Bifunctional Chromium-Doped Phenolic Porous Hydrothermal Carbon Catalysts for the Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural
Abstract
1. Introduction
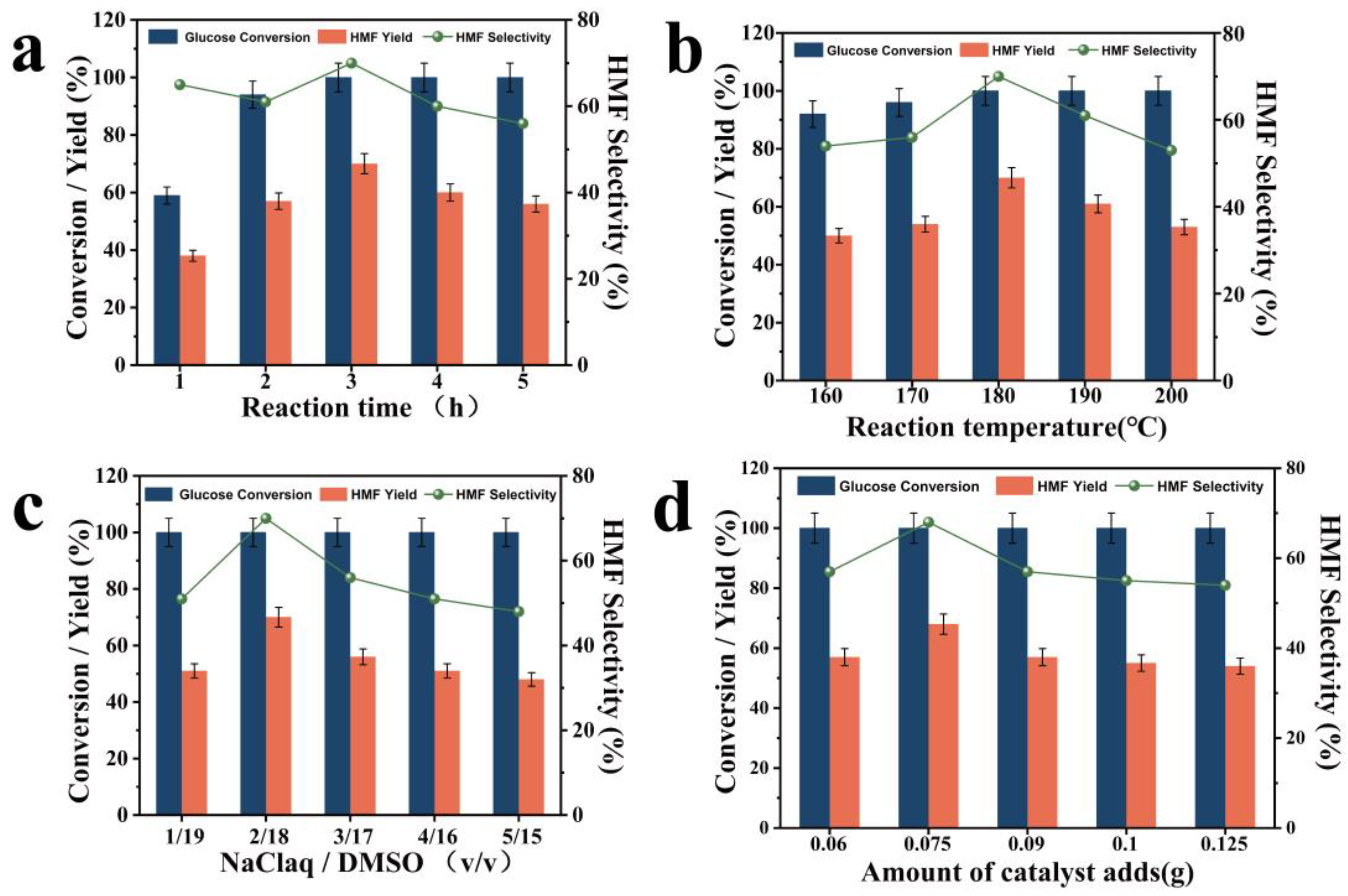
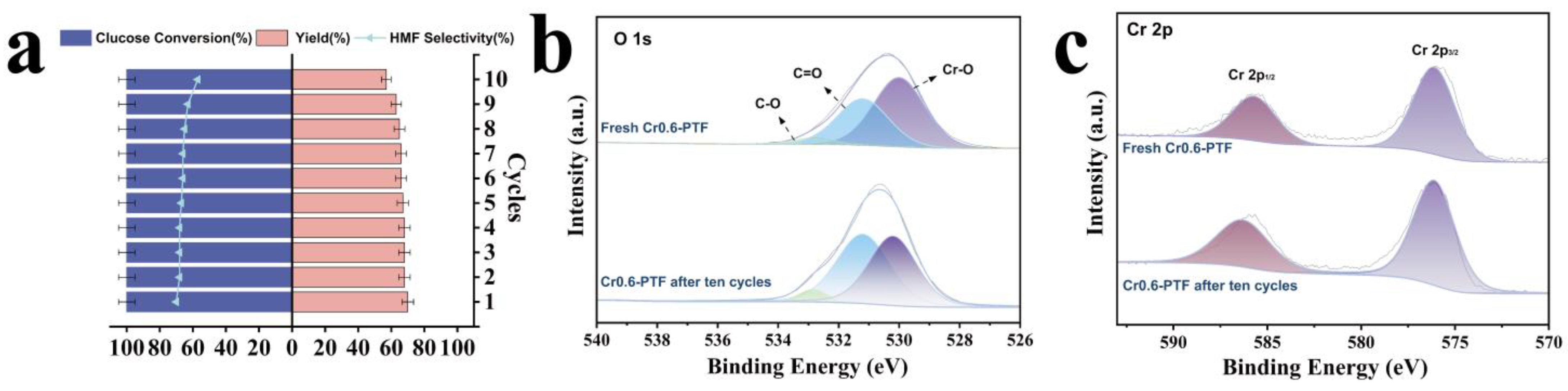
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalytic Reaction
3.4. Characterization
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Lai, J.; Liu, X.; Huang, G.; You, G.; Xu, Q.; Yin, D. Selective conversion of biomass-derived furfuryl alcohol into n-butyl levulinate over sulfonic acid functionalized TiO2 nanotubes. Green Energy Environ. 2022, 7, 257–265. [Google Scholar] [CrossRef]
- Dai, J. Synthesis of 2,5-diformylfuran from renewable carbohydrates and its applications: A review. Green Energy Environ. 2021, 6, 22–32. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N.; Kumar, D. Synergistic effect of modified activated carbon and ionic liquid in the conversion of microcrystalline cellulose to 5-hydroxymethyl furfural. Bioresour. Technol. 2018, 267, 326–332. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Jin, M. Towards efficient enzymatic saccharification of pretreated lignocellulose: Enzyme inhibition by lignin-derived phenolics and recent trends in mitigation strategies. Biotechnol. Adv. 2022, 61, 108044. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.; Zeng, X.; Sun, Y.; Ke, X.; Li, T.; Zhang, J.; Lin, L. Catalyst design strategy toward the efficient heterogeneously catalyzed selective oxidation of 5-hydroxymethylfurfural. Green. Energy Environ. 2022, 7, 900–932. [Google Scholar] [CrossRef]
- Yan, D.; Xin, J.; Shi, C.; Lu, X.; Ni, L.; Wang, G.; Zhang, S. Base-free conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid in ionic liquids. Chem. Eng. J. 2017, 323, 473–482. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Chen, P.; Yamaguchi, A.; Hiyoshi, N.; Mimura, N. Efficient continuous dehydration of fructose to 5-hydroxymethylfurfural in ternary solvent system. Fuel. 2023, 334, 126632. [Google Scholar] [CrossRef]
