Effects of Alnus japonica Pilot Scale Hot Water Extracts on a Model of Dexamethasone-Induced Muscle Loss and Muscle Atrophy in C57BL/6 Mice
Abstract
1. Introduction
2. Results
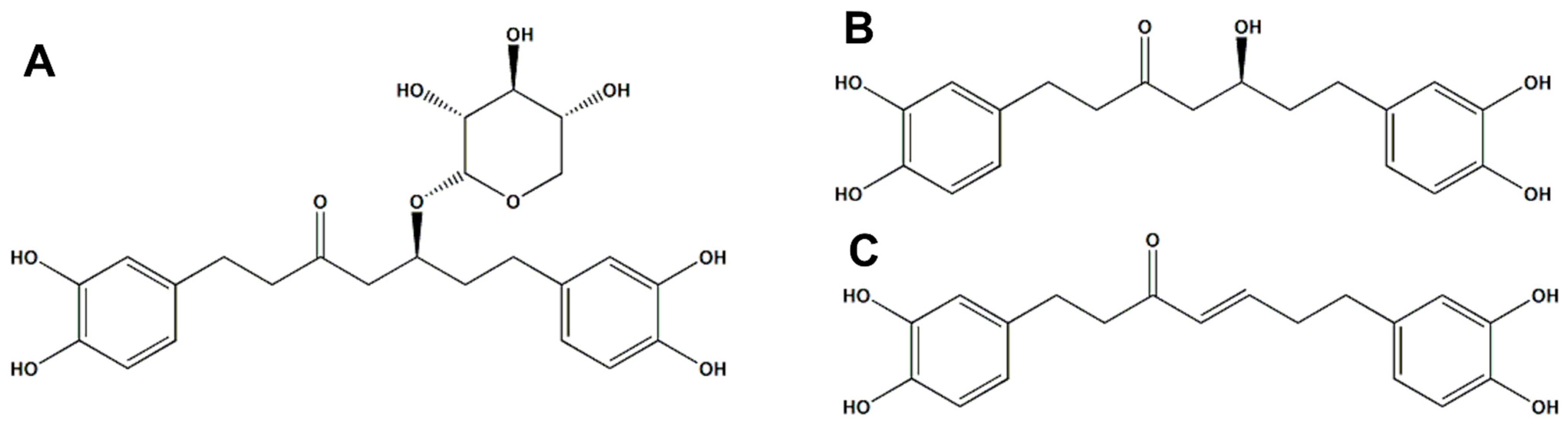
2.1. Phytochemical Analysis
2.1.1. Qualitative Analysis of AJHW
2.1.2. Quantitative Chromatographic Analysis of AJHW (HPLC)
2.1.3. Qualitative Analysis of AJHW on LC-MS/MS
2.2. Effects on Body Weight of Experimental Animals
2.3. Effects of Exercise Time and Exercise Capacity
2.4. Effects on Grip Strength
2.5. Effects on Fat Percentage and Lean Body Percentage
2.6. Effects on Muscle Weight
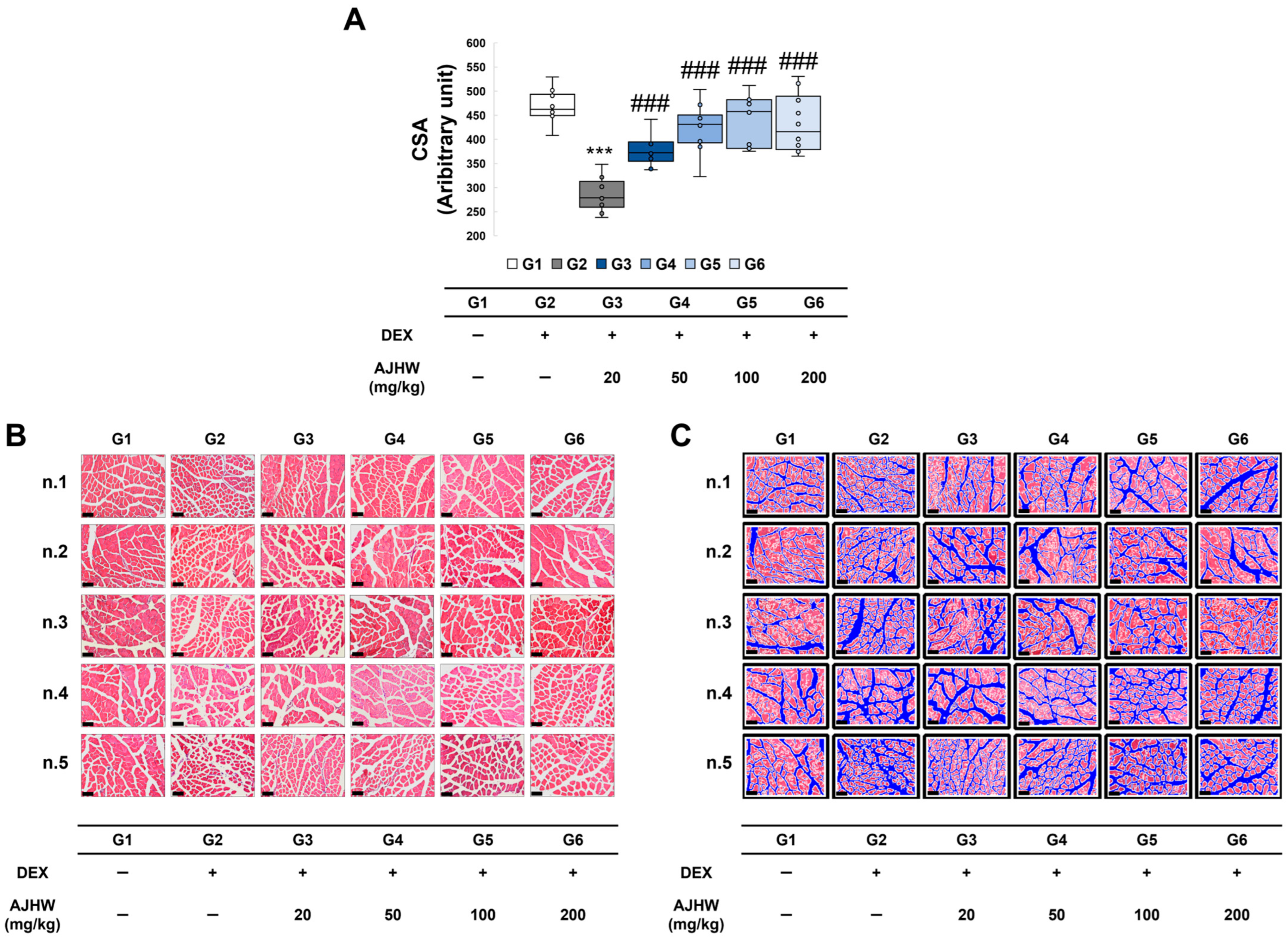
2.7. Effects on Muscle Fiber Cross-Sectional Area of the Tibialis Anterior (TA)
2.8. Effects on Protein Expression in Gastrocnemius (GA)
2.8.1. Effects on Muscle Degradation and Formation-Related Gene Expression (Protein)
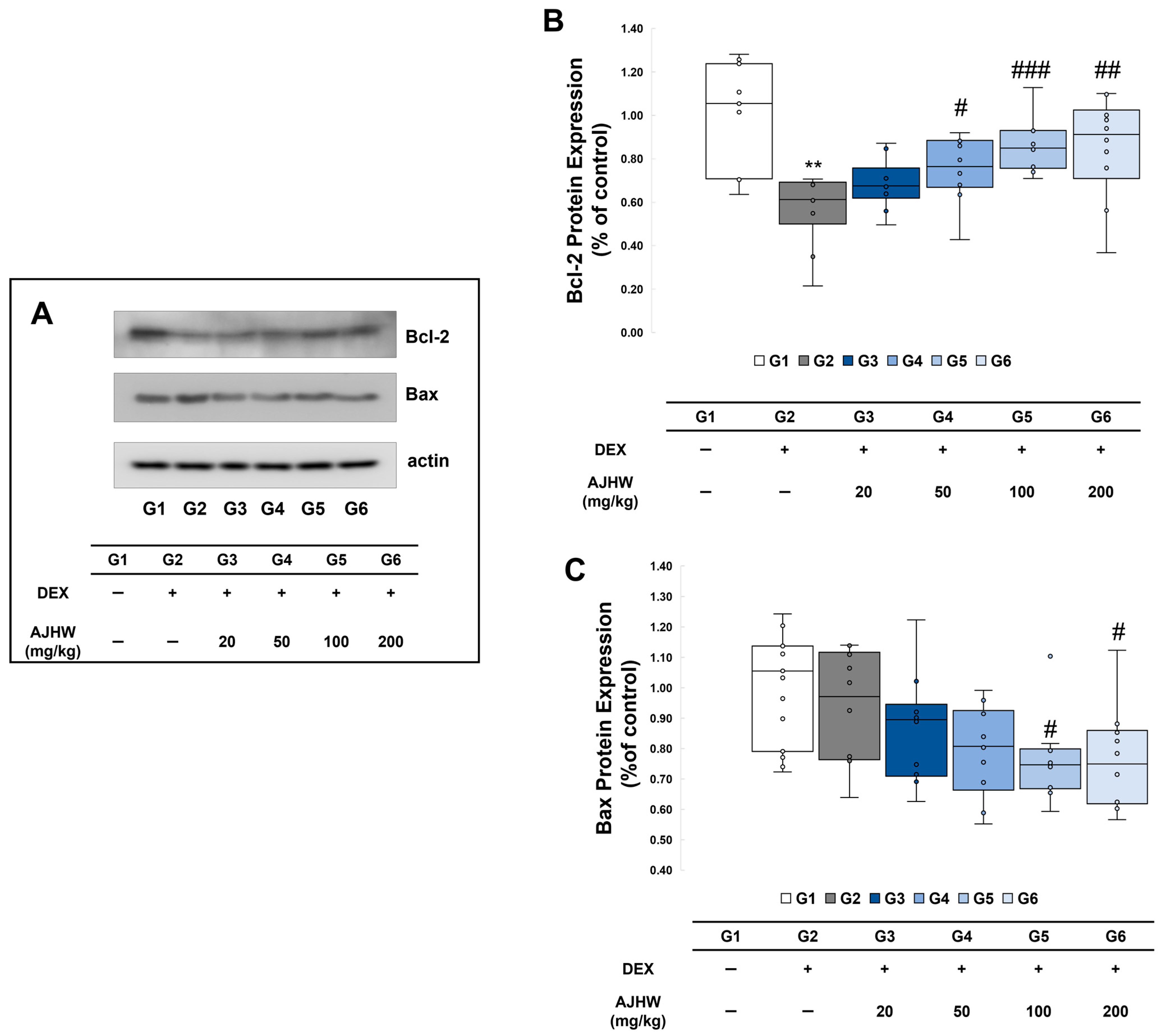
2.8.2. Effects on Expression of Apoptosis Regulatory Proteins
2.9. Effects on mRNA Expression in Muscle
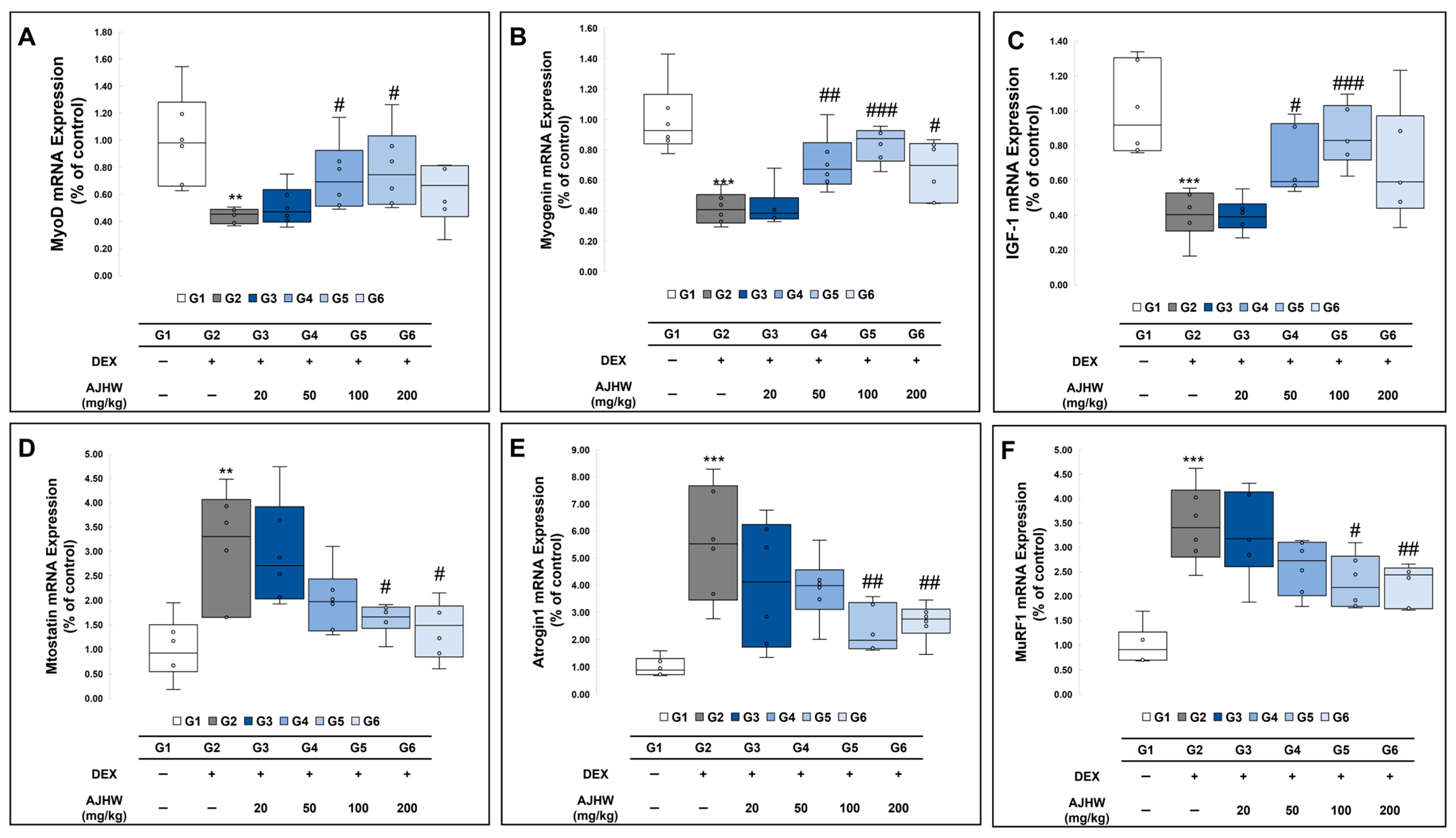
2.9.1. Effects on Muscle Degradation and Formation-Related Gene Expression (mRNA)
2.9.2. Effects on Inflammation-Related Gene Expression
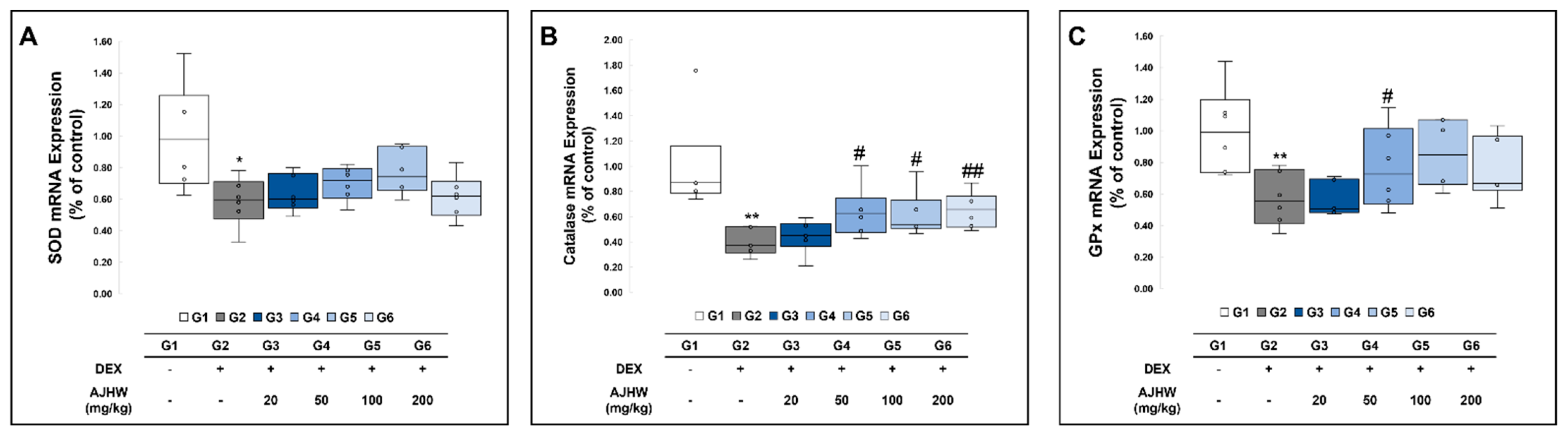
2.9.3. Effects on Antioxidant-Related Gene Expression
2.10. Effects on Serum Glutathione Content and Antioxidant Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Plant Extract Materials and Method
4.1.1. Plant Extract Materials
4.1.2. Plant Pilot-Scale Extraction Method
4.2. Phytochemical Analysis Methods
4.2.1. Standard Material for Phytochemical Analysis
- Oregonin (A) Brown amorphous powder, Negative LC-MS/MS: m/z 477 [M − H] −
- Hirsutanonol (B) Brown Oil, Negative LC/MS-MS: m/z 345 [M − H]−
- Hirsutenone (C) Brown Oil, Negative LC/MS-MS: m/z 326 [M − H] −
4.2.2. Qualitative Analysis of AJHWE (TLC)
4.2.3. Quantitative Chromatographic Analysis of AJHW (HPLC) Method
4.2.4. Measurement of the Molecular Weight of AJHW (LC-MS/MS)
4.3. Ethical Statement and Animals
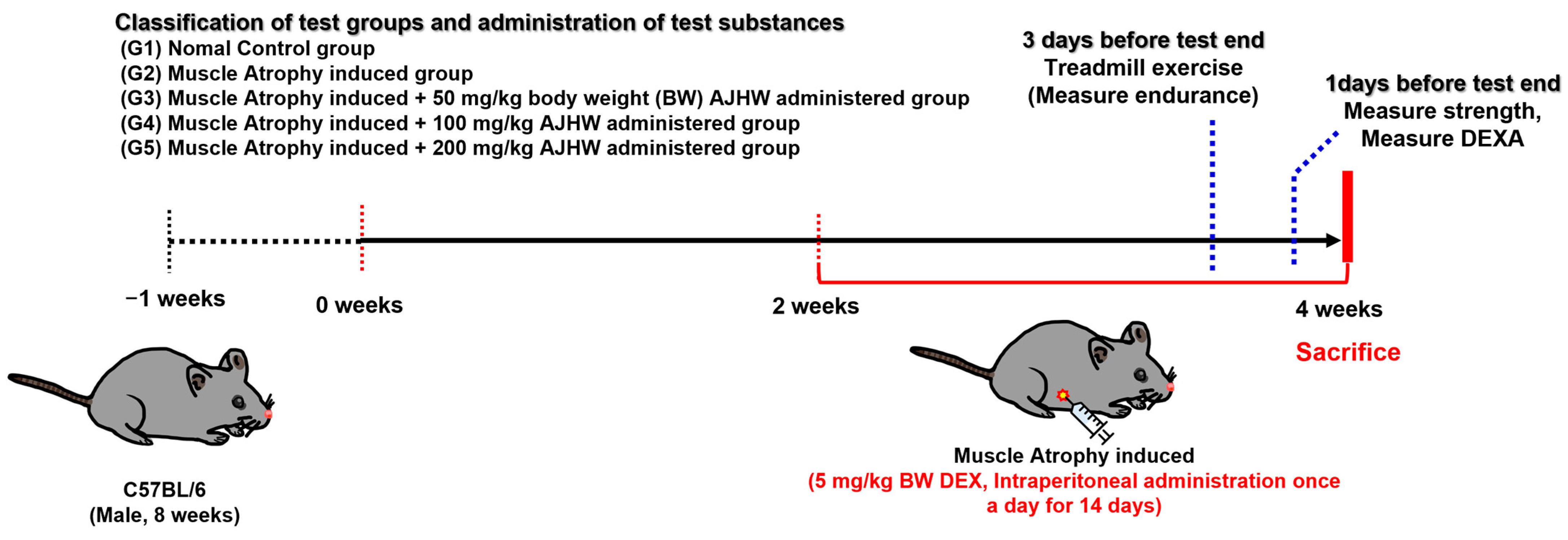
4.4. Experimental Design and Treatment
4.5. Measurement of Exercise Capacity
body weight (kg) × speed (m/s) × time (s) × grade × 9.8 m/s2
4.6. Measurement of Grip Strength
4.7. Measurement of Lean Body Percentage and Fat Percentage
4.8. Blood and Tissue Collection
