Abstract
There is increasing interest in the potential therapeutic role of 5-HT (serotonin) in the treatment of neurodegenerative diseases, which are characterized by the progressive degeneration and death of nerve cells. 5-HT is a vital neurotransmitter that plays a central role in regulating mood, cognition, and various physiological processes in the body. Disruptions in the 5-HT system have been linked to several neurological and psychiatric disorders, making it an attractive target for therapeutic intervention. Although the exact causes of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) are not fully understood, researchers believe that regulating the 5-HT system could help alleviate symptoms and potentially slow the progression of these diseases. Here, we delve into the potential of harnessing 5-HT as a therapeutic target for the treatment of neurodegenerative diseases. It is important to note that the current clinical drugs targeting 5-HT are still limited in the treatment of these complex diseases. Therefore, further research and clinical trials are needed to evaluate the feasibility and effectiveness of its clinical application.
1 Introduction
Neurodegenerative disease (NDs) is a chronic, progressive disease that causes a reduction in the number of neurons in the nervous system and loss of function, severely impairing the patient’s cognitive and motor function. These diseases include Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS), among others. In these diseases, patients often have varying degrees of anxiety, depression, and cognitive impairment [1,2,3]. The pathogenesis of NDs usually involves a variety of processes, such as abnormal protein aggregation, oxidative stress, and neuroinflammation [4,5,6]. At present, the clinical treatment of NDs mainly focuses on drug therapy, and most drugs cannot achieve a radical cure for NDs. Therefore, the scientific community is working hard to find new targets that can prevent NDs and develop more effective treatments. The serotonergic system is critical in key processes that regulate the central nervous system (CNS), such as in the brain, where the synthesis of neuroactive metabolites is regulated by serotonin-related metabolites [7], Tryptophan-related metabolic pathways are also implicated in CNS diseases [8].
5-hydroxytryptamine (5-HT, also known as serotonin), is a neurotransmitter synthesized from tryptophan in mammals and is widely distributed in most peripheral tissues and the brain [9]. As a key neurotransmitter in the CNS, 5-HT plays a crucial role in regulating emotions, sleep, and appetite through synaptic transmission between neurons, and it is closely associated with brain function [10]. An excess or deficiency of 5-HT may lead to various disorders, such as depression [11], anxiety [12], sleep disorders [13], and eating disorders [14]. Recent research discovered that 5-HT may also be related to NDs, such as PD, AD, and HD [8,15,16,17,18].
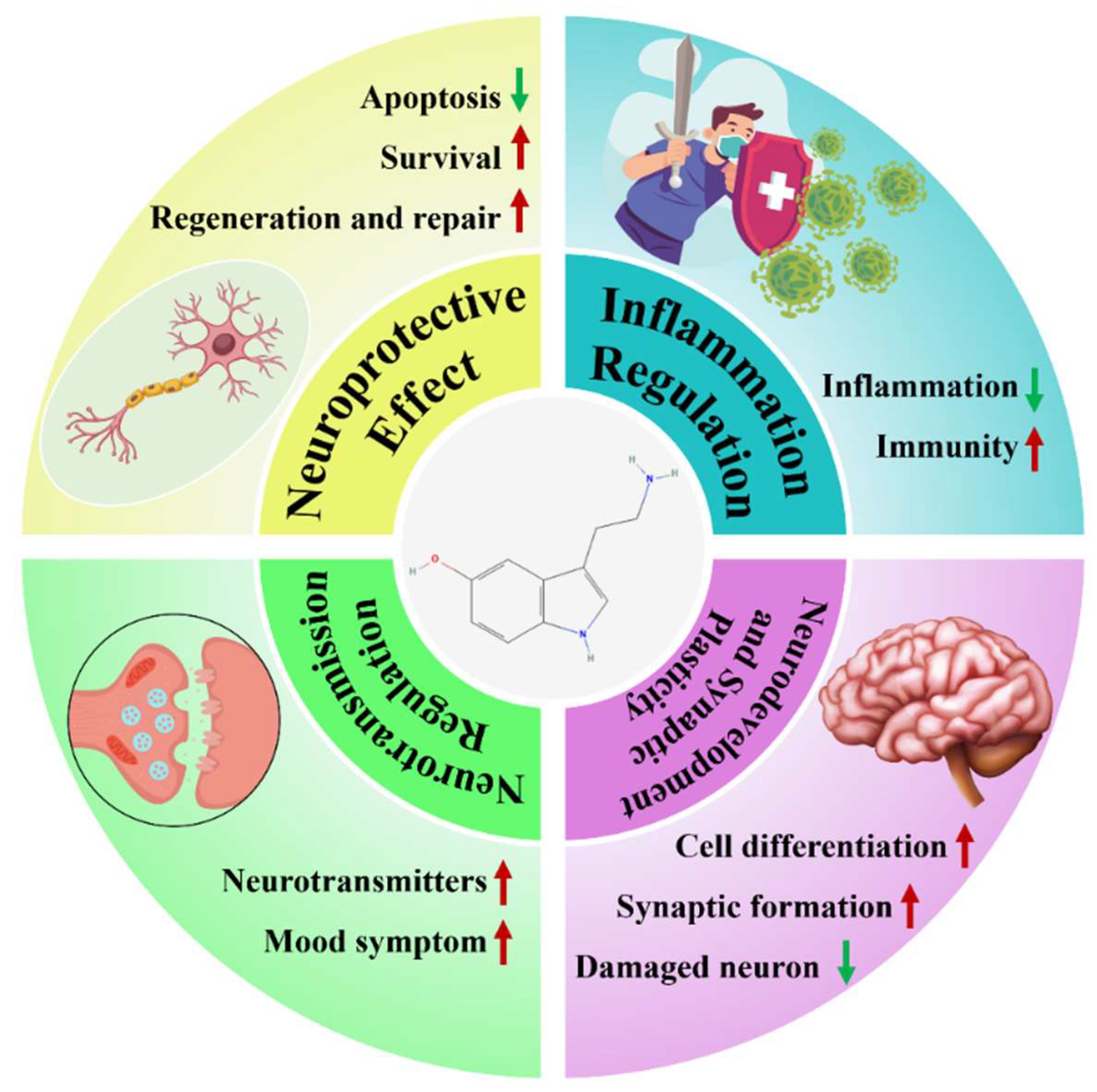
In summary, this review proposes the viewpoint that “5-HT can serve as a new target for the treatment of NDs”, (Figure 1) and this will be elaborated in detail below.
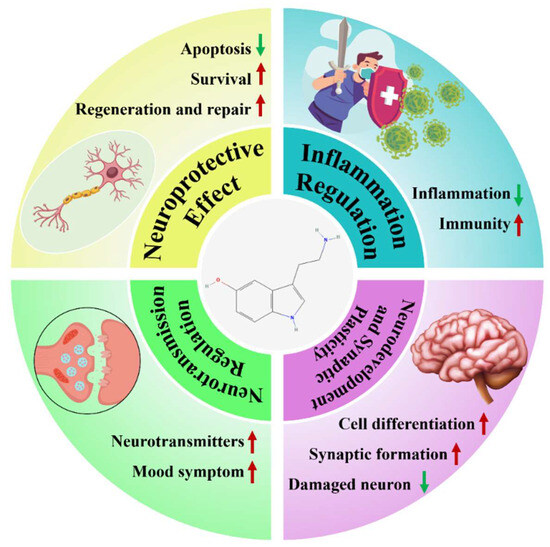
Figure 1.
Summary of the therapeutic mechanisms of 5-HT in NDs. Red arrows, upregulation; green arrows, downregulation.
2. The System of 5-HT
2.1. Characteristics of the 5-HT System
5-HT receptors are generally classified into 7 families and 14 subtypes [19]. Among them, 5-HT1 receptors are subdivided into 5-HT1A, 5-HT1B, and 5-HT1D subtypes, which are primarily distributed in the CNS and regulate the release of the 5-HT neurotransmitter. The 5-HT1A receptor is associated with anxiety and depression [20], while the 5-HT1B receptor is linked to appetite, sexual behavior, and temperature regulation [21,22,23]. The 5-HT2 receptors, which are divided into 5-HT2A, 5-HT2B, and 5-HT2C subtypes, are mainly found in the cerebral cortex and hypothalamus. The 5-HT2A receptor is related to hallucinations, delusions, and other psychotic symptoms [24]. The 5-HT3 receptor is primarily distributed in the CNS and the digestive system, and its agonists have emetic effects; thus, 5-HT3 receptor antagonists are used to treat nausea and vomiting [25]. The 5-HT4 receptor is mainly found in the brain and intestines, and its agonists can promote gastrointestinal motility and secretion, making them useful for treating intestinal motility disorders [26,27]. In addition to the aforementioned receptors, there are also 5-HT5, 5-HT6, and 5-HT7 receptors. The distribution and function of these receptors are not yet fully understood, but studies suggest they may be involved in memory, learning, sleep, and emotions. Below, we will introduce some common 5-HT receptors and write a table for easy reading (Table 1).

Table 1.
5-HT receptor, function, and known agonists/antagonist.
2.2. 5-HT Receptor and Its Function
2.2.1. 5-HT1R
The 5-HT1R family includes 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F [28,29]. These receptors, part of the serotonin receptor family, are widely distributed in the central nervous system and mainly act as inhibitory receptors. They reduce neuronal excitability by lowering intracellular cAMP levels through G protein-coupled signaling [30]. As autoreceptors on presynaptic terminals, they inhibit further serotonin release when serotonin levels rise, creating a negative feedback mechanism to maintain neurotransmitter balance. Additionally, 5-HT1R also function as heteroreceptors, regulating the release of other neurotransmitters such as norepinephrine and dopamine, thus playing a key role in regulating neural pathways and maintaining neurotransmission balance [31]. Among these, 5-HT1A is the most extensively studied subtype and is involved in various physiological and pathological processes. Its primary functions include the regulation of anxiety, mood, cognition, suicidal tendencies, appetite, sleep, and pain perception [32]. Currently, drugs targeting the 5-HT1A receptor, such as buspirone, have been applied in clinical treatments, primarily enhancing the receptor’s function to exert anxiolytic effects. The 5-HT1B/1D receptors are involved in regulating stress responses, mood, and motor control. Drugs such as naratriptan and sumatriptan are used to treat migraines by targeting these receptors [33,34,35]. The 5-HT1E and 5-HT1F receptors are involved in regulating physiological processes such as sleep, mood, and pain perception [29]. Lasmiditan, a 5-HT1F agonist approved by the FDA in 2019 for the treatment of migraines, specifically suppresses the expression of calcitonin gene-related protein (CGRP). This selective mechanism reduces the likelihood of cerebrovascular side effects compared to other migraine treatments [36].