- Yue, F.; Pedersen, C.M.; Yan, X.; Liu, Y.; Xiang, D.; Ning, C.; Wang, Y.; Qiao, Y. NMR studies of stock process water and reaction pathways in hydrothermal carbonization of furfural residue. Green Energy Environ. 2018, 3, 163–171. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, J.; Cai, T.; Liu, C.; Ye, J.; Zeng, X.; Wang, K. Advances in selective conversion of carbohydrates into 5-hydroxymethylfurfural. Green Energy Environ. 2024, 9, 1384–1406. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Y.; Wang, S.; Hou, S.; Fan, Y. Incorporation of tin oxide nanoparticles on sulfonated carbon microspheres as a bifunctional catalyst for efficient conversion of biomass-derived monosaccharides to 5-hydroxymethylfurfural and furfural. Ind. Crops Prod. 2024, 208, 117913. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Kim, Y.; Mittal, A.; Robichaud, D.J.; Pilath, H.M.; Etz, B.D.; St John, P.C.; Johnson, D.K.; Kim, S. Prediction of hydroxymethylfurfural yield in glucose conversion through investigation of Lewis acid and organic solvent effects. ACS Catal. 2020, 10, 14707–14721. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Z.-Y.; Dong, J.-J.; Li, H.; Han, R.-Z.; Xu, G.-C.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Bioresour. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef]
- Dallas Swift, T.D.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium (III) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef]
- Deng, T.; Cui, X.; Qi, Y.; Wang, Y.; Hou, X.; Zhu, Y. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2 in water. Chem. Commun. 2012, 48, 5494–5496. [Google Scholar] [CrossRef]
- Chung, N.H.; Oanh, V.T.; Thoa, L.K.; Hoang, P.H. Catalytic conversion of glucose into 5-hydroxymethyl furfural over Cu–Cr/ZSM-5 zeolite. Cat. Lett. 2020, 150, 170–177. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Srisuwanno, W.; Saenluang, K.; Prasertsab, A.; Salakhum, S.; Kidkhunthod, P.; Namuangruk, S.; Wattanakit, C. Isolated Hf-isomorphously substituted zeolites for one-pot HMF synthesis from glucose. Adv. Sustain. Syst. 2023, 7, 2200403. