Anti-Obesity Effects of LB-GABA
Abstract
1. Introduction
2. Results
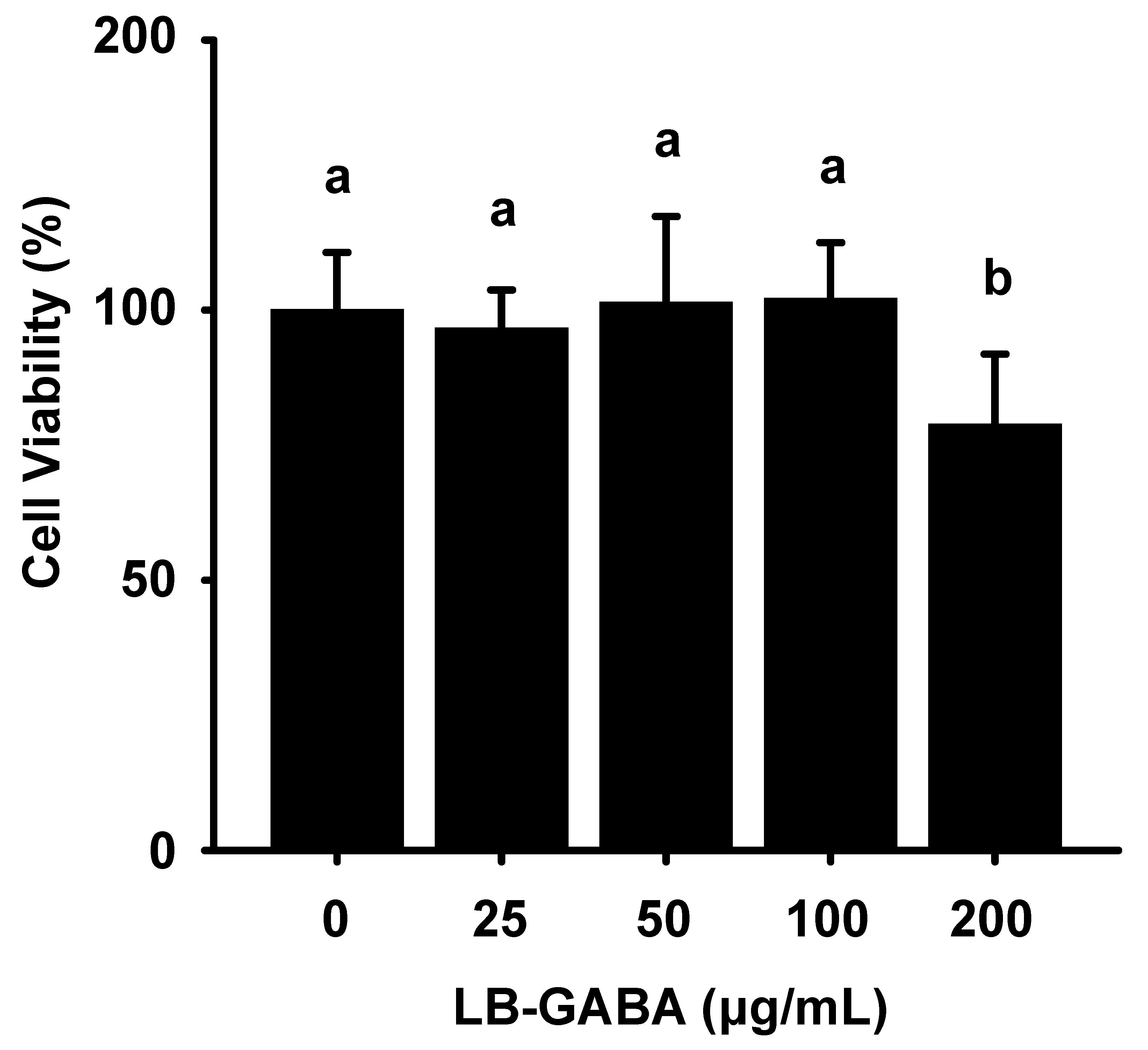
2.1. Effects of LB-GABA on the Viability of 3T3-L1 Adipocytes
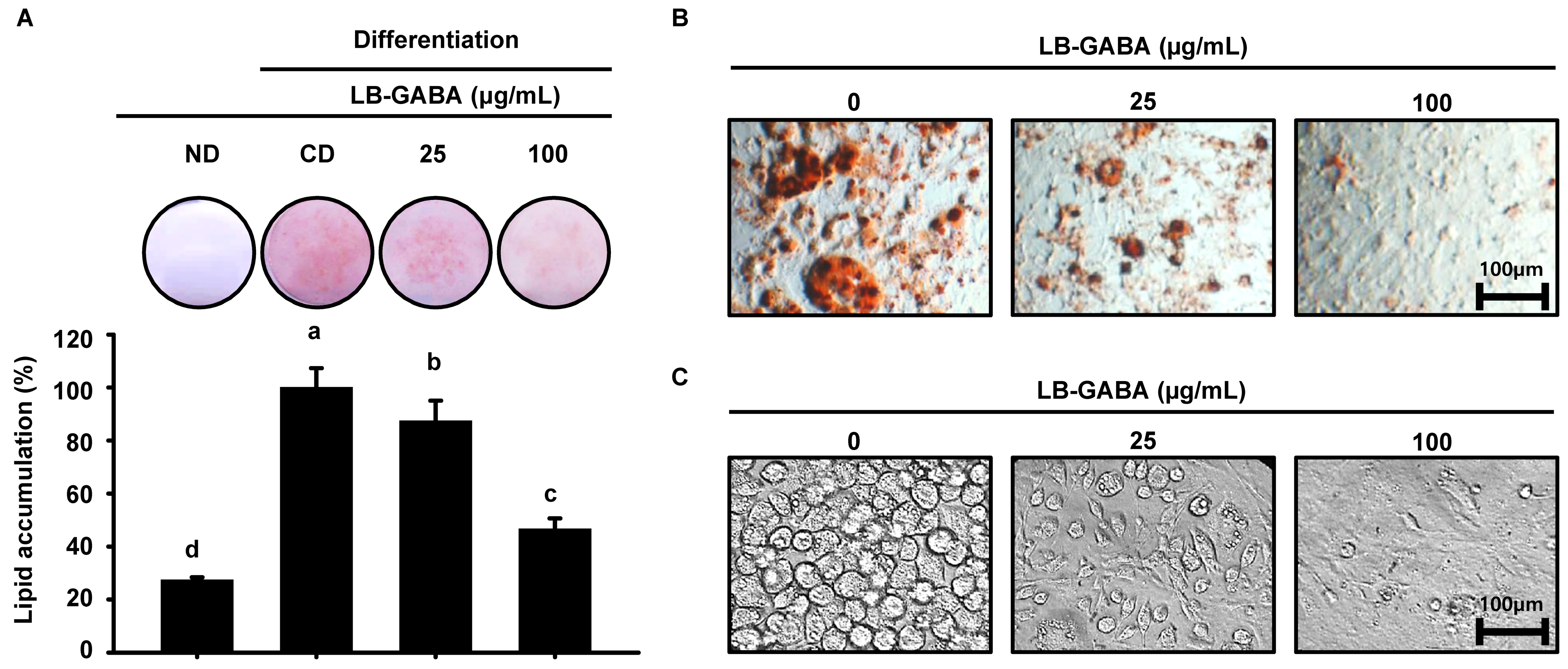
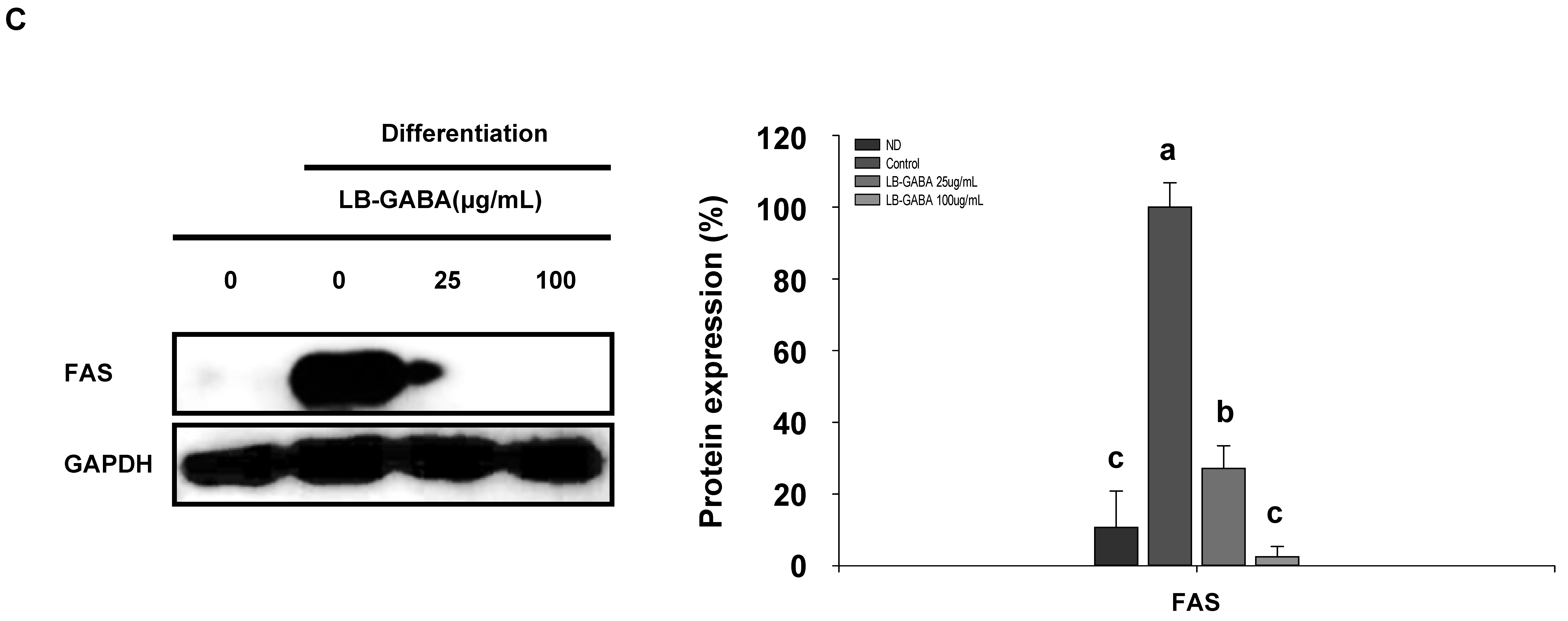
2.2. LB-GABA Inhibits Lipid Accumulation
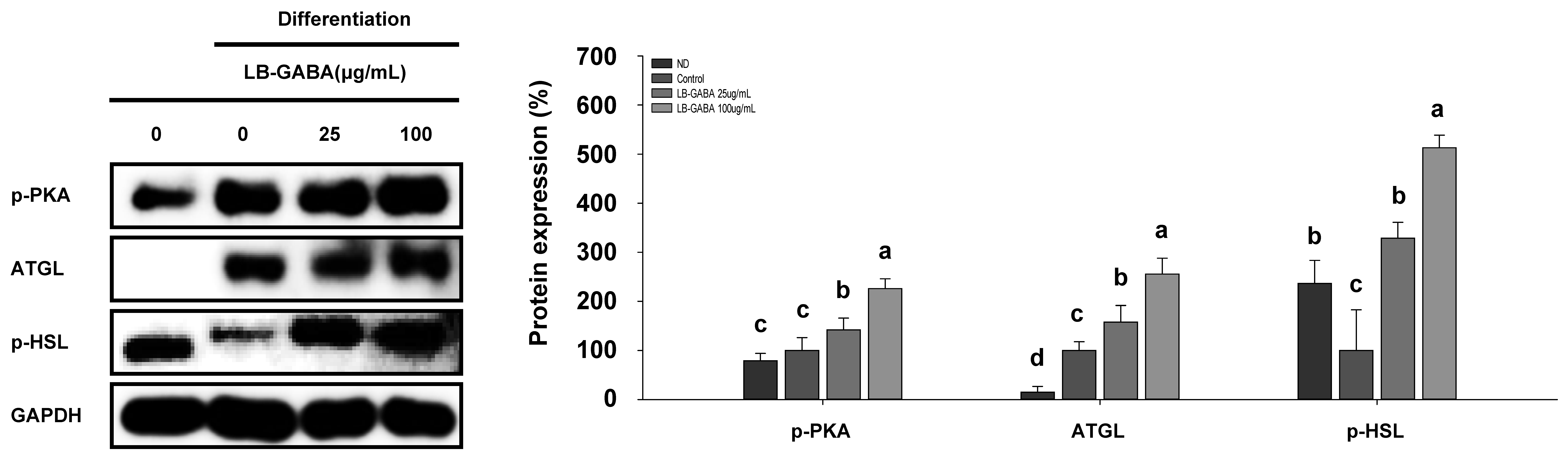
2.3. LB-GABA Increases Lipolysis in 3T3-L1 Adipocytes
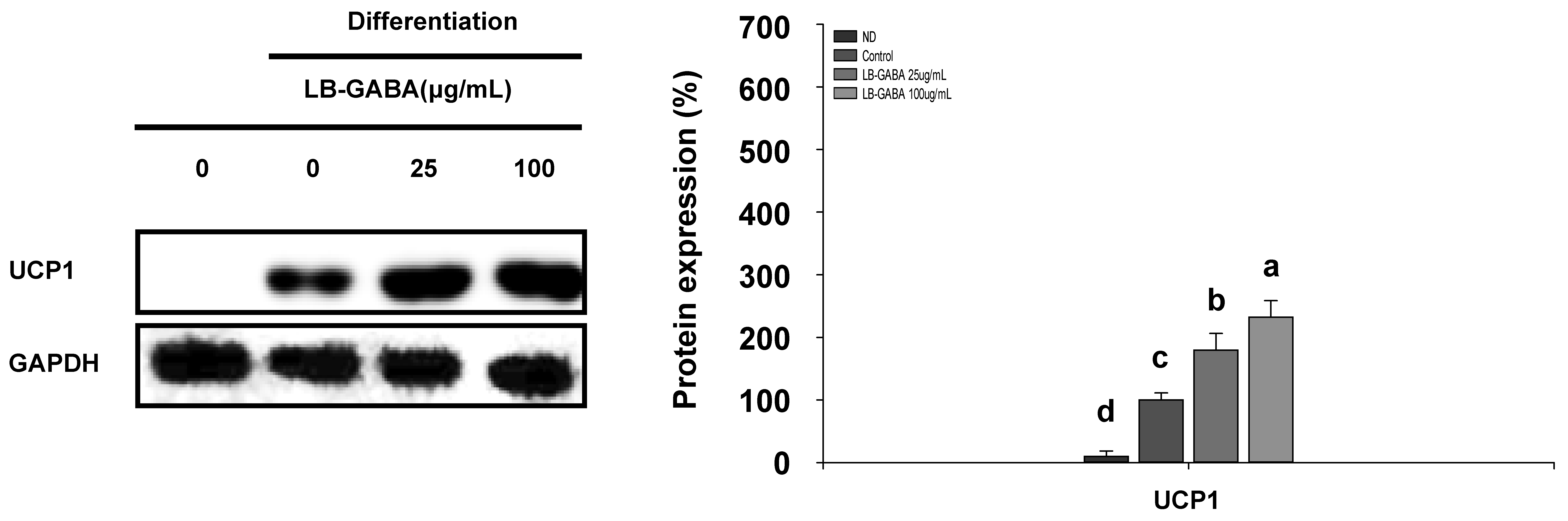
2.4. LB-GABA Activates Energy Metabolism by Promoting the Browning of 3T3-L1 Adipocytes
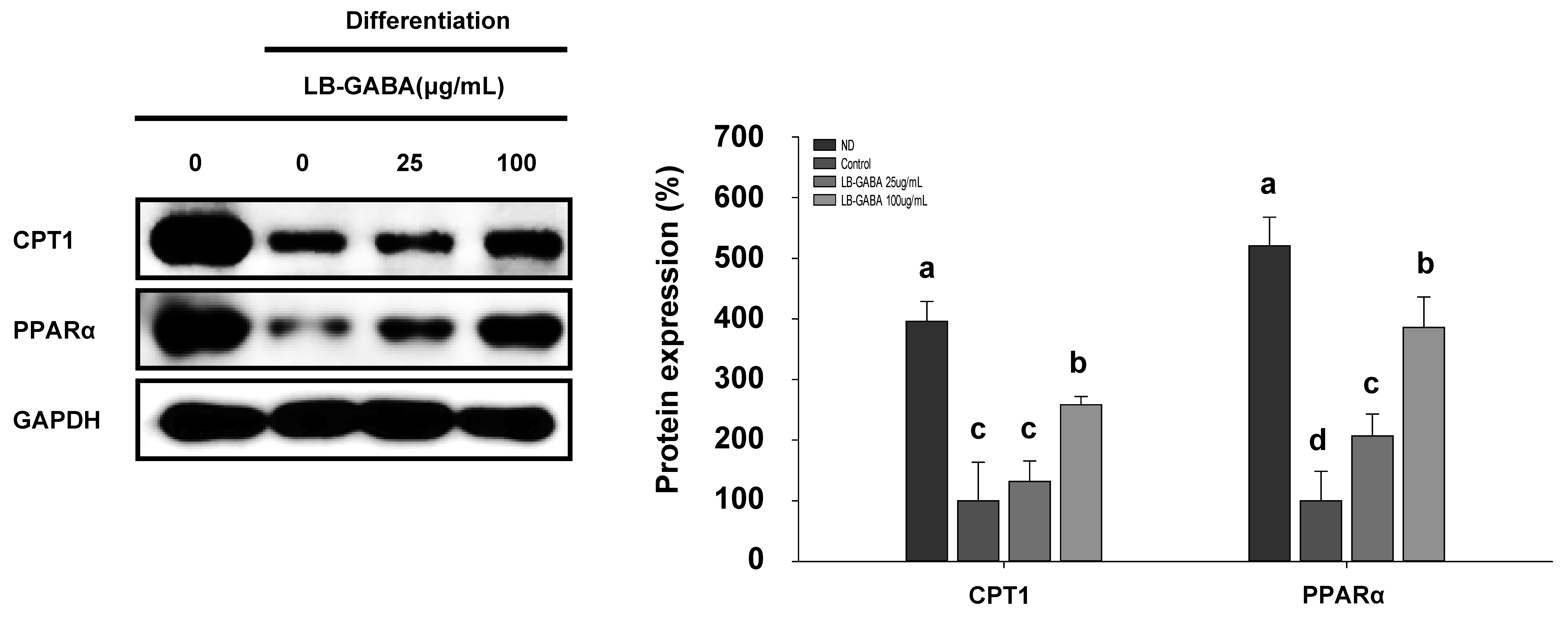
2.5. Investigation of LB-GABA’s Effect on Reducing Fatty Acid Oxidation in 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Materials
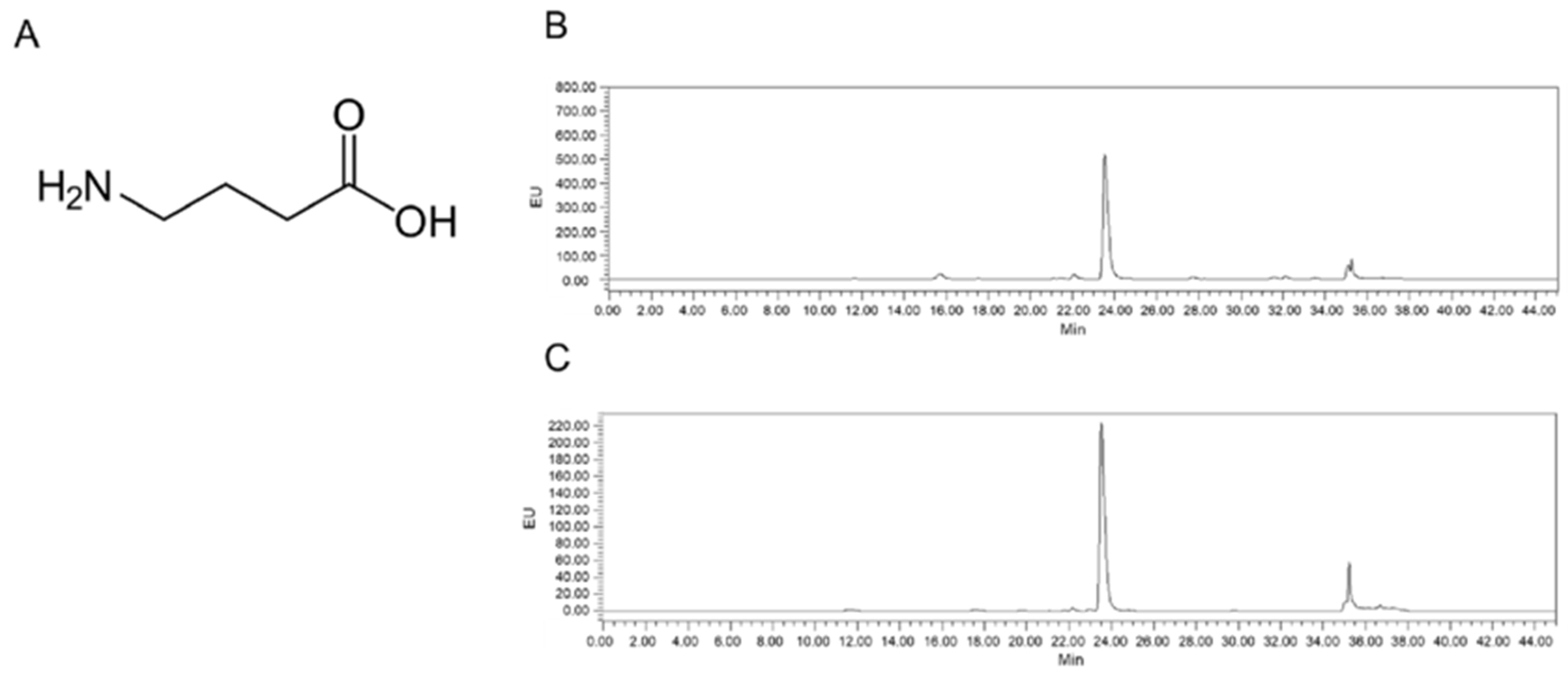
4.2. Preparation and Analysis of LB-GABA
4.3. Cell Culture
4.4. Cell Viability
4.5. Oil Red O Staining
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dietrich, M.O.; Horvath, T.L. Limitations in anti-obesity drug development: The critical role of hunger-promoting neurons. Nat. Rev. Drug Discov. 2012, 11, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jia, W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes 2018, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef]
