Lactobacillus helveticus HY7804 Modulates the Gut–Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model
Abstract
1. Introduction
2. Results
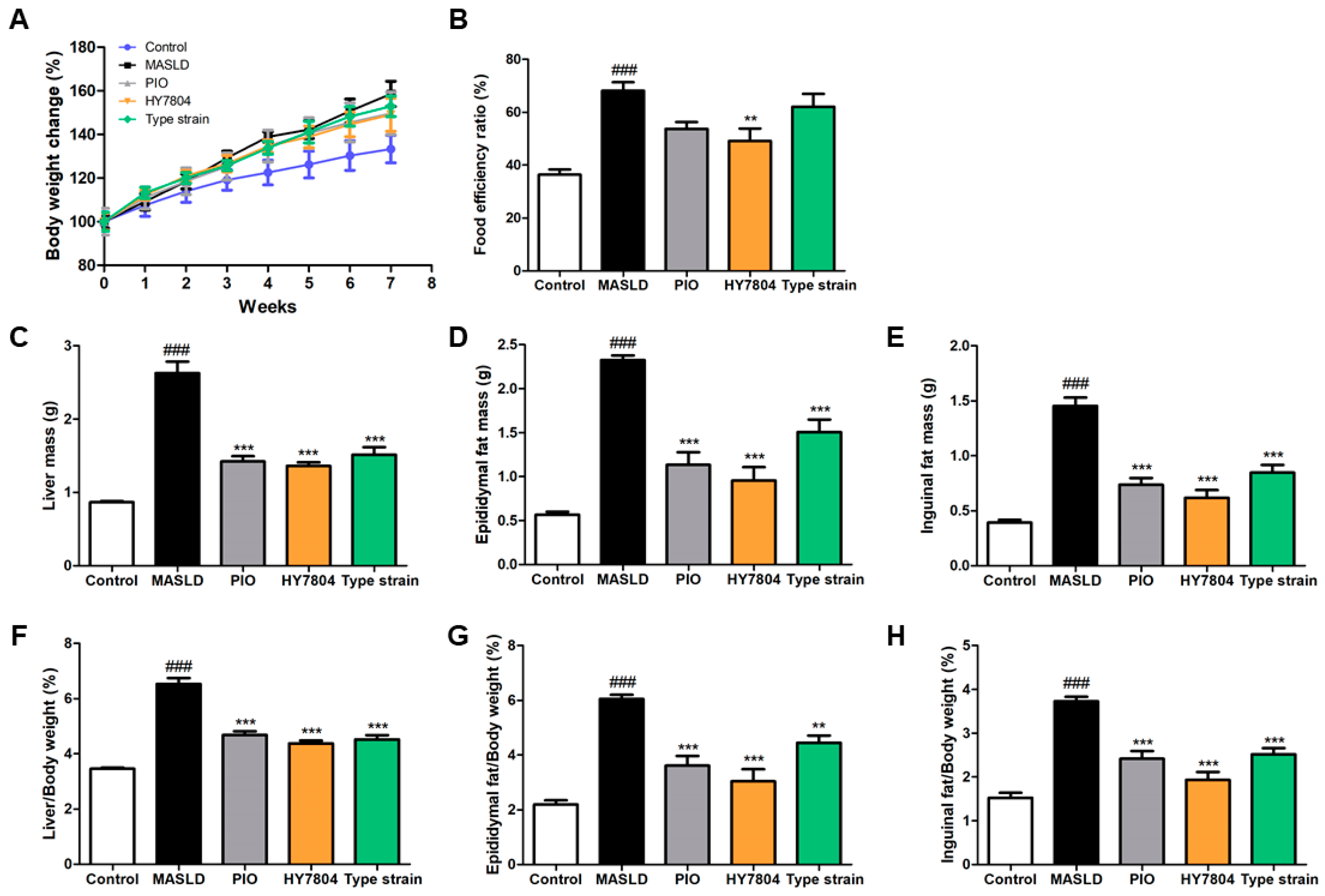
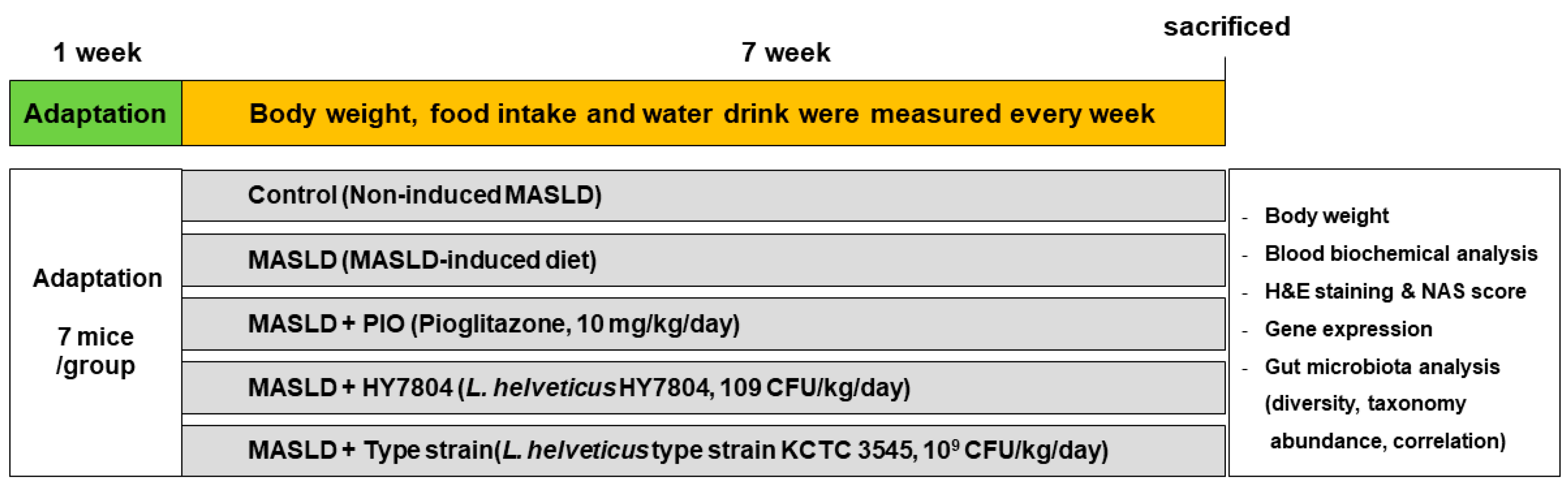
2.1. Effects of HY7804 on Physiological Indicators in MASLD-Induced Mice
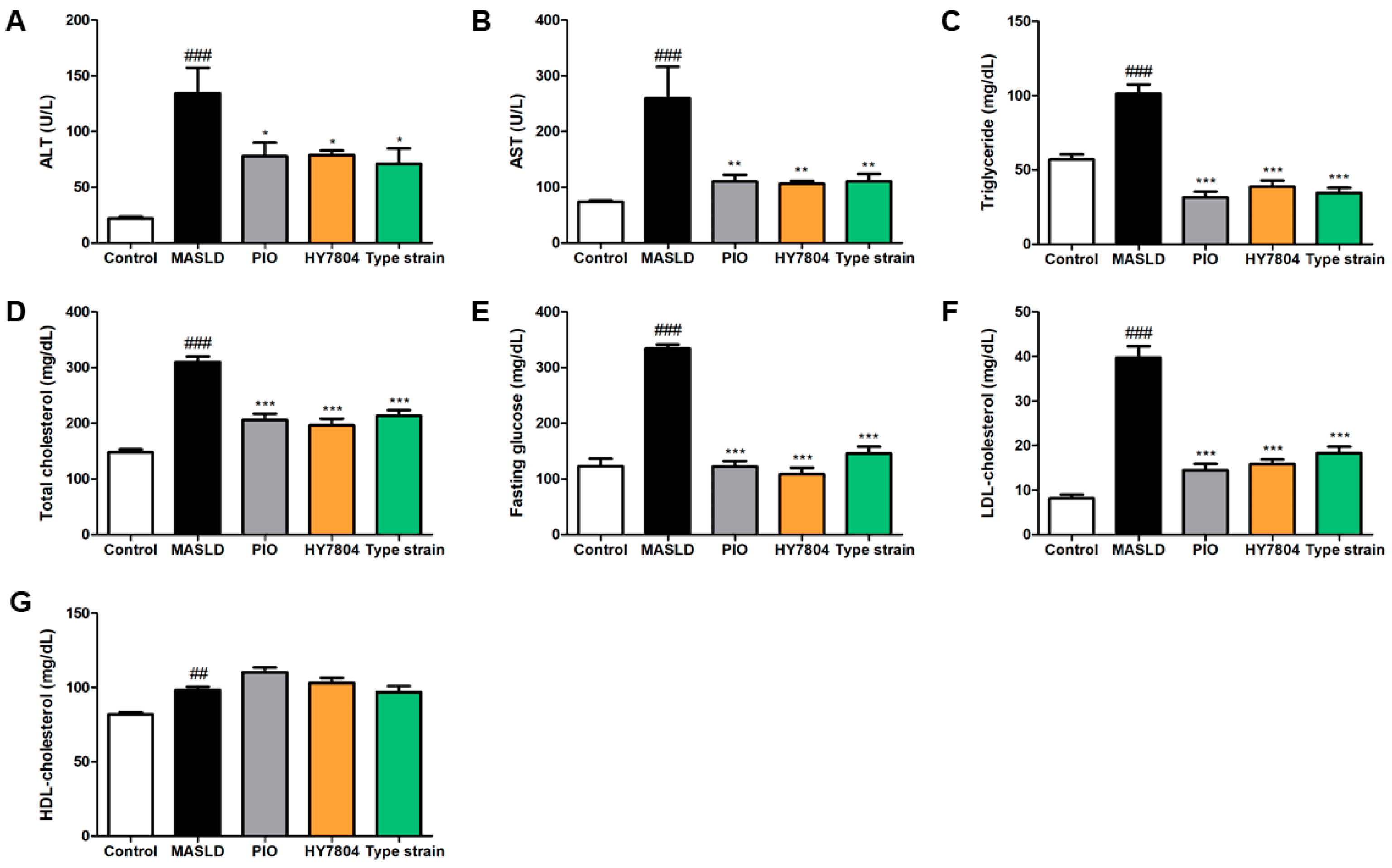
2.2. Effects of HY7804 on Blood Biochemical Analysis
2.3. Effects of HY7804 on Hepatic Histological Analysis
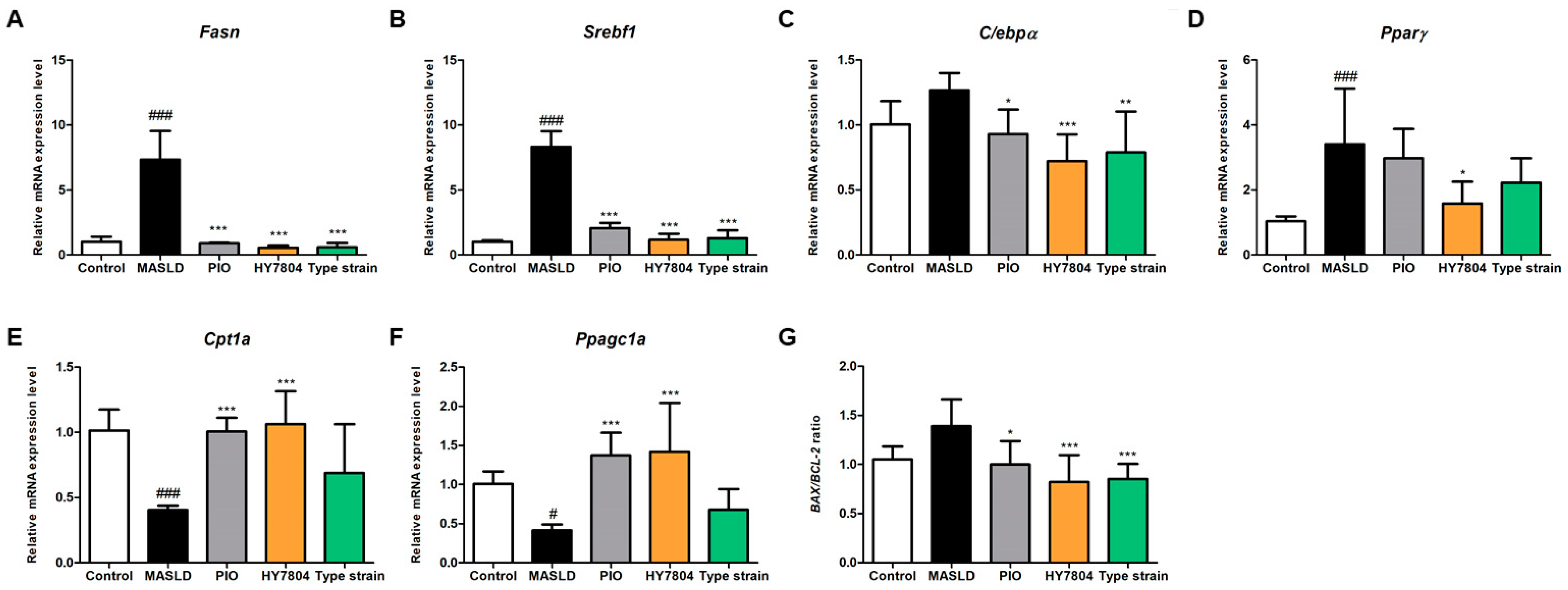
2.4. Effects of HY7804 on Hepatic Gene Expression
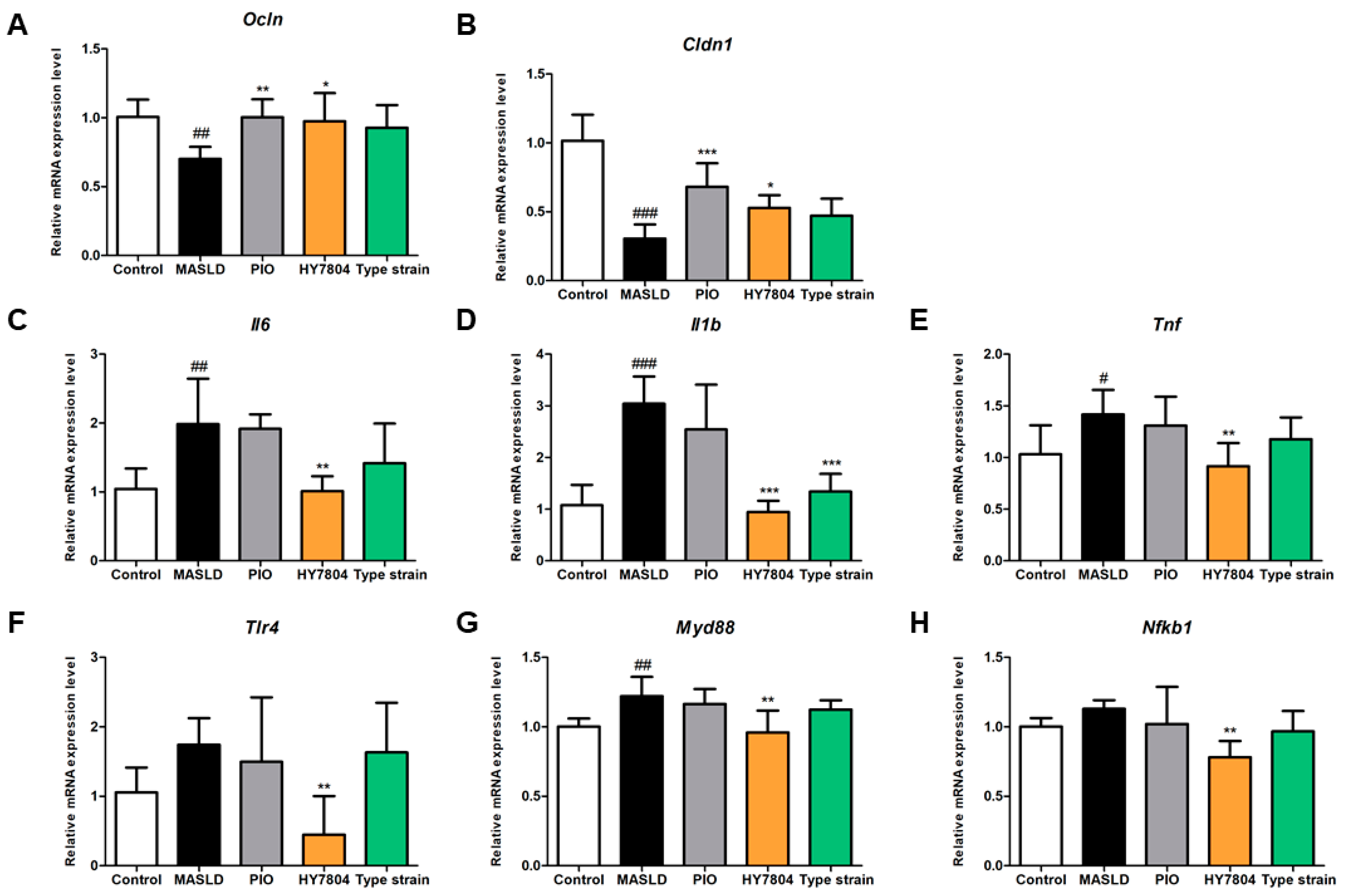
2.5. Effects of HY7804 on Expression of TJs and Inflammation-Related Genes in Colon Tissue
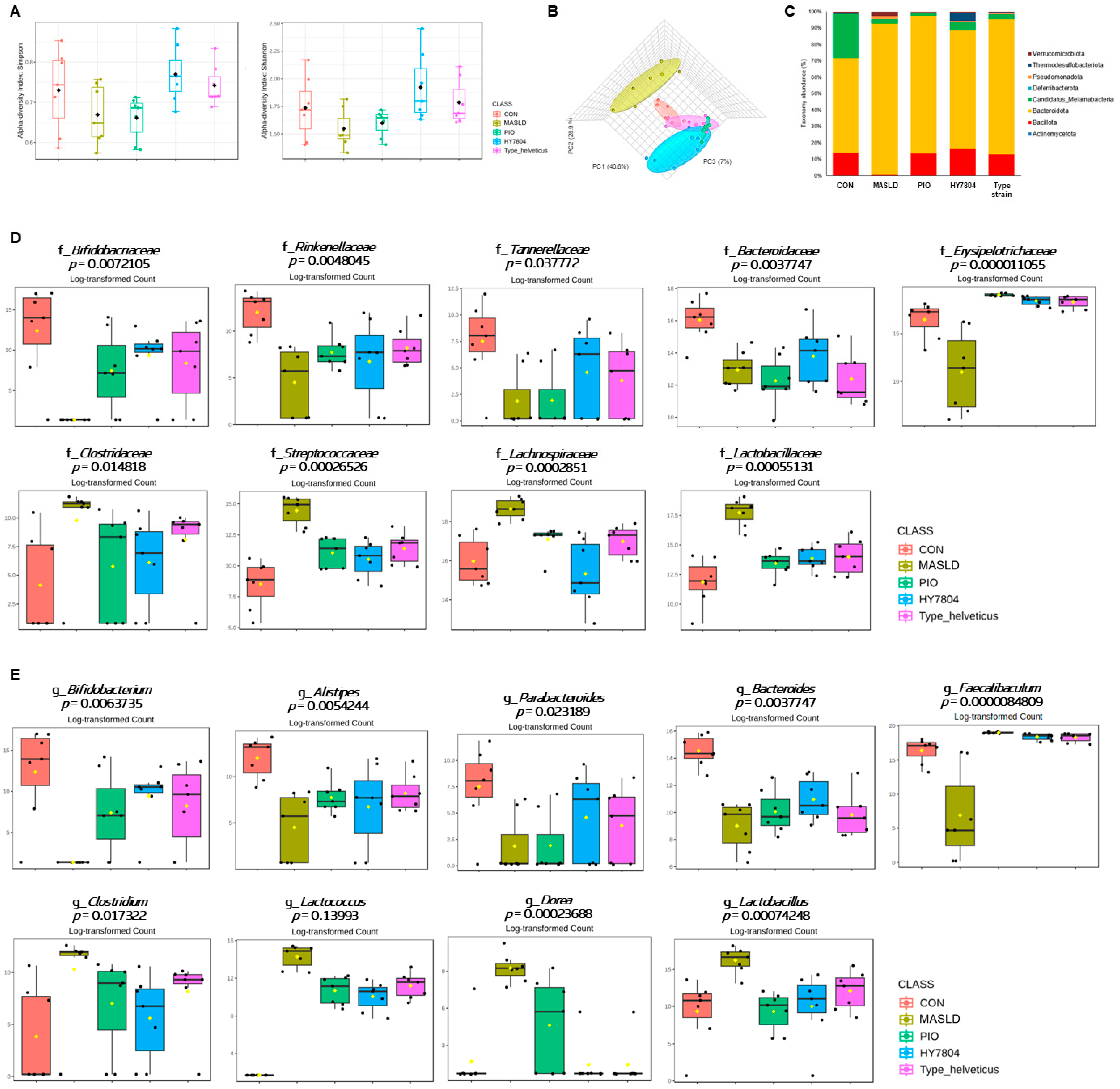
2.6. Effects of HY7804 on the Composition of the Gut Microbiota in the MASLD-Induced Mouse Model
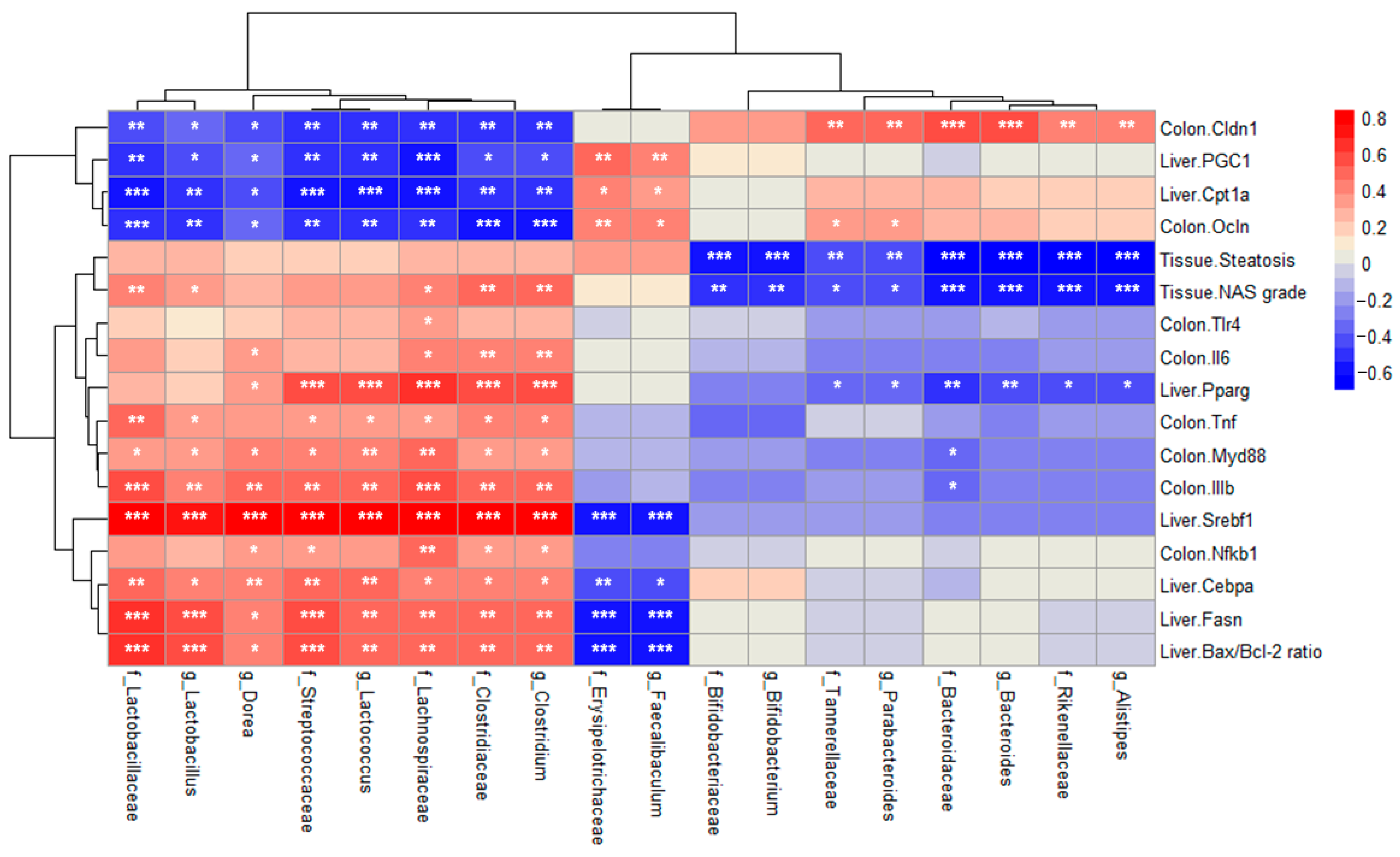
2.7. Correlation Between Relative Abundance and MASLD-Related Indicators
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture
4.2. Animal Experiments
4.3. Blood Sample Collection and Biochemical Analysis
4.4. H&E Staining and NAS Grading of Liver
4.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR Reaction
4.6. DNA Extraction and Sample Preparation for Next-Generation Sequencing (NGS)
- V3-Forward: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′,
- V4-Reverse: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′
4.7. Amplicon Sequence Variants (ASV) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Lirussi, F.; Mastropasqua, E.; Orando, S.; Orlando, R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst. Rev. 2007, 1, CD005165. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Li, L.; Yu, C.-H.; Shen, Z.; Chen, L.-H.; Li, Y.-M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. WJG 2013, 19, 6911. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Hung, H.-F.; Lu, C.-W.; Chang, H.-H.; Lee, L.-T.; Huang, K.-C. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci. Rep. 2016, 6, 27034. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Chung, C.-H.; Wang, Y.-H.; Hu, J.-M.; Chien, W.-C.; Cheng, Y.-C. Association between nonalcoholic fatty liver disease and colorectal cancer: A population-based study. Medicine 2023, 102, e33867. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Sartini, A.; Gitto, S.; Bianchini, M.; Verga, M.C.; Di Girolamo, M.; Bertani, A.; Del Buono, M.; Schepis, F.; Lei, B.; De Maria, N. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018, 9, 87. [Google Scholar] [CrossRef]
- Albillos, A.; De Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Liu, L.; Yin, M.; Gao, J.; Yu, C.; Lin, J.; Wu, A.; Zhu, J.; Xu, C.; Liu, X. Intestinal barrier function in the pathogenesis of nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 2022, 11, 452. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Farkas, A.E.; Hilgarth, R.S.; Krug, S.M.; Wolf, M.F.; Benedik, J.K.; Fromm, M.; Koval, M.; Parkos, C.; Nusrat, A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol. Biol. Cell 2014, 25, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. A J. Virtual Libr. 2009, 14, 2765. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Xin, D.; Zong-Shun, L.; Bang-Mao, W.; Lu, Z. Expression of intestinal tight junction proteins in patients with non-alcoholic fatty liver disease. Hepato-Gastroenterol. 2014, 61, 136–140. [Google Scholar]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81–88. [Google Scholar] [CrossRef]