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le Nguyen, D.A.L.; Phan, H.B.; Le, D.D.; Tran, P.H. Highly efficient and recyclable chromium/nitrogen-doped carbon nanotube catalysts with unexpected active sites for conversion of fructose into 5-hydroxymethylfurfural. Energy 2024, 305, 132317. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Wang, Y.; Cui, H.; Song, F.; Sun, X.; Zhang, L. Conversion of glucose into 5-hydroxymethylfurfural catalyzed by acid–base bifunctional heteropolyacid-based ionic hybrids. Green Chem. 2018, 20, 1551–1559. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Tao, Y.; Sun, W.; Wang, W. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silica-supported phosphotungstic acid heterogeneous catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Yan, Y.; Xue, S.; Zhang, Y. Efficient and selective conversion of hexose to 5-hydroxymethylfurfural with tin–zirconium-containing heterogeneous catalysts. Cat. Commun. 2014, 50, 38–43. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, Y.; Song, F.; Xie, Y.; Wang, M.; Cui, H.; Yi, W. N-doped carbon materials as heterogeneous catalysts for high efficiency isomerization glucose to fructose in aqueous media. Cat. Lett. 2020, 150, 493–504. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.; Cho, D.-W.; Song, H.; Tsang, D.C.; Tessonnier, J.-P.; Ok, Y.S.; Poon, C.S. Selective glucose isomerization to fructose via a nitrogen-doped solid base catalyst derived from spent coffee grounds. ACS Sustain. Chem. Eng. 2018, 6, 16113–16120. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Jin, C.E.S.; Sheng, K.; Zhang, X. Effect of swelling pretreatment on properties of cellulose-based hydrochar. ACS Sustain. Chem. Eng. 2019, 7, 10821–10829. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Liu, J.; Shuang, E.; Jin, C.; Xu, Z.; Zhang, X. Hydrothermal carbonization of cellulose and xylan into hydrochars and application on glucose isomerization. J. Cleaner Prod. 2019, 237, 117831. [Google Scholar] [CrossRef]