4.9. Measurement of Antioxidant Enzyme Activity and Glutathione Content in Serum
4.10. Hematoxylin and Eosin Stain
4.11. Western Blot Analysis
4.12. Real-Time Polymerase Chain Reaction (Real-Time PCR)
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJHW | Alnus japonica Hot Water Extract |
| AJ | Alnus japonica |
| EtOH | Ethanol |
| ROS | Reactive Oxygen Species |
| AAS | Anabolic Androgenic Steroids |
| HPLC | High Performance Liquid Chromatography |
| TLC | Thin -Layer Chromatography |
| LC-MS/MS | Liquid Chromatography-Mass Spectrometry |
| h | Hour |
| RT-PCR | Real -Time Polymerase Chain Reaction |
| GAPDH | Glyceraldehyde-3-phosphate Dehydrogenase |
| QF | Quadriceps Femoris |
| GA | Gastrocnemius |
| SOL | Soleus |
| EDL | Extensor Digitorum Longus |
| TA | Tibialis Anterior |
| CSA | Cross Sectional Area |
| PARP | Poly (ADP-ribose) Polymerase |
| Bcl-2 | B-cell Lymphoma-2 |
| Bax | Bcl-2-associated X protein |
| Akt | Protein Kinase B |
| mTOR | Mammalian Target of Rapamycin |
| FoxO3α | Forkhead Box O3α |
| MuRF1 | Muscle RING-finger Protein 1 |
| Atrogin-1 | F-box Only Protein 32 |
| MyoD | Myoblast Determination |
| IGF-1 | Insulin-Like Growth Factor-1 |
| PI3K | Phosphoinositide 3-Kinase |
| Myf5 | Myogenic Factor 5 |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| IL-1 | Interleukin-1 |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor α |
| TNF-β | Tumor Necrosis Factor β |
| BW | Body Weight |
| Gpx | Glutathione Peroxidase |
| SOD | Superoxide Dismutase |
References
- Vaupel, J.W.; Villavicencio, F.; Bergeron-Boucher, M.-P. Demographic perspectives on the rise of longevity. Proc. Natl. Acad. Sci. USA 2021, 118, e2019536118. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Andreev, K.; Dupre, M.E. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Canon, M.E.; Crimmins, E.M. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J. Nutr. Health Aging 2011, 15, 695–698. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia-newly emerging and high impact complications of diabetes. J. Diabetes Its Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef]
