Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases
Abstract
1. Introduction
1.1. Nanozyme Structure
- Metal-based nanozymes—these include metals, such as Fe, Au, Pt, Ag, Pd, and Ir, which possess catalytic activities resembling those of oxidases, peroxidases, catalases and SOD.
- Metal oxide-, metal-sulfide-, and metal–selenide-based nanozymes—examples include Fe3O4, MFe2O4 (M=Co, Ni, Cu, Mg, and Zn), CeO2, Co3O4, MnO2, V2O5, CuxO, Co9S8, MoS2, and MoSe2, known for their peroxidase, catalase and SOD mimicking abilities, utilizing metal sites to mimic the metal-heme redox center of metalloenzymes [8].
- Carbon-based nanozymes—these consist of fullerenes, carbon nanotubes, graphene oxide and carbon quantum dots, graphdiyne, and their doped derivatives, exhibiting peroxidase and SOD-mimicking activities.
- Metal–organic frameworks (MOF)—these are hybrid organic-inorganic porous crystalline materials with GPx-like activity; first reported in 2019 by Zhu et al. [28]
- Single-atom nanozymes (SAzymes)—these consist of single metal atoms (Fe, Ce, Mn, Pt, V, Cu) dispersed on suitable supports, such as carbon-based materials. These atoms serve as catalytic centers, closely mimicking the active site of natural enzymes and can exhibit peroxidase, oxidase, catalase, and SOD-like activities; they were first reported in 2019 by Lian et al. [29]
1.2. Nanozyme Applications
- 1.
- Biosensing and analytical applications
- 2.
- Biomedical applications
- 3.
- Environmental applications
1.3. Properties of Nanozymes for Biomedical Applications
- The use of biocompatible and completely nontoxic elements, or materials that biodegrade into excretable components without adverse effects.
- A hydrodynamic diameter sufficiently small to allow complete renal elimination from the body and to minimize excessive retention within the reticuloendothelial system.
- A zwitterionic or neutral surface coating to reduce nonspecific uptake by tissues and organs.
- High chemical stability in serum to ensure consistent performance.
- The capability to efficiently target diseased states following administration while being entirely eliminated from the body within a reasonable timeframe.
- Ease of scale-up and manufacturing facilitated by robust and reproducible procedures.
2. ROS Production
2.1. Types of ROS
2.2. Natural Antioxidants and Nanozymes with Antioxidant Activity
2.2.1. Catalases and Catalase-like Activity Enzymes
2.2.2. SOD and SOD-like Nanozymes
2.2.3. Glutathione Peroxidases and GPx-like Nanozymes
- GPx1, the most prevalent form of GPx, is located in the cytoplasm of all tissues and is particularly abundant in the heart. This tetrameric enzyme consists of four identical subunits, each containing a selenocysteine residue, and prefers hydrogen peroxide [95]. GPx1-GPx4 and GPx6 contain selenocysteine in the active site, while GPx5, GPx7, and GPx8 have cysteine residues.
- GPx2 is primarily found in the gastrointestinal tract and serves as the initial line of defense against the absorption of hydroperoxides from processed food. It also plays an important role in embryonic development and pathological processes such as cancer [96].
- GPx3 is the only extracellular form of GPx and functions in plasma to effectively remove hydroperoxides. It plays a dual role in cancer, acting either as a tumor suppressor or as a protein that promotes tumor survival [97].
- GPx5 is a crucial antioxidant enzyme that helps regulate oxidative stress in the epididymis, playing essential roles in the storage, maturation, and transport of sperm cells [100].
- GPx6 is mainly expressed in the olfactory system and is highly similar to GPx3. It is thought to play a role in the transmission and breakdown of odor-related signals [52].
- GPx7 does not contain a glutathione binding site [101], and therefore, it does not participate in redox reactions. Instead, it acts more as a protein disulfide isomerase present in the lumen of the endoplasmic reticulum.
- GPx8 also lacks a GSH-binding site and has limited GPx activity, regulating calcium efflux and storage in the endoplasmic reticulum [102].
3. Implication of Nanozymes in Neurodegenerative Diseases
- Antioxidant activity—Nanozymes mimic natural antioxidant enzymes like SOD, CAT, GPxs, facilitating the conversion of harmful ROS into less reactive species, thereby protecting neuronal cells from oxidative damage
- Modulation of inflammatory responses—Nanozymes interact with glial cells, such as microglia and astrocytes. These interactions can reduce the release of pro-inflammatory cytokines, which are often elevated in neurodegenerative conditions. By scavenging ROS and inhibiting inflammatory signaling pathways, nanozymes can shift the balance from a pro-inflammatory to an anti-inflammatory phenotype in microglia, promoting tissue repair and reducing neuronal damage.
- Metal ion chelation—Many nanozymes possess metal-chelating properties, allowing them to bind excess metal ions like iron and copper, which can catalyze the formation of harmful ROS through Fenton reactions. By sequestering these metal ions, nanozymes can reduce metal-induced oxidative stress and prevent the aggregation of misfolded proteins, such as amyloid-beta in AD.
- Enhancing cellular response—Surface modifications, such as bioconjugation with targeting ligands (e.g., antibodies or peptides), can promote specific interactions with neuronal receptors, leading to enhanced cellular responses.
- Facilitating neuroprotection—By mimicking the activity of natural enzymes and providing localized antioxidant effects, nanozymes can help maintain mitochondrial function, reduce apoptosis, and support neuronal health.