2.2.2. 5-HT2R
The 5-HT2R family includes the 5-HT2A, 5-HT2B, and 5-HT2C subtypes [28,29]. The 5-HT2A receptor is one of the targets of hallucinogens and is involved in the regulation of cognition, mood, perception, and behavior [37]. Additionally, dopamine D2 and 5-HT2A receptors have been shown to interact [38,39], playing a crucial role in neurotransmission. 5-HT2A receptor antagonists, such as risperidone and olanzapine, are clinically used to treat schizophrenia, mood disorders, and other psychiatric conditions [40,41]. The 5-HT2B receptor is expressed throughout the brain, particularly in the cerebellum, occipital cortex, and frontal cortex [42]. Recent studies found that 5-HT2B receptor agonists may be useful therapeutic tools for improving depression treatment [43]. The 5-HT2C receptor agonist lorcaserin is used to treat obesity [44].
2.2.3. 5-HT3R
The 5-HT3R family has only one subtype, 5-HT3, which is the only known 5-HT ion channel receptor [28,29]. It is distributed in both the peripheral and CNSs and is involved in physiological processes such as the vomiting reflex, cognition, and anxiety [45]. Currently, clinical drugs targeting the 5-HT3 receptor subtype are primarily anti-nausea medications, such as ondansetron, granisetron, and palonosetron. These drugs mainly inhibit the function of the 5-HT3 receptor, thereby reducing nausea, vomiting, and other symptoms [46,47,48]. Among antipsychotic drugs, some also exhibit inhibitory effects on the 5-HT3 receptor, which may be one of the mechanisms through which they alleviate psychotic symptoms [49].
2.2.4. 5-HT4R
5-HT4Rs are widely distributed in the CNS and peripheral organs. Their biological functions include regulating neurotransmission, influencing gastrointestinal motility and secretion, and participating in memory and learning processes [50,51]. Clinically, drugs targeting 5-HT4 receptors are primarily used to treat gastrointestinal diseases, such as alleviating dyspepsia, gastroesophageal reflux, and constipation. Currently listed 5-HT4 receptor agonists include cisapride, mosapride, and tegaserod [50,52,53]. Additionally, some studies found that 5-HT4 receptor agonists may have therapeutic effects on NDs and depression, but more research is needed to confirm these findings [54,55].
2.2.5. 5-HT5R
The 5-HT5R family includes the 5-HT5A and 5-HT5B subtypes, primarily distributed in regions such as the hippocampus, hypothalamus, and amygdala. However, the functions and roles of these subtypes in clinical applications are not yet clear and require further research and exploration [56].
2.2.6. 5-HT6R
The 5-HT6R family has only one subtype, 5-HT6, which is involved in regulating memory and learning, energy metabolism, and mood [57]. Currently, a drug targeting the 5-HT6 receptor, AVN-211, is in clinical trials. It acts as a 5-HT6 receptor antagonist and has great potential for treating AD [58].
2.2.7. 5-HT7R
The 5-HT7R family includes the 5-HT7A and 5-HT7B subtypes, which are primarily distributed in CNS regions such as the hippocampus, hypothalamus, and amygdala. These receptors are involved in various biological functions, including learning and memory, mood regulation, sleep, addictive behaviors, and pain [59,60,61,62]. Activation of 5-HT7 receptors can enhance hippocampal neuron plasticity and learning and memory abilities, while their antagonists can affect these functions [63]. Current research on 5-HT7 receptors mainly focuses on antipsychotic drugs, anxiolytics, and sleep-regulating medications. Several 5-HT7 receptor antagonists, such as vortioxetine and lurasidone, improve mood, cognition, and sleep. Vortioxetine enhances serotonin transmission and cognitive function, aiding depression treatment [64]. Lurasidone, used for schizophrenia and bipolar disorder, improves mood and cognitive deficits by blocking 5-HT7 receptors, helping alleviate negative symptoms [65].
3. The Diagnostic and Therapeutic Potential of 5-HT in NDs
NDs such as PD and AD affect the function and survival of 5-HT neurons, leading to dysregulation of the 5-HT system [66,67]. Recent research highlighted the significant scientific value of 5-HT in the progression of these diseases: in the early stages or even before the onset of NDs, regulating the 5-HT system may help delay the occurrence and progression of the disease. Some 5-HT-related biomarkers can aid in diagnosing NDs; after diagnosis, monitoring indicators of 5-HT and its related metabolites can help assess disease progression and regulate its course. Additionally, modulating the 5-HT system can serve as an adjunct to existing treatments, improving therapeutic outcomes and patient quality of life.
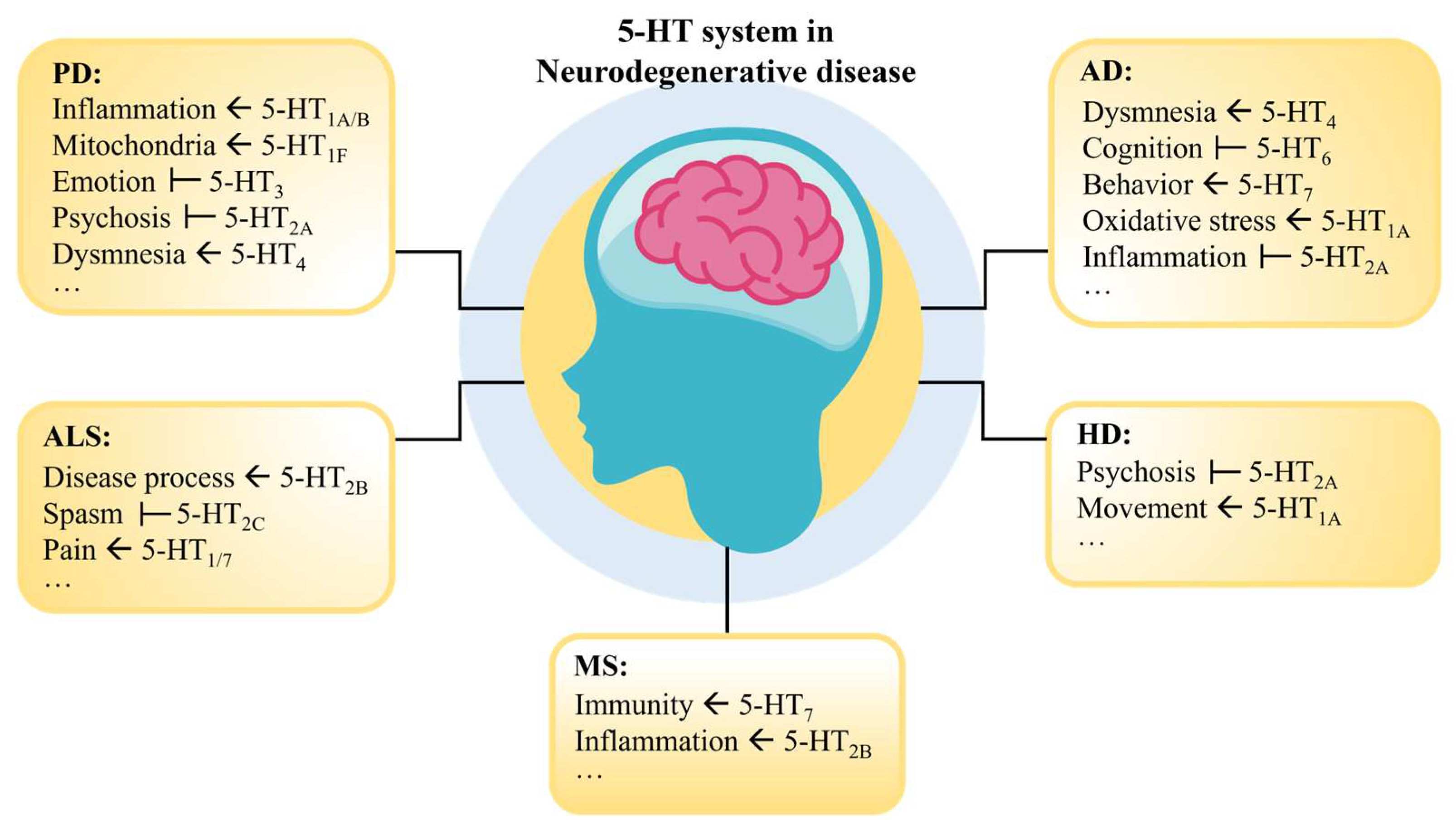
Various factors can lead to dysfunctions in the 5-HT system, causing an imbalance in bodily functions. For instance, mutations in genes such as SLC6A4 and HTR1A may lead to abnormalities in the 5-HT system, increasing the risk of depression, anxiety, and other mental disorders [68,69]. Stress, sleep deprivation, unhealthy diet, and lack of exercise can also affect the function of the 5-HT system [70,71,72]. Therefore, a deep understanding of the relationship between 5-HT and NDs can help us comprehend the pathology of these diseases and further explore therapeutic strategies for neurodegenerative disorders (Figure 2).
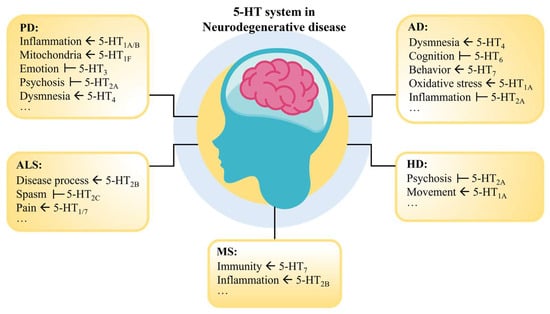
Figure 2.
Function of the 5-HT receptor in NDs. Where “ ” indicates that the activation of this receptor contributes to the recovery of the symptom; “
” indicates that the activation of this receptor contributes to the recovery of the symptom; “ ” indicates that inhibition of this receptor contributes to the recovery of the symptom.
” indicates that inhibition of this receptor contributes to the recovery of the symptom.