- Sanders, M.E.; Gibson, G.; Gill, H.S.; Guarner, F. Probiotics: Their potential to impact human health. Counc. Agric. Sci. Technol. Issue Pap. 2007, 36, 1–20. [Google Scholar]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Eslamparast, T.; Eghtesad, S.; Hekmatdoost, A.; Poustchi, H. Probiotics and nonalcoholic fatty liver disease. Middle East J. Dig. Dis. 2013, 5, 129. [Google Scholar]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The role of probiotics in skin health and related gut–skin axis: A review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Bistas, K.G.; Tabet, J.P.; Bistas, K. The benefits of prebiotics and probiotics on mental health. Cureus 2023, 15, e43217. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Kim, J.-Y.; Shim, J.-J.; Lim, J.; Kim, J.-Y.; Lee, J.-L. Lactobacillus helveticus isolated from raw milk improves liver function, hepatic steatosis, and lipid metabolism in non-alcoholic fatty liver disease mouse model. Microorganisms 2023, 11, 2466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, H.; Zhao, Y.; Ren, Y.; Ma, C.; Chen, H.; Li, M.; Tian, J.; Xue, C.; Long, G. Response to pioglitazone in non-alcoholic fatty liver disease patients with vs. without type 2 diabetes: A meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1111430. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Kemnitz, J.W.; Elson, D.F.; Roecker, E.B.; Baum, S.T.; Bergman, R.N.; Meglasson, M.D. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes 1994, 43, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Alavian, S.M.; Zare, A.; Daryani, N.E.; Fereshtehnejad, S.-M.; Daryani, N.E.; Keramati, M.R.; Abdollahzade, S.; taba Vakili, S.T. Non-alcoholic fatty liver disease and correlation of serum alanin aminotransferase level with histopathologic findings. Hepat. Mon. 2011, 11, 452. [Google Scholar]