- Srikanthan, P.; Horwich, T.B.; Tseng, C.H. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am. J. Cardiol. 2016, 117, 1355–1360. [Google Scholar] [CrossRef]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Liccini, A.P.; Malmstrom, T.K. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J. Am. Med. Dir. Assoc. 2016, 17, 846–851. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Marzetti, E.; Privitera, G.; Simili, V.; Wohlgemuth, S.E.; Aulisa, L.; Pahor, M.; Leeuwenburgh, C. Multiple pathways to the same end: Mechanisms of myonuclear apoptosis in sarcopenia of aging. Sci. World J. 2010, 10, 340–349. [Google Scholar] [CrossRef]
- Patel, J.; McLeod, L.E.; Vries, R.G.J.; Flynn, A.; Wang, X.; Proud, C.G. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 2002, 269, 3076–3085. [Google Scholar] [CrossRef]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease States. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Morley, J. Sarcopenia: Diagnosis and treatment. J. Nutr. Health Aging 2008, 12, 452–456. [Google Scholar] [CrossRef]
- Choi Hyon, K.; Atkinson, K.; Karlson Elizabeth, W.; Willett, W.; Curhan, G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef]
- Supasyndh, O.; Satirapoj, B.; Aramwit, P.; Viroonudomphol, D.; Chaiprasert, A.; Thanachatwej, V.; Vanichakarn, S.; Kopple, J.D. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.L.; Wood, R.I. Intracerebroventricular self-administration of commonly abused anabolic-androgenic steroids in male hamsters (Mesocricetus auratus): Nandrolone, drostanolone, oxymetholone, and stanozolol. Behav. Neurosci. 2005, 119, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef]
- Solimini, R.; Rotolo, M.; Mastrobattista, L.; Mortali, C.; Minutillo, A.; Pichini, S.; Pacifici, R.; Palmi, I. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 7–16. [Google Scholar] [PubMed]
- Albano, G.D.; Amico, F.; Cocimano, G.; Liberto, A.; Maglietta, F.; Esposito, M.; Rosi, G.L.; Di Nunno, N.; Salerno, M.; Montana, A. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare 2021, 9, 97. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide Survey of Fitness Trends for 2023. ACSM’s Health Fit. J. 2023, 27, 9–18. [Google Scholar] [CrossRef]
- Ko, G.-J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Momoh, R. Anabolic-Androgenic Steroid Abuse Causes Cardiac Dysfunction. Am. J. Men’s Health 2024, 18, 15579883241249647. [Google Scholar] [CrossRef]
- Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef]