 ” indicates that the activation of this receptor contributes to the recovery of the symptom; “
” indicates that the activation of this receptor contributes to the recovery of the symptom; “ ” indicates that inhibition of this receptor contributes to the recovery of the symptom.
” indicates that inhibition of this receptor contributes to the recovery of the symptom.
3.1. 5-HT Targeting AD Therapy
AD is caused by the extracellular deposition of beta-amyloid (Aβ) and the abnormal accumulation of hyperphosphorylated tau protein within brain cells, leading to neuronal death and loss of motor function [73]. In AD patients, there is a complex and regionally specific alteration in the expression and function of 5-HT receptors, these changes contribute to both the cognitive and neuropsychiatric symptoms of AD. Additionally, there is significant neuronal loss and dense neurofibrillary tangles in the raphe nuclei, which are responsible for producing 5-HT, in the brains of AD patients [74]. Activation of the serotonergic system can block the inflammatory response induced by Aβ oligomers in AD [75].
The main clinical treatments for AD include cholinesterase inhibitors and NMDA receptor antagonists [76,77]. Furthermore, new drugs such as antibodies against aggregated Aβ, BACE inhibitors that inhibit Aβ production, and tau aggregation inhibitors entered clinical trials [78,79,80]. Despite extensive research, currently available small-molecule drugs for AD have significant limitations: they only improve cognition temporarily and address only the symptoms of the disease without tackling its root causes [81]. These drugs primarily target two protein targets: inhibiting acetylcholinesterase (AChE), such as donepezil, or antagonizing NMDA receptors, such as memantine [82]. In 2021, the FDA approved the first disease-modifying option, the monoclonal antibody aducanumab, bringing hope for breakthroughs in AD treatment [83]. However, the FDA’s accelerated approval was mainly based on aducanumab’s ability to clear Aβ plaques, yet a correlation between Aβ clearance and cognitive impairment has not been demonstrated [84]. Additionally, treatment costs and safety concerns are prominent issues [85]. Therefore, there is an urgent need to discover novel small-molecule drugs targeting non-standard protein targets to meet this unmet clinical need.
Recent studies confirmed that 5-HT may influence the pathological features of AD by affecting oxidative stress and Aβ deposition. Compared to AD rats, those treated with 5-HT1A receptor antagonists and 5-HT2A receptor agonists showed significant biochemical improvements, including reduced levels of brain inflammation markers, oxidative stress indicators, and Aβ deposition [86]. Loratadine, a selective 5-HT2A receptor antagonist, can alleviate inflammation by improving microglial dysfunction, promoting the clearance of neurotoxic substances, reducing Aβ deposition, and improving the pathological process in AD mice [87]. Additionally, 5-HT4 receptor agonists may prevent AD by enhancing acetylcholine release [88] and inhibiting Aβ secretion [89].
5-HT-related drugs may also improve the most prominent cognitive impairment issues in AD. 5-HT6 receptor antagonists can enhance cognitive function by modulating cholinergic neurotransmission: studies have shown that 5-HT6 receptor antagonists (such as idalopirdine) have the potential to improve memory and learning abilities in clinical trials. 5-HT4 receptor agonists can enhance acetylcholine release, which helps improve cognitive function and memory formation. Some AD patients may exhibit behavioral and psychological symptoms such as hallucinations and delusions; 5-HT2A receptor antagonists, such as brexpiprazole and pimavanserin, have been shown to be effective in AD patients with severe psychosis. Brexpiprazole, in particular, acts through a more complex pharmacological profile, involving not only 5-HT2A receptor antagonism, but also modulation of other neurotransmitter systems, which contributes to its efficacy in alleviating psychotic symptoms in these patients [90,91].
Additionally, 5-HT6 receptor antagonists can not only improve cognitive function in AD patients, but also potentially provide neuroprotection and anti-neuroinflammatory effects by regulating neurotransmitter release, improving synaptic function, and reducing neuroinflammation [92]. Novel multi-target ligands designed for the 5-HT4 receptor can bind to the 5-HT4 receptor, increase neuronal survival rates, reduce oxidative stress-induced cellular damage, and improve synaptic plasticity, offering new avenues for future AD treatment [93].
3.2. 5-HT Targeted PD Therapy
PD involves the loss of dopaminergic neurons, which may be caused by mitochondrial dysfunction and oxidative stress [94]. Before motor symptoms appear, PD can also cause emotional, cognitive, and psychiatric issues [95]. The hallmark of this disease is the early and significant death of dopaminergic neurons in the substantia nigra pars compacta (SNpc), leading to dopamine deficiency in the basal ganglia (BG) and progressing to the characteristic motor disorders of PD [96]. Additionally, the aggregation of alpha-synuclein is a key pathological feature of PD, contributing to the formation of Lewy bodies [97]. Increasing evidence suggests that, in addition to dopamine, 5-HT also plays a role in PD [98]. The 5-HT system originates in the raphe nuclei and projects to the BG, including the SNpc and caudate-putamen [9]. In PD patients, 5-HT neurotransmission is reduced in the late stages of the disease due to degeneration of the dorsal raphe nucleus [99]. Moreover, decreased levels of 5-HT, its metabolite 5-HIAA, and 5-HT transporters (SERT) have been found in the BG of some patients [100,101]. Notably, PD patients often exhibit emotional symptoms such as anxiety and depression before motor symptoms arise [102]. PET studies have shown that 5-HT neurotransmission dysfunction may be related to resting tremor [103]. As the disease progresses, the dopamine deficiency in the striatum and the 5-HT defect in the raphe nuclei may jointly contribute to the occurrence of resting tremor. Therefore, 5-HT treatment may be beneficial for PD patients with tremor related to raphe nucleus dysfunction [103].
To date, dopamine replacement therapy with levodopa combined with peripheral decarboxylase inhibitors remains the most effective treatment for alleviating motor symptoms of PD [104]. However, many PD patients experience motor fluctuations due to the diminishing effectiveness of the treatment over time [105], with the most severe being levodopa-induced dyskinesia [104,106]. Motor fluctuations can be improved by reducing the dose of levodopa, but this affects the control of PD motor symptoms. Therefore, there is an urgent need to develop new effective therapies to prevent and manage motor complications and dyskinesia associated with dopamine replacement therapy. Currently, treatment for all PD patients is symptomatic, focusing on improving both motor (e.g., tremor, rigidity, and bradykinesia) and non-motor (e.g., constipation, cognition, mood, and sleep) symptoms, with no available treatments that modify the course of the disease [107].
The vast majority of scholars believe that neuroinflammation and oxidative stress are closely related to the progression of PD. 5-HT1A receptor agonists can alleviate levodopa-induced dyskinesia [108] and protect cells from MPTP-induced dopaminergic neurotoxicity [109], thus having neuroprotective potential. The 5-HT1A and 5-HT1B receptors also play roles in glial cell formation associated with PD and motor disorders: levodopa combined with 5-HT1A/1B receptor agonists treated PD-induced motor disorders in rats and delayed the occurrence of neuroinflammatory markers in the nigrostriatal system [110]. Additionally, activating the 5-HT1F receptor can promote mitochondrial biogenesis, reduce oxidative stress, and thus protect neuronal function and survival in PD mice [111]. Stimulating the 5-HT1A receptor can promote astrocyte proliferation and upregulate antioxidant molecules [112], and 5-HT6 receptor antagonists can also protect astrocytes from damage [113]. The selective 5-HT1A receptor agonist 8-OH-DPAT can protect hippocampal neurons from transient ischemia-induced damage in gerbils by regulating NMDA receptor NR1 subunit phosphorylation and increasing the expression of brain-derived neurotrophic factor (BDNF) [114].
Motor disorders significantly impact the quality of life for patients with PD. As PD progresses, common motor symptoms often reverse into L-DOPA-induced dyskinesia (LID), which may limit PD treatment [115]. In the 5-HT system, 5-hydroxytryptophan (5-HTP), a direct precursor of 5-HT, demonstrated significant anti-dyskinetic effects in PD rat models [116]. In PD mouse models, 5-HTP has been shown to reduce LID by modulating the AKT/mTOR/S6K signaling pathway [117], suggesting that 5-HT might have therapeutic effects on L-DOPA-induced motor disorders. Additionally, 5-HT2A receptor antagonists have been shown to alleviate motor disorders in MPTP-induced PD mice [118]. Ondansetron, a 5-HT3 receptor antagonist, can alleviate L-DOPA-induced motor disorders without affecting the therapeutic efficacy of L-DOPA, indicating that selective 5-HT3 blockade may be an effective strategy for mitigating L-DOPA-induced motor disorders and slowing their progression [119]. Dopamine agonists used to treat PD can relieve motor symptoms but often cause side effects such as nausea and vomiting [120]. Since 5-HT receptors play a role in the emetic reflex, 5-HT-targeting drugs can help mitigate these side effects. As such, 5-HT drugs may be used in combination with dopamine agonists to alleviate the adverse effects of dopamine therapy, improving the overall treatment approach for PD [121].
In clinical studies, non-motor symptoms of PD also received considerable attention. Depression and anxiety are common emotional disorders in PD patients. Selective serotonin reuptake inhibitors (SSRIs) can help alleviate depressive symptoms in PD patients [122]. Additionally, some studies suggest that 5-HT3 receptor antagonists may be a potential treatment for depression and anxiety [123,124,125]. Research has shown that the 5-HT2A receptor is associated with various psychiatric disorders, including psychosis. The highly selective 5-HT2A receptor antagonist EMD-281,014 effectively reduces LID and psychosis-like behaviors in MPTP-lesioned common marmosets without affecting L-DOPA’s therapeutic effects on PD, demonstrating its potential as an anti-dyskinetic and antipsychotic strategy [126]. Selective 5-HT2A antagonists/inverse agonists, such as Pimavanserin and M100907, can reverse psychosis-like behavior deficits in animal models of PD psychosis (PDP) without affecting motor behavior, suggesting that 5-HT2A antagonism/inverse agonism may aid in the treatment of PDP [127]. Notably, Parkinson’s patients treated with the 5-HT2A receptor antagonist Pimavanserin showed significant improvements in visual hallucinations and delusions [128]. Furthermore, pain is considered a significant non-motor manifestation of PD. Neuropathic pain is associated with changes in 5-HT levels [129]. Research indicates that inhibiting spinal 5-HT3 receptors and the excitability of spinal dorsal horn (SDH) neurons can reduce pain perception in PD rats [130]. Constipation and other gastrointestinal motility disorders also occur in many PD patients. Recent research found that electroacupuncture can significantly improve gut motility in PD mice, possibly through the stimulation of 5-HT4 receptor expression, which promotes the cAMP/PKA signaling pathway [131].