- Basak, S.; Raja, A.S.M.; Saxena, S.; Patil, P.G. Tannin based polyphenolic bio-macromolecules: Creating a new era towards sustainable flame retardancy of polymers. Polym. Degrad. Stab. 2021, 189, 109603. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B. 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Wei, J.; Wang, G.; Chen, F.; Bai, M.; Liang, Y.; Wang, H.; Zhao, D.; Zhao, Y. Sol-Gel Synthesis of Metal-Phenolic Coordination Spheres and Their Derived Carbon Composites. Angew. Chem. Int. Ed. Engl. 2018, 57, 9838–9843. [Google Scholar] [CrossRef]
- Liu, M.; Cai, C.; Li, J.; Zhao, J.; Teng, W.; Liu, R. Stöber synthesis of tannic acid–formaldehyde resin polymer spheres and their derived carbon nanospheres and nanocomposites for oxygen reduction reaction. J. Colloid Interface Sci. 2018, 528, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Qin, J.; Feng, Y.; Feng, B.; Yang, S.; Wang, Z.; Zhao, Y.; Wei, J. Sol–gel synthesis of spherical mesoporous high-entropy oxides. ACS Appl. Mater. Interfaces 2020, 12, 45155–45164. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Wang, Y.; Cui, H.; Zhang, Y.; Zhang, Y. Fructose dehydration to 5-HMF over three sulfonated carbons: Effect of different pore structures. J. Chem. Technol. Biotechnol. 2017, 92, 1454–1463. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, F.; Pu, C. Mechanism of formation of chromium acetate(Cr3+)/phenol-formaldehyde resin prepolymer(PRP) complex and its compound cross-linking reaction with polymer for conformance control. J. Petrol. Sci. Eng. 2019, 179, 675–683. [Google Scholar] [CrossRef]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Gibot, P.; Vidal, L. Original synthesis of chromium (III) oxide nanoparticles. J. Eur. Ceram. Soc. 2010, 30, 911–915. [Google Scholar] [CrossRef]
- Prashanth, K.S.; Mahesh, S.S.; Nanda Prakash, M.B.; Munirathnamma, L.M.; Ningaraju, S.; Ravikumar, H.B.; Somashekar, R.S.; Nagabhushana, B.M. Solution combustion synthesis of Cr2O3 nanoparticles and derived PVA/Cr2O3 nanocomposites-positron annihilation spectroscopic study. Mater. Today Proc. 2016, 3, 3646–3651. [Google Scholar] [CrossRef]
- Ramesh, C.; Mohan Kumar, K.T.; Latha, N.; Ragunathan, V. Green synthesis of Cr2O3 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Escherichia coli. Curr. Nanosci. 2012, 8, 603–607. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Lv, Q. Removal efficiency and mechanism of Cr(VI) from aqueous solution by maize straw biochars derived at different pyrolysis temperatures. Water 2019, 11, 781. [Google Scholar] [CrossRef]