- Wani, M.C.; Horwitz, S.B. Nature as a remarkable chemist: A personal story of the discovery and development of Taxol. Anti-Cancer Drugs 2014, 25, 482–487. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lee, E.-H.; Kim, C.-Y.; Shin, T.-Y.; Kim, S.-D.; Song, Y.-S.; Lee, K.-N.; Hong, S.-H.; Kim, H.-M. Anti-inflammatory Activity of Korean Folk Medicine Purple Bamboo Salt. Immunopharmacol. Immunotoxicol. 2003, 25, 377–384. [Google Scholar] [CrossRef]
- Boericke, W. Pocket Manual of Homoeopathic Materia Medica & Repertory: Comprising of the Characteristic and Guiding Symptoms of All Remedies (Clinical and Pahtogenetic [Sic]) Including Indian Drugs; B. Jain publishers: Noida, India, 2002; p. 24. [Google Scholar]
- Choi, S.E. Chemotaxonomic Significance of Oregonin in Alnus Species. Asian J. Chem. 2013, 25, 6989–6990. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Shimomae, M.; Nagashima, Y.; Muto, N.; Okuda, T.; Kawahara, N.; Nakane, T.; Sano, T. New Diarylheptanoids from Alnus japonica and Their Antioxidative Activity. Chem. Pharm. Bull. 2005, 53, 1519–1523. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, J.D.; Ahn, S.H.; Ahn, G.S.; Lee, Y.I.; Jeong, Y.S. Hepatoprotective and antioxidant effects of Alnus japonica extracts on acetaminophen-induced hepatotoxicity in rats. Phytother. Res. 2004, 18, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Park, K.H.; Jeong, M.S.; Kim, H.H.; Lee, D.I.; Joo, S.S.; Lee, C.S.; Bang, H.; Choi, Y.W.; Lee, M.-K.; et al. Effect of Alnus japonica extract on a model of atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011, 136, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-S.; Kim, M.-S.; Oh, W.-S.; Lee, D.-I. Enhancement of NK cytotoxicity, antimetastasis and elongation effect of survival time in B16-F10 melanoma cells by oregonin. Arch. Pharmacal Res. 2002, 25, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, M.; Choi, S.; Kim, M.; Kang, O.; Lee, Y.; Chae, H.; Obiang-Obounou, B.; Oh, Y.; Kim, M. Antibacterial activity of bark of Alnus pendula against methicillin-resistant Staphylococcus aureus. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 853–859. [Google Scholar]
- Lee, H.; Lee, K.S.; Jeong, J.H.; Yoon, J.S.; Hwang, S.H.; Kim, S.-Y.; Yeon, S.H.; Ryu, J.-H. Extract of Alnus japonica prevents dexamethasone-induced muscle atrophy in mice. J. Funct. Foods 2023, 101, 105419. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.; Kwon, Y.; Kim, T.; Lee, C.H.; Jang, H.; Kim, E.J.; Jung, J.I.; Min, S.; Park, K.-H.; et al. Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells. Curr. Issues Mol. Biol. 2024, 46, 8320–8339. [Google Scholar] [CrossRef]