The functional involvement of 5-HT receptors in learning and memory may improve cognitive impairments in PD patients. For example, the 5-HT7 receptor is involved in memory deficits induced by long-term isoflurane anesthesia [132] and the 5-HT4 receptor also shows potential in learning and memory [133]. A recent study suggests that memory processing in rats is associated with the 5-HT system, which may involve 5-HT1D and 5-HT1F receptors in the hippocampus [134].
3.3. 5-HT Targeting ALS Therapy
ALS, also known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder characterized by muscle weakness, atrophy, and stiffness, eventually leading to respiratory muscle weakness and death [135]. The etiology and pathogenesis of ALS remain unclear, but genetic variations and environmental factors are involved. Approximately 10% of cases are familial ALS (FALS), associated with genetic factors, while 90% are sporadic ALS (SALS) [135]. ALS primarily involves neuronal damage and death, and currently, there is no effective treatment for this devastating disease. Previous studies have shown that the pathogenesis of ALS is closely related to 5-HT [136,137,138]. While there is no cure for ALS, finding new therapeutic approaches is crucial. Although there is currently no evidence that 5-HT-targeted therapy can directly cure ALS, it has the potential to alleviate symptoms, improve quality of life, and reduce patient discomfort, which is significant for ALS patients.
The pathology of ALS mainly involves neuronal damage, glial cell abnormalities, neuroinflammatory responses, and abnormal protein aggregation. In these processes, 5-HT may act as a therapeutic target. Previous studies found that glutamate-induced neurotoxicity is triggered in rat nucleus accumbens neurons by reducing the binding of 5-HT to presynaptic 5-HT1B receptors, and ultimately leading to the rapid loss of motor neurons [139], 5-HT can achieve neuroprotective and anti-inflammatory effects by modulating glutamate excitability [138,140]. The 5-HT2B receptor is upregulated during ALS mouse spinal cord disease, and the deletion of the HTR2B gene encoding 5-HT2BR exacerbates the disease outcome in ALS mice [141]. Although treatment with a 5-HT2B receptor agonist did not improve the ultimate disease outcome in ALS mice, it was found to modulate microglial gene expression, slowing the progression of ALS, suggesting that targeting 5-HT2B might be a potential therapeutic approach [142]. After administration of 5-HT receptor antagonists, increased expression of TDP-43 (transcriptional regulator) and superoxide dismutase 1 (SOD1) proteins was observed, along with a significant increase in the number of astrocytes and microglia in certain anatomical regions and a reduction in the number of neurons. This may indicate that inhibition of certain 5-HT receptors promoted the onset of ALS [143]. Additionally, inflammation, including autoimmune inflammation, has been documented in the course of ALS, but treatments targeting inflammation have not yet been successful [144]. Previous studies have shown that the expression of the 5-HT7 receptor parallels the increased expression of TNF-α, IL-1β, and NF-κB, and 5-HT7 receptor agonists can produce anti-inflammatory effects [145], suggesting that 5-HT may slow the progression of ALS through anti-inflammatory mechanisms.
In the course of ALS, issues such as muscle spasms and pain, which significantly impact patients’ quality of life, need urgent solutions. Studies found that 5-HTP, a precursor of 5-HT metabolism, can increase 5-HT levels in ALS mice, improving motor function and survival rates [146]. The degeneration of 5-HT neurons may underlie spasms in ALS mice, and it has been found that 5-HT2B/C receptor inverse agonists can eliminate spasticity symptoms, suggesting that these agonists may improve motor function in ALS [147,148]. Pain, including neuropathic pain and spasticity-related pain, is a common yet overlooked symptom in ALS patients. Existing research confirmed that several 5-HT receptors play crucial roles in the progression and regulation of central and peripheral pain, particularly 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors [149]. Studying these receptors could potentially alleviate common pain symptoms in ALS patients. Sleep disruption is also prevalent among ALS patients [150] and 5-HT helps increase sleep propensity and regulate sleep disorders [151]. Additionally, early supplementation with 5-HT may alleviate or delay dysphagia symptoms, which occur in some ALS patients, thereby improving their quality of life [152].
Significant research has been conducted on survival prediction and disease prevention in ALS patients. Studies found that platelet 5-HT levels are associated with the survival of ALS patients, with higher platelet 5-HT levels correlating with longer survival, suggesting that platelet 5-HT levels may be a potential biomarker for predicting patient survival and are of great importance for the management and research of ALS [153]. Notably, research has shown that systemic injection of 5-HTP can delay hindlimb muscle weakness in ALS mice and reduce muscle tone. Additionally, high-dose administration of 5-HTP can significantly extend the lifespan of ALS model mice [138].
3.4. 5-HT Targeted HD Therapy
HD is an inherited neurodegenerative disorder affecting the nervous system, leading to the gradual loss of mental and motor functions, ultimately resulting in death [154]. It is caused by a defect in the Huntington gene (HTT) on chromosome 4, leading to the production of the Huntingtin protein. While this protein typically supports the development and function of the nervous system, in individuals with HD, it forms large abnormal aggregates within brain cells, eventually causing neuronal death and brain tissue degeneration [155]. Currently, there is no cure for HD, and only medications such as antipsychotics, antidepressants, anxiolytics, and psychotherapy can improve the quality of life for patients. Research has shown that there is a significant increase in SERT in the striatal tissue of grade 4 HD patients [156], and autopsy reports of HD patients also found significantly elevated levels of 5-HT [157], indicating a relationship between 5-HT and the pathogenesis of HD [156,158]. Thus, 5-HT is a potential therapeutic target for HD.
It is widely accepted that 5-HT receptor drugs can alleviate neuroinflammation, oxidative stress, and reduce apoptosis [159]. By modulating the 5-HT system, they may provide neuroprotective effects for HD patients. For example, N-acetyl-5-HT (NAS), a metabolite formed by the acetylation of 5-HT, has been shown to exert antioxidant and anti-apoptotic effects through the activation of the TrkB/CREB/BDNF pathway and the expression of antioxidant enzymes, demonstrating neuroprotective properties against oxidative stress-induced neurotoxicity [160]. HD patients often face severe motor disturbances, cognitive deficits, and psychological symptoms. Targeting the 5-HT system to regulate neurotransmission may improve these symptoms and enhance the quality of life for HD patients. Previous studies found that a single HD patient treated with perospirone, which acts as an antagonist for the 5-HT2A receptor and an agonist for the 5-HT1A receptor, showed significant control over involuntary movements and psychiatric symptoms [161], providing new avenues for future HD treatment research. Depression, anxiety, and irritability are common neuropsychiatric features in the preclinical stages of HD. Research indicates that altered 5-HT signaling underlies the occurrence of preclinical depression in HD [162], and several 5-HT receptors mediate anxiety behaviors [163]. Studies also found that many 5-HT receptors are associated with traits such as irritability and impulsivity [164].
3.5. 5-HT Targeted MS Therapy
MS is an autoimmune disease affecting the CNS, characterized by neuroprotective myelin damage and neuronal death. Myelin damage disrupts neural signal conduction, leading to motor, sensory, and cognitive issues. The etiology of MS is unclear, but likely involves genetic, environmental, and immune system factors [165]. Genes in the human leukocyte antigen (HLA) region may increase MS risk [166]. Sunlight, vitamin D, and geographic location also influence MS risk, with higher latitudes associated with greater risk [167]. Abnormal immune responses and infections may further contribute to MS development [168].
MS is a complex disease with limited treatment options. Previous studies noted reduced serum and platelet 5-HT levels in MS patients [169]. Among 5-HT receptors, the 5-HT7 receptor can activate initial T cells, influencing inflammatory responses. Research indicates that 5-HT7 is dysregulated in MS patients and can promote the release of IL-10, a potent immunosuppressive cytokine, suggesting that the 5-HT7 receptor may have immunoprotective effects [170]. Therefore, 5-HT7 can be considered an interesting therapeutic target for MS. Fluoxetine, a selective serotonin reuptake inhibitor, has been reported to have anti-inflammatory effects in MS [171]. Both fluoxetine and 5-HT can inhibit the production of IL-17, IFN-γ, and GM-CSF in stimulated CD4 T cells. Blocking the 5-HT2B receptor alters these effects, while activating the 5-HT2B receptor does not. These findings suggest that fluoxetine has anti-inflammatory effects in MS, possibly mediated through 5-HT2B receptor activation [172].
Similar to ALS patients, MS patients also experience widespread neuropathic pain and spasms [173], significantly affecting their quality of life. Research found that 5-HT reuptake inhibitors have some effect on central neuropathic pain [174] and 5-HT2B and 5-HT2C receptors on motor neurons can lead to the recovery of motor neuron function and spasticity post-injury [175]. These findings suggest that 5-HT can alleviate pain in MS patients.
Furthermore, studies have shown that the incidence of depression and anxiety is higher in MS patients than in the general population [176,177]. In individuals with one or two short allele copies of the 5-HT transporter-linked polymorphic region (5-HTTLPR), a stronger association was found between the burden of major life events and the severity of MS-related depression. This implies that polymorphisms in the 5-HT transporter gene modify the relationship between major life events and depression in MS patients [178].
Currently, research on 5-HT as a therapeutic target for MS is in its early stages, and its efficacy and safety need further investigation and clinical trials. Although there is still insufficient evidence to support 5-HT as an effective treatment strategy for MS, studying 5-HT and its receptor drugs may reveal potential benefits for MS symptoms, providing direction and rationale for future clinical research and drug development.
3.6. The Potential of 5-HT as a Biomarker in Diagnosing NDs.
In the treatment of any disease, early diagnosis and prognosis are crucial, and searching for biomarkers to diagnose AD and assess neurodegeneration is a popular topic. Studies found that 5-HT levels in the brains of AD patients are reduced [179], and measuring levels of 5-HT and its metabolite, 5-HIAA, in cerebrospinal fluid may aid in diagnosing and monitoring AD progression [180]. PET imaging of 5-HT1A receptors can evaluate mild neurodegenerative changes in AD [66] and lower 5-HT levels may be associated with poorer prognoses, such as cognitive decline and worsening emotional symptoms in both AD and PD patients.