- Modulating protein aggregation—Some nanozymes exhibit protease-like activity, enabling them to degrade misfolded or aggregated proteins associated with neurodegenerative diseases, such as tau or amyloid-beta. By facilitating the breakdown of these aggregates, nanozymes can help restore normal cellular function and prevent further neurotoxicity.
3.1. Implication of Nanozymes in Alzheimer’s Disease
3.2. Implication of Nanozymes in Parkinson’s Disease
3.3. Implication of Nanozymes in Multiple Sclerosis
3.4. Implication of Nanozymes in Amyotrophic Lateral Sclerosis
3.5. Implication of Nanozymes in Huntington’s Disease
3.6. Implication of Nanozymes in the Monitorig of Modulator H2S of Age Related Neurodegenerative Disease
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Jiang, Y.; Yang, A.; Meng, F.; Zhang, J. The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need. Aging Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Xia, Z.; Gao, M.; Sheng, P.; Shen, M.; Zhao, L.; Gao, L.; Yan, B. Fe3O4 Nanozymes Improve Neuroblast Differentiation and Blood-Brain Barrier Integrity of the Hippocampal Dentate Gyrus in D-Galactose-Induced Aged Mice. Int. J. Mol. Sci. 2022, 23, 6463. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Feng, W.; Dai, X.; Wang, J.; Geng, D.; Li, X.; Chen, Y.; Zhang, J. Cu2+-Chelatable and ROS-Scavenging MXenzyme as NIR-II-Triggered Blood–Brain Barrier-Crossing Nanocatalyst against Alzheimer’s Disease. Small 2022, 18, e2203031. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. Engl. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A clear definition with fuzzy edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Hu, Y.; Wu, J.; Wei, H. Introduction to Nanozymes. In Nanozymes: Next Wave of Artificial Enzymes; SpringerBriefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Mansur, H.S. Nanozyme-Based Cancer Nanotheranostics: Emerging Applications and Challenges in Brain Cancer Therapeutics. J. Nanotheranostics 2025, 6, 4. [Google Scholar] [CrossRef]
- Peng, F.F.; Zhang, Y.; Gu, N. Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chin. Chem. Lett. 2008, 19, 730–733. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal. Chim. Acta 2013, 776, 79–86. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Cheng, X.; Zhao, Y.; Luo, W.; Elzatahry, A.A.; Alghamdi, A.; He, X.; Su, J.; Deng, Y. Highly dispersed Pt nanoparticles on ultrasmall EMT zeolite: A peroxidase-mimic nanoenzyme for detection of H2O2 or glucose. J. Colloid Interface Sci. 2020, 570, 300–311. [Google Scholar] [CrossRef]
- Li, P.; Klet, R.C.; Moon, S.-Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.-J.; Al-Juaid, S.S.; Hupp, J.T.; et al. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, J.; Wu, Y.; Cao, P.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Shape-dependent enzyme-like activity of Co3O4 nanoparticles and their conjugation with his-tagged EGFR single-domain antibody. Colloids Surf. B Biointerfaces 2017, 154, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef]
- Singh, N.; Geethika, M.; Eswarappa, S.M.; Mugesh, G. Manganese-Based Nanozymes: Multienzyme Redox Activity and Effect on the Nitric Oxide Produced by Endothelial Nitric Oxide Synthase. Chemistry 2018, 24, 8393–8403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, X.; Li, S.; Dong, W.; Huang, Y. Tuning the oxidase mimics activity of manganese oxides via control of their growth conditions for highly sensitive detection of glutathione. Sens. Actuators B Chem. 2018, 258, 80–87. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Liu, J.; Lu, K.; Zhang, T.; Xie, Y.; Ji, Y.; Wu, X. Highly sensitive and robust peroxidase-like activity of Au-Pt core/shell nanorod-antigen conjugates for measles virus diagnosis. J. Nanobiotechnol. 2018, 16, 46. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd-Ir Core-Shell Nanocubes: A Type of Highly Efficient and Versatile Peroxidase Mimic. ACS Nano 2015, 9, 9994–10004. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, H.; Zang, H.; Ye, F.; Quan, X. Three-Dimensional Graphene Supported Bimetallic Nanocomposites with DNA Regulated-Flexibly Switchable Peroxidase-Like Activity. ACS Appl. Mater. Interfaces 2016, 8, 9855–9864. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Fan, A. Improvement of mimetic peroxidase activity of gold nanoclusters on the luminol chemiluminescence reaction by surface modification with ethanediamine. Luminescence 2018, 33, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ma, D.; Mei, L.; Gao, Q.; Yin, W.; Zhang, X.; Yan, L.; Gu, Z.; Ma, X.; Zhao, Y. Peroxidase-like activity of MoS2 nanoflakes with different modifications and their application for H2O2 and glucose detection. J. Mater. Chem. B 2018, 6, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Thao, N.T.M.; Do, H.D.K.; Nam, N.N.; Tran, N.K.S.; Dan, T.T.; Trinh, K.T.L. Antioxidant Nanozymes: Mechanisms, Activity Manipulation, and Applications. Micromachines 2023, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Park, T.J.; Kim, M.I. Recent Research Trends and Future Prospects in Nanozymes. J. Nanomater. 2015, 2015, 756278. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, Y.; Zhang, Y.; Zhang, A.; Jia, M.; Yin, J.; Shen, G. Nanozymes: A bibliometrics review. J. Nanobiotechnol. 2024, 22, 704. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, Y.; Jin, Q.; Chao, Y.; Tian, L.; Liu, J.; Dong, Z.; Liu, Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemo-photodynamic cancer therapy. Nano Res. 2019, 12, 1307–1312. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Sci Adv. 2019, 5, eaav5490. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhao, Y. Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy. Nano-Micro Lett. 2022, 14, 95. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Sharma, G.; Chatterjee, S.; Chakraborty, C.; Kim, J.