. Targeted Metabolomics of CSF in Healthy Individuals and Patients with Secondary Progressive Multiple Sclerosis Using High-Resolution Mass Spectrometry. Metabolomics 2020, 16, 26. [Google Scholar] [CrossRef]
- Haghikia, A.; Kayacelebi, A.A.; Beckmann, B.; Hanff, E.; Gold, R.; Haghikia, A.; Tsikas, D. Serum and Cerebrospinal Fluid Concentrations of Homoarginine, Arginine, Asymmetric and Symmetric Dimethylarginine, Nitrite and Nitrate in Patients with Multiple Sclerosis and Neuromyelitis Optica. Amino Acids 2015, 47, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.; Morbidelli, L.; Bazzani, L.; Rossi, A. Influence of Circulating Endothelin-1 and Asymmetric Dimethylarginine on Whole Brain Circulation Time in Multiple Sclerosis. Biomark. Insights 2017, 12, 1177271917712514. [Google Scholar] [CrossRef]
- Onmaz, D.E.; Isık, S.M.T.; Abusoglu, S.; Ekmekci, A.H.; Sivrikaya, A.; Abusoglu, G.; Ozturk, S.; Aydemir, H.Y.; Unlu, A. Serum ADMA Levels Were Positively Correlated with EDSS Scores in Patients with Multiple Sclerosis. J. Neuroimmunol. 2021, 353, 577497. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Kim, J.; Saxena, N.; Choi, S.; Islam, S.M.T.; Singh, A.K.; Khan, M.; Won, J. Vascular and Immunopathological Role of Asymmetric Dimethylarginine (ADMA) in Experimental Autoimmune Encephalomyelitis. Immunology 2021, 164, 602–616. [Google Scholar] [CrossRef]
- Bystrická, Z.; Laubertová, L.; Ďurfinová, M.; Paduchová, Z. Methionine Metabolism and Multiple Sclerosis. Biomarkers 2017, 22, 747–754. [Google Scholar] [CrossRef]