- Lee, C.H.; Kwon, Y.; Park, S.; Kim, T.; Kim, M.S.; Kim, E.J.; Jung, J.I.; Min, S.; Park, K.-H.; Jeong, J.H.; et al. The Impact of Ulmus macrocarpa Extracts on a Model of Sarcopenia-Induced C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 6197. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Choi, S.E. Extraction method and physiological activity of high content oregonin derived from plant of Alnus sibirica Fisch. ex Turcz. Korean J. Pharmacogn. 2019, 50, 165–174. [Google Scholar]
- Sivanandham, V. Phytochemical Techniques—A Review. World J. Sci. Res. 2015, 1, 80–91. [Google Scholar]
- Klarić, M.; Pervan, S.; Biošić, M. Influence of Lyophilisation and Oven-Drying on Extraction Yield of Oregonin from European Black Alder (Alnus glutinosa (L.) Gaertn.) Bark. Wood Ind./Drv. Ind. 2017, 68, 205–210. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Choi, S.E.; Kim, K.H.; Kwon, J.H.; Kim, S.B.; Kim, H.W.; Lee, M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharmacal Res. 2008, 31, 1287–1289. [Google Scholar] [CrossRef]
- Choi, S.E.; Park, K.H.; Kim, M.H.; Song, J.H.; Jin, H.Y.; Lee, M.W. Diarylheptanoids from the Bark of Alnus pendula Matsumura. Nat. Prod. Sci. 2012, 18, 106–110. [Google Scholar]
- Georgescu-Roegen, N. The Economics of Production. Am. Econ. Rev. 1970, 60, 1–9. [Google Scholar]
- Bjornsson, T.D.; Callaghan, J.T.; Einolf, H.J.; Fischer, V.; Gan, L.; Grimm, S.; Kao, J.; King, S.P.; Miwa, G.; Ni, L.; et al. The Conduct of In Vitro and In Vivo Drug-Drug Interaction Studies: A Pharmaceutical Research and Manufacturers of America (PhRMA) Perspective. Drug Metab. Dispos. 2003, 31, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-Q.; He, M.; Yu, D.-J.; Wu, Y.-X.; Wang, X.-H.; Lv, S.; Xiao, W.-F.; Li, Y.-S. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- An, D.H.; Lee, C.H.; Kwon, Y.; Kim, T.H.; Kim, E.J.; Jung, J.I.; Min, S.; Cheong, E.J.; Kim, S.; Kim, H.K.; et al. Effects of Alnus japonica Hot Water Extract and Oregonin on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells. Pharmaceuticals 2024, 17, 1661. [Google Scholar] [CrossRef]
- de Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef]
