Longitudinal Evaluation of the Detection Potential of Serum Oligoelements Cu, Se and Zn for the Diagnosis of Alzheimer’s Disease in the 3xTg-AD Animal Model
Abstract
1. Introduction
2. Results
2.1. Behavior Evaluation
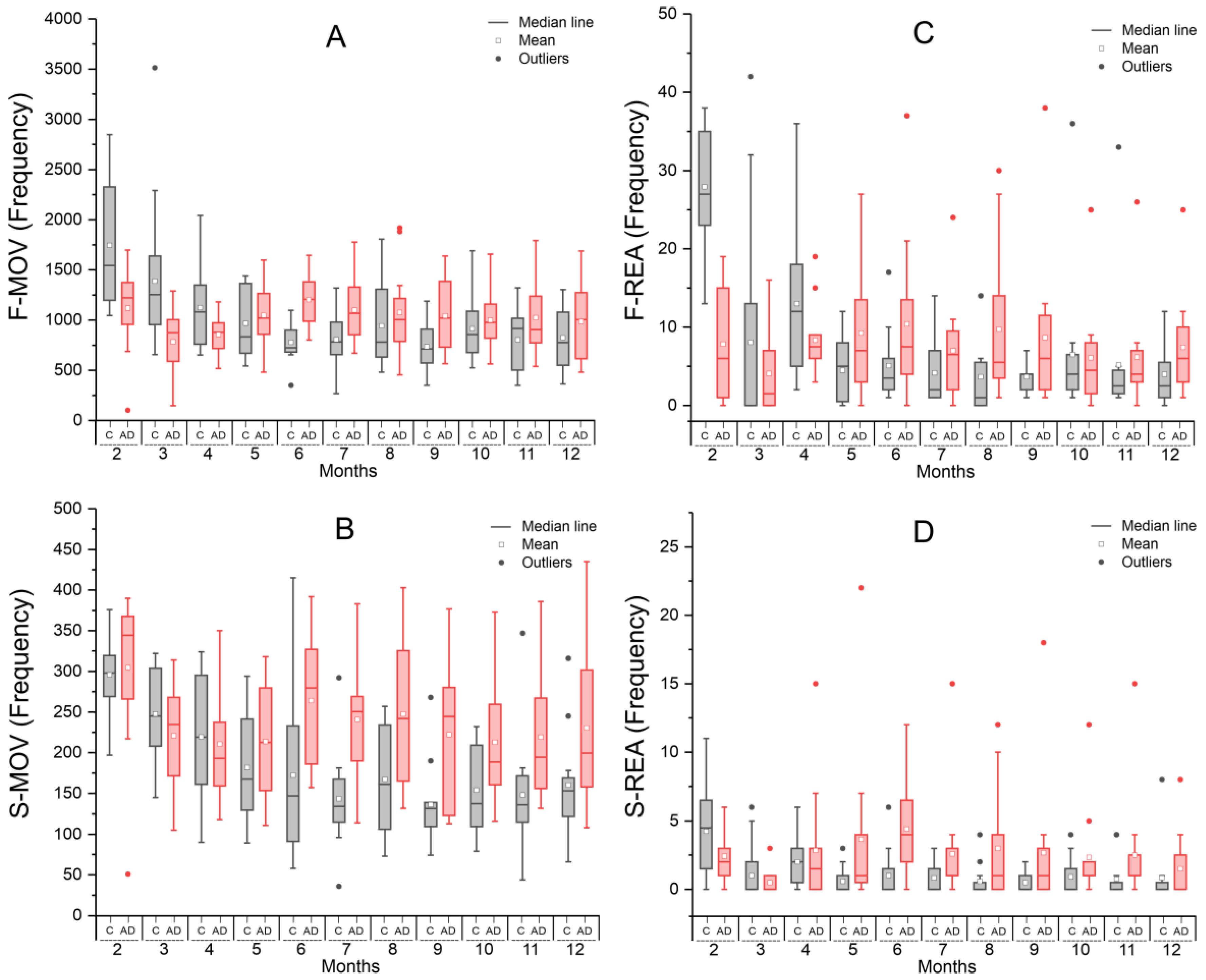
2.1.1. Spontaneous Locomotion in the Open Field Test—Actimeter
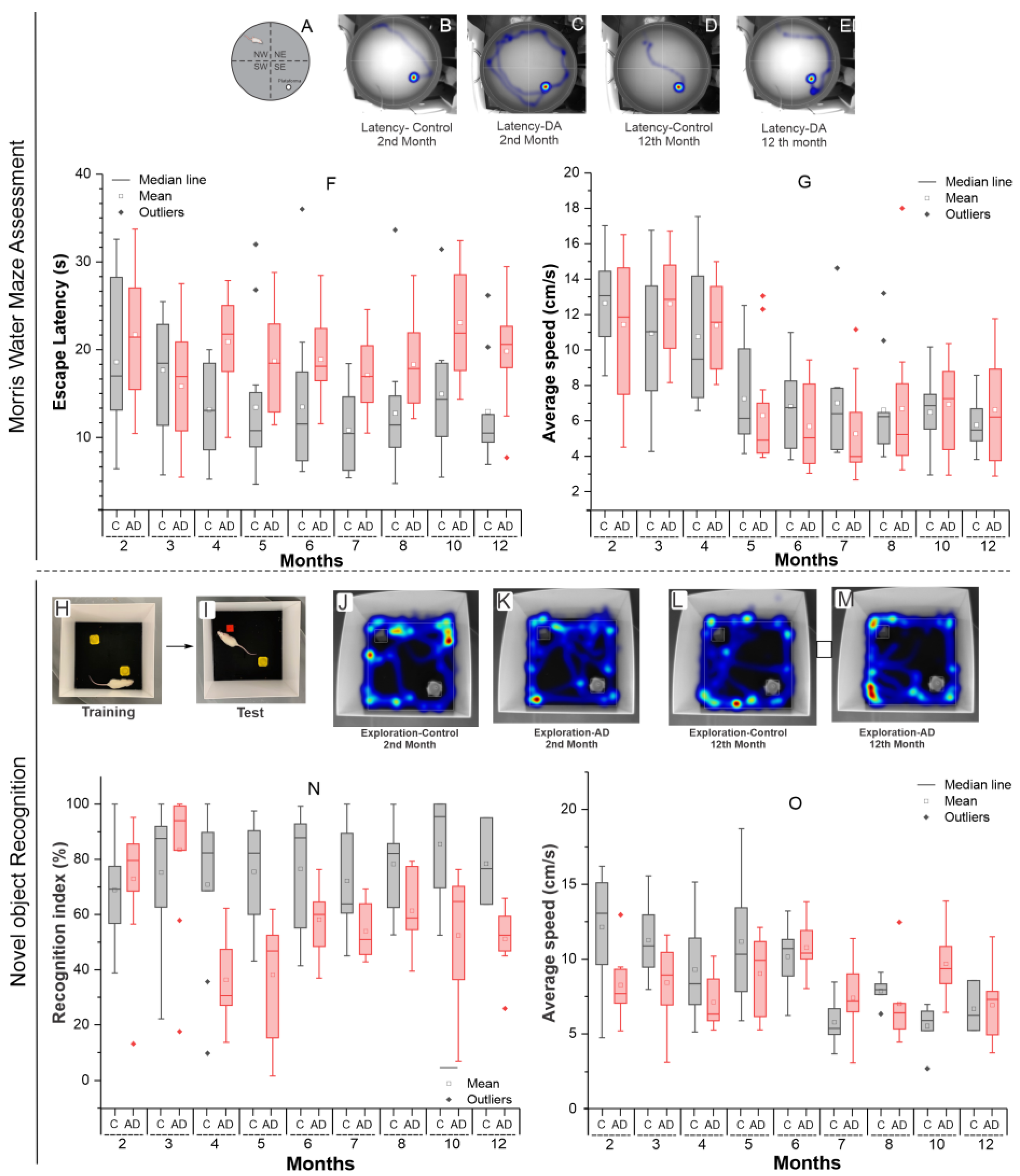
2.1.2. Morris Water Maze Evaluation
2.1.3. Assessment of Novel Object Recognition
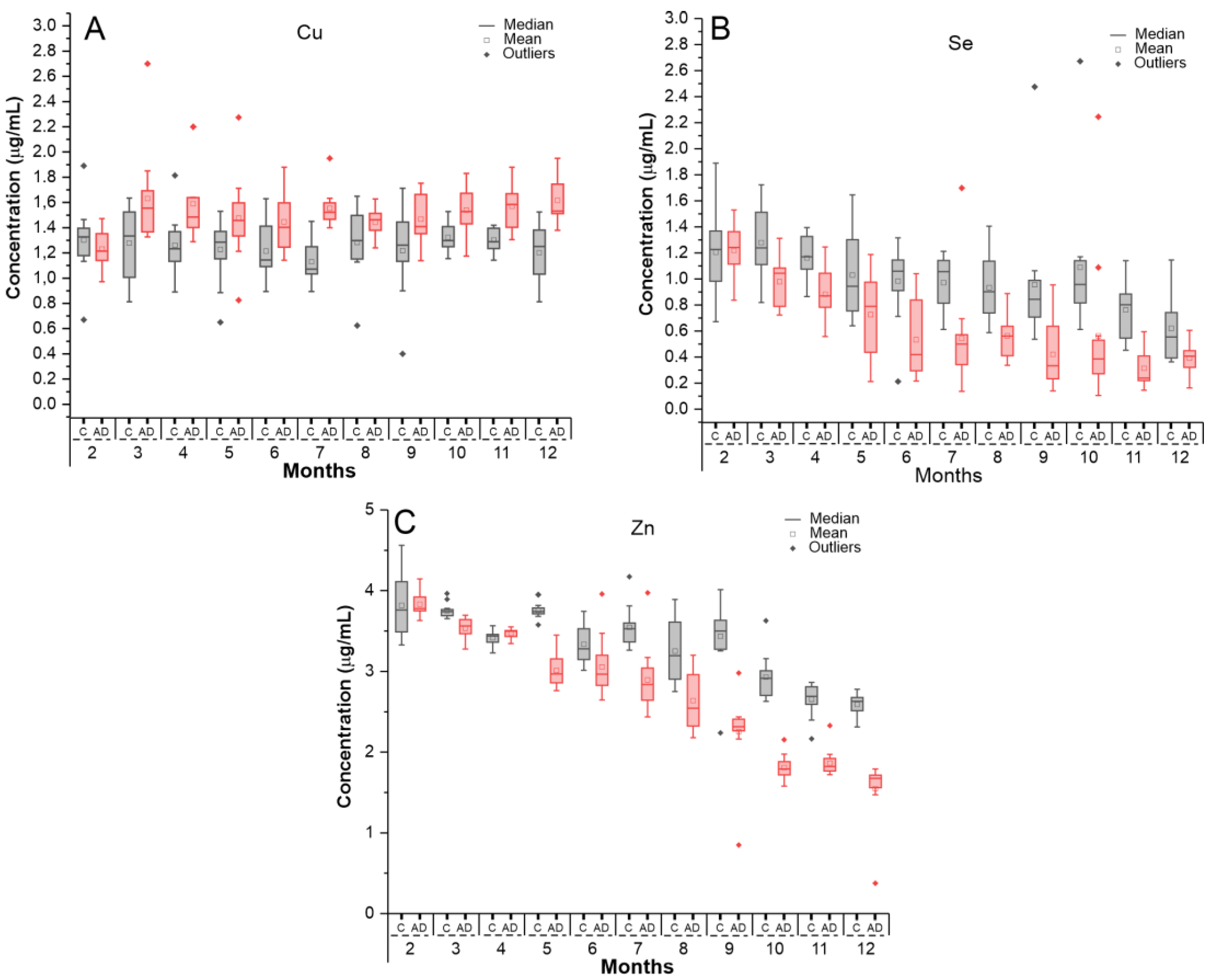
2.2. Evaluation of Oligoelements Levels in Serum Samples by ICP-MS
2.2.1. Calibration Curve for Oligoelement Quantification in Serum Samples
2.2.2. Quantification of Oligoelements in Serum Samples Using ICP-MS Technique
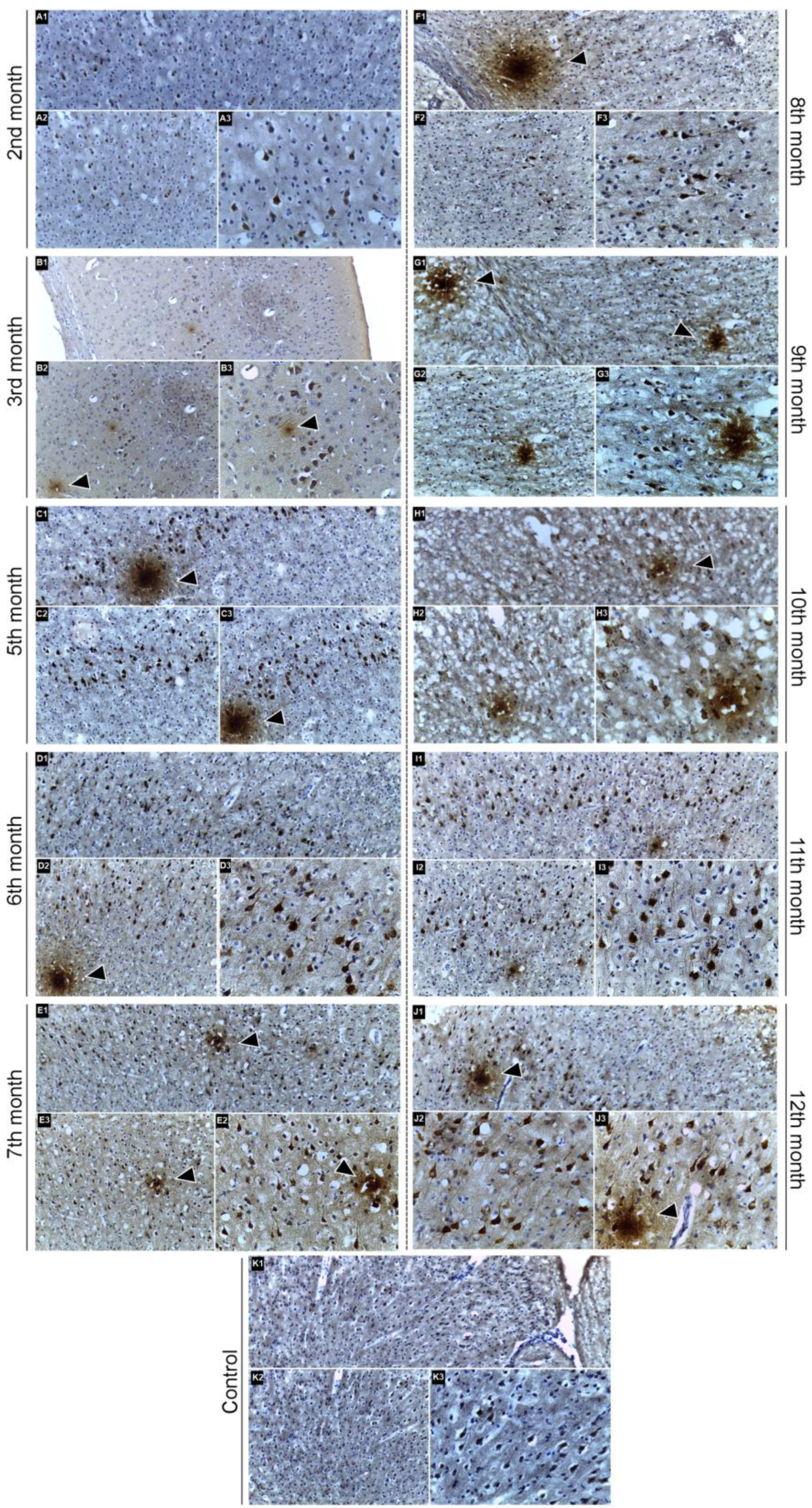
2.3. Immunohistochemical Confirmation of Aβ Peptide Presence in the CNS
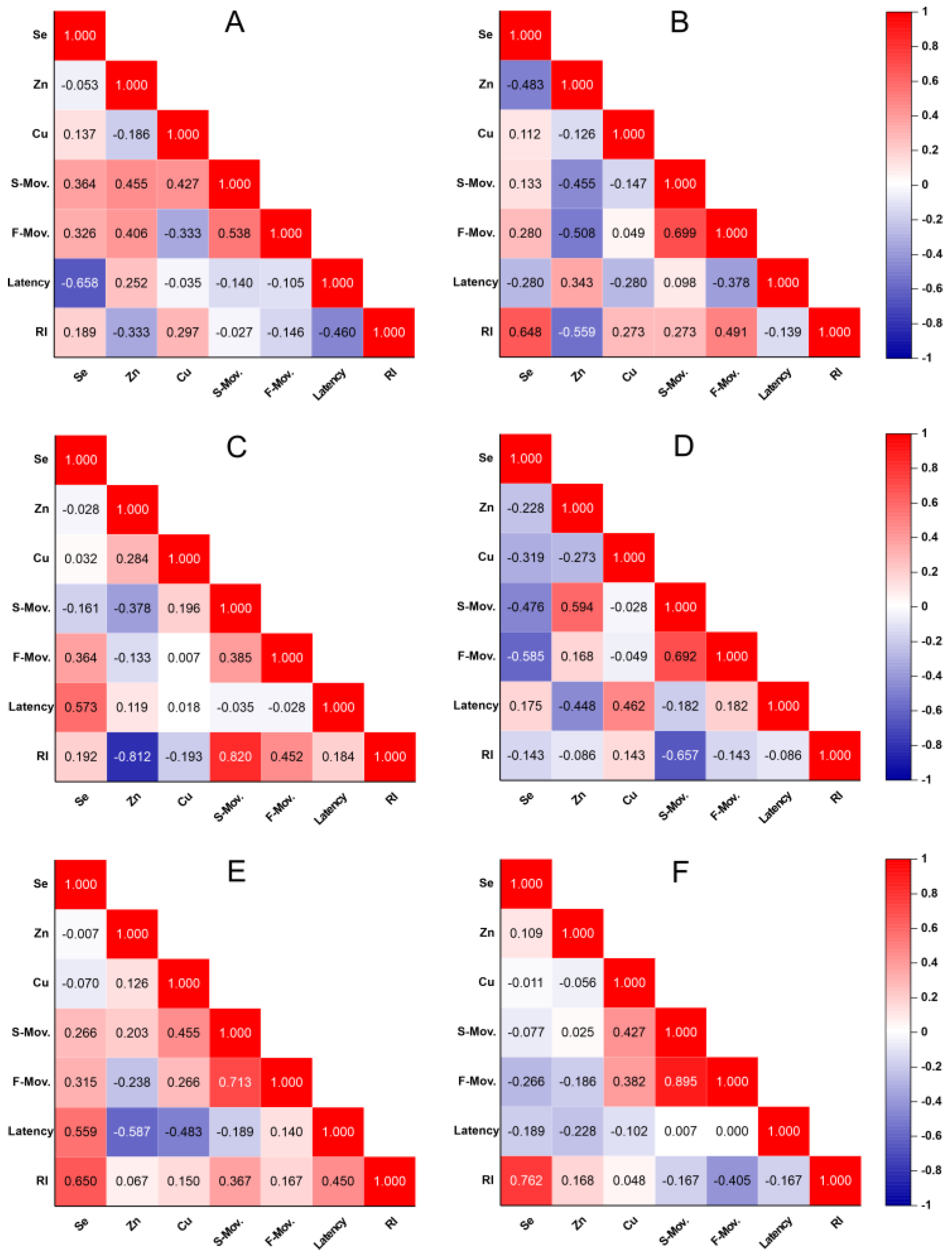
3. Discussion
4. Materials and Methods
4.1. Animals
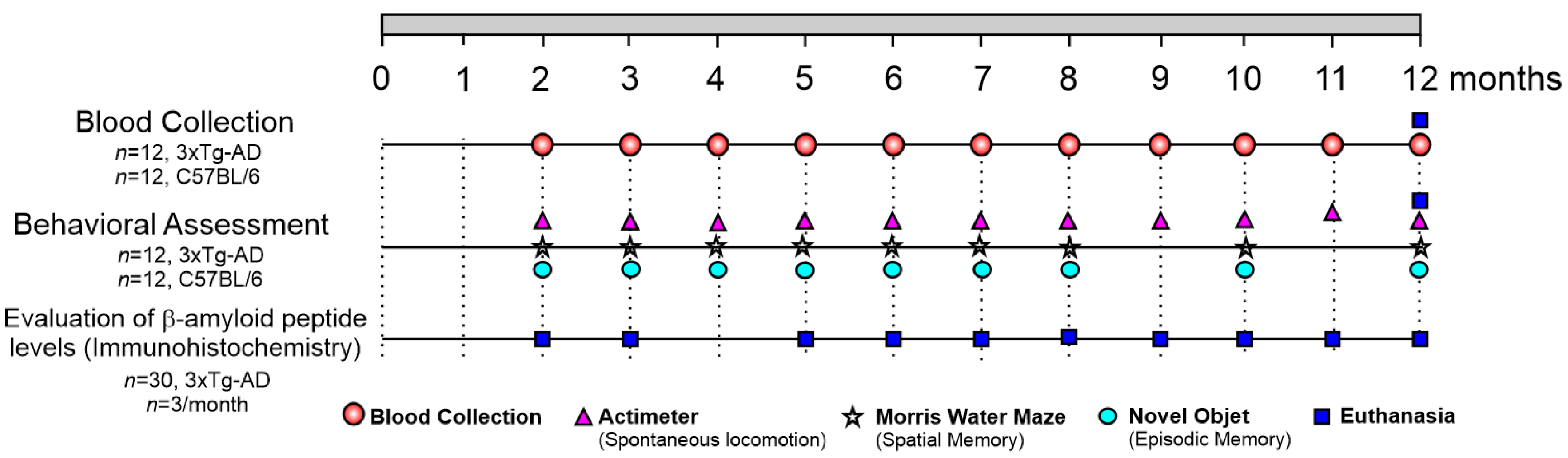
4.2. Experimental Design
4.3. Behavioral Assessment
4.3.1. Spontaneous Locomotion in the Open Field—Actimeter
4.3.2. Morris Water Maze
4.3.3. Novel Object Recognition
4.4. Determination of Oligoelements in Serum Samples by ICP-MS
4.4.1. Blood Sample Collection and Pre-Preparation
4.4.2. Preparation of Samples for Quantitative Measurements by ICP-MS
4.4.3. Quantitative Analysis of Oligoelements in Serum Samples by ICP-MS
4.5. Identification of Aβ Peptide Deposition in the CNS by Immunohistochemistry
4.6. Statistcal Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assfaw, A.D.; Schindler, S.E.; Morris, J.C. Advances in blood biomarkers for Alzheimer disease (AD): A review. Kaohsiung J. Med. Sci. 2024, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Abi-Ghanem, C.; Kelly, R.D.; Riccio, D.A.; Mansour, F.M.; Zuloaga, K.L. Sex differences in metabolic phenotype and hypothalamic inflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Biol. Sex Differ. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- GBD. The Lancet Neurology; Global Burden of Disease Study: Seattle, WA, USA, 2021. [Google Scholar]
- PAHO. The Economic Impact of Alzheimer’s Disease in Latin America and the Caribbean; Pan American Health Organization: Washington, DC, USA, 2021. [Google Scholar]
- Sandoval-Hernández, A.G.; Hernández, H.G.; Restrepo, A.; Muñoz, J.I.; Bayon, G.F.; Fernández, A.F.; Fraga, M.F.; Cardona-Gómez, G.P.; Arboleda, H.; Arboleda, G.H. Liver X Receptor Agonist Modifies the DNA Methylation Profile of Synapse and Neurogenesis-Related Genes in the Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2016, 58, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, T.S.; Ayo, J.O. Pathophysiology of Alzheimer’s disease: An insight into the genetic factors, hypotheses, redox imbalance, and antioxidant intervention. Comp. Clin. Pathol. 