Dementia and Sleep Disorders: The Effects of Drug Therapy in a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
- A.
- Low risk of bias: A plausible bias is unlikely to significantly alter the results (categorized as “No” in the risk of bias table).
- B.
- High risk of bias: A plausible bias that significantly weakens confidence in the results (categorized as “Yes” in the risk of bias table).
- C.
- Unclear risk of bias: A plausible bias that raises some doubt about the results (categorized as “Unclear” in the risk of bias table).
2.4. Data Synthesis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BZD | Benzodiazepines |
| LBD | Lewy body dementia |
| EEG | Electroencephalogram |
| FTD | Frontotemporal dementia |
| GABA | Gamma-aminobutyric acid |
| ICSD-3 | International Classification of Sleep Disorders |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NAMCS | National Ambulatory Medical Care Survey |
| NREM | Non-rapid eye movement |
| PDD | Parkinson’s disease dementia |
| PSQI | Pittsburgh Sleep Quality Index |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement sleep |
| SD | Standard deviation |
| 5 HT | 5-hydroxytryptamine |
References
- Salud OM de la OMS. Demencia. 2023. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dementia (accessed on 30 June 2024).
- Juarez-Cedillo, T.; Gonzelez-Figueroa, E.; Gutierez-Gutierez, L.; Aguilar-Navarro, S.G.; Garcia-Cruz, J.C.; Escobedo de la Peña, J.; Suerna-Hernandez, A. Prevalence of Dementia and Main Subtypes in Mexico: The Study on Aging and Dementia in Mexico (SADEM). J. Alzheimers Dis. 2022, 89, 931–941. [Google Scholar] [CrossRef]
- Stang, C.D.; Mullan, A.F.; Hajeb, M.; Camerucci, E.; Turcano, P.; Martin, P.; Mielke, M.M.; Josephs, K.A.; Bower, J.H.; St Louis, E.K.; et al. Timeline of Rapid Eye Movement Sleep Behavior Disorder in Overt Alpha-Synucleinopathies. Ann. Neurol. 2021, 89, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, D.; Calo, S.; Dichter, M.N.; Meyer, G.; Möhler, R.; Köpke, S. Non-pharmacological interventions for sleep disturbances in people with dementia. Cochrane Database Syst. Rev. 2023, 1, CD011881. [Google Scholar] [CrossRef]
- De Luca, R.; Todd, W.D.; Burgess, C.R. Editorial: Novel pharmacological treatments in sleep disorders. Front. Neurosci. 2022, 16, 1086983. [Google Scholar] [CrossRef]
- Cabaj, J.; Bargieł, J.; Soroka, E. Benzodiazepines and z-drugs-between treatment effectiveness and the risk of addiction. J. Educ. Health Sport 2023, 46, 468–480. [Google Scholar] [CrossRef]