- Kim, J.W.; Ku, S.-K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chun, Y.-S.; Kim, J.-K.; Lee, J.-O.; Ku, S.-K.; Shim, S.-M. Curcumin Attenuates Sarcopenia in Chronic Forced Exercise Executed Aged Mice by Regulating Muscle Degradation and Protein Synthesis with Antioxidant and Anti-inflammatory Effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Jang, E.-R.; Kim, Y.J.; Lee, M.S.; Seo, S.J.; Lee, M.W. Hirsutenone inhibits lipopolysaccharide-activated NF-κB-induced inflammatory mediator production by suppressing Toll-like receptor 4 and ERK activation. Int. Immunopharmacol. 2010, 10, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, H.J.; Jung, S.Y.; Yook, C.-S.; Jin, C.; Lee, Y.S. A New Diarylheptanoid Glycoside from the Stem Bark of Alnus hirsuta and Protective Effects of Diarylheptanoid Derivatives in Human HepG2 Cells. Chem. Pharm. Bull. 2010, 58, 238–241. [Google Scholar] [CrossRef]
- Jeong, M.S.; Choi, S.E.; Kim, J.Y.; Kim, J.S.; Kim, E.J.; Park, K.H.; Lee, D.I.; Joo, S.S.; Lee, C.S.; Bang, H.; et al. Atopic dermatitis-like skin lesions reduced by topical application and intraperitoneal injection of Hirsutenone in NC/Nga mice. Clin. Dev. Immunol. 2010, 2010, 618517. [Google Scholar] [CrossRef]
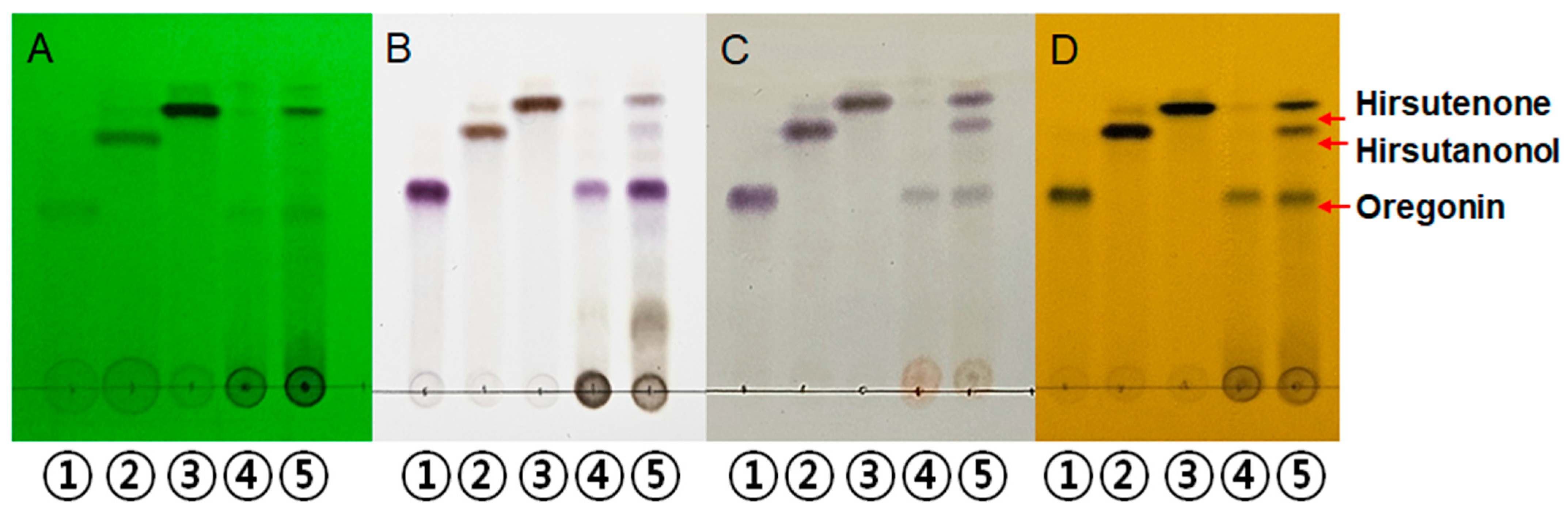
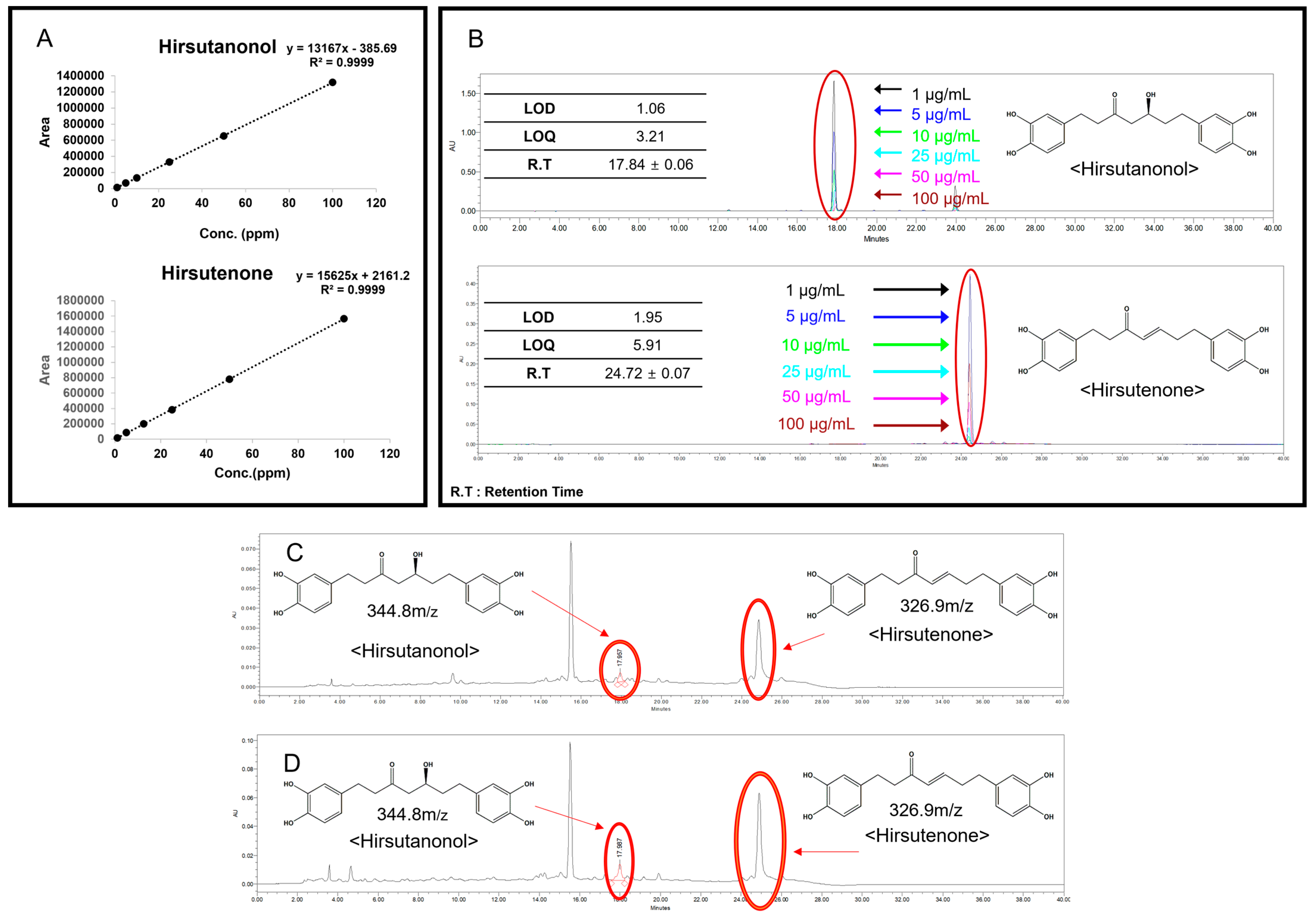
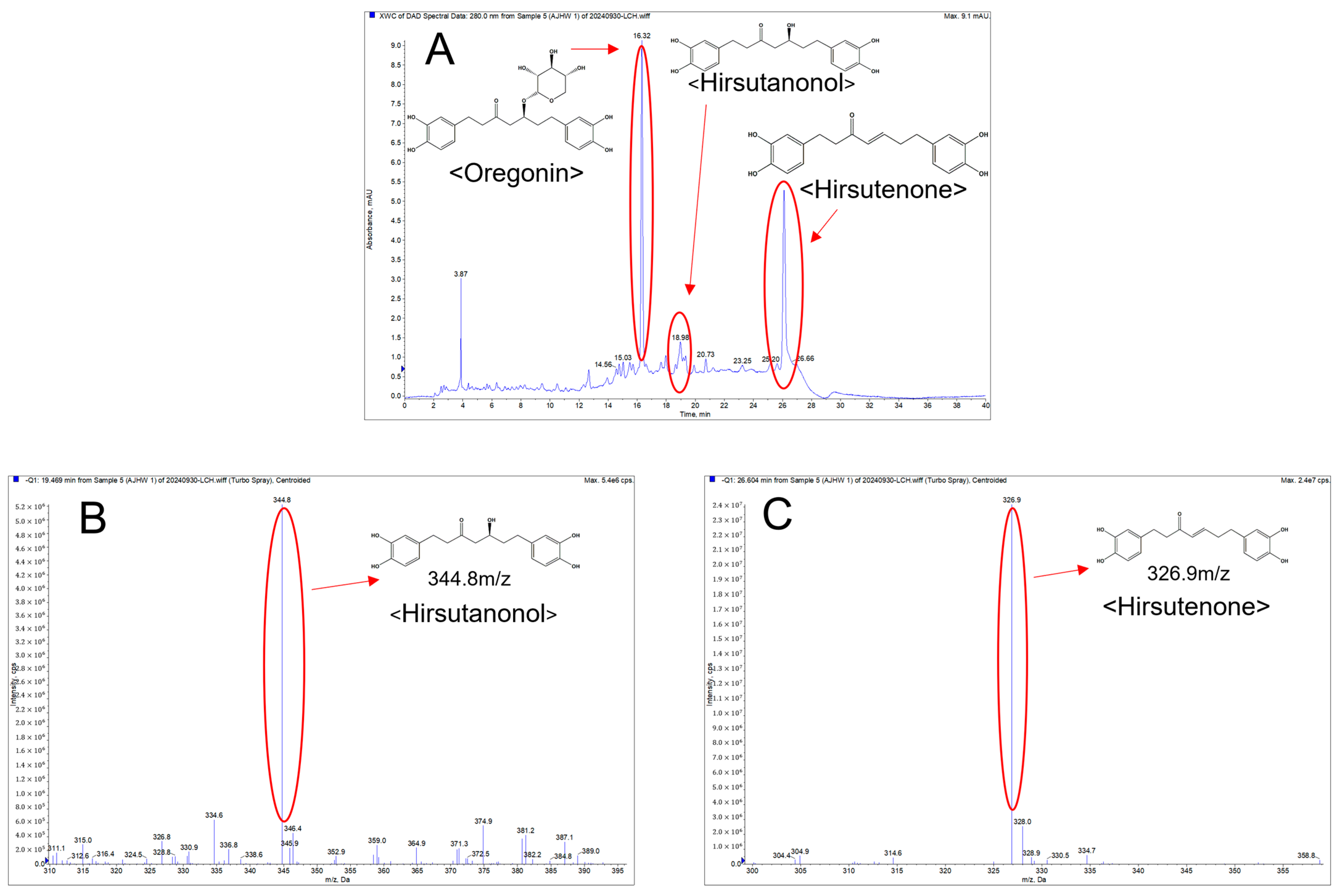
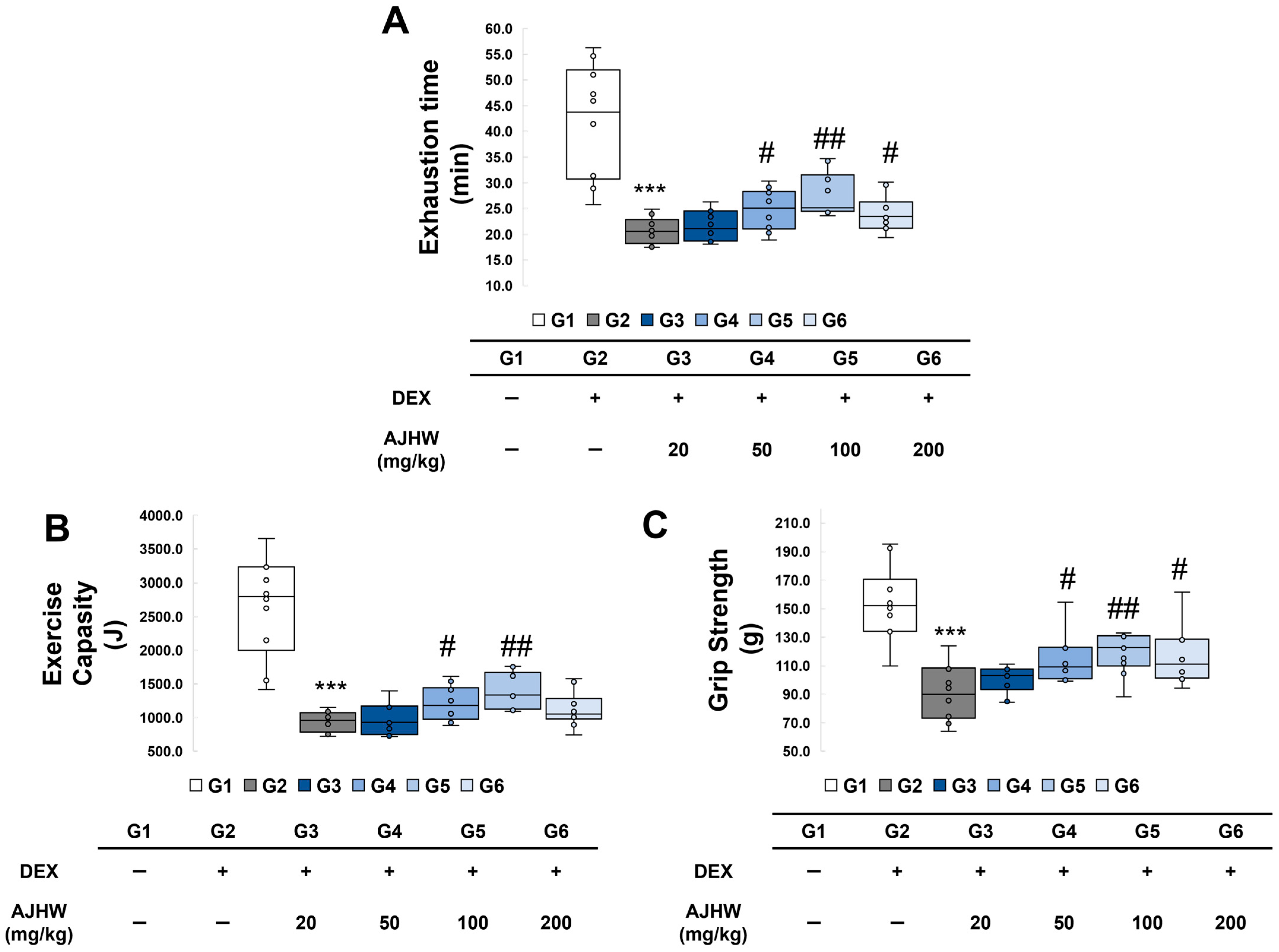



| 0 Week | 1 Week | 2 Week | 3 Week | 4 Week | |
|---|---|---|---|---|---|
| G1 | 25.1 ± 0.4 | 27.5 ± 0.3 | 28.1 ± 0.4 | 29.8 ± 0.6 | 30.2 ± 0.5 |
| G2 | 25.1 ± 0.3 | 27.1 ± 0.2 | 28.1 ± 0.2 | 27.6 ± 0.3 ** | 27.1 ± 0.4 *** |
| G3 | 25.2 ± 0.3 | 27.2 ± 0.4 | 28.0 ± 0.3 | 27.0 ± 0.5 | 27.5 ± 0.7 |
| G4 | 25.2 ± 0.3 | 27.5 ± 0.6 | 27.7 ± 0.6 | 27.9 ± 0.8 | 27.8 ± 0.6 |
| G5 | 25.1 ± 0.2 | 27.2 ± 0.4 | 28.3 ± 0.4 | 26.8 ± 0.6 | 28.5 ± 0.7 |
| G6 | 25.1 ± 0.2 | 26.8 ± 0.3 | 27.9 ± 0.4 | 26.6 ± 0.5 | 27.3 ± 0.5 |
| G1 | G2 | G3 | G4 | G5 | G6 | ||
|---|---|---|---|---|---|---|---|
| Muscle weight (g) | QF | 0.390 ± 0.015 | 0.305 ± 0.008 *** | 0.313 ± 0.013 | 0.338 ± 0.010 # | 0.365 ± 0.020 # | 0.333 ± 0.015 |
| GA | 0.325 ± 0.007 | 0.268 ± 0.006 *** | 0.273 ± 0.008 | 0.285 ± 0.008 | 0.298 ± 0.010 # | 0.286 ± 0.005 # | |
| SOL | 0.0202 ± 0.0003 | 0.0171 ± 0.0002 *** | 0.0174 ± 0.0008 | 0.0176 ± 0.0005 | 0.0189 ± 0.0004 ## | 0.0176 ± 0.0004 | |