2024, 33, 811–821. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Blennow, K.; Hansson, O. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association of Plasma Neurofilament Light with Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2017, 74, 557–566. [Google Scholar] [CrossRef]
- Mandal, P.K.; Maroon, J.C.; Garg, A.; Arora, N.K.; Bansal, R.; Kaushik, A.; Samkaria, A.; Kumaran, G.; Arora, Y. Blood Biomarkers in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3975–3978. [Google Scholar] [CrossRef]
- Ciavardelli, D.; Consalvo, A.; Caldaralo, V.; Di Vacri, M.L.; Nisi, S.; Corona, C.; Frazzini, V.; Sacchetta, P.; Urbani, A.; Di Ilio, C.; et al. Characterisation of element profile changes induced by long-term dietary supplementation of zinc in the brain and cerebellum of 3xTg-AD mice by alternated cool and normal plasma ICP-MS. Metallomics 2012, 4, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960. [Google Scholar] [CrossRef]
- Du, B.; Chen, K.; Wang, W.; Lei, P. Targeting Metals in Alzheimer’s Disease: An Update. J. Alzheimer’s Dis. 2024, 101, S141–S154. [Google Scholar] [CrossRef]
- Abduljabbar, T.N.; Sharp, B.L.; Reid, H.J.; Barzegar-Befroeid, N.; Peto, T.; Lengyel, I. Determination of Zn, Cu and Fe in human patients’ serum using micro-sampling ICP-MS and sample dilution. Talanta 2019, 204, 663–669. [Google Scholar] [CrossRef]
- Barber, A.J.; del Genio, C.L.; Swain, A.B.; Pizzi, E.M.; Watson, S.C.; Tapiavala, V.N.; Zanazzi, G.J.; Gaur, A.B. Age, sex and Alzheimer’s disease: A longitudinal study of 3xTg-AD mice reveals sex-specific disease trajectories and inflammatory responses mirrored in postmortem brains from Alzheimer’s patients. Alzheimer’s Res. Ther. 2024, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gloria, Y.; Ceyzériat, K.; Tsartsalis, S.; Millet, P.; Tournier, B.B. Dopaminergic dysfunction in the 3xTg-AD mice model of Alzheimer’s disease. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Billings, L.M.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Learning decreases Aβ*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J. Neurosci. 2007, 27, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Sterniczuk, R.; Antle, M.C.; LaFerla, F.M.; Dyck, R.H. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res. 2010, 1348, 149–155. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Botton, P.H.; Costa, M.S.; Ardais, A.P.; Mioranzza, S.; Souza, D.O.; da Rocha, J.B.; Porciúncula, L.O. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav. Brain Res. 2010, 214, 254–259. [Google Scholar] [CrossRef]