- Letukienė, A.; Hendrixson, V.; Ginevičienė, V. Current knowledge and scientific trends in myokines and exercise research in the context of obesity. Front. Med. 2024, 11, 1421962. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Sørensen, T.I.A. Obesity Defined as Excess Storage of Inert Triglycerides—Do We Need a Paradigm Shift? Obes. Facts 2011, 4, 91–94. [Google Scholar] [CrossRef]
- Nedergaard, J.; Golozoubova, V.; Matthias, A.; Asadi, A.; Jacobsson, A.; Cannon, B. UCP1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic ine§ciency. Biochim. Biophys. Acta (BBA)—Bioenerg. 2001, 1504, 82–106. [Google Scholar] [CrossRef]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.; Alzamendi, A.; Moreno, G.; Portales, A.; Castrogiovanni, D.; Spinedi, E.; Giovambattista, A. Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model. Nutrients 2016, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Cerk, I.K.; Wechselberger, L.; Oberer, M. Adipose Triglyceride Lipase Regulation: An Overview. CPPS 2017, 19, 221–233. [Google Scholar] [CrossRef]
- Henique, C.; Mansouri, A.; Fumey, G.; Lenoir, V.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; Cohen, I. Increased Mitochondrial Fatty Acid Oxidation Is Sufficient to Protect Skeletal Muscle Cells from Palmitate-induced Apoptosis. J. Biol. Chem. 2010, 285, 36818–36827. [Google Scholar] [CrossRef]
- Tardelli, M. Monoacylglycerol lipase reprograms lipid precursors signaling in liver disease. WJG 2020, 26, 3577–3585. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; Da Silva, D.S.; Farias, G.R.; De Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Plutzky, J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab. J. 2016, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Frankel, S. γ-Aminobutyric Acid in Brain: Its Formation from Glutamic Acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Liu, L.; Yang, R.; Wang, Z. Microbial production of gamma-aminobutyric acid: Applications, state-of-the-art achievements, and future perspectives. Crit. Rev. Biotechnol. 2021, 41, 491–512. [Google Scholar] [CrossRef]
- Puts, N.A.J.; Edden, R.A.E. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. npj Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Park, C.; Chen, X.; Tian, C.-L.; Park, G.N.; Chenouard, N.; Lee, H.; Yeo, X.Y.; Jung, S.; Bi, G.; Tsien, R.W.; et al. Inhibitory synaptic vesicles have unique dynamics and exocytosis properties. bioRix 2020. [Google Scholar] [CrossRef]