| EDL | 0.026 ± 0.002 | 0.019 ± 0.001 ** | 0.018 ± 0.001 | 0.021 ± 0.001 | 0.022 ± 0.001 # | 0.020 ± 0.001 | |
| TA | 0.126 ± 0.003 | 0.102 ± 0.005 ** | 0.105 ± 0.011 | 0.111 ± 0.003 | 0.115 ± 0.006 | 0.110 ± 0.003 | |
| Relative muscle weight (g/100 g BW) | QF | 1.295 ± 0.048 | 1.126 ± 0.027 ** | 1.144 ± 0.055 | 1.225 ± 0.045 | 1.279 ± 0.065 # | 1.223 ± 0.060 |
| GA | 1.079 ± 0.030 | 0.990 ± 0.025 * | 0.997 ± 0.038 | 1.032 ± 0.039 | 1.053 ± 0.050 | 1.049 ± 0.017 | |
| SOL | 0.067 ± 0.001 | 0.063 ± 0.001 * | 0.063 ± 0.002 | 0.063 ± 0.002 | 0.066 ± 0.001 # | 0.065 ± 0.001 | |
| EDL | 0.088 ± 0.006 | 0.069 ± 0.004 * | 0.066 ± 0.003 | 0.075 ± 0.003 | 0.076 ± 0.003 | 0.075 ± 0.003 | |
| TA | 0.418 ± 0.013 | 0.376 ± 0.017 | 0.389 ± 0.044 | 0.402 ± 0.015 | 0.405 ± 0.020 | 0.404 ± 0.015 |
| G1 | G2 | G3 | G4 | G5 | G6 | |
|---|---|---|---|---|---|---|
| Glutathione (μM) | 12.43 ± 2.57 | 4.38 ± 0.75 ** | 8.25 ± 0.68 ## | 7.47 ± 0.78 # | 8.59 ± 0.68 ### | 8.71 ± 0.87 ## |
| SOD (U/mL) | 3.59 ± 0.23 | 2.33 ± 0.28 * | 1.96 ± 0.22 | 2.63 ± 0.17 | 2.68 ± 0.19 | 3.68 ± 0.25 # |
| Catalase (nM/min/mL) | 41.0 ± 4.0 | 26.1 ± 3.6 * | 31.9 ± 3.0 | 32.9 ± 3.2 | 37.3 ± 3.7 # | 36.7 ± 5.0 |
| GPx (nM/min/mL) | 198.7 ± 5.0 | 138.9 ± 5.4 ** | 159.1 ± 13.9 | 175.1 ± 10.4 ## | 189.1 ± 6.2 ### | 189.2 ± 6.9 ### |
| Antibodies | Details | Manufacturer |
|---|---|---|
| Antibody: phospho-mTOR (Ser253) | #5536 | Cell Signaling Technology (Danvers, MA, USA) |
| Antibody: mTOR | #2972 | Cell Signaling Technology |
| Antibody: phospho-FoxO3α (Ser253) | #9466 | Cell Signaling Technology |
| Antibody: FoxO3α | #2497 | Cell Signaling Technology |
| Antibody: phospho-Akt (Ser) | #4051 | Cell Signaling Technology |
| Antibody: Akt | #9272 | Cell Signaling Technology |
| Antibody: Bax | #2772 | Cell Signaling Technology |
| Antibody: Bcl-2 | #3498 | Cell Signaling Technology |
| Antibody: Actin | #3700 | Cell Signaling Technology |
| mRNA | Primer Sequences | |
|---|---|---|
| Atrogin-1 | Forward | 5′-GCCCTCCACACTAGTTGACC-3′ |
| Reverse | 5‘-GACGGATTGACAGCCAGGAA-3′ | |
| Catalase | Forward | 5′-GAACGAGGAGGAGAGGAAAC-3′ |
| Reverse | 5′-TGAAATTCTTGACCGCTTTC-3′ | |
| GPx1 | Forward | 5′-CAGGTCGGACGTACTTGAG-3′ |
| Reverse | 5′-CAGGTCGGACGTACTTGAG-3′ | |
| IGF-1 | Forward | 5′-GTGGATGCTCTTCAGTTCGTGTG-3′ |
| Reverse | 5′-TCCAGTCTCCTCAGATCACAGC-3′ | |
| IL-1β | Forward | 5′-TGGACCTTCCAGGATGAGGACA-3′ |