- Zou, Y.; Zhong, L.; Hu, C.; Sheng, G. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: A population-based longitudinal study. Lipids Health Dis. 2020, 19, 245. [Google Scholar] [CrossRef]
- Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Metabolic syndrome and non-alcoholic fatty liver disease. Ann. Hepatol. 2009, 8, 18–24. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, H.S.; Cho, A.-R.; Lee, Y.-J.; Kwon, Y.-J. Non-alcoholic fatty liver disease is an independent risk factor for LDL cholesterol target level. Int. J. Environ. Res. Public Health 2021, 18, 3442. [Google Scholar] [CrossRef]
- Estep, M.J.; Matharoo, G.; Hossain, N.; Nguyenngo, C.; Goodman, Z.; Baranova, A.; Younossi, Z. Elevated fasting serum glucose in non-alcoholic fatty liver disease (NAFLD) patients is correlated with the degree of steatosis: 393. Off. J. Am. Coll. Gastroenterol. ACG 2012, 107, S166. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. 2016, 91, 452–468. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, fatty acid oxidation. In StatPearls [Internet]; Statpearls Publishing: Tresure Island, FL, USA, 2023. [Google Scholar]
- Weber, M.; Mera, P.; Casas, J.; Salvador, J.; Rodríguez, A.; Alonso, S.; Sebastián, D.; Soler-Vázquez, M.C.; Montironi, C.; Recalde, S. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. FASEB J. 2020, 34, 11816–11837. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Wang, X.; Li, A.; Shan, A.; Ma, J. The promotion of fatty acid β-oxidation by hesperidin via activating SIRT1/PGC1α to improve NAFLD induced by a high-fat diet. Food Funct. 2024, 15, 372–386. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; He, S.; Li, P.; Zhong, X. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genet. Mol. Res. 2014, 13, 3991–3999. [Google Scholar] [CrossRef] [PubMed]