- Fifel, K.; Videnovic, A. Chronotherapies for Parkinson’s disease. Prog. Neurobiol. 2019, 174, 16–27. [Google Scholar] [CrossRef]
- Marchi, N.A.; Ramponi, C.; Hirotsu, C.; Hirotsu, C.; Haba-Rubio, J.; Lutti, A.; Preisig, M.; Marques-Vidal, P.; Vollenweider, P.; Kherif, F.; et al. Mean Oxygen Saturation during Sleep Is Related to Specific Brain Atrophy Pattern. Ann. Neurol. 2020, 87, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Lutsey, P.L.; Benveniste, H.; Brown, D.L.; Full, K.M.; Lee, J.M.; Osorio, R.S.; Pase, M.P.; Redeker, N.S.; Redline, S.; et al. Impact of Sleep Disorders and Disturbed Sleep on Brain Health: A Scientific Statement From the American Heart Association. Stroke 2024, 55, e61–e76. [Google Scholar] [CrossRef]
- Contreras, A.; Perez, C. Insomnio, en busca del tratamiento ideal: Fármacos y medidas no farmacológicas. Rev. Med. Clin. Las Condez 2021, 32, 591–602. [Google Scholar] [CrossRef]
- Reuben, C.; Elgaddal, N.; Black, L.I. Sleep medication use in adults aged 18 and over: United States, 2020. NCHS Data Brief. 2023, 462, 1–8. [Google Scholar]
- Álamo González, C.; Alonso Álvarez, M.L.; Cañellas Dols, F.; Martín Águeda, B.; Pérez Díaz, H.; Romero Santo-Tomás, O.; Terán Santos, J. Pautas de Actuación y Seguimiento: Insomnio; Fundación para la Formación de la Organización Médica Colegial (FFOMC): Madrid, Spain, 2016; pp. 31–41. [Google Scholar]
- Soyka, M.; Wild, I.; Caulet, B.; Leontiou, C.; Lugoboni, F.; Haak, G. Long-term use of benzodiazepines in chronic insomnia: A European perspective. Front. Psychiatry 2023, 14, 1212028. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Benzodiazepines: Uses, Dangers, and Clinical Considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.J.; Shin, W.; Jung, H.J.; Kim, B.; Lee, B.K.; Jung, Y.S. Impact of Sleep Disorder as a Risk Factor for Dementia in Men and Women. Biomol. Ther. 2020, 28, 58–73. [Google Scholar] [CrossRef]
- Ferini-Strambi, L. Sleep disorders and increased risk of dementia. Eur. J. Neurol. 2022, 29, 3484–3485. [Google Scholar] [CrossRef]
- Stefani, A.; Santamaria, J.; Iranzo, A.; Hackner, H.; Schenck, C.H.; Högl, B. Nelotanserin as symptomatic treatment for rapid eye movement sleep behavior disorder: A double-blind randomized study using video analysis in patients with dementia with Lewy bodies or Parkinson’s disease dementia. Sleep Med. 2021, 81, 180–187. [Google Scholar] [CrossRef]