- Xia, L.; Li, B.; Zhang, Y.; Zhang, R.; Ji, L.; Chen, H.; Cui, G.; Zheng, H.; Sun, X.; Xie, F.; et al. Cr2O3 Nanoparticle-Reduced Graphene Oxide Hybrid: A Highly Active Electrocatalyst for N2 Reduction at Ambient Conditions. Inorg. Chem. 2019, 58, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dai, H.; Zhang, L.; Deng, J.; He, H.; Au, C.T. Ultrasound-assisted nanocasting fabrication and excellent catalytic performance of three-dimensionally ordered mesoporous chromia for the combustion of formaldehyde, acetone, and methanol. Appl. Cat. B 2010, 100, 229–237. [Google Scholar] [CrossRef]
- Tu, S.; Yu, X.; Ji, Q.; Ma, Q.; Zhou, C.; Chen, L.; Okonkwo, C.E. Exploration of lower critical solution temperature DES in a thermoreversible aqueous two-phase system for integrating glucose conversion and 5-HMF separation. Renew. Energy 2022, 189, 392–401. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Liu, X.; Xia, Q.; Wang, Y. Comprehensive understanding of the role of Brønsted and Lewis acid sites in glucose conversion into 5-hydromethylfurfural. ChemCatChem 2017, 9, 2739–2746. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, G.; Zhou, H.; Cao, S.; Zhang, Y.; Wang, S.; Pang, H. Enhanced active sites and stability in nano-MOFs for electrochemical energy storage through dual regulation by tannic acid. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311075. [Google Scholar] [CrossRef] [PubMed]
- Swift, T.D.; Nguyen, H.; Erdman, Z.; Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis acid/Brønsted acid-catalyzed conversion of carbohydrates to 5-hydroxymethylfurfural using zeolite beta. J. Cat 2016, 333, 149–161. [Google Scholar] [CrossRef]
- Ding, D.; Xi, J.; Wang, J.; Liu, X.; Lu, G.; Wang, Y. Production of methyl levulinate from cellulose: Selectivity and mechanism study. Green Chem. 2015, 17, 4037–4044. [Google Scholar] [CrossRef]