- Singla, T.; Muneshwar, K.N.; Pathade, A.G.; Yelne, S. Hepatocytic ballooning in non-alcoholic steatohepatitis: Bridging the knowledge gap and charting future avenues. Cureus 2023, 15, e45884. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Fukui, H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk of inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mizugaki, A.; Inoue, Y.; Kato, H.; Murakami, H. Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells. Amino Acids 2021, 53, 1021–1032. [Google Scholar] [CrossRef]
- Baek, J.; Kim, J.-H.; Nam, Y.; Kim, G.-E.; Ryu, K.; Sa, S.; Han, J.-S.; Kim, W. Allulose enhances epithelial barrier function by tight junction regulation via the TLR4/MyD88/NF-κB immune signaling pathway in an intestinal Caco-2 cell model. J. Funct. Foods 2023, 108, 105721. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Dheer, R.; Santaolalla, R.; Davies, J.M.; Lang, J.K.; Phillips, M.C.; Pastorini, C.; Vazquez-Pertejo, M.T.; Abreu, M.T. Intestinal epithelial toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infect. Immun. 2016, 84, 798–810. [Google Scholar] [CrossRef]
- Luo, H.; Guo, P.; Zhou, Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS ONE 2012, 7, e46291. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Gupta, H.; Min, B.-H.; Ganesan, R.; Gebru, Y.A.; Sharma, S.P.; Park, E.; Won, S.-M.; Jeong, J.-J.; Lee, S.-B.; Cha, M.-G. Gut microbiome in non-alcoholic fatty liver disease: From mechanisms to therapeutic role. Biomedicines 2022, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.-J.; Wang, X.-Y.; Fan, J.-G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, E.J.; Chaharlangi, D.; Wong, E.O.-Y.; Kim, D.; Navarre, W.W. Acids produced by lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut Microbes 2022, 14, 2046452. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Park, H.J.; Cha, M.G.; Park, E.; Won, S.-M.; Ganesan, R.; Gupta, H.; Gebru, Y.A.; Sharma, S.P.; Lee, S.B. The Lactobacillus as a probiotic: Focusing on liver diseases. Microorganisms 2022, 10, 288. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875.e863. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Ho, C.L. Recent development of probiotic bifidobacteria for treating human diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Gutierrez, A.; Pucket, B.; Engevik, M.A. Bifidobacterium and the intestinal mucus layer. Microbiome Res. Rep. 2023, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Sun, W.-H.; Chen, T.-H.; Tsai, P.-C.; Chen, C.-C.; Huang, S.-L. Gut mucosal microbiome is perturbed in rheumatoid arthritis mice and partly restored after TDAG8 deficiency or suppression by salicylanilide derivative. Int. J. Mol. Sci. 2022, 23, 3527. [Google Scholar] [CrossRef]
- Morgan, D.M.; Cao, Y.; Miller, K.; McGoldrick, J.; Bellavance, D.; Chin, S.M.; Halvorsen, S.; Maxner, B.; Richter, J.M.; Sassi, S. Microscopic colitis is characterized by intestinal dysbiosis. Clin. Gastroenterol. Hepatol. 2020, 18, 984–986. [Google Scholar] [CrossRef]
- Khiabani, S.A.; Haghighat, S.; Khosroshahi, H.T.; Asgharzadeh, M.; Kafil, H.S. Diversity of Bacteroidaceae family in gut microbiota of patients with chronic kidney disease and end stage renal disease. Health Promot. Perspect. 2023, 13, 237. [Google Scholar] [CrossRef]
- Price, C.E.; Valls, R.A.; Ramsey, A.R.; Loeven, N.A.; Jones, J.T.; Barrack, K.E.; Schwartzman, J.D.; Royce, D.B.; Cramer, R.A.; Madan, J.C. Intestinal Bacteroides modulates inflammation, systemic cytokines, and microbial ecology via propionate in a mouse model of cystic fibrosis. MBio 2024, 15, e03144-23. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, L.; Zhai, Q.; Zhao, J.; Tian, F. Anti-inflammatory, barrier maintenance, and gut microbiome modulation effects of Saccharomyces cerevisiae QHNLD8L1 on DSS-induced ulcerative colitis in mice. Int. J. Mol. Sci. 2023, 24, 6721. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, L.; Zheng, N.; Blecker, C.; Delcenserie, V.; Li, H.; Wang, J. 2′-fucosyllactose ameliorates inflammatory bowel disease by modulating gut microbiota and promoting MUC2 expression. Front. Nutr. 2022, 9, 822020. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Kubota, T.; Kumagai, H.; Itoh, S.; Satoh, H.; Yano, W.; Ogata, H.; Tokuyama, K.; Takamoto, I. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and-independent pathways. J. Biol. Chem. 2006, 281, 8748–8755. [Google Scholar] [CrossRef] [PubMed]

| Histological Features | Description | Score |
|---|---|---|
| Steatosis | <5% | 1 |
| 5–33% | 2 | |
| 34–65% | 3 | |
| >66% | 4 | |
| Lobular inflammation | No foci | 0 |
| <2 foci /×200 field | 1 | |
| 2–4 foci/×200 field | 2 | |
| >4 foci/×200 field | 3 | |
| Hepatocyte ballooning | None | 0 |
| Few ballooned cells | 1 | |
| Many ballooned cells/prominent ballooning | 2 |
| Gene | Gene Name | Catalog Number |
|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 |
| Fasn | Fatty acid synthase | Mm00433237_m1 |
| Pparγ | Peroxisome proliferator-activated receptor gamma | Mm00440945_m1 |
| Srebp-1c | Sterol regulatory element-binding protein 1 | Mm00550338_m1 |
| C/ebpα | CCAAT/enhancer-binding protein alpha | Mm00514283_m1 |
| Cpt1a | Carnitine palmitoyltransferase 1a | Mm01231183_m1 |
| Ppargc1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | Mm01208831_m1 |
| Bax | BCL2 associated X, apoptosis regulator | Mm00432051_m1 |