For PD, effective clinical biomarkers are currently lacking. Research indicates that plasma levels of 5-HT and 5-HIAA are decreased in PD patients, suggesting these could serve as peripheral markers for non-motor symptoms of PD [181]. Additionally, reduced expression of the SERT is linked to the neurodegenerative process in PD. PET imaging has shown that the binding levels of SERT in the brains of clinically advanced, non-depressed PD patients are significantly reduced in multiple brain regions, indicating that serotonergic neuron damage may be a common feature in advanced PD [182].
Regarding HD, genetic testing is typically required for diagnosis, but early diagnosis and reliable biomarkers are essential for disease management and the development of treatment strategies. Recent studies have shown significantly reduced 5-HT levels in HD patients, indicating that this reduction could serve as a potential biomarker for HD, providing new directions for the diagnosis and treatment of HD [183].
4. Progress of Clinical Research on Targeting 5-HT for NDs
In the treatment of PD, the clinical application of 5-HT as a drug target is relatively limited. Pimavanserin, a popular drug, played a significant role in several clinical studies. Pimavanserin is a selective 5-HT2A receptor inverse agonist primarily used to treat hallucinations and delusions in PD patients, particularly those associated with PDP. It alleviates these non-motor symptoms by reducing the activity of 5-HT2A receptors. In addition to Pimavanserin, other studies explored the potential applications of various 5-HT system modulators in PD treatment.
Similarly, the clinical application of 5-HT-targeted drugs in AD treatment is also relatively limited. Currently, no specific 5-HT drugs have been approved for treating core symptoms of AD, such as cognitive decline and memory impairment. However, some studies are investigating methods to improve AD symptoms and disease progression by modulating the 5-HT system. These drugs mainly aim to alleviate AD-related behavioral and psychological symptoms, such as anxiety and depression, by modulating 5-HT receptors and influencing neurotransmitter activity. In the context of HD, ALS, and MS, 5-HT is rarely used as a drug target in clinical research. Current research primarily focuses on the potential of these drugs to improve motor behavior in these diseases.
The increasing number of clinical studies targeting 5-HT indicates that many institutions and research units can no longer overlook the significant role of 5-HT in the nervous system (Table 2). This recognition marks a new starting point for the treatment of NDs.

Table 2.
Clinical research of 5-HT in ND in the past 5 years. In status, “+” represents completed; “=” represents recruiting; and “−” represents not yet recruiting.
5. Conclusions
In conclusion, substantial research has been conducted on the role of 5-HT in NDs, but ongoing exploration is essential for further advancements. 5-HT exerts neuroprotective effects by promoting neuronal survival and function. It also has anti-inflammatory and immunomodulatory properties, regulating immune cell function and the extent of inflammatory responses. Furthermore, 5-HT plays a crucial role in modulating neural transmission through its interaction with receptors, which is significant in neurodegenerative conditions. Additionally, 5-HT influences neuronal differentiation, migration, and synapse formation, playing a key regulatory role in neural network formation and function.
Current studies indicate that 5-HT receptor agonists and antagonists may positively impact symptom relief and disease progression in NDs, potentially alleviating some side effects associated with current clinical treatments. This suggests that exploring the relationship between 5-HT and NDs can unveil new therapeutic strategies and drug targets. However, translating these findings into clinical outcomes remains limited.
While targeting the 5-HT system for treating NDs shows promise, it is important to note that it cannot yet serve as a standalone treatment method. The role of 5-HT in the brain is highly complex, and solely targeting 5-HT may lead to unforeseen side effects. Moreover, long-term use of 5-HT drugs can lead to tolerance, reducing their efficacy over time. Many potential 5-HT-targeting drugs struggle to cross the blood–brain barrier, limiting their effects on the CNS. Addressing these issues, future research could develop drugs that act on multiple neurotransmitter systems, reducing side effects associated with single-target treatments. For instance, modulators combining dopamine and 5-HT could offer more comprehensive therapeutic effects. Advanced drug delivery systems such as lipid nanoparticles, biomimetic nanoparticles, or other innovative methods could enhance the efficiency of crossing the blood–brain barrier.
Interestingly, studying the changes in 5-HT during neurodegenerative disease progression revealed potential biomarkers and indicators for early diagnosis and assessment. These biomarkers can significantly impact the treatment of NDs by monitoring disease progression, evaluating therapeutic efficacy, aiding clinical decisions, and potentially preventing disease onset. Consequently, more fundamental research is required to support these concepts, facilitating the translation of findings into clinical practice and genuinely improving patient outcomes and quality of life.
Author Contributions
H.C. and C.X. wrote the manuscript. T.L. and W.B. designed and organized the review. C.X., H.D. and Z.H. supervised the work and contributed to the design and organization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (32300682 to C.X.) and the Fundamental Research Funds for the Central Universities (FRF-TP-22-007A1 to C.X.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge our host institutions’ excellent environment and support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-HT | Serotonin |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic lateral sclerosis |
| MS | Multiple sclerosis |
| ND | Neurodegenerative disease |
| CNS | central nervous system |
| AChE | acetylcholinesterase |
| Aβ | beta-amyloid |
| SNpc | substantia nigra pars compacta |
| BG | basal ganglia |
| SERT | 5-HT transporters |
| BDNF | brain-derived neurotrophic factor |
| LID | L-DOPA-induced dyskinesia |
| 5-HTP | 5-hydroxytryptophan |
| SSRIs | Selective serotonin reuptake inhibitors |
| PDP | PD psychosis |
| SDH | spinal dorsal horn |
| FALS | familial ALS |
| SALS | sporadic ALS |
| TDP-43 | TAR DNA-binding protein 43 |
| SOD1 | superoxide dismutase 1 |
| NAS | N-acetyl-5-HT |
| HLA | human leukocyte antigen |
| 5-HTTLPR | 5-HT transporter-linked polymorphic region |
| ICD | Impulse control disorder |
References
- Pentkowski, N.S.; Rogge-Obando, K.K.; Donaldson, T.N.; Bouquin, S.J.; Clark, B.J. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neurosci. Biobehav. Rev. 2021, 127, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Agarwal, P. Depression and Anxiety in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.; van Duijn, E. Anxiety in Huntington’s Disease. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol. Aging 1987, 8, 345–346. [Google Scholar] [CrossRef]
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C.; et al. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging Dis. 2023, 14, 858–878. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Sahu, A.; Gopalakrishnan, L.; Gaur, N.; Chatterjee, O.; Mol, P.; Modi, P.K.; Dagamajalu, S.; Advani, J.; Jain, S.; Keshava Prasad, T.S. The 5-Hydroxytryptamine signaling map: An overview of serotonin-serotonin receptor mediated signaling network. J. Cell Commun. Signal. 2018, 12, 731–735. [Google Scholar] [CrossRef]
- Coppen, A.J.; Doogan, D.P. Serotonin and its place in the pathogenesis of depression. J. Clin. Psychiatry 1988, 49 (Suppl. S49), 4–11. [Google Scholar] [PubMed]
- Baldwin, D.; Rudge, S. The role of serotonin in depression and anxiety. Int. Clin. Psychopharmacol. 1995, 9 (Suppl. S4), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R. Serotonin and sleep. Sleep. Med. Rev. 2002, 6, 55–67. [Google Scholar] [CrossRef]
- Haleem, D.J. Serotonin neurotransmission in anorexia nervosa. Behav. Pharmacol. 2012, 23, 478–495. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Central nervous system effects of 5-HT(7) receptors: A potential target for neurodegenerative diseases. Mol. Med. 2022, 28, 70. [Google Scholar] [CrossRef]
- Hsam, O.; Kohl, Z. Serotonin in synucleinopathies. Behav. Brain Res. 2023, 445, 114367. [Google Scholar] [CrossRef]
- Huot, P.; Sgambato-Faure, V.; Fox, S.H.; McCreary, A.C. Serotonergic Approaches in Parkinson’s Disease: Translational Perspectives, an Update. ACS Chem. Neurosci. 2017, 8, 973–986. [Google Scholar] [CrossRef]
- Eremin, D.V.; Kondaurova, E.M.; Rodnyy, A.Y.; Molobekova, C.A.; Kudlay, D.A.; Naumenko, V.S. Serotonin Receptors as a Potential Target in the Treatment of Alzheimer’s Disease. Biochemistry 2023, 88, 2023–2042. [Google Scholar]
- Ohno, Y.; Tatara, A.; Shimizu, S.; Sasa, M. Management of cognitive impairments in schizophrenia: The therapeutic role of 5-HT receptors. In Schizophrenia Research: Recent Advances; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 321–336. [Google Scholar]
- Garcia-Garcia, A.L.; Newman-Tancredi, A.; Leonardo, E.D. P5-HT1A receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 2014, 231, 623–636. [Google Scholar] [CrossRef]