- Xing, X.; Shi, X.; Ruan, M.; Wei, Q.; Li, J.; Guan, Y.; Gao, H.; Xu, S. Efficient synthesis of 5-hydroxymethylfurfural from carbohydrates via SO3H-containing dendrimer β zeolite catalyst. Fuel 2024, 358, 130106. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Efficient catalytic conversion of fructose into 5-hydroxymethylfurfural in ionic liquids at room temperature. ChemSusChem 2009, 2, 944–946. [Google Scholar] [CrossRef]
- Chen, D.; Liang, F.; Feng, D.; Du, F.; Zhao, G.; Liu, H.; Xian, M. Sustainable utilization of lignocellulose: Preparation of furan derivatives from carbohydrate biomass by bifunctional lignosulfonate-based catalysts. Catal. Commun. 2016, 84, 159–162. [Google Scholar] [CrossRef]
- Zhang, M.; Su, K.; Song, H.; Li, Z.; Cheng, B. The excellent performance of amorphous Cr2O3, SnO2, SrO and graphene oxide–ferric oxide in glucose conversion into 5-HMF. Catal. Commun. 2015, 69, 76–80. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Liu, X.; Chang, F.; Zhang, Y.; Xue, W.; Yang, S. Immobilizing Cr3+ with SO3H-functionalized solid polymeric ionic liquids as efficient and reusable catalysts for selective transformation of carbohydrates into 5-hydroxymethylfurfural. Bioresour. Technol. 2013, 144, 21–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Production of 5-hydroxymethylfurfural from glucose catalyzed by hydroxyapatite supported chromium chloride. Bioresour. Technol. 2011, 102, 3970–3972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.; Liu, W.; Yao, Y.; Wang, H.; Xia, X.-F.; Li, W. Conversion of glucose into 5-hydroxymethylfurfural catalyzed by chromium (III) Schiff base complexes and acidic ionic liquids immobilized on mesoporous silica. RSC Adv. 2015, 5, 60736–60744. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, Y.; Wu, C.; Liu, X.; Xia, Y.; Wang, H. MCM-41 Immobilized Acidic Functional Ionic Liquid and Chromium (III) Complexes Catalyzed Conversion of Hexose into 5-Hydroxymethylfurfural. Chin. J. Chem. 2017, 35, 1739–1748. [Google Scholar] [CrossRef]