| Bcl-2 | BCL2, apoptosis regulator | Mm00477631_m1 |
| Il-6 | Interleukin 6 | Mm00446190_m1 |
| Il-1β | Interleukin 1 beta | Mm00434228_m1 |
| Tnfα | Tumor necrosis factor | Mm00443258_m1 |
| Tlr4 | Toll-like receptor 4 | Mm00445273_m1 |
| Nfκb1 | Nuclear factor kappa B subunit1 | Mm00476361_m1 |
| Myd88 | Myeloid differentiation primary response 88 | Mm00440338_m1 |
| Ocln | Occludin | Mm00500910_m1 |
| Cldn1 | Claudin-1 | Mm01342184_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jeon, H.-J.; Jeong, J.-W.; Lee, K.; Gwon, H.; Lee, D.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. Lactobacillus helveticus HY7804 Modulates the Gut–Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model. Int. J. Mol. Sci. 2025, 26, 3557. https://doi.org/10.3390/ijms26083557
Kim H, Jeon H-J, Jeong J-W, Lee K, Gwon H, Lee D, Kim J-Y, Shim J-J, Lee J-H. Lactobacillus helveticus HY7804 Modulates the Gut–Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model. International Journal of Molecular Sciences. 2025; 26(8):3557. https://doi.org/10.3390/ijms26083557
Chicago/Turabian StyleKim, Hyeonji, Hye-Jin Jeon, Ji-Woong Jeong, Kippeum Lee, Hyeonjun Gwon, Daehyeop Lee, Joo-Yun Kim, Jae-Jung Shim, and Jae-Hwan Lee. 2025. "Lactobacillus helveticus HY7804 Modulates the Gut–Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model" International Journal of Molecular Sciences 26, no. 8: 3557. https://doi.org/10.3390/ijms26083557
APA StyleKim, H., Jeon, H.-J., Jeong, J.-W., Lee, K., Gwon, H., Lee, D., Kim, J.-Y., Shim, J.-J., & Lee, J.-H. (2025). Lactobacillus helveticus HY7804 Modulates the Gut–Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model. International Journal of Molecular Sciences, 26(8), 3557. https://doi.org/10.3390/ijms26083557