- Voigt, J.P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Giuliano, F. 5-Hydroxytryptamine in premature ejaculation: Opportunities for therapeutic intervention. Trends Neurosci. 2007, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hagan, J.J.; Slade, P.D.; Gaster, L.; Jeffrey, P.; Hatcher, J.P.; Middlemiss, D.N. Stimulation of 5-HT1B receptors causes hypothermia in the guinea pig. Eur. J. Pharmacol. 1997, 331, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.E.; Ettinger, D.S. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs 1998, 55, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M.; Goto, K.; Kawahara, I. The 5-hydroxytryptamine 4 Receptor Agonist-induced Actions and Enteric Neurogenesis in the Gut. J. Neurogastroenterol. Motil. 2014, 20, 17–30. [Google Scholar] [CrossRef]
- Beglinger, C. Tegaserod: A novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int. J. Clin. Pract. 2002, 56, 47–51. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Aguiar, R.P.; Newman-Tancredi, A.; Prickaerts, J.; Oliveira, R.M.W. The 5-HT(1A) receptor as a serotonergic target for neuroprotection in cerebral ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110210. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Albert, P.R.; Lemonde, S. 5-HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist 2004, 10, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Massiou, H. Naratriptan. Curr. Med. Res. Opin. 2001, 17 (Suppl. S1), s51–s53. [Google Scholar] [CrossRef] [PubMed]
- Boers, P.M.; Donaldson, C.; Zagami, A.S.; Lambert, G.A. Naratriptan has a selective inhibitory effect on trigeminovascular neurones at central 5-HT1A and 5-HT(1B/1D) receptors in the cat: Implications for migraine therapy. Cephalalgia 2004, 24, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Moen, M.D.; Keating, G.M. Sumatriptan fast-disintegrating/rapid-release tablets. Drugs 2006, 66, 883–890. [Google Scholar] [CrossRef]
- Lamb, Y.N. Lasmiditan: First Approval. Drugs 2019, 79, 1989–1996. [Google Scholar] [CrossRef]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Lukasiewicz, S.; Polit, A.; Kędracka-Krok, S.; Wędzony, K.; Maćkowiak, M.; Dziedzicka-Wasylewska, M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta 2010, 1803, 1347–1358. [Google Scholar] [CrossRef]
- Kapur, S.; Remington, G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry 1996, 153, 466–476. [Google Scholar]
- Germann, D.; Kurylo, N.; Han, F. Risperidone. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 313–361. [Google Scholar]
- Nagai, N.; Watanabe, K. [Olanzapine]. Nihon Rinsho 2013, 71, 666–672. [Google Scholar]
- Bonhaus, D.W.; Bach, C.; DeSouza, A.; Salazar, F.H.; Matsuoka, B.D.; Zuppan, P.; Chan, H.W.; Eglen, R.M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: Comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995, 115, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Devroye, C.; Cathala, A.; Piazza, P.V.; Spampinato, U. The central serotonin(2B) receptor as a new pharmacological target for the treatment of dopamine-related neuropsychiatric disorders: Rationale and current status of research. Pharmacol. Ther. 2018, 181, 143–155. [Google Scholar] [CrossRef]
- Higgins, G.A.; Fletcher, P.J.; Shanahan, W.R. Lorcaserin: A review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 2020, 205, 107417. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lummis, S.C. 5-HT3 receptors. Curr. Pharm. Des. 2006, 12, 3615–3630. [Google Scholar] [CrossRef] [PubMed]
- Roila, F.; Del Favero, A. Ondansetron clinical pharmacokinetics. Clin. Pharmacokinet. 1995, 29, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.T.; Curran, M.P. Transdermal granisetron. Drugs 2009, 69, 2597–2605. [Google Scholar] [CrossRef]
- Celio, L.; Niger, M.; Ricchini, F.; Agustoni, F. Palonosetron in the prevention of chemotherapy-induced nausea and vomiting: An evidence-based review of safety, efficacy, and place in therapy. Core Evid. 2015, 10, 75–87. [Google Scholar] [CrossRef]
- Wang, R.Y.; Ashby, C.R., Jr.; Edwards, E.; Zhang, J.Y. The role of 5-HT3-like receptors in the action of clozapine. J. Clin. Psychiatry 1994, 55 (Suppl. B), 23–26. [Google Scholar]
- Serotonin 5-HT4 Receptor Agonists. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 39–51. [Google Scholar] [CrossRef]
- Maclennan, S.; Augood, C.; Cash-Gibson, L.; Logan, S.; Gilbert, R.E. Cisapride treatment for gastro-oesophageal reflux in children. Cochrane Database Syst. Rev. 2010, 2010, Cd002300. [Google Scholar] [CrossRef]
- Curran, M.P.; Robinson, D.M. Mosapride in gastrointestinal disorders. Drugs 2008, 68, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Castro, E.; Pilar-Cuéllar, F.; Pascual-Brazo, J.; Díaz, A.; Rojo, M.L.; Linge, R.; Martín, A.; Valdizán, E.M.; Pazos, A. Serotonin 5-HT4 receptors: A new strategy for developing fast acting antidepressants? Curr. Pharm. Des. 2014, 20, 3751–3762. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; Robert, S.J.; Lezoualc’h, F. New insights into serotonin 5-HT4 receptors: A novel therapeutic target for Alzheimer’s disease? Curr. Alzheimer Res. 2004, 1, 79–85. [Google Scholar] [CrossRef]
- Nelson, D.L. 5-HT5 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 53–58. [Google Scholar] [CrossRef]
- Woolley, M.L.; Marsden, C.A.; Fone, K.C. 5-ht6 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 59–79. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Ivanenkov, Y.A. AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer’s Disease. Mol. Pharm. 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Gasbarri, A.; Pompili, A. Serotonergic 5-HT7 receptors and cognition. Rev. Neurosci. 2014, 25, 311–323. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci. Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev. Neurosci. 2014, 25, 429–437. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci. Lett. 2007, 414, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Højer, A.M.; Areberg, J.; Nomikos, G. Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 2018, 57, 673–686. [Google Scholar] [CrossRef]
- Miura, I.; Horikoshi, S.; Ichinose, M.; Suzuki, Y.; Watanabe, K. Lurasidone for the Treatment of Schizophrenia: Design, Development, and Place in Therapy. Drug Des. Devel Ther. 2023, 17, 3023–3031. [Google Scholar] [CrossRef]
- Mattsson, P.; Cselényi, Z.; Andrée, B.; Borg, J.; Nag, S.; Halldin, C.; Farde, L. Decreased 5-HT(1A) binding in mild Alzheimer’s disease-A positron emission tomography study. Synapse 2022, 76, e22235. [Google Scholar] [CrossRef]
- Politis, M.; Wu, K.; Loane, C.; Quinn, N.P.; Brooks, D.J.; Oertel, W.H.; Björklund, A.; Lindvall, O.; Piccini, P. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci. Transl. Med. 2012, 4, 128ra41. [Google Scholar] [CrossRef]
- Wendland, J.R.; DeGuzman, T.B.; McMahon, F.; Rudnick, G.; Detera-Wadleigh, S.D.; Murphy, D.L. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr. Genet. 2008, 18, 31–39. [Google Scholar] [CrossRef]
- Lemonde, S.; Turecki, G.; Bakish, D.; Du, L.; Hrdina, P.D.; Bown, C.D.; Sequeira, A.; Kushwaha, N.; Morris, S.J.; Basak, A.; et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 2003, 23, 8788–8799. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef]
- McEwen, B.S. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001, 933, 265–277. [Google Scholar] [CrossRef]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. 2007, 32, 394–399. [Google Scholar]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Delatour, B.; Potier, M.C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Ledo, J.H.; Azevedo, E.P.; Beckman, D.; Ribeiro, F.C.; Santos, L.E.; Razolli, D.S.; Kincheski, G.C.; Melo, H.M.; Bellio, M.; Teixeira, A.L.; et al. Cross Talk Between Brain Innate Immunity and Serotonin Signaling Underlies Depressive-Like Behavior Induced by Alzheimer’s Amyloid-β Oligomers in Mice. J. Neurosci. 2016, 36, 12106–12116. [Google Scholar] [CrossRef] [PubMed]
- Areosa, S.A.; Sherriff, F.; McShane, R. Memantine for dementia. Cochrane Database Syst. Rev. 2005, 2, Cd003154. [Google Scholar]
- Pinheiro, L.; Faustino, C. Therapeutic Strategies Targeting Amyloid-β in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Moussa-Pacha, N.M.; Abdin, S.M.; Omar, H.A.; Alniss, H.; Al-Tel, T.H. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med. Res. Rev. 2020, 40, 339–384. [Google Scholar] [CrossRef]
- Davidowitz, E.J.; Krishnamurthy, P.K.; Lopez, P.; Jimenez, H.; Adrien, L.; Davies, P.; Moe, J.G. In Vivo Validation of a Small Molecule Inhibitor of Tau Self-Association in htau Mice. J. Alzheimers Dis. 2020, 73, 147–161. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Beshir, S.A.; Aadithsoorya, A.M.; Parveen, A.; Goh, S.S.L.; Hussain, N.; Menon, V.B. Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review. Int. J. Alzheimers Dis. 2022, 2022, 9343514. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.; Gandy, S.; Saini, V.; George, D.R.; Larson, E.B.; Alexander, G.C.; Avorn, J.; Brownlee, S.; Camp, C.; Chertkow, H.; et al. Making the Case for Accelerated Withdrawal of Aducanumab. J. Alzheimers Dis. 2022, 87, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, I.P.C.; Retinasamy, T.; Shaikh, M.F. Is Aducanumab for LMICs? Promises and Challenges. Brain Sci. 2021, 11, 1547. [Google Scholar] [CrossRef]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Soleimani Asl, S.; Komaki, A. Protective effects of 5-HT(1A) receptor antagonist and 5-HT(2A) receptor agonist on the biochemical and histological features in a rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2019, 96, 140–147. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Lv, J.; Zhu, X.; Jiang, X.; Lu, W.; Lu, Y.; Tang, Z.; Wang, J.; Shen, X. Antiallergic drug desloratadine as a selective antagonist of 5HT(2A) receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell 2021, 20, e13286. [Google Scholar] [CrossRef]