-C. Advances in Nanozymes as a Paradigm for Viral Diagnostics and Therapy. Pharmacol. Rev. 2023, 75, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Golchin, J.; Golchin, K.; Alidadian, N.; Ghaderi, S.; Eslamkhah, S.; Eslamkhah, M.; Akbarzadeh, A. Nanozyme applications in biology and medicine: An overview. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Wójciuk, K.; Karczmarczyk, U. Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Appl. Sci. 2021, 11, 9019. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Singh, S.; Banerjee, R.; Pal, K. Emerging trends in biodegradable polymer-metal nanoconjugates for cancer therapeutics. Eur. Polym. J. 2024, 207, 112835. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Huang, K.; Cheng, N. Nanozyme as a rising star for metabolic disease management. J. Nanobiotechnol. 2024, 22, 226. [Google Scholar] [CrossRef]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines. Discov. Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Fouling Surface—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/fouling-surface (accessed on 3 April 2025).
- Tian, Y.; Lv, H.; Ju, Y.; Hao, J.; Cui, J. Zwitterionic Poly(ethylene glycol) Nanoparticles Minimize Protein Adsorption and Immunogenicity for Improved Biological Fate. ACS Appl. Mater. Interfaces 2025, 17, 6125–6133. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Ali, S.; Sikdar, S.; Basak, S.; Rajbanshi, B.; Mondal, M.; Roy, D.; Dutta, A.; Kumar, A.; Dakua, V.K.; Chakrabarty, R.; et al. β-Cyclodextrin-Stabilized Biosynthesis Nanozyme for Dual Enzyme Mimicking and Fenton Reaction with a High Potential Anticancer Agent. ACS Omega 2022, 7, 4457–4470. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, W.; Wu, R.; Guo, J.; Liu, X.; Shi, B.; Zhang, C.; Wu, L.; Zheng, Y.; Liu, A.; et al. Chitosan-Stabilized PtAu Nanoparticles with Multienzyme-Like Activity for Mixed Bacteria Infection Wound Healing and Insights into Its Antibacterial Mechanism. Small Struct. 2024, 5, 2300553. [Google Scholar] [CrossRef]
- Ren, L.; Liu, X.; Tang, S.; Wang, Y.; Yang, M.; Guo, L.; Li, J.; Jiao, K.; Wang, L. DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems. Biosensors 2025, 15, 205. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef]
- He, S.; Ma, L.; Zheng, Q.; Wang, Z.; Chen, W.; Yu, Z.; Yan, X.; Fan, K. Peptide nanozymes: An emerging direction for functional enzyme mimics. Bioact. Mater. 2024, 42, 284–298. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 429–435. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological Defense Mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Bierbaum, V.M.; Ellison, G.B.; Kato, S. Superoxide Does React with Peroxides: Direct Observation of the Haber–Weiss Reaction in the Gas Phase. Angew. Chem. Int. Ed. Engl. 2007, 46, 4948–4950. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Wood, N.D.; Dyster, S. Ionisation constants of ˙OH and HO˙2 in aqueous solution up to 200 °C. A pulse radiolysis study. J. Chem. Soc. Faraday Trans. 1988, 84, 1113–1121. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Brezova, V.; Valko, M.; Breza, M.; Morris, H.; Telser, J.; Dvoranova, D.; Kaiserova, K.; Varecka, L.; Mazur, M.; Leibfritz, D. Role of Radicals and Singlet Oxygen in Photoactivated DNA Cleavage by the Anticancer Drug Camptothecin: An Electron Paramagnetic Resonance Study. J. Phys. Chem. B 2003, 107, 2415–2425. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Azzi, A. Oxidative Stress: What Is It? Can It Be Measured? Where Is It Located? Can It Be Good or Bad? Can It Be Prevented? Can It Be Cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Lazarow, P.B.; de Duve, C. The Synthesis and Turnover of Rat Liver Peroxisomes: IV. Intracellular Pathway of Catalase Synthesis. J. Cell Biol. 1973, 59, 507–524. [Google Scholar] [CrossRef]
- Middelkoop, E.; Wiemer, E.A.C.; Schoenmaker, D.E.T.; Strijland, A.; Tager, J.M. Topology of catalase assembly in human skin fibroblasts. Biochim. Biophys. Acta 1993, 1220, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Djordjević, V.V.; Kostić, J.; Krivokapić, Ž.; Krtinić, D.; Ranković, M.; Petković, M.; Ćosić, V. Decreased Activity of Erythrocyte Catalase and Glutathione Peroxidase in Patients with Schizophrenia. Medicina 2022, 58, 1491. [Google Scholar] [CrossRef]
- Gomes, P.; Simão, S.; Lemos, V.; Amaral, J.S.; Soares-da-Silva, P. Loss of oxidative stress tolerance in hypertension is linked to reduced catalase activity and increased c-Jun NH2-terminal kinase activation. Free Radic. Biol. Med. 2013, 56, 112–122. [Google Scholar] [CrossRef]
- Góth, L. Catalase Deficiency and Type 2 Diabetes. Diabetes Care 2008, 31, e93. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.; Gao, J.; Li, K.; Zhang, R.; Wang, G.; Li, C.; Gao, T. Promoter variant in the catalase gene is associated with vitiligo in Chinese people. J. Investig. Dermatol. 2010, 130, 2647–2653. [Google Scholar] [CrossRef]
- Gromadzka, G.; Przybyłkowski, A.; Litwin, T.; Karpińska, A. Antioxidant Capacity Is Decreased in Wilson’s Disease and Correlates to Liver Function. Biol. Trace Elem. Res. 2023, 201, 1582–1587. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, H.; Dai, Y.; Su, Z.; Wang, C.; Lei, L.; Lin, D.; Li, X.; Chen, H.; Fan, K.; et al. In Vivo Regenerable Cerium Oxide Nanozyme-Loaded pH/H2O2-Responsive Nanovesicle for Tumor-Targeted Photothermal and Photodynamic Therapies. ACS Appl. Mater. Interfaces. 2021, 13, 233–244. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.-J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Hou, Y.; Wang, X.; Xue, C.; Li, W.; Cai, K.; Zhao, Y.; Luo, Z. State-of-the-art iron-based nanozymes for biocatalytic tumor therapy. Nanoscale Horiz. 2020, 5, 202–217. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Liang, Q.; Xi, J.; Zhao, H.; Jin, Y.; Gao, X.; Yan, X.; Gao, L.; Fan, K. Unveiling the active sites on ferrihydrite with apparent catalase-like activity for potentiating radiotherapy. Nano Today 2021, 41, 101317. [Google Scholar] [CrossRef]