- Louzada, L.L.; Machado, F.V.; Quintas, J.L.; Ribeiro, G.A.; Silva, M.V.; Mendonça-Silva, D.L.; Gonçalves, B.S.B.; Nóbrega, O.T.; Camargos, E.F. The efficacy and safety of zolpidem and zopiclone to treat insomnia in Alzheimer’s disease: A randomized, triple-blind, placebo-controlled trial. Neuropsychopharmacology 2022, 47, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Cheng, L.; Li, S.; Xu, F. Effects of eszopiclone on sleep quality and cognitive function in elderly patients with Alzheimer’s disease and sleep disorder: A randomized controlled trial. Brain Behav. 2022, 12, e2488. [Google Scholar] [CrossRef] [PubMed]
- Franjić, S. Dementia is a Serious Disorder of Memory and other Intellectual Abilities. Int. J. Clin. Nephrol. 2023, 5, 1–5. [Google Scholar] [CrossRef]
- Nasta, B.; Hill-Strathy, M.; Biskup, E.; Rauen, K. Sleep disorders and dementia. In Sex and Gender Differences in Alzheimer’s Disease; Academic Press: Cambridge, MA, USA, 2021; pp. 207–232. [Google Scholar]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer’s disease with depression as the first symptom: A case report with literature review. Front. Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Pineda-León, M.; Searle-León, J.; Sandoval-Huenchual, D. Reporte de serie de casos de prescripción de benzodiacepinas en personas mayores con insomnio. Rev. Chil. Atención Primaria Salud Familiar 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Joyce, G.; Ferido, P.; Thunell, J.; Tysinger, B.; Zissimopoulos, J. Benzodiazepine use and the risk of dementia. Alzheimers Dement. 2022, 8, e12309. [Google Scholar] [CrossRef] [PubMed]
- Montes-Castrejon, A.; Moncayo-Samperio, L.; Flores-Ramos, M. Benzodiazepine consumption, functionality, cognition, and somnolence in older adults at a tertiary care psychiatric hospital in Mexico City. Cureus 2024, 16, e53252. [Google Scholar] [CrossRef]
- Borda, M.G.; Jaramillo-Jimenez, A.; Oesterhus, R.; Santacruz, J.M.; Tovar-Rios, D.A.; Soennesyn, H.; Cano-Gutierrez, C.A.; Vik-Mo, A.O.; Aarsland, D. Benzodiazepines and antidepressants: Effects on cognitive and functional decline in Alzheimer’s disease and Lewy body dementia. Int. J. Geriatr. Psychiatry 2021, 36, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Hrehova, L.; Mezian, K. Nonpharmacologic treatment of insomnia in primary care settings. Int. J. Clin. Pract. 2021, 75, e14084. [Google Scholar] [CrossRef]