- Consolo, S.; Arnaboldi, S.; Giorgi, S.; Russi, G.; Ladinsky, H. 5-HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport 1994, 5, 1230–1232. [Google Scholar] [CrossRef]
- Cho, S.; Hu, Y. Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp. Neurol. 2007, 203, 274–278. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Darcy, S.; Jackson, H.; White, T.; Rosenberg, P. Psychedelics as Novel Therapeutics in Alzheimer’s Disease: Rationale and Potential Mechanisms. Curr. Top. Behav. Neurosci. 2022, 56, 287–317. [Google Scholar]
- Caraci, F.; Santagati, M.; Caruso, G.; Cannavò, D.; Leggio, G.M.; Salomone, S.; Drago, F. New antipsychotic drugs for the treatment of agitation and psychosis in Alzheimer’s disease: Focus on brexpiprazole and pimavanserin. F1000Res 2020, 9, F1000, Faculty Rev-686. [Google Scholar] [CrossRef]
- de Jong, I.E.M.; Mørk, A. Antagonism of the 5-HT(6) receptor—Preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology 2017, 125, 50–63. [Google Scholar] [CrossRef]
- Lanthier, C.; Payan, H.; Liparulo, I.; Hatat, B.; Lecoutey, C.; Since, M.; Davis, A.; Bergamini, C.; Claeysen, S.; Dallemagne, P.; et al. Novel multi target-directed ligands targeting 5-HT(4) receptors with in cellulo antioxidant properties as promising leads in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 182, 111596. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Burton, E.A.; Ross, G.W.; Huang, X.; Savica, R.; Abbott, R.D.; Ascherio, A.; Caviness, J.N.; Gao, X.; Gray, K.A.; et al. Research on the premotor symptoms of Parkinson’s disease: Clinical and etiological implications. Environ. Health Perspect. 2013, 121, 1245–1252. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathobiology of non-motor symptoms in Parkinson disease. J. Neural Transm. 2015, 122, 1429–1440. [Google Scholar] [CrossRef]
- de Natale, E.R.; Wilson, H.; Politis, M. Serotonergic imaging in Parkinson’s disease. Prog. Brain Res. 2021, 261, 303–338. [Google Scholar]
- Rylander, D.; Parent, M.; O’Sullivan, S.S.; Dovero, S.; Lees, A.J.; Bezard, E.; Descarries, L.; Cenci, M.A. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 2010, 68, 619–628. [Google Scholar] [CrossRef]
- Reijnders, J.S.; Ehrt, U.; Weber, W.E.; Aarsland, D.; Leentjens, A.F. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189. [Google Scholar] [CrossRef]
- Pasquini, J.; Ceravolo, R.; Qamhawi, Z.; Lee, J.Y.; Deuschl, G.; Brooks, D.J.; Bonuccelli, U.; Pavese, N. Progression of tremor in early stages of Parkinson’s disease: A clinical and neuroimaging study. Brain 2018, 141, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Stocchi, F. Levodopa: A new look at an old friend. Mov. Disord. 2018, 33, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Morgante, F.; Merola, A.; Fasano, A.; Marsili, L.; Fox, S.H.; Bezard, E.; Picconi, B.; Calabresi, P.; Lang, A.E. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Ohno, Y. Therapeutic role of 5-HT1A receptors in the treatment of schizophrenia and Parkinson’s disease. CNS Neurosci. Ther. 2011, 17, 58–65. [Google Scholar] [CrossRef]
- Bezard, E.; Gerlach, I.; Moratalla, R.; Gross, C.E.; Jork, R. 5-HT1A receptor agonist-mediated protection from MPTP toxicity in mouse and macaque models of Parkinson’s disease. Neurobiol. Dis. 2006, 23, 77–86. [Google Scholar] [CrossRef]
- Pinna, A.; Costa, G.; Serra, M.; Contu, L.; Morelli, M. Neuroinflammation and L-dopa-induced abnormal involuntary movements in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease are counteracted by combined administration of a 5-HT(1A/1B) receptor agonist and A(2A) receptor antagonist. Neuropharmacology 2021, 196, 108693. [Google Scholar] [CrossRef]
- Scholpa, N.E.; Lynn, M.K.; Corum, D.; Boger, H.A.; Schnellmann, R.G. 5-HT(1F) receptor-mediated mitochondrial biogenesis for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2018, 175, 348–358. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Serotonin 1A Receptors on Astrocytes as a Potential Target for the Treatment of Parkinson’s Disease. Curr. Med. Chem. 2016, 23, 686–700. [Google Scholar] [CrossRef]
- Grychowska, K.; Chaumont-Dubel, S.; Kurczab, R.; Koczurkiewicz, P.; Deville, C.; Krawczyk, M.; Pietruś, W.; Satała, G.; Buda, S.; Piska, K.; et al. Dual 5-HT(6) and D(3) Receptor Antagonists in a Group of 1H-Pyrrolo[3,2-c]quinolines with Neuroprotective and Procognitive Activity. ACS Chem. Neurosci. 2019, 10, 3183–3196. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Colocho, P.; Del Río, J.; Frechilla, D. Neuroprotective effects of serotonin 5-HT 1A receptor activation against ischemic cell damage in gerbil hippocampus: Involvement of NMDA receptor NR1 subunit and BDNF. Brain Res. 2008, 1199, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Puligheddu, M.; Sanna, F.; Cannas, A.; Farris, R.; Tronci, E.; Figorilli, M.; Defazio, G.; Carta, M. Efficacy and safety of 5-Hydroxytryptophan on levodopa-induced motor complications in Parkinson’s disease: A preliminary finding. J. Neurol. Sci. 2020, 415, 116869. [Google Scholar] [CrossRef]
- Choi, Y.; Huh, E.; Lee, S.; Kim, J.H.; Park, M.G.; Seo, S.Y.; Kim, S.Y.; Oh, M.S. 5-Hydroxytryptophan Reduces Levodopa-Induced Dyskinesia via Regulating AKT/mTOR/S6K and CREB/ΔFosB Signals in a Mouse Model of Parkinson’s Disease. Biomol. Ther. 2023, 31, 402. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Nayyar, T.; Deutch, A.Y.; Ansah, T.A. 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2010, 59, 31–36. [Google Scholar] [CrossRef]
- Kwan, C.; Frouni, I.; Bédard, D.; Hamadjida, A.; Huot, P. Ondansetron, a highly selective 5-HT(3) receptor antagonist, reduces L-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat model of Parkinson’s disease. Eur. J. Pharmacol. 2020, 871, 172914. [Google Scholar] [CrossRef]
- Osinski, M.A.; Uchic, M.E.; Seifert, T.; Shaughnessy, T.K.; Miller, L.N.; Nakane, M.; Cox, B.F.; Brioni, J.D.; Moreland, R.B. Dopamine D2, but not D4, receptor agonists are emetogenic in ferrets. Pharmacol. Biochem. Behav. 2005, 81, 211–219. [Google Scholar] [CrossRef]
- Johnston, K.D.; Lu, Z.; Rudd, J.A. Looking beyond 5-HT(3) receptors: A review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 13–25. [Google Scholar] [CrossRef]
- Lemke, M.R.; Fuchs, G.; Gemende, I.; Herting, B.; Oehlwein, C.; Reichmann, H.; Rieke, J.; Volkmann, J. Depression and Parkinson’s disease. J. Neurol. 2004; 251, (Suppl. S6), vi24–vi27. [Google Scholar]
- Bhatt, S.; Devadoss, T.; Manjula, S.N.; Rajangam, J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr. Neuropharmacol. 2021, 19, 1545–1559. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.R.; Ricci, L.A.; Melloni, R.H., Jr. Aggression and anxiety in adolescent AAS-treated hamsters: A role for 5HT3 receptors. Pharmacol. Biochem. Behav. 2015, 134, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hamadjida, A.; Nuara, S.G.; Bédard, D.; Gaudette, F.; Beaudry, F.; Gourdon, J.C.; Huot, P. The highly selective 5-HT(2A) antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology 2018, 139, 61–67. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.; Price, D.L.; Bonhaus, D.W. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav. Pharmacol. 2011, 22, 681–692. [Google Scholar] [CrossRef]
- Isaacson, S.H.; Ballard, C.G.; Kreitzman, D.L.; Coate, B.; Norton, J.C.; Fernandez, H.H.; Ilic, T.V.; Azulay, J.P.; Ferreira, J.J.; Abler, V.; et al. Efficacy results of pimavanserin from a multi-center, open-label extension study in Parkinson’s disease psychosis patients. Park. Relat. Disord. 2021, 87, 25–31. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Reyes-Long, S.; Bandala, C.; Morraz-Varela, A.; Bonilla-Jaime, H.; Alfaro-Rodriguez, A. Neuropathic Pain in Parkinson’s Disease. Neurol. India 2022, 70, 1879–1886. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, L.G.; Liu, L.B.; An, M.Q.; Dong, L.G.; Gu, H.Y.; Dai, Y.P.; Wang, F.; Mao, C.J.; Liu, C.F. Inhibition of Spinal 5-HT3 Receptor and Spinal Dorsal Horn Neuronal Excitability Alleviates Hyperalgesia in a Rat Model of Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 7253–7264. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Z.; Huang, R.; Wu, L.; Huang, Y.; Qi, Q.; Zhong, R.; Chen, Y.; Li, L.; Wu, H. Electroacupuncture Modulates 5-HT(4R)-Mediated cAMP/PKA Signaling to Improve Intestinal Motility Disorders in a Thy1-αSyn Parkinson’s Mouse Model. Evid. Based Complement. Altern. Med. 2022, 2022, 8659462. [Google Scholar] [CrossRef]
- Liu, T.; Song, J.; Zhou, Q.; Chu, S.; Liu, Y.; Zhao, X.; Ma, Z.; Xia, T.; Gu, X. The role of 5-HT(7)R in the memory impairment of mice induced by long-term isoflurane anesthesia. Neurobiol. Learn. Mem. 2022, 188, 107584. [Google Scholar] [CrossRef]
- Roux, C.M.; Leger, M.; Freret, T. Memory Disorders Related to Hippocampal Function: The Interest of 5-HT(4)Rs Targeting. Int. J. Mol. Sci. 2021, 22, 12082. [Google Scholar] [CrossRef]
- Afshar, S.; Shahidi, S.; Baooshi, H.; Hoseini, M.; Esmaeili, M.; Hashemi-Firouzi, N.; Komaki, A. The role of hippocampal 5-HT(1D) and 5-HT(1F) receptors on learning and memory in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R. Serotonergic mechanisms in amyotrophic lateral sclerosis. Int. J. Neurosci. 2006, 116, 775–826. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Cazenave, W.; Allain, A.E.; Cattaert, D.; Branchereau, P. Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 1107. [Google Scholar] [CrossRef]