- Ren, C.; Hu, X.; Zhou, Q. Graphene Oxide Quantum Dots Reduce Oxidative Stress and Inhibit Neurotoxicity In Vitro and In Vivo through Catalase-Like Activity and Metabolic Regulation. Adv. Sci. 2018, 5, 1700595. [Google Scholar] [CrossRef]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim. Biophys. Acta 2010, 1804, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Prasad, N.; Ramteke, P.; Dholia, N.; Yadav, U.C.S. Chapter 27—Therapeutic Interventions to Block Oxidative Stress-Associated Pathologies. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 341–362. [Google Scholar] [CrossRef]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions of c60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Damle, M.A.; Jakhade, A.P.; Chikate, R.C. Modulating Pro- and Antioxidant Activities of Nanoengineered Cerium Dioxide Nanoparticles against Escherichia coli. ACS Omega 2019, 4, 3761–3771. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 14, 1056–1058. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shi, Y.; Eyring, E.M.; Ernst, R.D. Concentration of Ce3+ and Oxygen Vacancies in Cerium Oxide Nanoparticles. Chem. Mater. 2006, 18, 5144–5146. [Google Scholar] [CrossRef]
- D’Angelo, A.M.; Liu, A.C.Y.; Chaffee, A.L. Oxygen Uptake of Tb–CeO2: Analysis of Ce3+ and Oxygen Vacancies. J. Phys. Chem. C 2016, 120, 14382–14389. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Zhao, H.; Qi, H.; Li, J.; Li, J.-F.; Zhou, X.; Wang, A.; Fan, K.; Yan, X.; et al. Bioinspired copper single-atom nanozyme as a superoxide dismutase-like antioxidant for sepsis treatment. Exploration 2022, 2, 20210267. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Faryabi, K.; Talaei, A.J.; Shekha, M.S.; Ale-Ebrahim, M.; Salihi, A.; Nanakali, N.M.Q.; Aziz, F.M.; Rasti, B.; Hasan, A.; et al. Antioxidant properties of gold nanozyme: A review. J. Mol. Liq. 2020, 297, 112004. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L. Unraveling the Multi-Enzyme-Like Activities of Iron Oxide Nanozyme via a First-Principles Microkinetic Study. J. Phys. Chem. C 2019, 123, 30318–30334. [Google Scholar] [CrossRef]
- Liu, Y.; Qing, Y.; Jing, L.; Zou, W.; Guo, R. Platinum-Copper Bimetallic Colloid Nanoparticle Cluster Nanozymes with Multiple Enzyme-like Activities for Scavenging Reactive Oxygen Species. Langmuir 2021, 37, 7364–7372. [Google Scholar] [CrossRef]
- Dong, J.; Song, L.; Yin, J.-J.; He, W.; Wu, Y.; Gu, N.; Zhang, Y. Co3O4 Nanoparticles with Multi-Enzyme Activities and Their Application in Immunohistochemical Assay. ACS Appl. Mater. Interfaces 2014, 6, 1959–1970. [Google Scholar] [CrossRef]
- Dashtestani, F.; Ghourchian, H.; Najafi, A. Silver-gold-apoferritin nanozyme for suppressing oxidative stress during cryopreservation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 831–840. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Sook Kim-Han, J.; Erlanger, B.F.; Huang, T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Robson, A.; Houghton, B.C.; Jepson, C.A.; Ford, W.C.L.; Frayne, J. Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Hum. Reprod. 2013, 28, 2332–2342. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Yoboue, E.D.; Rimessi, A.; Anelli, T.; Pinton, P.; Sitia, R. Regulation of Calcium Fluxes by GPX8, a Type-II Transmembrane Peroxidase Enriched at the Mitochondria-Associated Endoplasmic Reticulum Membrane. Antioxid. Redox Signal. 2017, 27, 583–595. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Aranda, R.; Martín, M.G.; Doroshow, J.H.; Binder, S.W.; Chu, F.-F. Mice with combined disruption of Gpx1 andGpx2 genes have colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G848–G855. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.-F.; Esworthy, R.S.; Chu, P.G.; Longmate, J.A.; Huycke, M.M.; Wilczynski, S.; Doroshow, J.H. Bacteria-Induced Intestinal Cancer in Mice with Disrupted Gpx1 and Gpx2 Genes. Cancer Res. 2004, 64, 962–968. [Google Scholar] [CrossRef]
- An, B.C.; Jung, N.-K.; Park, C.Y.; Oh, I.-J.; Choi, Y.-D.; Park, J.-I.; Lee, S.-W. Epigenetic and Glucocorticoid Receptor-Mediated Regulation of Glutathione Peroxidase 3 in Lung Cancer Cells. Mol. Cells 2016, 39, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.-F.; Hu, T.-L.; Schneider, B.G.; Chen, Z.; Xu, Z.-K.; El-Rifai, W. Silencing of glutathione peroxidase 3 through DNA hypermethylation is associated with lymph node metastasis in gastric carcinomas. PLoS ONE 2012, 7, e46214. [Google Scholar] [CrossRef]
- Yu, Y.P.; Yu, G.; Tseng, G.; Cieply, K.; Nelson, J.; Defrances, M.; Zarnegar, R.; Michalopoulos, G.; Luo, J.-H. Glutathione Peroxidase 3, Deleted or Methylated in Prostate Cancer, Suppresses Prostate Cancer Growth and Metastasis. Cancer Res. 2007, 67, 8043–8050. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Z.; Kim, K.-Y.; Jin, X.; Roh, M.R.; Jin, Z. Hypermethylation and downregulation of glutathione peroxidase 3 are related to pathogenesis of melanoma. Oncol. Rep. 2016, 36, 2737–2744. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.-L. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023, 559, 216119. [Google Scholar] [CrossRef]