- Khalili, M.; Soltani, M.; Amiri Moghadam, S.; Dehghan, P.; Azimi, A.; Abbaszadeh, O. Effect of Alpha-Lipoic Acid on Asymmetric Dimethylarginine and Disability in Multiple Sclerosis Patients: A Randomized Clinical Trial. Electron. Physician 2017, 9, 4899–4905. [Google Scholar] [CrossRef][Green Version]

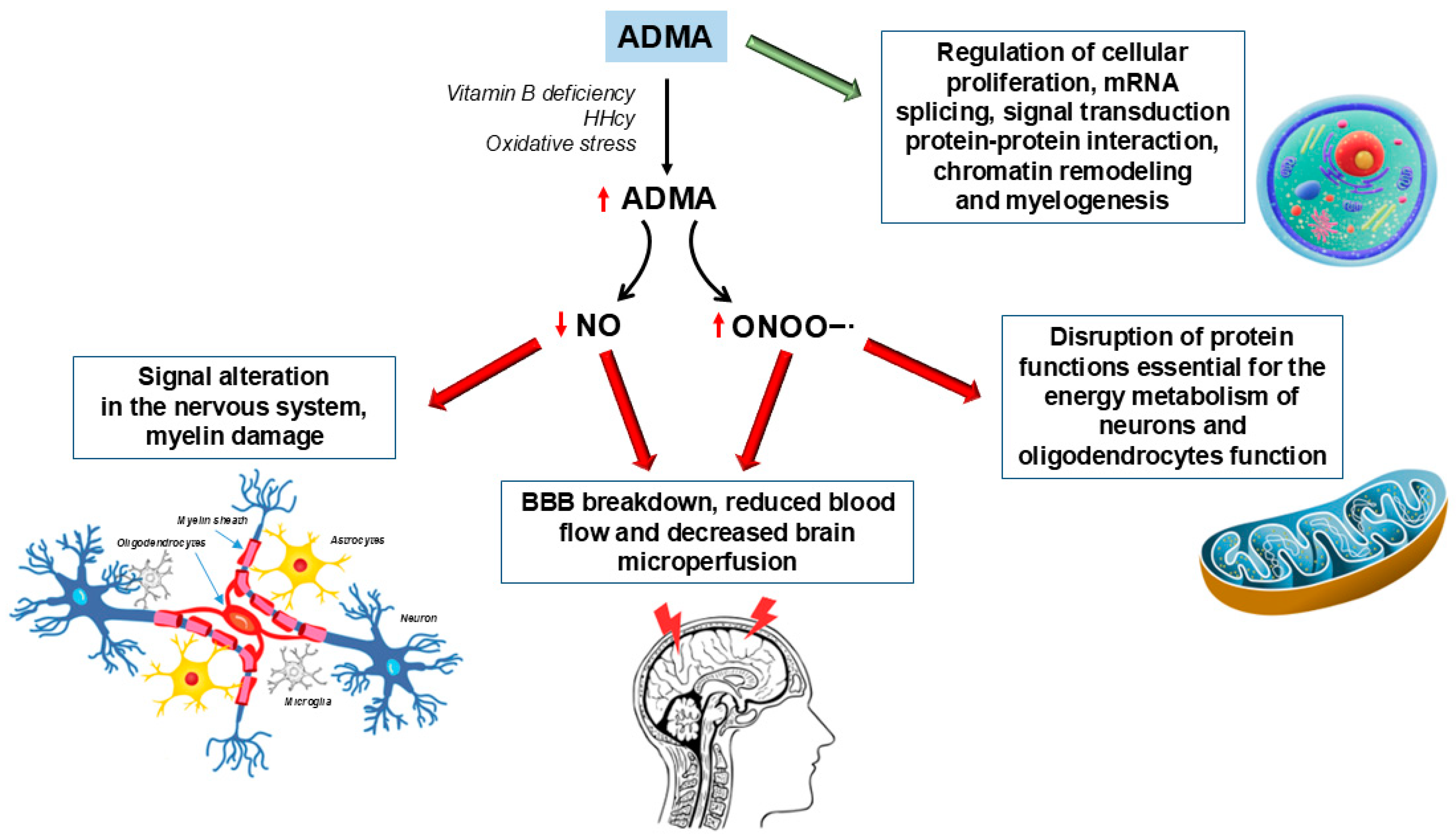
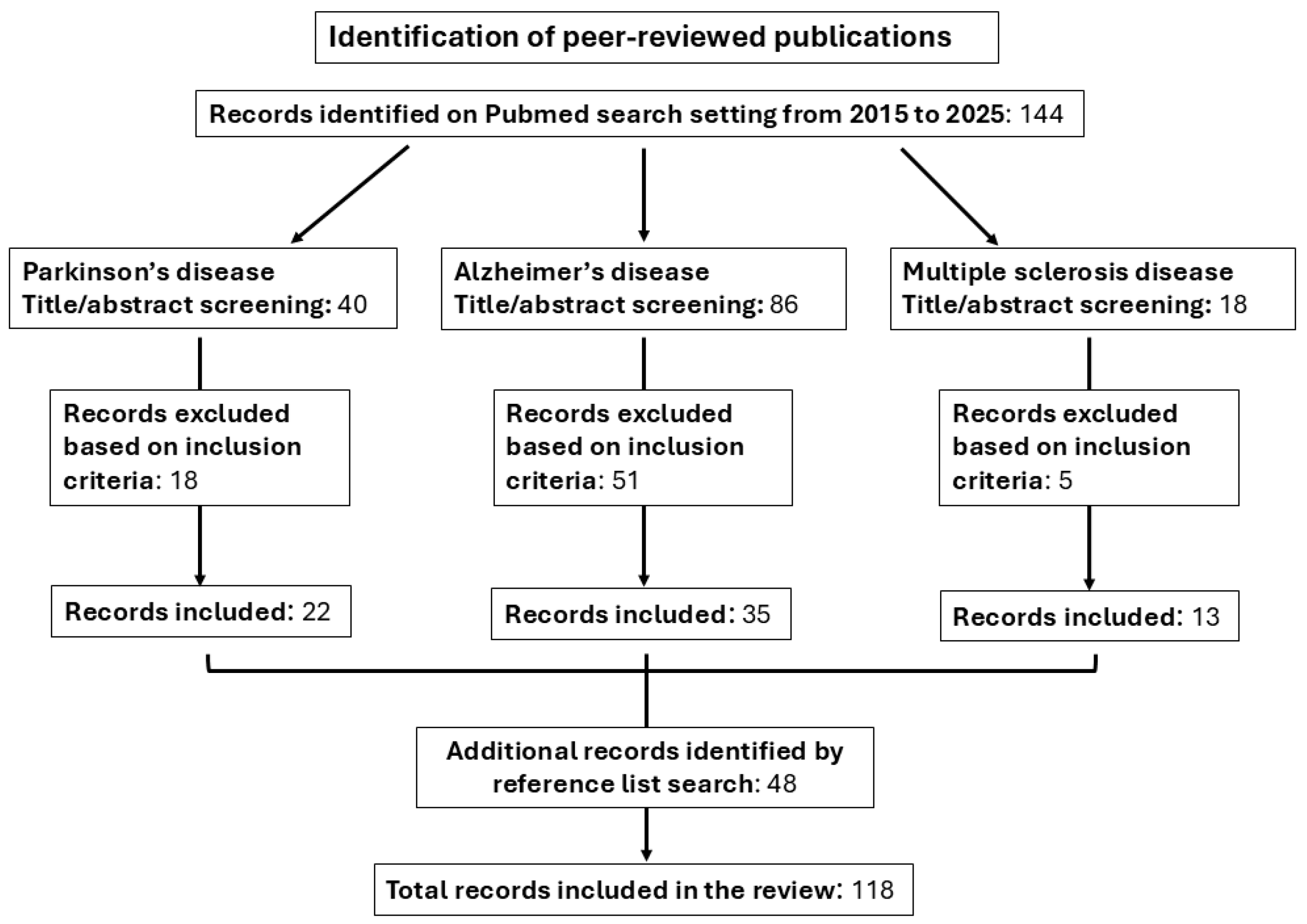
| Vitamins | Parkinson’s Disease | Alzheimer’s Disease | Multiple Sclerosis |
|---|---|---|---|
| Vitamin B12 | Vitamin B12 was deficient in PD patients [16,25] and correlated negatively with tHcy levels [1,17,26,27]. Supplementation could have positive effects in reducing tHcy, ADMA levels, and in neuroprotection [22,26,27] or be ineffective [9,16,37]. | Reduced B12 concentrations were associated with cognitive decline [47,48,49]. Supplementation in AD patients could be beneficial in reducing tHcy, improving cognitive functions and visual impairments [38,50,51,60], or be ineffective [52,53]. | Vitamin B12 levels in MS patients were associated with HHcy and disease relapses [89], but other studies did not observe this association [81,82,83,84,87]. |
| Vitamin B9 | HHcy correlated negatively with B9 levels [1,17]. Supplementation could have positive effects on reducing tHcy, ADMA levels, and vascular events [22,23] or be ineffective [9,16,37]. | Reduced B9 concentrations were associated with cognitive decline [50]. Supplementation in AD patients could be beneficial in reducing tHcy, APP, PS1, and Aβ protein levels, improving neuronal and visual functions [38,50,51,55,56,60], or be ineffective [52,53]. | No significant differences were observed compared to the control group and in children with early-onset MS [87,89]. |