- Babić Leko, M.; Langer Horvat, L.; Španić Popovački, E.; Zubčić, K.; Hof, P.R.; Šimić, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Kochanowicz, J. Dietary Habits, Selenium, Copper, Zinc and Total Antioxidant Status in Serum in Relation to Cognitive Functions of Patients with Alzheimer’s Disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tan, L.; Wang, H.F.; Ma, J.; Liu, J.; Tan, M.S.; Sun, J.H.; Zhu, X.C.; Jiang, T.; Yu, J.T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimer’s Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef]
- Bucossi, S.; Ventriglia, M.; Panetta, V.; Salustri, C.; Pasqualetti, P.; Mariani, S.; Siotto, M.; Rossini, P.M.; Squitti, R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 2011, 24, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Xu, W.; Lin, Y.; He, J.; Huang, M. Oxidative Stress Markers and Metal Ions are Correlated with Cognitive Function in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 2017, 32, 353–359. [Google Scholar] [CrossRef]
- Squitti, R.; Ghidoni, R.; Simonelli, I.; Ivanova, I.D.; Colabufo, N.A.; Zuin, M.; Benussi, L.; Binetti, G.; Cassetta, E.; Rongioletti, M.; et al. Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J. Trace Elem. Med. Biol. 2018, 45, 181–188. [Google Scholar] [CrossRef]
- Vaz, F.N.C.; Fermino, B.L.; Haskel, M.V.L.; Wouk, J.; de Freitas, G.B.L.; Fabbri, R.; Montagna, E.; Rocha, J.B.T.; Bonini, J.S. The Relationship Between Copper, Iron, and Selenium Levels and Alzheimer Disease. Biol. Trace Elem. Res. 2018, 181, 185–191. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals toxicity and its correlation with the gene expression in Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 3245–3252. [Google Scholar] [CrossRef]
- Toba, K.; Mitra, M.; Shahnaz, R.; Farshad, S.; Saeed, S.; Masoumeh, K.; Tahereh, F. Trace Element Concentration and Cognitive Dysfunction in Elderly Residents in Birjand. Curr. Alzheimer Res. 2022, 19, 674–680. [Google Scholar] [CrossRef]
- Li, K.; Li, A.; Mei, Y.; Zhao, J.; Zhou, Q.; Li, Y.; Yang, M.; Xu, Q. Trace elements and Alzheimer dementia in population-based studies: A bibliometric and meta-analysis. Environ. Pollut. 2023, 318, 120782. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.; Verhey, F.R.; Sijben, J.W.; Bouwman, F.H.; Dautzenberg, P.L.; Lansink, M.; Sipers, W.M.; van Asselt, D.Z.; van Hees, A.M.; Stevens, M.; et al. Differences in nutritional status between very mild Alzheimer’s disease patients and healthy controls. J. Alzheimer’s Dis. 2014, 41, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef] [PubMed]