- Shema, R.; Kulicke, R.; Cowley, G.S.; Stein, R.; Root, D.E.; Heiman, M. Synthetic lethal screening in the mammalian central nervous system identifies Gpx6 as a modulator of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 268–272. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, P.; Karmodak, N.; Jemmis, E.D.; Mugesh, G. Nanoisozymes: Crystal-Facet-Dependent Enzyme-Mimetic Activity of V2 O5 Nanomaterials. Angew. Chem. Int. Ed. Engl. 2018, 57, 4510–4515. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 2014, 5, 5301. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale 2019, 11, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Qu, A.; Xu, L.; Sun, M.; Zhang, H.; Xu, C.; Kuang, H. Chiral Molecule-mediated Porous CuxO Nanoparticle Clusters with Antioxidation Activity for Ameliorating Parkinson’s Disease. J. Am. Chem. Soc. 2019, 141, 1091–1099. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Adhikari, A.; Mondal, S.; Das, M.; Biswas, P.; Pal, U.; Darbar, S.; Bhattacharya, S.S.; Pal, D.; Saha-Dasgupta, T.; Das, A.K.; et al. Incorporation of a Biocompatible Nanozyme in Cellular Antioxidant Enzyme Cascade Reverses Huntington’s Like Disorder in Preclinical Model. Adv. Healthc. Mater. 2021, 10, e2001736. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Sun, S.; Yang, J.; Long, W.; Wang, J.; Mu, X.; Li, Q.; Hao, W.; Zhang, S.; Liu, H.; et al. Nanozyme-Based Bandage with Single-Atom Catalysis for Brain Trauma. ACS Nano 2019, 13, 11552–11560. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Pu, F.; Liu, Z.; Ren, J.; Qu, X. A GO–Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem. Commun. 2017, 53, 3082–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, N.; Zhang, J.; Zhu, J.; Guo, Y.; Xu, L. A novel nanozyme based on selenopeptide-modified gold nanoparticles with a tunable glutathione peroxidase activity. RSC Adv. 2020, 10, 8685–8691. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Impaired Redox Signaling in Huntington’s Disease: Therapeutic Implications. Front. Mol. Neurosci. 2019, 12, 68. [Google Scholar] [CrossRef]
- Baillet, A.; Chanteperdrix, V.; Trocmé, C.; Casez, P.; Garrel, C.; Besson, G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson’s disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yuan, X.; Wei, J.-A.; Guo, X.; Gong, Y.; Li, J.; Zhou, H.; Zhang, L.; Liu, J. A Functionalized Octahedral Palladium Nanozyme as a Radical Scavenger for Ameliorating Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2021, 13, 49602–49613. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials 2020, 232, 119752. [Google Scholar] [CrossRef]
- Ma, M.; Gao, N.; Li, X.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. A Biocompatible Second Near-Infrared Nanozyme for Spatiotemporal and Non-Invasive Attenuation of Amyloid Deposition through Scalp and Skull. ACS Nano 2020, 14, 9894–9903. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.; Gao, N.; Pi, Z.; Du, X.; Ren, J.; Qu, X. Self-Protecting Biomimetic Nanozyme for Selective and Synergistic Clearance of Peripheral Amyloid-β in an Alzheimer’s Disease Model. J. Am. Chem. Soc. 2020, 142, 21702–21711. [Google Scholar] [CrossRef]
- Ge, K.; Mu, Y.; Liu, M.; Bai, Z.; Liu, Z.; Geng, D.; Gao, F. Gold Nanorods with Spatial Separation of CeO2 Deposition for Plasmonic-Enhanced Antioxidant Stress and Photothermal Therapy of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2022, 14, 3662–3674. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A Redox Modulatory Mn3O4 Nanozyme with Multi-Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson’s Disease Model. Angew. Chem. Int. Ed. Engl. 2017, 129, 14455–14459. [Google Scholar] [CrossRef]
- Ma, X.; Hao, J.; Wu, J.; Li, Y.; Cai, X.; Zheng, Y. Prussian Blue Nanozyme as a Pyroptosis Inhibitor Alleviates Neurodegeneration. Adv. Mater. 2022, 34, 2106723. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Xu, X.; Yang, X.; Chen, L.; Jiang, C.; Wang, Y.; Hu, W.; Wei, X.; Yang, Z. Catalytic-Enhanced Lactoferrin-Functionalized Au-Bi2Se3 Nanodots for Parkinson’s Disease Therapy via Reactive Oxygen Attenuation and Mitochondrial Protection. Adv. Healthc. Mater. 2021, 10, 2100316. [Google Scholar] [CrossRef]
- Feng, W.; Han, X.; Hu, H.; Chang, M.; Ding, L.; Xiang, H.; Chen, Y.; Li, Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat. Commun. 2021, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Li, Y.; Peng, H.; Zhao, R.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Lu, Z.; Yang, J.; et al. Self-Catalytic Small Interfering RNA Nanocarriers for Synergistic Treatment of Neurodegenerative Diseases. Adv. Mater. 2022, 34, e2105711. [Google Scholar] [CrossRef]
- Martínez-Camarena, Á.; Merino, M.; Sánchez-Sánchez, A.V.; Blasco, S.; Llinares, J.M.; Mullor, J.L.; García-España, E. An antioxidant boehmite amino-nanozyme able to disaggregate Huntington’s inclusion bodies. Chem. Commun. 2022, 58, 5021–5024. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Oswalia, J.; Yadav, S.; Vachher, M.; Nigam, A. Recent trends in nanozyme research and their potential therapeutic applications. Curr. Res. Biotechnol. 2024, 7, 100205. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, M.; Li, P. The applications of nanozymes in neurological diseases: From mechanism to design. Theranostics 2023, 13, 2492–2514. [Google Scholar] [CrossRef]
- Efthymiou, A.G.; Goate, A.M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 2017, 12, 43. [Google Scholar] [CrossRef]
- Canevelli, M.; Piscopo, P.; Talarico, G.; Vanacore, N.; Blasimme, A.; Crestini, A.; Tosto, G.; Troili, F.; Lenzi, G.L.; Confaloni, A.; et al. Familial Alzheimer’s disease sustained by presenilin 2 mutations: Systematic review of literature and genotype–phenotype correlation. Neurosci. Biobehav. Rev. 2014, 42, 170–179. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.-P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomedicine 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef]