- Rusanen, A.; Lahti, R.; Lappalainen, K.; Kärkkäinen, J.; Hu, T.; Romar, H.; Lassi, U. Catalytic conversion of glucose to 5-hydroxymethylfurfural over biomass-based activated carbon catalyst. Catal. Today 2020, 357, 94–101. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Zuo, M.; Niu, Q.; Zhu, Y.; Li, S.; Jia, W.; Zhou, Z.; Zeng, X.; Lin, L. Biochar catalysts for efficiently 5-hydroxymethylfurfural (HMF) synthesis in aqueous natural deep eutectic solvent (A-NADES). Ind. Crops Prod. 2023, 192, 115953. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C.; Wang, H.; He, Z.-H.; Li, L.; Ma, Y.; Guo, N.; Liu, Z.-T. Boosting transformation of glucose and fructose to 5-hydroxymethylfurfural by a strategy of dual catalyst in biphasic solvent system with salt. Ind. Crops Prod. 2024, 216, 118702. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Hu, F.; Wu, Y.; Wang, H.; Chen, Y.; Yuan, H.; Gao, L.; Xiao, G. Highly efficient Cr/β zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]

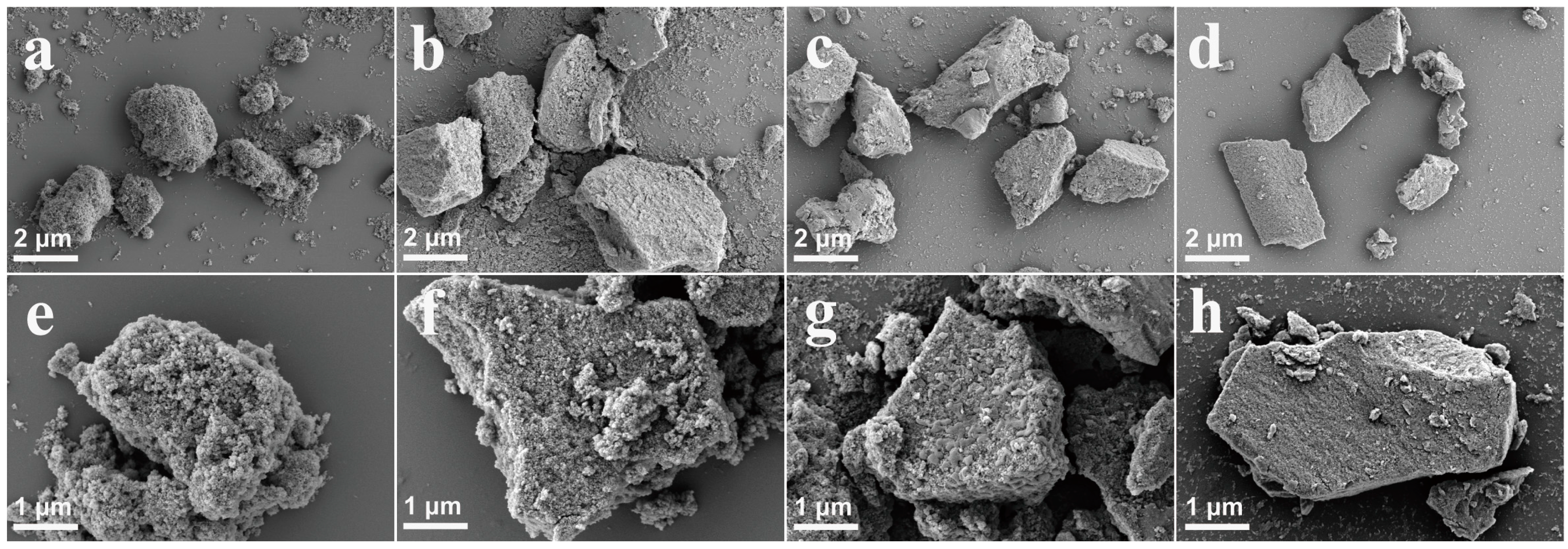



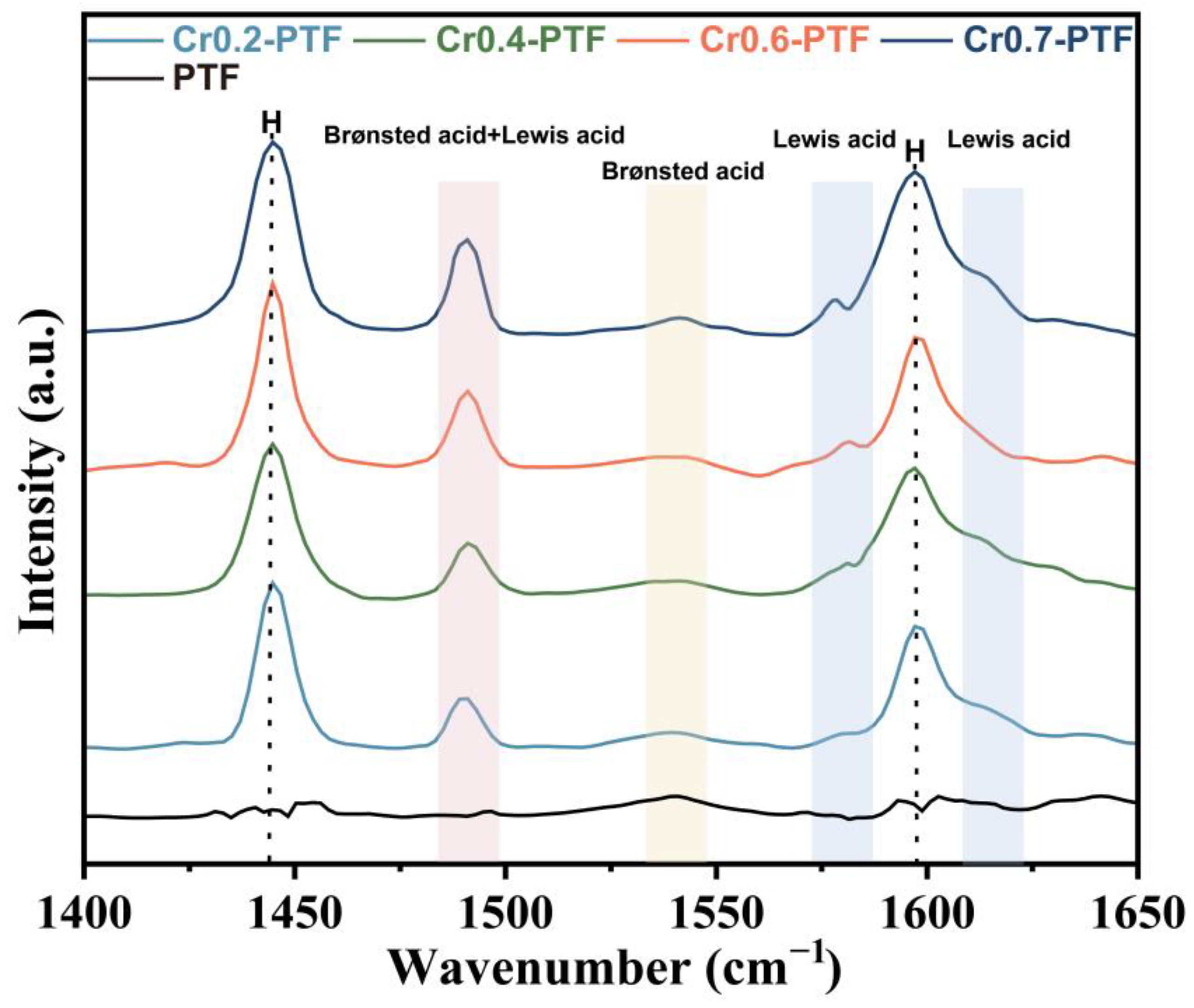
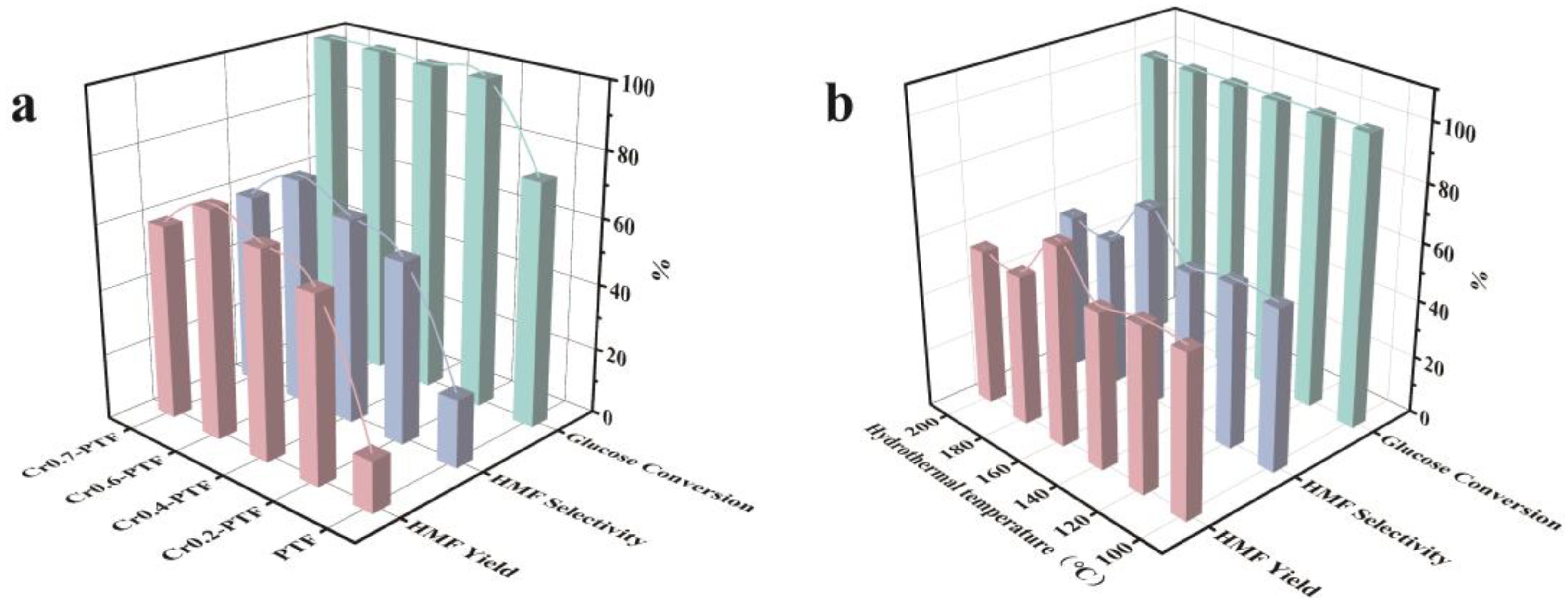
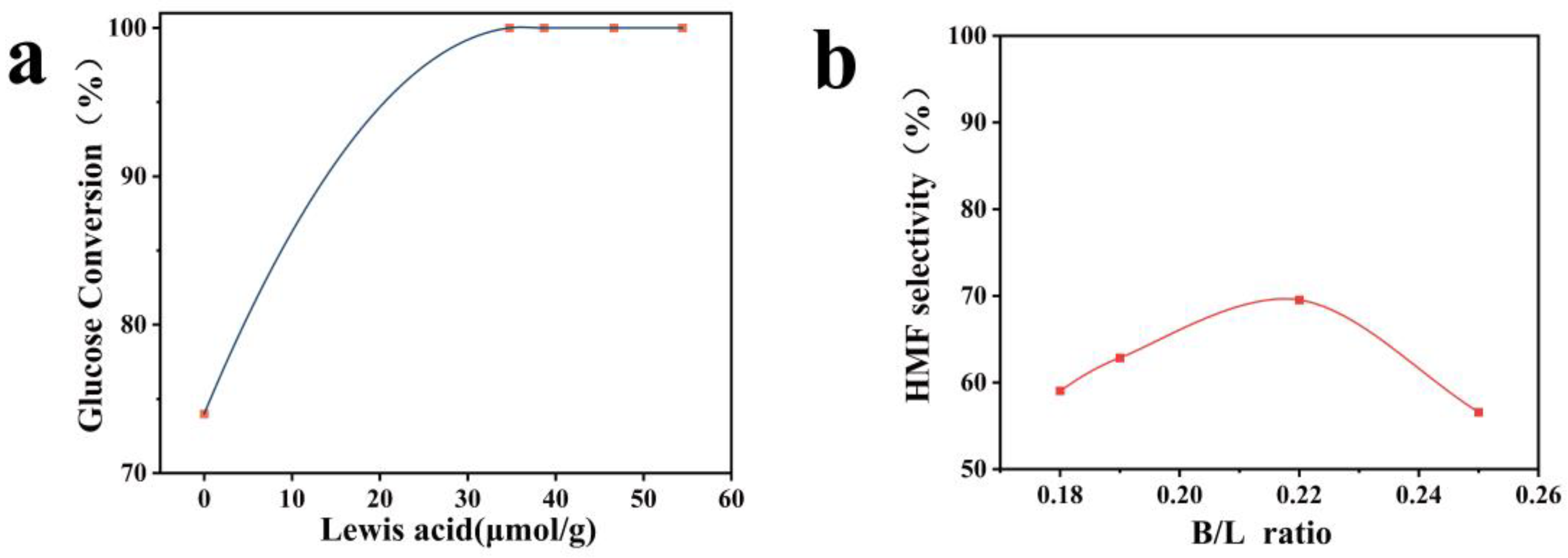
| Sample | SBET (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|---|
| Cr0.2-PTF | 126.54 | 0.57 | 0.0013 | 0.57 | 18.09 |
| Cr0.4-PTF | 97.31 | 0.21 | 0.0024 | 0.21 | 8.47 |
| Cr0.6-PTF | 39.75 | 0.06 | 0 | 0.06 | 6.14 |
| Cr0.7-PTF | 33.63 | 0.05 | 0 | 0.05 | 4.11 |
| PTF | 21.54 | 0.12 | 0 | 0.12 | 17.61 |
| Sample | Brønsted Acidity (μmol/g) | Lewis Acidity (μmol/g) | Total Acidity (μmol/g) | B/L Ratio |
|---|---|---|---|---|
| PTF | 10.54 | 0 | 10.54 | |
| Cr0.2-PTF | 8.64 | 34.73 | 43.37 | 0.25 |
| Cr0.4-PTF | 7.38 | 38.68 | 46.06 | 0.19 |
| Cr0.6-PTF | 10.37 | 46.62 | 56.99 | 0.22 |
| Cr0.7-PTF | 9.83 | 54.43 | 64.25 | 0.18 |
| Catalyst | Solvent | T (°C) | Reaction Time (h) | HMF Yield (%) | HMF Selectivity (%) | References |
|---|---|---|---|---|---|---|
| Cr0.6-PTF | NaClaq/DMSO | 180 | 3 | 69.5 | 69.5 | This work |
| LS-Cr | NaClaq/THF | 190 | 4 | 60.4 | 60.4 | [51] |
| GO-Cr2O3 | [EMIM]Br | 140 | 4 | 67 | - | [52] |
| FPIL2a | H2O/DMSO | 150 | 2 | 48.7 | - | [53] |
| Cr-HAP | [EMIM]Br | 150 | 0.5 | 32.5 | 37.7 | [54] |
| Cr(salen)-IM-HSO4-MCM-41 | DMSO | 140 | 4 | 43.5 | 43.5 | [55] |