- Chmatalova, Z.; Vyhnalek, M.; Laczo, J.; Hort, J.; Pospisilova, R.; Pechova, M.; Skoumalova, A. Relation of plasma selenium and lipid peroxidation end products in patients with Alzheimer’s disease. Physiol. Res. 2017, 66, 1049–1056. [Google Scholar] [CrossRef]
- Strumylaite, L.; Kregzdyte, R.; Kucikiene, O.; Baranauskiene, D.; Simakauskiene, V.; Naginiene, R.; Damuleviciene, G.; Lesauskaite, V.; Zemaitiene, R. Alzheimer’s Disease Association with Metals and Metalloids Concentration in Blood and Urine. Int. J. Environ. Res. Public Health 2022, 19, 7309. [Google Scholar] [CrossRef]
- Baum, L.; Chan, I.H.S.; Cheung, S.K.-K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. BioMetals 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Yan, X.-D.; Qu, X.-S.; Yin, J.; Qiao, J.; Zhang, J.; Qi, J.-S.; Wu, M.-N. Adiponectin Ameliorates Cognitive Behaviors and In Vivo Synaptic Plasticity Impairments in 3xc Mice. J. Alzheimer’s Dis. 2022, 85, 343–357. [Google Scholar] [CrossRef]
- Guo, Q.; Fu, W.; Sopher, B.L.; Miller, M.W.; Ware, C.B.; Martin, G.M.; Mattson, M.P. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med 1999, 5, 101–106. [Google Scholar] [CrossRef]
- Mastrangelo, M.A.; Bowers, W.J. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Dins, A.; Bayer, T.A. AβPP Accumulation and/or Intraneuronal Amyloid-β Accumulation? The 3xTg-AD Mouse Model Revisited. J. Alzheimer’s Dis. 2012, 28, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar] [CrossRef]
- Rosenberg, R.N.; Fu, M.; Lambracht-Washington, D. Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology. Alzheimer’s Res. Ther. 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yu, K.Y.; Cui, Y.J.; Wang, Z.J.; Cai, H.Y.; Cao, J.M.; Wu, M.N. Orexin-A aggravates cognitive deficits in 3xTg-AD mice by exacerbating synaptic plasticity impairment and affecting amyloid β metabolism. Neurobiol. Aging 2023, 124, 71–84. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Feng, W.; Wang, C.; Chen, M.; Li, Y.; Chen, J.; Liu, X.; Liu, Q.; Tian, J. Berberine ameliorates iron levels and ferroptosis in the brain of 3 × Tg-AD mice. Phytomedicine 2023, 118, 154962. [Google Scholar] [CrossRef]
- Son, H.J.; Jeong, Y.J.; Yoon, H.J.; Lee, S.Y.; Choi, G.-E.; Park, J.-A.; Kim, M.H.; Lee, K.C.; Lee, Y.J.; Kim, M.K.; et al. Assessment of brain beta-amyloid deposition in transgenic mouse models of Alzheimer’s disease with PET imaging agents 18F-flutemetamol and 18F-florbetaben. BMC Neurosci. 2018, 19, 45. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Guzmán-Ramos, K.; Moreno-Castilla, P.; Castro-Cruz, M.; McGaugh, J.L.; Martínez-Coria, H.; LaFerla, F.M.; Bermúdez-Rattoni, F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 2012, 19, 453–460. [Google Scholar] [CrossRef][Green Version]