- Nazem, A.; Mansoori, G.A. Nanotechnology solutions for Alzheimer’s disease: Advances in research tools, diagnostic methods and therapeutic agents. J. Alzheimer’s Dis. 2008, 13, 199–223. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.L.; Deramecourt, V.; Auger, F.; Durieux, N.; Cordonnier, C.; Devos, D.; Defebvre, L.; Moreau, C.; Caparros-Lefebvre, D.; Leys, D.; et al. Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: A semi-quantitative 7.0 T magnetic resonance imaging study. Eur. J. Neurol. 2014, 21, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular Basis of Familial and Sporadic Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Kelleher, R.J.; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Zoltowska, K.M.; Berezovska, O. Dynamic Nature of presenilin1/γ-Secretase: Implication for Alzheimer’s Disease Pathogenesis. Mol. Neurobiol. 2018, 55, 2275–2284. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Laske, C.; Sohrabi, H.R.; Frost, S.M.; López-De-Ipiña, K.; Garrard, P.; Buscema, M.; Dauwels, J.; Soekadar, S.R.; Mueller, S.; Linnemann, C.; et al. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 561–578. [Google Scholar] [CrossRef]
- Huang, W.; Xia, Q.; Zheng, F.; Zhao, X.; Ge, F.; Xiao, J.; Liu, Z.; Shen, Y.; Ye, K.; Wang, D.; et al. Microglia-Mediated Neurovascular Unit Dysfunction in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, S335–S354. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, A.; Guo, X.; Jia, Z.; Chen, X.; Zhu, X.; Xia, Y.; Liu, J.; Xu, Y.; Qin, X. Selenium-core nanozymes dynamically regulates Aβ & neuroinflammation circulation: Augmenting repair of nervous damage. Chem. Eng. J. 2021, 418, 129345. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, X.; Wang, L.; Yin, M.; Wang, L.; Chen, N.; Fan, C.; Song, H. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv. Mater. 2016, 28, 1387–1393. [Google Scholar] [CrossRef]
- Cheng, F.; Kotha, S.; Fu, M.; Yang, Q.; Wang, H.; He, W.; Mao, X. Nanozyme enabled protective therapy for neurological diseases. Nano Today 2024, 54, 102142. [Google Scholar] [CrossRef]
- Bai, Z.; Ge, K.; Fu, J.; Yu, D.; Hua, Z.; Xue, S.; Li, Z.; Sheng, W.; Wu, X.; Gao, F.; et al. Engineered urinary-derived extracellular vesicles loaded nanoenzymes as Trojan horses to regulate the inflammatory microenvironment for treatment of Alzheimer’s disease. Chem. Eng. J. 2023, 465, 142955. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Role of zinc and copper ions in the pathogenetic mechanisms of traumatic brain injury and Alzheimer’s disease. Rev. Neurosci. 2019, 31, 233–243. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, S.; Zhang, N.; Zhou, Y.; Cheng, N.; Wang, M.; Xu, M.; Feng, Z.; Niu, X.; Cheng, Y.; et al. Single-Atom Nanozymes Linked Immunosorbent Assay for Sensitive Detection of A β 1-40: A Biomarker of Alzheimer’s Disease. Research 2020, 2020, 4724505. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimeŕs Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, L.; Weber, G.E.; Parfitt, G.M.; Cordeiro, A.P.; Sahoo, S.K.; Fantini, C.; Klosterhoff, M.C.; Romano, L.A.; Furtado, C.A.; Santos, A.P.; et al. Biopersistence of PEGylated carbon nanotubes promotes a delayed antioxidant response after infusion into the rat hippocampus. PLoS ONE 2015, 10, e0129156. [Google Scholar] [CrossRef]
- Yu, D.; Ma, M.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. MOF-encapsulated nanozyme enhanced siRNA combo: Control neural stem cell differentiation and ameliorate cognitive impairments in Alzheimer’s disease model. Biomaterials 2020, 255, 120160. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef]
- Li, M.; Shi, P.; Xu, C.; Ren, J.; Qu, X. Cerium oxide caged metal chelator: Anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. α-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef]
- Brahmachari, S.; Lee, S.; Kim, S.; Yuan, C.; Karuppagounder, S.S.; Ge, P.; Shi, R.; Kim, E.J.; Liu, A.; Kim, D.; et al. Parkin interacting substrate zinc finger protein 746 is a pathological mediator in Parkinson’s disease. Brain 2019, 142, 2380–2401. [Google Scholar] [CrossRef]
- Butler, Y.R.; Liu, Y.; Kumbhar, R.; Zhao, P.; Gadhave, K.; Wang, N.; Li, Y.; Mao, X.; Wang, W. α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice. Nat. Commun. 2022, 13, 4060. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, X.; Mao, X.; Xu, E.; Qi, C.; Wang, H.; Brahmachari, S.; York, B.; Sriparna, M.; Li, A.; et al. Lymphocyte Activation Gene 3 (Lag3) Contributes to α-Synucleinopathy in α-Synuclein Transgenic Mice. Front. Cell. Neurosci. 2021, 15, 656426. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, Y.; Wang, W.; Wang, Y.; Liu, H.; Liu, F.; Chen, R.; Dawson, V.L.; Dawson, T.M.; Lu, F.; et al. Molecular Mediation of Prion-like α-Synuclein Fibrillation from Toxic PFFs to Nontoxic Species. ACS Appl. Bio Mater. 2020, 3, 6096–6102. [Google Scholar] [CrossRef]
- Jo, A.; Lee, Y.; Kam, T.I.; Kang, S.U.; Neifert, S.; Karuppagounder, S.S.; Khang, R.; Kang, H.; Park, H.; Chou, S.-C.; et al. PARIS farnesylation prevents neurodegeneration in models of Parkinson’s disease. Sci. Transl. Med. 2021, 13, eaax8891. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.-H.; et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374. [Google Scholar] [CrossRef]
- Panicker, N.; Kam, T.I.; Wang, H.; Neifert, S.; Chou, S.C.; Kumar, M.; Brahmachari, S.; Jhaldiyal, A.; Hinkle, J.T.; Akkentli, F.; et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron 2022, 110, 2422–2437.e9. [Google Scholar] [CrossRef]