- Richardson, K.; Loke, Y.K.; Fox, C.; Maidment, I.; Howard, R.; Steel, N.; Arthur, A.; Boyd, P.J.; Aldus, C.; Ballard, C.; et al. Adverse effects of Z-drugs for sleep disturbance in people living with dementia: A populationbased cohort study. BMC Med. 2020, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Harbourt, K.; Nevo, O.N.; Zhang, R.; Chan, V.; Croteau, D. Association of eszopiclone, zaleplon, or zolpidem with complex sleep behaviors resulting in serious injuries, including death. Pharmacoepidemiol. Drug Saf. 2020, 29, 684–691. [Google Scholar] [CrossRef]
- Heinemann, S.; Klemperer, J.; Hummers, E.; Nau, R.; Himmel, W. Reducing the use of sleep-inducing drugs during hospitalisation by a multi-faceted intervention: A pilot study. Eur. J. Hosp. Pharm. Sci. Pract. 2024, 31, 117–123. [Google Scholar] [CrossRef]
- Guo, T.; Mao, G.; Zhao, L.; Xia, D.; Yang, L. Comparative pharmacokinetics of zolpidem tartrate in five ethnic populations of China. Acta Pharm. Sin. B. 2014, 4, 146–150. [Google Scholar] [CrossRef]
- Schaller, B. Zopiclone: Some remarks on pharmacokinetics and pharmacodynamics. Swiss Med. Wkly. 2003, 133, 100. [Google Scholar] [CrossRef]
- Kuang, H.; Zhu, Y.G.; Zhou, Z.F.; Yang, M.W.; Hong, F.F.; Yang, S.L. Sleep disorders in Alzheimer’s disease: The predictive roles and potential mechanisms. Neural Regen. Res. 2021, 16, 1965–1972. [Google Scholar] [CrossRef]
- Garbarino, S.; Bragazzi, N.L. Revolutionizing sleep health: The emergence and impact of personalized sleep medicine. J. Pers. Med. 2024, 14, 598. [Google Scholar] [CrossRef] [PubMed]
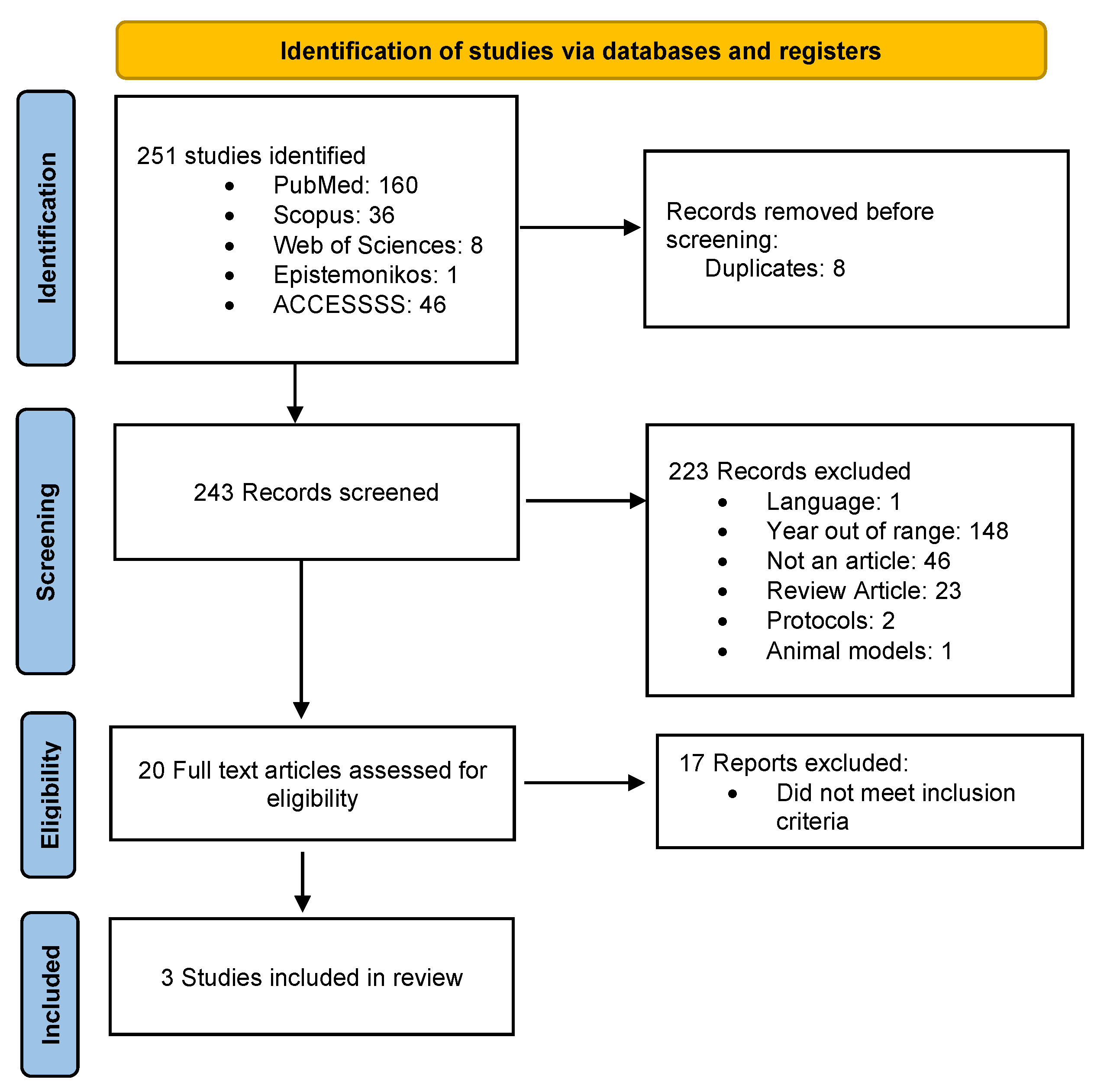
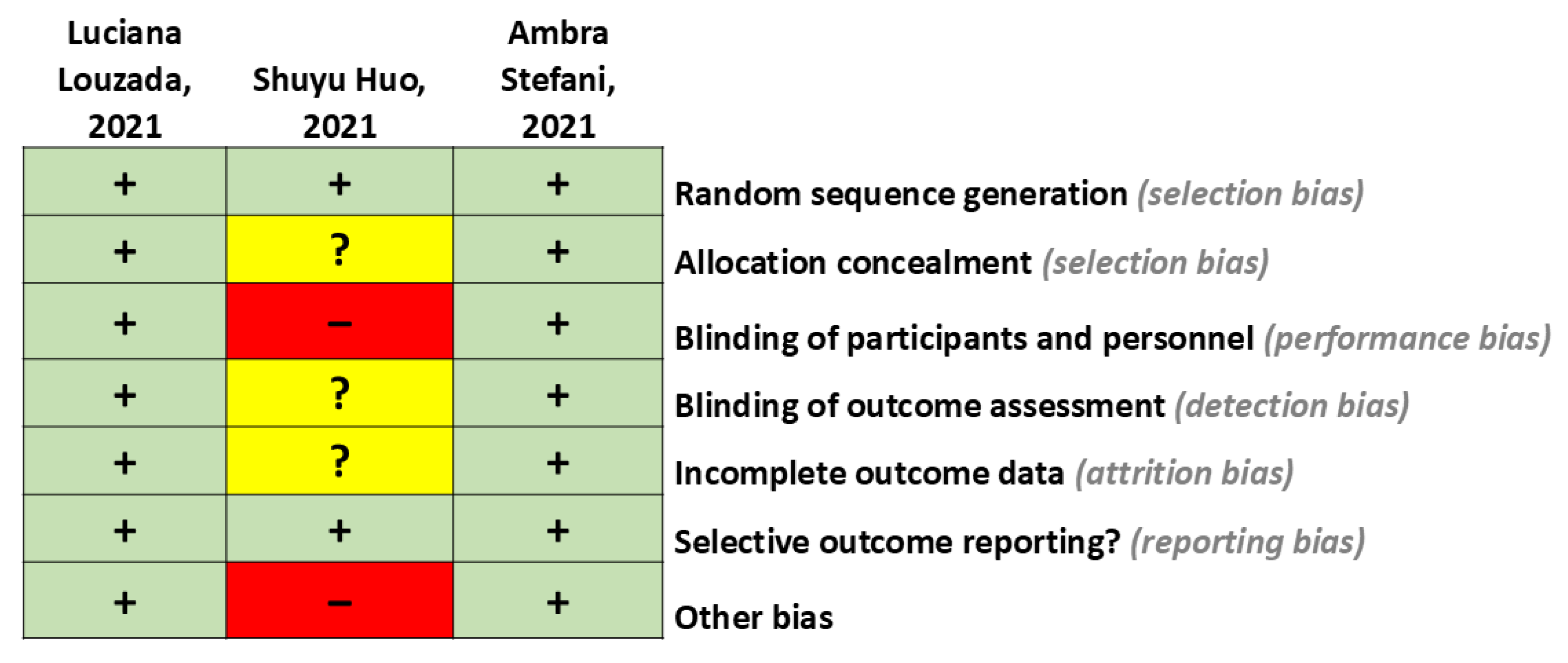

| Reference | Study Design | Follow-Up Duration | Sample | Objective Sleep Measure | Intervention/Comparison | Outcome |
|---|---|---|---|---|---|---|
| Stefani et al. (2021), USA [17] | Randomized, double-blind, placebo-controlled trial. | 4 weeks | 34 older adults (≥50 years) diagnosed with LBD or PDD and recurrent RBD (MMSE ≥ 18 or MoCA ≥ 12). | Video polysomnography (v PSG) was used to assess the number and duration of REM episodes and the nature of RBD. Movements and vocalizations in REM were classified according to the Innsbruck classification system. | n = 16: Nelotanserin 80 mg n = 18: Placebo | No significant difference was found between Nelotanserin and placebo in the change from baseline to week 4 in sleep behavior disorder events during simple/minor, simple/major, and complex REM phases per 10 min assessment frequency in REM sleep. |