- Barros-Viegas, A.T.; Carmona, V.; Ferreiro, E.; Guedes, J.; Cardoso, A.M.; Cunha, P.; Pereira de Almeida, L.; Resende de Oliveira, C.; Pedro de Magalhães, J.; Peça, J.; et al. miRNA-31 Improves Cognition and Abolishes Amyloid-β Pathology by Targeting APP and BACE1 in an Animal Model of Alzheimer’s Disease. Mol. Ther. Nucleic Acids 2020, 19, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R.; Strazielle, C. Neurobehavioral characteristics of mice with modified intermediate filament genes. Rev. Neurosci. 2003, 14, 369–385. [Google Scholar] [CrossRef]
- Baglietto-Vargas, D.; Chen, Y.; Suh, D.; Ager, R.R.; Rodriguez-Ortiz, C.J.; Medeiros, R.; Myczek, K.; Green, K.N.; Baram, T.Z.; LaFerla, F.M. Short-term modern life-like stress exacerbates Aβ-pathology and synapse loss in 3xTg-AD mice. J. Neurochem. 2015, 134, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Herline, K.; Prelli, F.; Mehta, P.; MacMurray, C.; Goñi, F.; Wisniewski, T. Immunotherapy to improve cognition and reduce pathological species in an Alzheimer’s disease mouse model. Alzheimer’s Res. Ther. 2018, 10, 54. [Google Scholar] [CrossRef]
- Ha, J.; Kwon, G.E.; Son, Y.; Jang, S.A.; Cho, S.Y.; Park, S.J.; Kim, H.; Lee, J.; Lee, J.; Seo, D.; et al. Cholesterol profiling reveals 7β-hydroxycholesterol as a pathologically relevant peripheral biomarker of Alzheimer’s disease. Psychiatry Clin. Neurosci. 2024, 78, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Santos, D.A.; Karaahmet, B.; Belcher, E.K.; Owlett, L.D.; Trojanczyk, L.A.; Olschowka, J.A.; O’Banion, M.K. Evaluating Effects of Glatiramer Acetate Treatment on Amyloid Deposition and Tau Phosphorylation in the 3xTg Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 758677. [Google Scholar] [CrossRef]
- Mulder, C.K.; Gerkema, M.P.; Van Der Zee, E.A. Circadian clocks and memory: Time-place learning. Front. Mol. Neurosci. 2013, 6, 8. [Google Scholar] [CrossRef]
- Castro-Alvarez, J.F.; Alejandro Uribe-Arias, S.; Kosik, K.S.; Cardona-Gómez, G.P. Long- and short-term CDK5 knockdown prevents spatial memory dysfunction and tau pathology of triple transgenic Alzheimer’s mice. Front. Aging Neurosci. 2014, 6, 243. [Google Scholar] [CrossRef]
- Carvalho da Silva, A.M.; Lemos, C.; Silva, H.B.; Ferreira, I.L.; Tomé, A.R.; Rego, A.C.; Cunha, R.A. Simultaneous Alteration of the Circadian Variation of Memory, Hippocampal Synaptic Plasticity, and Metabolism in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 835885. [Google Scholar] [CrossRef]
- Nelson, R.J.; Bumgarner, J.R.; Walker, W.H., 2nd; DeVries, A.C. Time-of-day as a critical biological variable. Neurosci. Biobehav. Rev. 2021, 127, 740–746. [Google Scholar] [CrossRef]