- Park, C.; Chen, X.; Tian, C.-L.; Park, G.N.; Chenouard, N.; Lee, H.; Yeo, X.Y.; Jung, S.; Tsien, R.W.; Bi, G.-Q.; et al. Unique dynamics and exocytosis properties of GABAergic synaptic vesicles revealed by three-dimensional single vesicle tracking. Proc. Natl. Acad. Sci. USA 2021, 118, e2022133118. [Google Scholar] [CrossRef]
- Yoto, A.; Murao, S.; Motoki, M.; Yokoyama, Y.; Horie, N.; Takeshima, K.; Masuda, K.; Kim, M.; Yokogoshi, H. Oral intake of γ-aminobutyric acid affects mood and activities of central nervous system during stressed condition induced by mental tasks. Amino Acids 2012, 43, 1331–1337. [Google Scholar] [CrossRef]
- Simmonite, M.; Carp, J.; Foerster, B.R.; Ossher, L.; Petrou, M.; Weissman, D.H.; Polk, T.A. Age-Related Declines in Occipital GABA are Associated with Reduced Fluid Processing Ability. Acad. Radiol. 2019, 26, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Jin, Y.; Zhuo, J.; Cao, X.; Liu, G.; Du, H.; Liu, L.; Wang, J.; Xiao, H. Exogenous GABA improves the antioxidant and anti-aging ability of silkworm (Bombyx mori). Food Chem. 2022, 383, 132400. [Google Scholar] [CrossRef] [PubMed]