| Louzada et al. (2022), Brazil [18] | Randomized, triple-blind, placebo-controlled trial | 2 weeks | 62 older adults (≥65 years) with probable late-onset Alzheimer’s dementia and insomnia (MMSE 0–24). | The main nocturnal sleep duration, duration of nocturnal awakenings, number of nocturnal awakenings, total daytime sleep duration, and number of daily naps were assessed. | n = 21: Zolpidem 10 mg/day n = 18: Zopiclone 7.5 mg/day n = 20: Placebo | Zopiclone led to an increase of 81 min in main nocturnal sleep duration; Zolpidem showed no significant difference. For the nocturnal awakening duration, Zopiclone and Zolpidem decreased awakenings by 26 and 21 min, respectively. Patients treated with Zopiclone had a reduced frequency and intensity of insomnia symptoms. |
| Huo et al. (2022), China [19] | Randomized trial controlled with alprazolam | 4 weeks | n = 96 older adults (≥60 years) diagnosed with Alzheimer’s disease and sleep disorders per ICSD-3. | Pittsburgh Sleep Quality Index and EEG were used to record sleep progression and architecture. | n = 48: Eszopiclone 3 mg/day n = 48: Alprazolam 0.4 mg/day | Eszopiclone significantly improves sleep quality, benefits sleep progression and architecture, reduces mental symptoms such as fear or anxiety, significantly increases cognitive functions, and improves patients’ quality of life. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez-Mendoza, L.F.; Vázquez-Alvarez, A.O.; Torres-Mendoza, B.M.; Trujillo-Rangel, W.A.; Torres-Sánchez, E.D.; Bracho-Valdés, I.; Delgado-Lara, D.L.C. Dementia and Sleep Disorders: The Effects of Drug Therapy in a Systematic Review. Int. J. Mol. Sci. 2025, 26, 5654. https://doi.org/10.3390/ijms26125654
Chavez-Mendoza LF, Vázquez-Alvarez AO, Torres-Mendoza BM, Trujillo-Rangel WA, Torres-Sánchez ED, Bracho-Valdés I, Delgado-Lara DLC. Dementia and Sleep Disorders: The Effects of Drug Therapy in a Systematic Review. International Journal of Molecular Sciences. 2025; 26(12):5654. https://doi.org/10.3390/ijms26125654
Chicago/Turabian StyleChavez-Mendoza, Luis Fernando, Alan O. Vázquez-Alvarez, Blanca Miriam Torres-Mendoza, Walter A. Trujillo-Rangel, Erandis D. Torres-Sánchez, Ismael Bracho-Valdés, and Daniela L. C. Delgado-Lara. 2025. "Dementia and Sleep Disorders: The Effects of Drug Therapy in a Systematic Review" International Journal of Molecular Sciences 26, no. 12: 5654. https://doi.org/10.3390/ijms26125654
APA StyleChavez-Mendoza, L. F., Vázquez-Alvarez, A. O., Torres-Mendoza, B. M., Trujillo-Rangel, W. A., Torres-Sánchez, E. D., Bracho-Valdés, I., & Delgado-Lara, D. L. C. (2025). Dementia and Sleep Disorders: The Effects of Drug Therapy in a Systematic Review. International Journal of Molecular Sciences, 26(12), 5654. https://doi.org/10.3390/ijms26125654