| Time (Months) | Groups | Statistic p-Value | |
|---|---|---|---|
| Control | Alzheimer Disease | ||
| Mean ± SD | Mean ± SD | ||
| F-MOV | |||
| 2 | 1744.58 ± 658.25 | 1119.92 ± 413.06 | 0.011 |
| 3 | 1387.16 ± 671.87 | 783.33 ± 335.06 | 0.007 |
| 4 | 1125.08 ± 413.62 | 856.08 ± 198.87 | 0.055 |
| 5 | 968.58 ± 344.11 | 1048.08 ± 334.43 | 0.572 |
| 6 | 777.83 ± 199.82 | 1204.00 ± 279.25 | <0.001 |
| 7 | 806.83 ± 268.97 | 1100.92 ± 322.09 | 0.024 |
| 8 | 944.50 ± 423.63 | 1076.58 ± 445.10 | 0.464 |
| 9 | 736.25 ± 238.16 | 1044.42 ± 367.28 | 0.023 |
| 10 | 914.58 ± 343.90 | 1000.33 ± 292.59 | 0.517 |
| 11 | 802.67 ± 316.87 | 1025.75 ± 382.31 | 0.134 |
| 12 | 824.83 ± 321.93 | 986.42 ± 391.42 | 0.281 |
| S-MOV | |||
| 2 | 295.67 ± 47.53 | 304.667 ± 96.51 | 0.775 |
| 3 | 247.53 ± 54.71 | 220.667 ± 65.23 | 0.226 |
| 4 | 219.33 ± 76.05 | 210.667 ± 71.24 | 0.776 |
| 5 | 181.58 ± 66.33 | 213.58 ± 69.22 | 0.260 |
| 6 | 172.42 ± 109.04 | 264.08 ± 84.29 | 0.031 |
| 7 | 143.33 ± 61.01 | 240.75 ± 80.81 | 0.003 |
| 8 | 167.25 ± 70.15 | 247.50 ± 93.33 | 0.026 |
| 9 | 136.42 ± 50.41 | 222.25 ± 91.55 | 0.009 |
| 10 | 153.83 ± 54.89 | 212.83 ± 78.16 | 0.044 |
| 11 | 148.08 ± 74.76 | 218.92 ± 86.68 | 0.043 |
| 12 | 160.17 ± 64.96 | 230.33 ± 99.07 | 0.052 |
| F-REA | |||
| 2 | 27.917 ± 7.73 | 7.833 ± 7.29 | 0.005 |
| 3 | 8.053 ± 12.84 | 4.083 ± 5.16 | 0.001 |
| 4 | 13.000 ± 9.91 | 8.333 ± 4.52 | 0.014 |
| 5 | 4.500 ± 4.14 | 9.250 ± 8.51 | 0.754 |
| 6 | 5.083 ± 4.50 | 10.417 ± 10.21 | 0.095 |
| 7 | 4.167 ± 4.15 | 7.000 ± 6.55 | 0.088 |
| 8 | 3.667 ± 5.24 | 9.750 ± 9.77 | 0.967 |
| 9 | 3.667 ± 1.87 | 8.667 ± 10.23 | 0.007 |
| 10 | 6.500 ± 9.59 | 6.083 ± 6.71 | 0.297 |
| 11 | 5.167 ± 8.88 | 6.167 ± 6.73 | 0.475 |
| 12 | 4.000 ± 3.93 | 7.417 ± 6.64 | 0.113 |
| S-REA | |||
| 2 | 4.25 ± 3.42 | 2.42 ± 1.98 | 0.122 |
| 3 | 1.00 ± 1.83 | 0.50 ± 0.90 | 0.387 |
| 4 | 2.00 ± 1.86 | 2.83 ± 4.32 | 0.546 |
| 5 | 0.58 ± 0.99 | 3.67 ± 6.17 | 0.101 |
| 6 | 1.00 ± 1.86 | 4.42 ± 3.45 | 0.006 |
| 7 | 0.83 ± 1.19 | 2.58 ± 4.12 | 0.172 |
| 8 | 0.58 ± 1.24 | 3.00 ± 4.07 | 0.068 |
| 9 | 0.50 ± 0.79 | 2.67 ± 5.05 | 0.156 |
| 10 | 0.92 ± 1.38 | 2.33 ± 3.31 | 0.185 |
| 11 | 0.75 ± 1.54 | 2.50 ± 4.10 | 0.180 |
| 12 | 0.83 ± 2.29 | 1.50 ± 2.47 | 0.500 |
| Time (Months) | Groups | Statistics p-Value | |||
|---|---|---|---|---|---|
| Control | Alzheimer Disease | ||||
| N | Mean ± SD | N | Mean ± SD | ||
| Escape latency (s) | |||||
| 2 | 9 | 18.61 ± 9.32 | 7 | 21.705 ± 7.561 | 0.487 |
| 3 | 11 | 17.66 ± 6.62 | 12 | 15.845 ± 6.827 | 0.526 |
| 4 | 8 | 13.19 ± 5.51 | 10 | 20.915 ± 5.554 | 0.010 |