- Seo, B.A.; Kim, D.; Hwang, H.; Kim, M.S.; Ma, S.X.; Kwon, S.H.; Kweon, S.H.; Wang, H.; Yoo, J.M.; Choi, S.; et al. TRIP12 ubiquitination of glucocerebrosidase contributes to neurodegeneration in Parkinson’s disease. Neuron 2021, 109, 3758–3774.e11. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.Q.; Jia, C.; Lim, Y.J.; Feng, G.; Xu, E.; Long, H.; Kimura, Y.; Tao, Y.; Zhao, C.; et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2011196118. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, J.; Zhou, H.; Liu, Q.; Jia, L.; Zhang, X.; Ge, D.; Shi, W.; Sun, Y. Polydopamine-Based Nanocomposite as a Biomimetic Antioxidant with a Variety of Enzymatic Activities for Parkinson’s Disease. ACS Appl. Mater. Interfaces 2022, 14, 32901–32913. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Mao, Y.; Xu, E.; Jia, H.; Zhang, S.; Dawson, V.L.; Dawson, T.M.; Li, Y.-M.; Zheng, Z.; He, W.; et al. Nanozyme scavenging ROS for prevention of pathologic α-synuclein transmission in Parkinson’s disease. Nano Today 2021, 36, 101027. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, L.; Miao, X.; Chen, C.; Su, S. Prussian Blue Nanoparticle Supported MoS2 Nanocomposites as a Peroxidase-Like Nanozyme for Colorimetric Sensing of Dopamine. Biosensors 2022, 12, 260. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, D.; Seo, K.; Kim, Y.G.; Han, S.I.; Kang, T.; Soh, M.; Hyeon, T. Ceria Nanoparticle Systems for Selective Scavenging of Mitochondrial, Intracellular, and Extracellular Reactive Oxygen Species in Parkinson’s Disease. Angew. Chem. Int. Ed. Engl. 2018, 130, 9552–9556. [Google Scholar] [CrossRef]
- Liu, H.; Han, Y.; Wang, T.; Zhang, H.; Xu, Q.; Yuan, J.; Li, Z. Targeting Microglia for Therapy of Parkinson’s Disease by Using Biomimetic Ultrasmall Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21730–21742. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Ghaeidamini, M.; Bernson, D.; Sasanian, N.; Kumar, R.; Esbjörner, E.K. Graphene oxide sheets and quantum dots inhibit α-synuclein amyloid formation by different mechanisms. Nanoscale 2020, 12, 19450–19460. [Google Scholar] [CrossRef]
- Shang, Q.; Zhang, L.; Chen, C.; Tang, W.; Han, M.; Chen, Q.; Liu, W. EDTA–Fe2+ Complex-Functionalized Fe3O4 Nanozyme as Tyrosine Hydroxylase Mimics for the Production of l-DOPA. ACS Appl. Nano Mater. 2022, 5, 2678–2687. [Google Scholar] [CrossRef]
- Zéphir, H. Progress in understanding the pathophysiology of multiple sclerosis. Rev. Neurol. 2018, 174, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, H.A.; Ferrari, A.J.; Degenhardt, L.; Feigin, V.; Vos, T. The global burden of mental, neurological and substance use disorders: An analysis from the global burden of disease study 2010. PLoS ONE 2015, 10, e0116820. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- Quintas, R.; Raggi, A.; Giovannetti, A.M.; Pagani, M.; Sabariego, C.; Cieza, A.; Leonardi, M. Psychosocial difficulties in people with epilepsy: A systematic review of literature from 2005 until 2010. Epilepsy Behav. 2012, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- Heckman, K.L.; DeCoteau, W.; Estevez, A.; Reed, K.J.; Costanzo, W.; Sanford, D.; Leiter, J.C.; Clauss, J.; Knapp, K.; Gomez, C.; et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013, 7, 10582–10596. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Stauffer, J.E.; Schulte, D.J.; Ravits, J. Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. Neurol. Clin. 2015, 33, 855–876. [Google Scholar] [CrossRef]
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef]
- Deng, H.X.; Zhai, H.; Bigio, E.H.; Yan, J.; Fecto, F.; Ajroud, K.; Mishra, M.; Ajroud-Driss, S.; Heller, S.; Sufit, R.; et al. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann. Neurol. 2010, 67, 739–748. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Jones, A.; Troakes, C.; King, A.; Al-Sarraj, S.; Van Den Berg, L.H. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 2012, 124, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Lasiene, J.; Yamanaka, K. Glial cells in amyotrophic lateral sclerosis. Neurol. Res. Int. 2011, 2011, 718987. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 2002, 26, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Mead, R.J.; Shaw, P.J. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta 2006, 1762, 1051–1067. [Google Scholar] [CrossRef]
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef]
- Singh, N.; NaveenKumar, S.K.; Geethika, M.; Mugesh, G. A Cerium Vanadate Nanozyme with Specific Superoxide Dismutase Activity Regulates Mitochondrial Function and ATP Synthesis in Neuronal Cells. Angew. Chem. Int. Ed. Engl. 2021, 60, 3121–3130. [Google Scholar] [CrossRef]
- Schilling, G. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum. Mol. Genet. 1999, 8, 397–407, Erratum in Hum. Mol. Genet. 1999, 8, 943. [Google Scholar] [CrossRef]
- Cattaneo, E.; Rigamonti, D.; Goffredo, D.; Zuccato, C.; Squitieri, F.; Sipione, S. Loss of normal huntingtin function: New developments in Huntington’s disease research. Trends Neurosci. 2001, 24, 182–188. [Google Scholar] [CrossRef]
- Davies, S.W.; Turmaine, M.; Cozens, B.A.; DiFiglia, M.; Sharp, A.H.; Ross, C.A.; Scherzinger, E.; Wanker, E.E.; Mangiarini, L.; Bates, G.P. Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 1997, 90, 537–548. [Google Scholar] [CrossRef]
- Giorgini, F.; Guidetti, P.; Nguyen, Q.V.; Bennett, S.C.; Muchowski, P.J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat. Genet. 2005, 37, 526–531. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Rodríguez-Colman, M.J.; Tamarit, J.; Ortega, Z.; Lucas, J.J.; Ferrer, I.; Ferrer, I.; Ros, J.; Cabiscol, E. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic. Biol. Med. 2010, 49, 612–621. [Google Scholar] [CrossRef]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Bai, R.; Li, Y.F.; Wang, L.; Chen, C. Selenium Nanoparticles as an Efficient Nanomedicine for the Therapy of Huntington’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 34725–34735. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, J.; Wang, J.; Wei, Y.; Huang, D.; Liang, Z. Application of Nanozymes in the Treatment of Brain Diseases. Prog. Chem. 2024, 36, 18–26. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, W.; Liu, J.; Lu, M.; Li, K.; Lin, Y. Integrating Prussian Blue Analog-Based Nanozyme and Online Visible Light Absorption Approach for Continuous Hydrogen Sulfide Monitoring in Brains of Living Rats. Anal. Chem. 2020, 92, 662–667. [Google Scholar] [CrossRef]