- Zuppichini, M.D.; Hamlin, A.M.; Zhou, Q.; Kim, E.; Rajagopal, S.; Beltz, A.M.; Polk, T.A. GABA levels decline with age: A longitudinal study. Imaging Neurosci. 2024, 2, 1–15. [Google Scholar] [CrossRef]
- Jin, H.; Han, H.; Song, G.; Oh, H.-J.; Lee, B.-Y. Anti-Obesity Effects of GABA in C57BL/6J Mice with High-Fat Diet-Induced Obesity and 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2024, 25, 995. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.C.; Yang, H.; Soni, K.G.; Wang, S.P.; Mitchell, G.A.; Wu, J.W. An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis. Cells 2019, 8, 395. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 358–369. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Sebastián, D.; Fucho, R.; Weber, M.; Mir, J.F.; García-Casarrubios, E.; Obregón, M.J.; Zorzano, A.; Valverde, Á.M.; Serra, D.; et al. Carnitine Palmitoyltransferase 1 Increases Lipolysis, UCP1 Protein Expression and Mitochondrial Activity in Brown Adipocytes. PLoS ONE 2016, 11, e0159399. [Google Scholar] [CrossRef]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome Proliferator-Activated Receptor γ and C/EBPα Synergistically Activate Key Metabolic Adipocyte Genes by Assisted Loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Jin, H.; Oh, H.-J.; Kim, J.; Lee, K.-P.; Han, X.; Lee, O.-H.; Lee, B.-Y. Effects of Ecklonia stolonifera extract on the obesity and skeletal muscle regeneration in high-fat diet-fed mice. J. Funct. Foods 2021, 82, 104511. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-J.; Jin, H.; Lee, K.; Song, J.-H.; Chei, S.; Oh, H.-J.; Oh, J.-H.; Lee, B.-Y. Cardamonin suppresses lipogenesis by activating protein kinase A-mediated browning of 3T3-L1 cells. Phytomedicine 2019, 65, 153064. [Google Scholar] [CrossRef] [PubMed]
- Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006, 53, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.C.; Betz, M.J.; Blondin, D.P.; Augustin, R.; Sharma, A.K.; Tseng, Y.-H.; Scheele, C.; Zimdahl, H.; Mark, M.; Hennige, A.M.; et al. Challenges in tackling energy expenditure as obesity therapy: From preclinical models to clinical application. Mol. Metab. 2021, 51, 101237. [Google Scholar] [CrossRef]