| 5 | 12 | 13.44 ± 8.12 | 12 | 18.724 ± 5.844 | 0.081 |
| 6 | 11 | 13.46 ± 8.88 | 10 | 18.889 ± 4.825 | 0.103 |
| 7 | 8 | 10.82 ± 4.85 | 12 | 17.073 ± 4.194 | 0.007 |
| 8 | 11 | 12.76 ± 7.69 | 12 | 18.301 ± 4.919 | 0.050 |
| 10 | 11 | 14.95 ± 7.11 | 12 | 23.076 ± 6.535 | 0.009 |
| 12 | 9 | 12.96 ± 6.28 | 12 | 19.818 ± 5.636 | 0.017 |
| Average speed (cm/s) | |||||
| 2 | 9 | 12.65 ± 2.79 | 7 | 11.44 ± 4.15 | 0.496 |
| 3 | 11 | 10.96 ± 3.94 | 12 | 12.61 ± 2.99 | 0.268 |
| 4 | 8 | 10.76 ± 4.27 | 10 | 11.38 ± 2.62 | 0.705 |
| 5 | 12 | 7.26 ± 2.76 | 12 | 6.30 ± 3.19 | 0.442 |
| 6 | 11 | 6.80 ± 2.39 | 10 | 5.69 ± 2.38 | 0.297 |
| 7 | 8 | 7.01 ± 3.43 | 12 | 5.27 ± 2.57 | 0.211 |
| 8 | 11 | 6.61 ± 2.79 | 12 | 6.68 ± 4.12 | 0.959 |
| 10 | 11 | 6.49 ± 2.18 | 12 | 6.93 ± 2.45 | 0.651 |
| 12 | 9 | 5.77 ± 1.49 | 12 | 6.62 ± 3.03 | 0.445 |
| Time (Months) | Groups | Statistics p-Value | |||
|---|---|---|---|---|---|
| Control | Alzheimer Disease | ||||
| N | Mean ± SD | N | Mean ± SD | ||
| Recognition index | |||||
| 2 | 12 | 68.89 ± 18.99 | 10 | 72.93 ± 24.16 | 0.665 |
| 3 | 12 | 75.20 ± 24.76 | 11 | 83.49 ± 25.11 | 0.435 |
| 4 | 9 | 70.87 ± 29.37 | 9 | 36.30 ± 16.30 | 0.007 |
| 5 | 11 | 75.53 ± 18.60 | 10 | 38.18 ± 22.78 | <0.001 |
| 6 | 8 | 76.50 ± 23.38 | 9 | 58.10 ± 13.22 | 0.061 |
| 7 | 12 | 72.18 ± 18.57 | 9 | 53.96 ± 10.21 | 0.016 |
| 8 | 7 | 78.25 ± 15.82 | 6 | 61.36 ± 15.00 | 0.075 |
| 10 | 6 | 85.50 ± 20.01 | 9 | 52.43 ± 23.42 | 0.014 |
| 12 | 3 | 78.42 ± 15.78 | 8 | 51.13 ± 12.17 | 0.013 |
| Average speed (cm/s) | |||||
| 2 | 12 | 12.14 ± 3.49 | 10 | 8.27 ± 2.08 | 0.006 |
| 3 | 12 | 11.27 ± 2.42 | 11 | 8.42 ± 2.84 | 0.017 |
| 4 | 9 | 9.31 ± 3.39 | 9 | 7.14 ± 1.75 | 0.107 |
| 5 | 11 | 11.18 ± 3.92 | 10 | 9.04 ± 2.72 | 0.167 |
| 6 | 8 | 10.15 ± 2.15 | 9 | 10.79 ± 1.91 | 0.529 |
| 7 | 12 | 5.78 ± 1.41 | 9 | 7.41 ± 2.36 | 0.063 |
| 8 | 7 | 7.87 ± 0.84 | 6 | 7.02 ± 2.82 | 0.459 |
| 10 | 6 | 5.54 ± 1.53 | 9 | 9.69 ± 2.38 | 0.002 |
| 12 | 3 | 6.69 ± 1.71 | 8 | 6.93 ± 2.47 | 0.882 |
| Time (Months) | Control (µg/mL) | AD (µg/mL) | t-Student |
|---|---|---|---|
| Copper (Cu) | Mean ± SD | Mean ± SD | p-Value |
| 2 | 1.30 ± 0.28 | 1.23 ± 0.14 | 0.440 |
| 3 | 1.28 ± 0.29 | 1.63 ± 0.38 | 0.017 |
| 4 | 1.26 ± 0.23 | 1.59 ± 0.30 | 0.007 |
| 5 | 1.23 ± 0.25 | 1.48 ± 0.34 | 0.054 |
| 6 | 1.21 ± 0.22 | 1.45 ± 0.24 | 0.022 |
| 7 | 1.13 ± 0.18 | 1.55 ± 0.15 | <0.001 |
| 8 | 1.28 ± 0.27 | 1.44 ± 0.10 | 0.071 |
| 9 | 1.22 ± 0.33 | 1.47 ± 0.19 | 0.034 |
| 10 | 1.32 ± 0.11 | 1.54 ± 0.19 | 0.002 |
| 11 | 1.30 ± 0.09 | 1.57 ± 0.18 | <0.001 |
| 12 | 1.20 ± 0.24 | 1.62 ± 0.173 | <0.001 |
| Selenium (Se) | |||
| 2 | 1.21 ± 0.31 | 1.22 ± 0.21 | 0.895 |