- Wang, C.; Ren, G.; Yuan, B.; Zhang, W.; Lu, M.; Liu, J.; Li, K.; Lin, Y. Enhancing Enzyme-like Activities of Prussian Blue Analog Nanocages by Molybdenum Doping: Toward Cytoprotecting and Online Optical Hydrogen Sulfide Monitoring. Anal. Chem. 2020, 92, 7822–7830. [Google Scholar] [CrossRef]
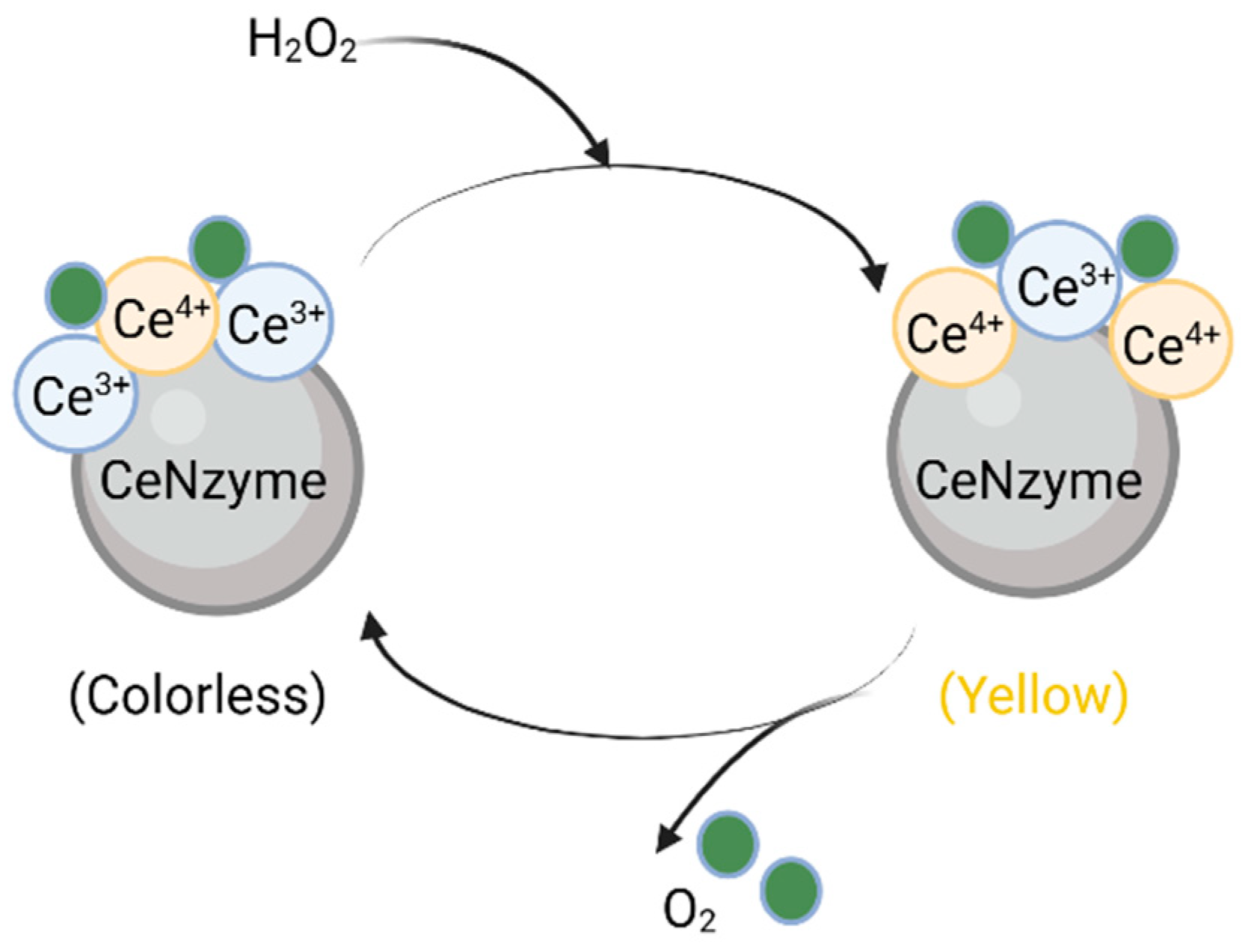
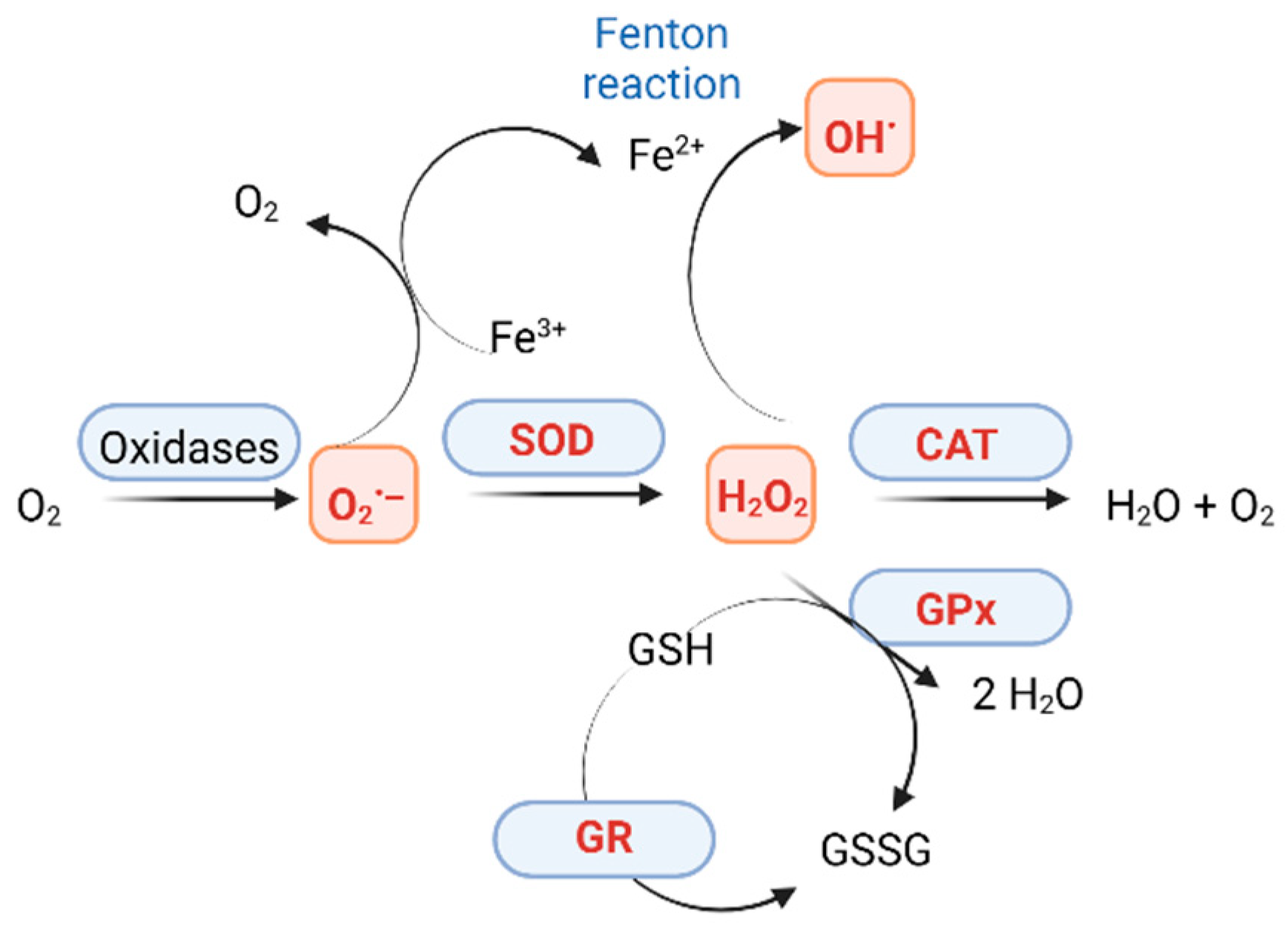
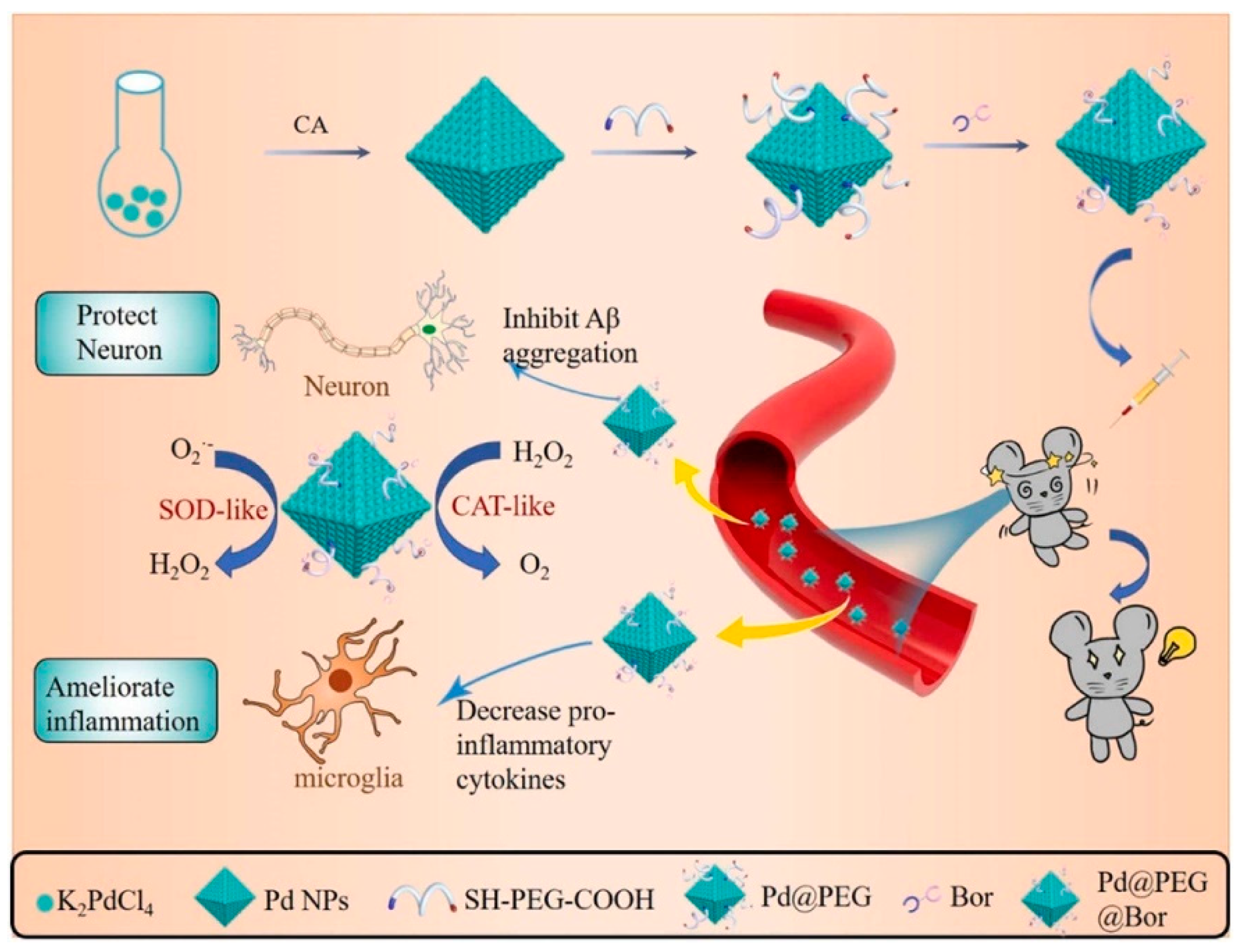
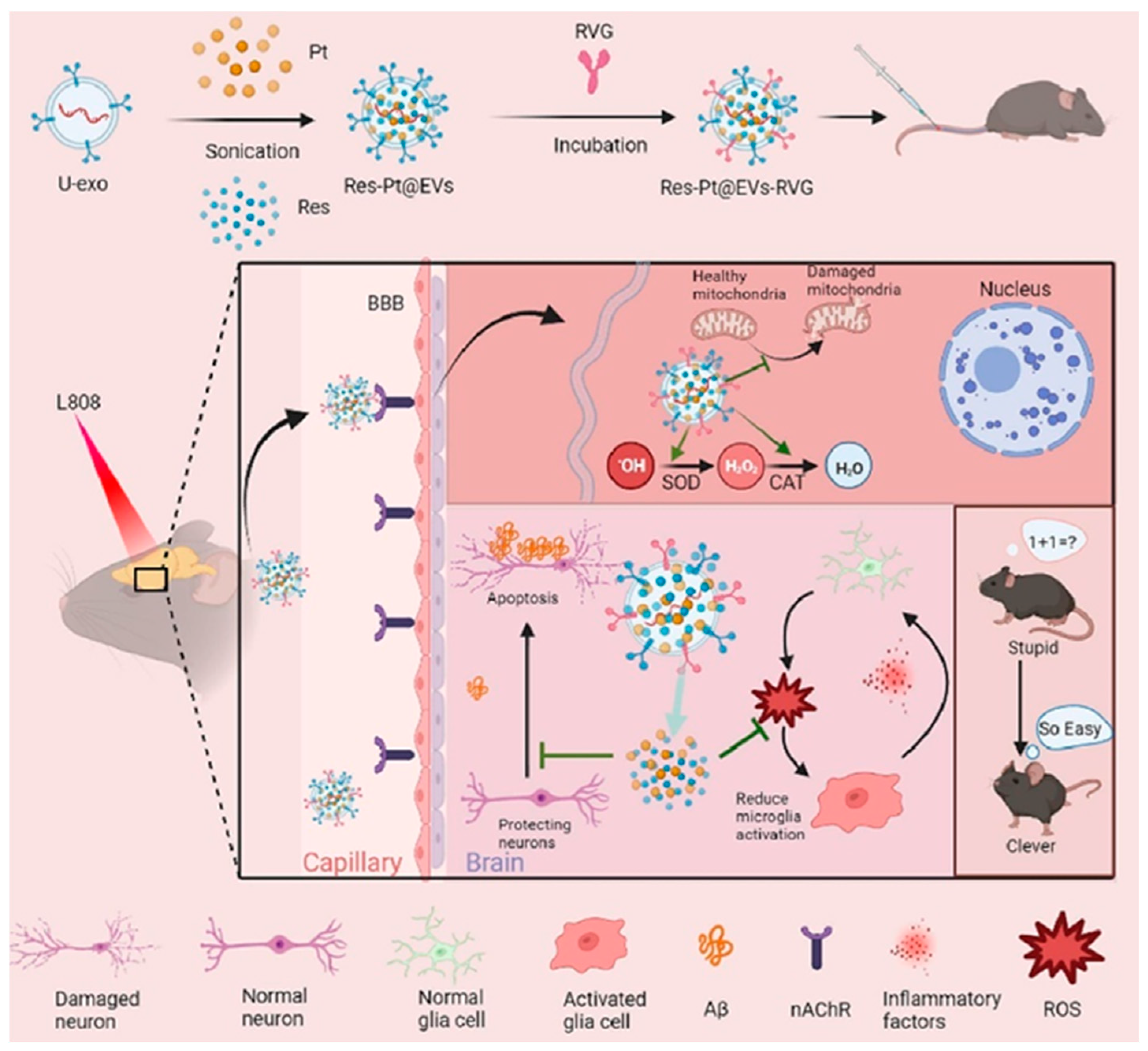
| Advantages | Limitations and Challenges 1 |
|---|---|
| Simpler preparation process | The specificity, selectivity, and biocatalytic activity of most nanozymes are expected to be improved and controllable |
| Better cost-effectiveness; relatively low cost of production | Research on more kinds of enzyme-like activities (most nanozymes reported have oxidoreductase activity) |
| Higher chemical and thermal stability | More efforts and studies are needed to clarify their catalytic mechanisms (structure-activity relationships) and kinetics |
| Better controllability of properties due to their easy modification | As nanosized materials, biosafety and potential toxicity remain challenging and should be diligently researched (usually highly stable materials with a composition comprising inherent elements of organisms and with multiple enzyme-like activities) |
| Applicability to various health problems, including diagnostics and therapy, and the possibility of targeting cells, tissues, and organs, mostly through surface modification | Novel application scenarios should be developed |
| Easier to synthesize in large quantities; scalable to industry | Multi-enzyme-like activities should be rationally designed for cascade reactions |
| More versatile and tunable catalytic capabilities (function of composition, size, morphology, crystal face, valence, active site, etc.) | Surface modification methods designed for targeting should be optimized to reduce the influence on the activity of nanozymes (for example, hinder the interaction of the substrate with the nanozyme surface |
| Long-term storage stability, with superior recyclability and reusability | |
| Superior versatility and applicability to various health problems | |
| Nanozyme activities can often be controlled through the modulation of the pH, temperature, light, magnetic field, or other external stimuli, which can render them more effective in the treatment of diseases | |
| Superior sustainability in terms of renewable precursor sources | |
| As nanozymes combine characteristics of nanomaterials and enzymes, in addition to catalytic activity, they can also exhibit interesting physicochemical properties, such as photoluminescence, supermagnetism, photothermal, and other properties |
| Disease | Nanozyme | Function | Models | Therapeutic Effects | References |
|---|---|---|---|---|---|
| Alzheimer’s disease | Pd@PEG@ Bor | SOD, CAT | 3× Tg-AD mice | ROS↓; inhibits Aβ plaque deposition, reduces neuronal loss, alleviates neuroinflammation, enhances cognitive function. | [127] |
| PEG-Fe3O4 | SOD, CAT | D-galactose induced aged mice | ROS↓; PECAM-1↑; Claudin5↑; ZO-1↑; promotes neuroblast differentiation in the hippocampal dentate gyrus | [2] | |
| TPP-MoS2 QDs | SOD, CAT | APP/PS1 mice | ROS↓; IL-1β↓; IL-6↓; TNF-α↓; TGF-β↑; prevents Aβ deposits; prevents inflammation | [128] | |
| KD8@N-MCNs | SOD, CAT | 3× Tg-AD mice | ROS↓; IL-1β↓; TNF-α↓; decreases Aβ deposits, improves memory, and alleviate neuroinflammation | [129] | |
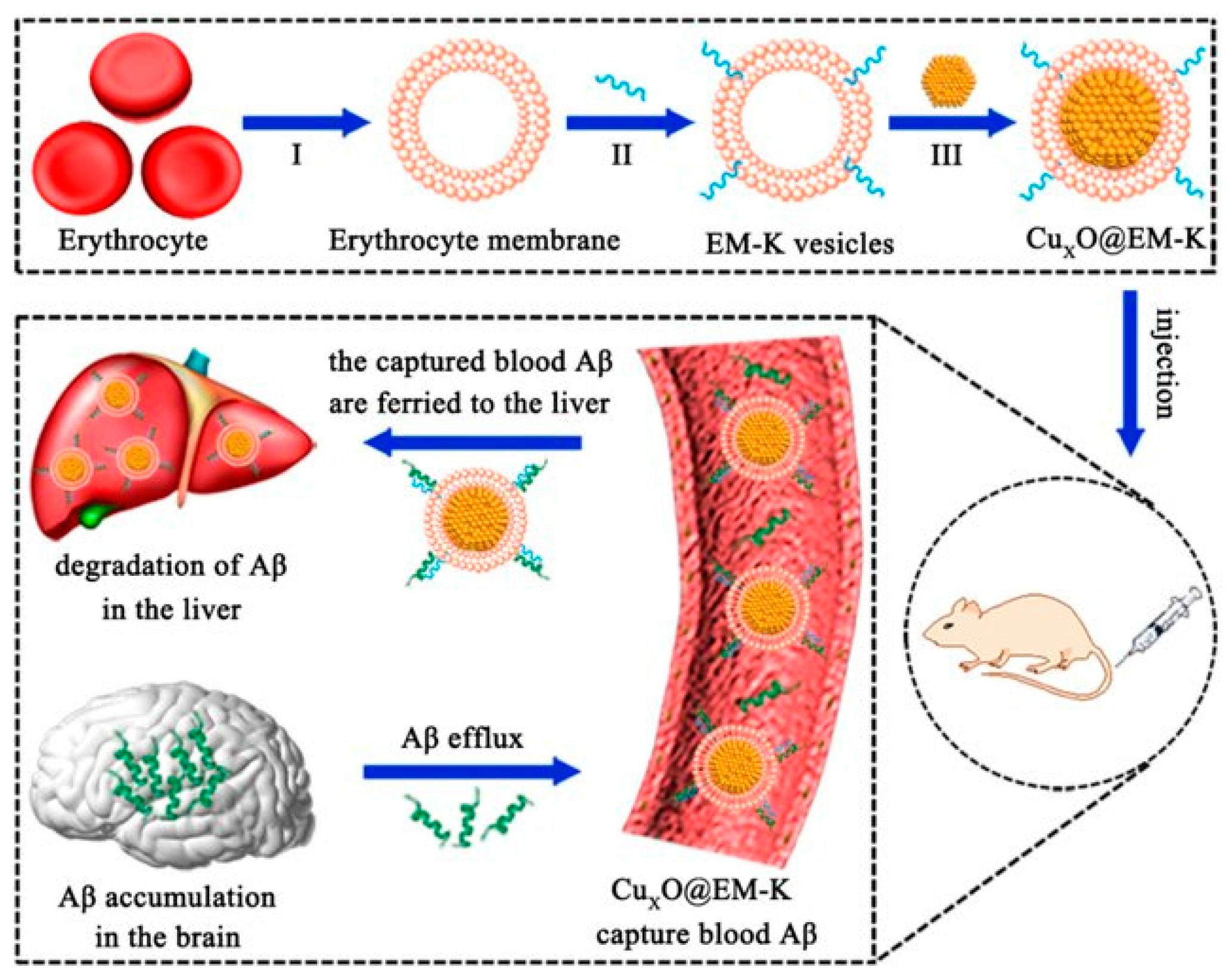
| CuxO@EM-K | SOD, CAT | 3× Tg-AD mice | ROS↓; reduces Aβ load; ameliorates memory deficits | [130] | |
| Nb2C MXenzyme | SOD, CAT | APP/PS1 Transgenic mice | ROS↓; capture Cu2+; decreases Aβ deposits; and alleviates mitochondrial and neuroglial damage while improving cognitive deficits. | [3] | |
| KLVFF@Au-CeO2 | SOD, CAT | APP/PS1 Transgenic mice | ROS↓; inhibits Aβ aggregation and degrade Aβ fibril; improve the cognitive function | [131] | |
| Parkinson’s disease | Mnf | SOD, CAT, and GPx | 1-methyl-4-phenylpyridinium (MPP+) induced PD-like cellular model | ROS↓; inhibits caspases-3/7 activation, providing neuroprotection | [132] |
| PBzyme | SOD, CAT | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model of mice. | ROS↓; NLRP3 inflammasomes↓; caspase-1↓; GSDMD↓; protects dopaminergic neurons, alleviates motor deficits, and mitigates mitochondrial damage | [133] | |
| Lf-Au-Bi2Se3 | SOD, CAT, and GPx | MPTP-induced PD model of mice | ROS↓; enhances memory and mobility, protects mitochondria, and prevents loss of dopaminergic neurons in substantia nigra pars compacta | [134] | |
| 2D V2C MXenzyme | SOD, CAT, and GPx | MPTP-induced PD model of mice | ROS↓; tyrosine hydroxylase↑; IBA-1↓; inhibits inflammation | [135] | |
| S/Ce-PABMS | SOD, CAT, and GPx | MPTP-induced PD model of mice | ROS↓; IL-10↑; IL-1β↓; inhibits inflammation; reduces α-synuclein aggregation, and improves motor coordination | [136] | |
| Huntington’s disease | BNPs | SOD | mHTT deposits induced cell model | Captures Cu2+; reduces mitochondria oxidative stress, disaggregating mutant huntingtin proteins. | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duță, C.; Dogaru, C.B.; Muscurel, C.; Stoian, I. Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 3522. https://doi.org/10.3390/ijms26083522
Duță C, Dogaru CB, Muscurel C, Stoian I. Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(8):3522. https://doi.org/10.3390/ijms26083522
Chicago/Turabian StyleDuță, Carmen, Carmen Beatrice Dogaru, Corina Muscurel, and Irina Stoian. 2025. "Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 8: 3522. https://doi.org/10.3390/ijms26083522
APA StyleDuță, C., Dogaru, C. B., Muscurel, C., & Stoian, I. (2025). Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(8), 3522. https://doi.org/10.3390/ijms26083522