| Cr(Salten)-MCM-41-[(CH2)-3SO3HVIm]HSO4 | DMSO | 140 | 4 | 50.2 | 50.2 | [56] |
| Catalyst | Solvent | T (°C) | Reaction Time (h) | HMF Yield Using Recycled Catalyst (%) | Catalyst Recycling Runs | References |
|---|---|---|---|---|---|---|
| Cr0.6-PTF | NaClaq/DMSO | 180 | 3 | 62 | 10 | This work |
| Zn-modified activated carbon | NaClaq/THF | 160 | 8 | 51 | 3 | [57] |
| Sn-Mont | THF/DMSO | 160 | 3 | 53.5 | 4 | [58] |
| CnS700-Sn | A-NADES | 140 | 2 | 22.3 | 6 | [59] |
| SiO2-Zr-P | NaClaq/THF | 180 | 3 | 69 | 5 | [60] |
| 0.4-Cr/β zeolite | NaClaq/THF | 150 | 1.5 | 58 | 3 | [61] |
| Catalyst | NH3·H2O (mL) | HCHO (mL) | TA (g) | CrCl3·6H2O (g) | TA/CrCl3·6H2O |
|---|---|---|---|---|---|
| PTF | 0.5 | 0.4 | 0.2 | 0 | |
| Cr0.2-PTF | 0.5 | 0.4 | 0.2 | 0.2 | 1:1 |
| Cr0.4-PTF | 0.5 | 0.4 | 0.2 | 0.4 | 1:2 |
| Cr0.6-PTF | 0.5 | 0.4 | 0.2 | 0.6 | 1:3 |
| Cr0.7-PTF | 0.5 | 0.4 | 0.2 | 0.7 | 1:3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, P.; Mao, W.; Wu, Z.; Gao, H.; Ling, C.; Zhou, J. Bifunctional Chromium-Doped Phenolic Porous Hydrothermal Carbon Catalysts for the Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. Int. J. Mol. Sci. 2025, 26, 3648. https://doi.org/10.3390/ijms26083648
Xiao P, Mao W, Wu Z, Gao H, Ling C, Zhou J. Bifunctional Chromium-Doped Phenolic Porous Hydrothermal Carbon Catalysts for the Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. International Journal of Molecular Sciences. 2025; 26(8):3648. https://doi.org/10.3390/ijms26083648
Chicago/Turabian StyleXiao, Pize, Wei Mao, Zhiming Wu, Huimin Gao, Chutong Ling, and Jinghong Zhou. 2025. "Bifunctional Chromium-Doped Phenolic Porous Hydrothermal Carbon Catalysts for the Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural" International Journal of Molecular Sciences 26, no. 8: 3648. https://doi.org/10.3390/ijms26083648
APA StyleXiao, P., Mao, W., Wu, Z., Gao, H., Ling, C., & Zhou, J. (2025). Bifunctional Chromium-Doped Phenolic Porous Hydrothermal Carbon Catalysts for the Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. International Journal of Molecular Sciences, 26(8), 3648. https://doi.org/10.3390/ijms26083648