- He, P.; He, B.; Li, S.; Chai, W.; Rao, W.; Zhu, Y.; Chen, W.; Zhang, P.; Zhang, X.; Pan, H.; et al. Distribution Features and Potential Effects of Serotonin in the Cerebrum of SOD1 G93A Transgenic Mice. eNeuro 2022, 9, ENEURO.0001-22.2022. [Google Scholar] [CrossRef]
- Muramatsu, M.; Lapiz, M.D.; Tanaka, E.; Grenhoff, J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur. J. Neurosci. 1998, 10, 2371–2379. [Google Scholar] [CrossRef]
- Ciranna, L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: Implications in physiological functions and in pathology. Curr. Neuropharmacol. 2006, 4, 101–114. [Google Scholar] [CrossRef]
- El Oussini, H.; Bayer, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Sinniger, J.; Dirrig-Grosch, S.; Dieterlé, S.; Echaniz-Laguna, A.; Larmet, Y.; Müller, K.; et al. Serotonin 2B receptor slows disease progression and prevents degeneration of spinal cord mononuclear phagocytes in amyotrophic lateral sclerosis. Acta Neuropathol. 2016, 131, 465–480. [Google Scholar] [CrossRef]
- Arnoux, A.; Ayme-Dietrich, E.; Dieterle, S.; Goy, M.A.; Schann, S.; Frauli, M.; Monassier, L.; Dupuis, L. Evaluation of a 5-HT(2B) receptor agonist in a murine model of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 23582. [Google Scholar] [CrossRef]
- Jiang, S.S.; Gong, M.N.; Rao, W.; Chai, W.; Chen, W.Z.; Zhang, X.; Nie, H.B.; Xu, R.S. 5-Hydroxytryptamine: A potential therapeutic target in amyotrophic lateral sclerosis. Neural Regen. Res. 2023, 18, 2047–2055. [Google Scholar]
- Lam, L.; Halder, R.C.; Montoya, D.J.; Rubbi, L.; Rinaldi, A.; Sagong, B.; Weitzman, S.; Rubattino, R.; Singh, R.R.; Pellegrini, M.; et al. Anti-inflammatory therapies of amyotrophic lateral sclerosis guided by immune pathways. Am. J. Neurodegener. Dis. 2015, 4, 28–39. [Google Scholar] [PubMed]
- Guseva, D.; Holst, K.; Kaune, B.; Meier, M.; Keubler, L.; Glage, S.; Buettner, M.; Bleich, A.; Pabst, O.; Bachmann, O.; et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm. Bowel Dis. 2014, 20, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.J.; Lopes, E.C.; Cheema, S.S. The serotonin precursor 5-hydroxytryptophan delays neuromuscular disease in murine familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2003, 4, 171–176. [Google Scholar] [CrossRef]
- Dentel, C.; Palamiuc, L.; Henriques, A.; Lannes, B.; Spreux-Varoquaux, O.; Gutknecht, L.; René, F.; Echaniz-Laguna, A.; Gonzalez de Aguilar, J.L.; Lesch, K.P.; et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: A link to spasticity. Brain 2013, 136 Pt. 2, 483–493. [Google Scholar] [CrossRef]
- El Oussini, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Marques, C.; Dirrig-Grosch, S.; Dieterlé, S.; Picchiarelli, G.; Sinniger, J.; Rouaux, C.; Dupuis, L. Degeneration of serotonin neurons triggers spasticity in amyotrophic lateral sclerosis. Ann. Neurol. 2017, 82, 444–456. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yao, X.X.; Gao, S.H.; Li, R.; Li, B.J.; Yang, W.; Cui, R.J. Role of 5-HT receptors in neuropathic pain: Potential therapeutic implications. Pharmacol. Res. 2020, 159, 104949. [Google Scholar] [CrossRef]
- Boentert, M. Sleep and Sleep Disruption in Amyotrophic Lateral Sclerosis. Curr. Neurol. Neurosci. Rep. 2020, 20, 25. [Google Scholar] [CrossRef]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Haney, M.M.; Sinnott, J.; Osman, K.L.; Deninger, I.; Andel, E.; Caywood, V.; Mok, A.; Ballenger, B.; Cummings, K.; Thombs, L.; et al. Mice Lacking Brain-Derived Serotonin Have Altered Swallowing Function. Otolaryngol. Head. Neck Surg. 2019, 161, 468–471. [Google Scholar] [CrossRef]
- Dupuis, L.; Spreux-Varoquaux, O.; Bensimon, G.; Jullien, P.; Lacomblez, L.; Salachas, F.; Bruneteau, G.; Pradat, P.F.; Loeffler, J.P.; Meininger, V. Platelet serotonin level predicts survival in amyotrophic lateral sclerosis. PLoS ONE 2010, 5, e13346. [Google Scholar] [CrossRef]
- Li, S.H.; Li, X.J. Huntingtin-protein interactions and the pathogenesis of Huntington’s disease. Trends Genet. 2004, 20, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Young, A.B. Huntingtin in health and disease. J. Clin. Investig. 2003, 111, 299–302. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Suofu, Y.; Yablonska, S.; Wang, X.; Larkin, T.M.; Kim, J.; Carlisle, D.L.; Friedlander, R.M. Increased Serotonin Transporter Expression in Huntington’s Disease Patients Is Not Consistently Replicated in Murine Models. J. Huntingt. Dis. 2019, 8, 449–457. [Google Scholar] [CrossRef]
- Reynolds, G.P.; Pearson, S.J. Decreased glutamic acid and increased 5-hydroxytryptamine in Huntington’s disease brain. Neurosci. Lett. 1987, 78, 233–238. [Google Scholar] [CrossRef]
- Bédard, C.; Wallman, M.J.; Pourcher, E.; Gould, P.V.; Parent, A.; Parent, M. Serotonin and dopamine striatal innervation in Parkinson’s disease and Huntington’s chorea. Park. Relat. Disord. 2011, 17, 593–598. [Google Scholar] [CrossRef]
- Muñoz-Castañeda, J.R.; Montilla, P.; Padillo, F.J.; Bujalance, I.; Muñoz, M.C.; Muntané, J.; Túnez, I. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol. Exp. 2006, 66, 1–6. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lee, B.D.; Sok, D.E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef]
- Roppongi, T.; Togo, T.; Nakamura, S.; Asami, T.; Yoshimi, A.; Shiozaki, K.; Kato, D.; Kawanishi, C.; Hirayasu, Y. Perospirone in treatment of Huntington’s disease: A first case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 308–310. [Google Scholar] [CrossRef]
- Pang, T.Y.; Du, X.; Zajac, M.S.; Howard, M.L.; Hannan, A.J. Altered serotonin receptor expression is associated with depression-related behavior in the R6/1 transgenic mouse model of Huntington’s disease. Hum. Mol. Genet. 2009, 18, 753–766. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Tudor, L.; Nikolac Perkovic, M.; Podobnik, J.; Dodig Curkovic, K.; Curkovic, M.; Svob Strac, D.; Cusek, M.; Bortolato, M.; Pivac, N. Serotonin 5-HT(2A) receptor polymorphisms are associated with irritability and aggression in conduct disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 117, 110542. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Hoffjan, S.; Akkad, D.A. The genetics of multiple sclerosis: An update 2010. Mol. Cell. Probes 2010, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun. Rev. 2009, 8, 621–626. [Google Scholar] [CrossRef]
- Baidina, T.V.; Trushnikova, T.N.; Danilova, M.A. Interferon-induced depression and peripheral blood serotonin in patients with multiple sclerosis. Zh Nevrol. Psikhiatr Im. S S Korsakova 2018, 118, 77–81. [Google Scholar] [CrossRef]
- Reverchon, F.; Guillard, C.; Mollet, L.; Auzou, P.; Gosset, D.; Madouri, F.; Valéry, A.; Menuet, A.; Ozsancak, C.; Pallix-Guyot, M.; et al. T Lymphocyte Serotonin 5-HT(7) Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients. Biomedicines 2022, 10, 2418. [Google Scholar] [CrossRef]
- Foley, P.; Lawler, A.; Chandran, S.; Mead, G. Potential disease-modifying effects of selective serotonin reuptake inhibitors in multiple sclerosis: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 709–710. [Google Scholar] [CrossRef]
- Sviridova, A.; Rogovskii, V.; Kudrin, V.; Pashenkov, M.; Boyko, A.; Melnikov, M. The role of 5-HT(2B)-receptors in fluoxetine-mediated modulation of Th17- and Th1-cells in multiple sclerosis. J. Neuroimmunol. 2021, 356, 577608. [Google Scholar] [CrossRef]
- Iannitti, T.; Kerr, B.J.; Taylor, B.K. Mechanisms and pharmacology of neuropathic pain in multiple sclerosis. Curr. Top. Behav. Neurosci. 2014, 20, 75–97. [Google Scholar]
- Gurba, K.N.; Chaudhry, R.; Haroutounian, S. Central Neuropathic Pain Syndromes: Current and Emerging Pharmacological Strategies. CNS Drugs 2022, 36, 483–516. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.; Laursen, B.; Stenager, E.N.; Stenager, E. Psychiatric co-morbidity in multiple sclerosis: The risk of depression and anxiety before and after MS diagnosis. Mult. Scler. 2016, 22, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Rickards, H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S1), i48–i52. [Google Scholar] [CrossRef]
- Saul, A.; Taylor, B.; Simpson, S., Jr.; Ponsonby, A.L.; Blizzard, L.; Dwyer, T.; McMorran, B.; Wood, B.; van der Mei, I.A. Polymorphism in the serotonin transporter gene polymorphisms (5-HTTLPR) modifies the association between significant life events and depression in people with multiple sclerosis. Mult. Scler. 2019, 25, 848–855. [Google Scholar] [CrossRef]
- Nazarali, A.J.; Reynolds, G.P. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: A postmortem study. Cell. Mol. Neurobiol. 1992, 12, 581–587. [Google Scholar] [CrossRef]
- Babić Leko, M.; Nikolac Perković, M.; Španić, E.; Švob Štrac, D.; Pleić, N.; Vogrinc, Ž.; Gunjača, I.; Bežovan, D.; Nedić Erjavec, G.; Klepac, N.; et al. Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease. Biomedicines 2022, 10, 3118. [Google Scholar] [CrossRef]
- Tong, Q.; Zhang, L.; Yuan, Y.; Jiang, S.; Zhang, R.; Xu, Q.; Ding, J.; Li, D.; Zhou, X.; Zhang, K. Reduced plasma serotonin and 5-hydroxyindoleacetic acid levels in Parkinson’s disease are associated with nonmotor symptoms. Park. Relat. Disord. 2015, 21, 882–887. [Google Scholar] [CrossRef]
- Guttman, M.; Boileau, I.; Warsh, J.; Saint-Cyr, J.A.; Ginovart, N.; McCluskey, T.; Houle, S.; Wilson, A.; Mundo, E.; Rusjan, P.; et al. Brain serotonin transporter binding in non-depressed patients with Parkinson’s disease. Eur. J. Neurol. 2007, 14, 523–528. [Google Scholar] [CrossRef]
- Cheng, M.L.; Chang, K.H.; Wu, Y.R.; Chen, C.M. Metabolic disturbances in plasma as biomarkers for Huntington’s disease. J. Nutr. Biochem. 2016, 31, 38–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).