- Song, N.-J.; Chang, S.-H.; Li, D.Y.; Villanueva, C.J.; Park, K.W. Induction of thermogenic adipocytes: Molecular targets and thermogenic small molecules. Exp. Mol. Med. 2017, 49, e353. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Suh, H.J.; Choi, H.-S. Lactobacillus brevis-Fermented Gamma-Aminobutyric Acid Ameliorates Depression-and Anxiety-Like Behaviors by Activating the Brain-Derived Neurotrophic Factor-Tropomyosin Receptor Kinase B Signaling Pathway in BALB/C Mice. J. Agric. Food Chem. 2024, 72, 2977–2988. [Google Scholar] [CrossRef]
- Jeong, A.-H.; Hwang, J.; Jo, K.; Kim, S.; Ahn, Y.; Suh, H.J.; Choi, H.-S. Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models. Int. J. Mol. Sci. 2021, 22, 3537. [Google Scholar] [CrossRef]
- Khan, I.; Preeti, K.; Kumar, R.; Khatri, D.K.; Singh, S.B. Activation of SIRT1 by silibinin improved mitochondrial health and alleviated the oxidative damage in experimental diabetic neuropathy and high glucose-mediated neurotoxicity. Arch. Physiol. Biochem. 2024, 130, 420–436. [Google Scholar] [CrossRef]
- Khan, I.; Kaur, S.; Rishi, A.K.; Boire, B.; Aare, M.; Singh, M. Cannabidiol and Beta-Caryophyllene Combination Attenuates Diabetic Neuropathy by Inhibiting NLRP3 Inflammasome/NFκB through the AMPK/sirT3/Nrf2 Axis. Biomedicines 2024, 12, 1442. [Google Scholar] [CrossRef]
- Khan, I.; Preeti, K.; Kumar, R.; Kumar Khatri, D.; Bala Singh, S. Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int. Immunopharmacol. 2023, 116, 109793. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Kalinovich, A.V.; De Jong, J.M.A.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: Two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Song, G.; Kim, J.; Jin, H.; Lee, B.-Y. Anti-Obesity Effects of LB-GABA. Int. J. Mol. Sci. 2025, 26, 3554. https://doi.org/10.3390/ijms26083554
Han H, Song G, Kim J, Jin H, Lee B-Y. Anti-Obesity Effects of LB-GABA. International Journal of Molecular Sciences. 2025; 26(8):3554. https://doi.org/10.3390/ijms26083554
Chicago/Turabian StyleHan, Hyein, Gunju Song, Jongwon Kim, Heegu Jin, and Boo-Yong Lee. 2025. "Anti-Obesity Effects of LB-GABA" International Journal of Molecular Sciences 26, no. 8: 3554. https://doi.org/10.3390/ijms26083554
APA StyleHan, H., Song, G., Kim, J., Jin, H., & Lee, B.-Y. (2025). Anti-Obesity Effects of LB-GABA. International Journal of Molecular Sciences, 26(8), 3554. https://doi.org/10.3390/ijms26083554