| Reverse | 5′-GTTCATCTCGGAGCCTGTAGTG-3′ | |
| IL-6 | Forward | 5′-CCTCTGGTCTTCTGGAGTACC-3′ |
| Reverse | 5′-ACTCCTTCTGTGACTCCAGC-3′ | |
| MuRF1 | Forward | 5′-GAGGGCCATTGACTTTGGGA-3′ |
| Reverse | 5′-TTTACCCTCTGTGGTCACGC-3′ | |
| MyoD1 | Forward | 5′-GCACTACAGTGGCGACTCAGAT-3′ |
| Reverse | 5′-TAGTAGGCGGTGTCGTAGCCAT-3′ | |
| Myogenin | Forward | 5′-CCATCCAGTACATTGAGCGCCT-3′ |
| Reverse | 5′-CTGTGGGAGTTGCATTCACTGG-3′ | |
| Myostatin | Forward | 5′-ACTGGACCTCTCGATAGAACACTC-3′ |
| Reverse | 5′-ACTTAGTGCTGTGTGTGTGGAGAT-3′ | |
| SOD2 | Forward | 5′-ATCAGGACCCATTGCAAGGA-3′ |
| Reverse | 5′-AGGTTTCACTTCTTGCAAGCT-3′ | |
| TNF-α | Forward | 5′-GGTGCCTATGTCTCAGCCTCTT-3′ |
| Reverse | 5′-GCCATAGAACTGATGAGAGGGAG-3′ | |
| GAPDH | Forward | 5′-TGGGTGTGAACCATGAGAAG-3′ |
| Reverse | 5′-GCTAAGCAGTTGGTGGTGC-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.D.; Lee, C.H.; Kwon, Y.E.; Kim, T.H.; Kim, E.J.; Jung, J.I.; Min, S.I.; Cheong, E.J.; Jang, T.Y.; Kim, H.K.; et al. Effects of Alnus japonica Pilot Scale Hot Water Extracts on a Model of Dexamethasone-Induced Muscle Loss and Muscle Atrophy in C57BL/6 Mice. Int. J. Mol. Sci. 2025, 26, 3656. https://doi.org/10.3390/ijms26083656
Jang HD, Lee CH, Kwon YE, Kim TH, Kim EJ, Jung JI, Min SI, Cheong EJ, Jang TY, Kim HK, et al. Effects of Alnus japonica Pilot Scale Hot Water Extracts on a Model of Dexamethasone-Induced Muscle Loss and Muscle Atrophy in C57BL/6 Mice. International Journal of Molecular Sciences. 2025; 26(8):3656. https://doi.org/10.3390/ijms26083656
Chicago/Turabian StyleJang, Hyeon Du, Chan Ho Lee, Ye Eun Kwon, Tae Hee Kim, Eun Ji Kim, Jae In Jung, Sang Il Min, Eun Ju Cheong, Tae Young Jang, Hee Kyu Kim, and et al. 2025. "Effects of Alnus japonica Pilot Scale Hot Water Extracts on a Model of Dexamethasone-Induced Muscle Loss and Muscle Atrophy in C57BL/6 Mice" International Journal of Molecular Sciences 26, no. 8: 3656. https://doi.org/10.3390/ijms26083656
APA StyleJang, H. D., Lee, C. H., Kwon, Y. E., Kim, T. H., Kim, E. J., Jung, J. I., Min, S. I., Cheong, E. J., Jang, T. Y., Kim, H. K., & Choi, S. E. (2025). Effects of Alnus japonica Pilot Scale Hot Water Extracts on a Model of Dexamethasone-Induced Muscle Loss and Muscle Atrophy in C57BL/6 Mice. International Journal of Molecular Sciences, 26(8), 3656. https://doi.org/10.3390/ijms26083656