| 3 | 1.28 ± 0.27 | 0.98 ± 0.19 | 0.006 |
| 4 | 1.16 ± 0.17 | 0.88 ± 0.19 | 0.001 |
| 5 | 1.03 ± 0.33 | 0.73 ± 0.32 | 0.033 |
| 6 | 0.98 ± 0.29 | 0.53 ± 0.29 | 0.001 |
| 7 | 0.97 ± 0.21 | 0.54 ± 0.40 | 0.004 |
| 8 | 0.93 ± 0.26 | 0.56 ± 0.17 | <0.001 |
| 9 | 0.96 ± 0.50 | 0.42 ± 0.26 | 0.003 |
| 10 | 1.090 ± 0.528 | 0.563 ± 0.57 | 0.030 |
| 11 | 0.763 ± 0.235 | 0.314 ± 0.152 | <0.001 |
| 12 | 0.621 ± 0.281 | 0.393 ± 0.118 | 0.016 |
| Zinc (Zn) | |||
| 2 | 3.82 ± 0.38 | 3.83 ± 3.83 | 0.890 |
| 3 | 3.75 ± 0.09 | 3.53 ± 3.53 | <0.001 |
| 4 | 3.41 ± 0.08 | 3.47 ± 3.47 | 0.080 |
| 5 | 3.75 ± 0.10 | 3.01 ± 3.01 | <0.001 |
| 6 | 3.33 ± 0.25 | 3.06 ± 3.06 | <0.001 |
| 7 | 3.54 ± 0.25 | 2.89 ± 2.89 | <0.001 |
| 8 | 3.25 ± 0.39 | 2.64 ± 2.64 | <0.001 |
| 9 | 3.43 ± 0.44 | 2.25 ± 2.25 | <0.001 |
| 10 | 2.93 ± 0.27 | 1.81 ± 1.81 | <0.001 |
| 11 | 2.65 ± 0.20 | 1.86 ± 1.87 | <0.001 |
| 12 | 2.59 ± 0.14 | 1.55 ± 1.55 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, O.F.M.; Valle, N.M.E.; Mamani, J.B.; Costa, C.J.S.; Alves, A.H.; Oliveira, F.A.; Rego, G.N.A.; Galanciak, M.C.S.; Felix, K.; Nucci, M.P.; et al. Longitudinal Evaluation of the Detection Potential of Serum Oligoelements Cu, Se and Zn for the Diagnosis of Alzheimer’s Disease in the 3xTg-AD Animal Model. Int. J. Mol. Sci. 2025, 26, 3657. https://doi.org/10.3390/ijms26083657
Dias OFM, Valle NME, Mamani JB, Costa CJS, Alves AH, Oliveira FA, Rego GNA, Galanciak MCS, Felix K, Nucci MP, et al. Longitudinal Evaluation of the Detection Potential of Serum Oligoelements Cu, Se and Zn for the Diagnosis of Alzheimer’s Disease in the 3xTg-AD Animal Model. International Journal of Molecular Sciences. 2025; 26(8):3657. https://doi.org/10.3390/ijms26083657
Chicago/Turabian StyleDias, Olivia F. M., Nicole M. E. Valle, Javier B. Mamani, Cicero J. S. Costa, Arielly H. Alves, Fernando A. Oliveira, Gabriel N. A. Rego, Marta C. S. Galanciak, Keithy Felix, Mariana P. Nucci, and et al. 2025. "Longitudinal Evaluation of the Detection Potential of Serum Oligoelements Cu, Se and Zn for the Diagnosis of Alzheimer’s Disease in the 3xTg-AD Animal Model" International Journal of Molecular Sciences 26, no. 8: 3657. https://doi.org/10.3390/ijms26083657
APA StyleDias, O. F. M., Valle, N. M. E., Mamani, J. B., Costa, C. J. S., Alves, A. H., Oliveira, F. A., Rego, G. N. A., Galanciak, M. C. S., Felix, K., Nucci, M. P., & Gamarra, L. F. (2025). Longitudinal Evaluation of the Detection Potential of Serum Oligoelements Cu, Se and Zn for the Diagnosis of Alzheimer’s Disease in the 3xTg-AD Animal Model. International Journal of Molecular Sciences, 26(8), 3657. https://doi.org/10.3390/ijms26083657