| Vitamin B6 | It was deficient in PD subjects [16,32]. Dietary intake of vitamin B6 exhibited preventive effect of developing PD [16] or be ineffective [9,37]. | No significant differences were observed in children with early-onset MS [87]. | |
| Vitamin B2 | Supplementation could neutralize the effects of the MTHFR C677T polymorphism and decrease Hcy levels [34,35]. | ||
| Vitamin D | Vitamin D deficiency may be involved in learning and memory. Co-supplementation with vitamins B could have positive effects on vitamin D and SAM metabolism [54]. | Evidence has associated lower vitamin D levels with an elevated risk of developing MS or symptom relapse [95,96,97,98]. | |
| Vitamin A | Vitamin A supplement seems to be useful in neuronal plasticity and could improve cognitive ability [105,106]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saija, C.; Currò, M.; Ientile, R.; Caccamo, D.; Bertuccio, M.P. Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3672. https://doi.org/10.3390/ijms26083672
Saija C, Currò M, Ientile R, Caccamo D, Bertuccio MP. Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(8):3672. https://doi.org/10.3390/ijms26083672
Chicago/Turabian StyleSaija, Caterina, Monica Currò, Riccardo Ientile, Daniela Caccamo, and Maria Paola Bertuccio. 2025. "Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review" International Journal of Molecular Sciences 26, no. 8: 3672. https://doi.org/10.3390/ijms26083672
APA StyleSaija, C., Currò, M., Ientile, R., Caccamo, D., & Bertuccio, M. P. (2025). Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review. International Journal of Molecular Sciences, 26(8), 3672. https://doi.org/10.3390/ijms26083672






