Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly
Abstract
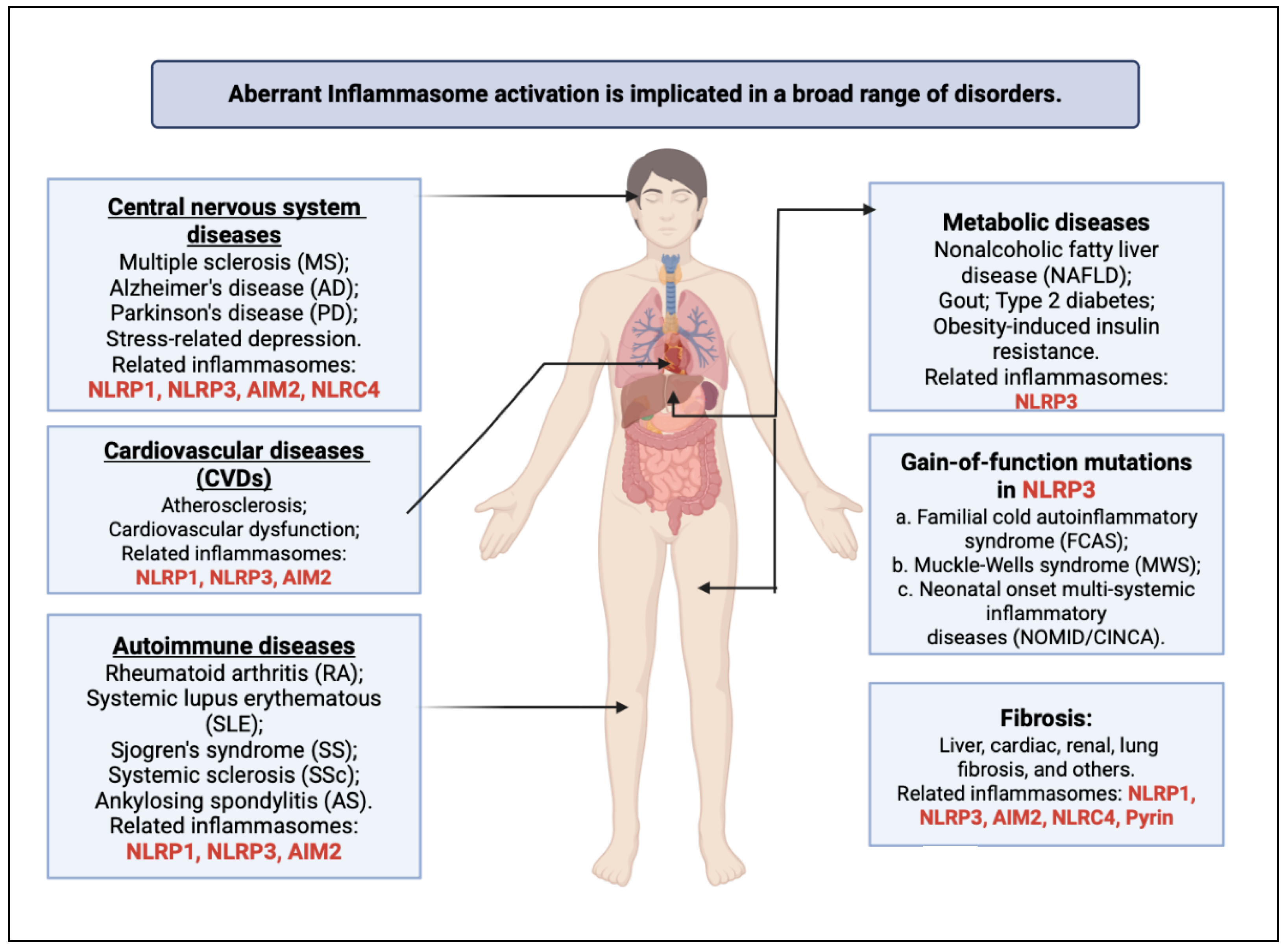
1. Introduction
2. Inflammasome Structure and Activation Pathways
2.1. Signal 1: Priming
2.2. Signal 2: Activation
2.3. Other Inflammasome Pathways
3. The Role of Inflammasomes in Cardio-Rheumatic Diseases
3.1. Familial Mediterranean Fever
3.2. Rheumatic Heart Disease
3.3. Kawasaki Disease
3.4. Ankylosing Spondylitis
3.5. Rheumatoid Arthritis
| Disease | Inflammasome/Related Protein and Pathway | References |
|---|---|---|
| Familial Mediterranean fever | Pyrin and IL-1β: FMF is characterized by mutations in the MEFV gene encoding pyrin. These mutations result in a defective pyrin protein that cannot properly regulate IL-1β production, leading to excessive inflammation and episodes of fever. Pyrin dysfunction is a primary driver of the disease’s autoinflammatory episodes. | [95,96] |
| Rheumatic heart disease | NLRP3 inflammasome and Streptococcus-triggered responses: in RHD, the immune response to Streptococcus bacteria can aberrantly activate NLRP3 inflammasomes. This activation contributes to inflammation and subsequent damage to heart valves. | [59,97] |
| Kawasaki disease | NLRP3 inflammasome: this complex plays a critical role in activating IL-1β, a pro-inflammatory cytokine. Its activation in Kawasaki disease is associated with the severe inflammation seen in blood vessels, which is central to the disease’s pathology. | [78,79] |
| Ankylosing spondylitis | NLRP3, IL-1β, and IL-18: ankylosing spondylitis is associated with dysregulation of the inflammasome pathway, particularly the NLRP3 inflammasome, leading to increased production of pro-inflammatory cytokines such as interleukin-1β and IL-18, contributing to the chronic inflammation and spinal involvement characteristic of the disease. Targeting the inflammasome pathway is a focus of research for potential therapeutic interventions in ankylosing spondylitis. | [52,53,56] |
| Rheumatoid arthritis | Immune dysregulation in RA activates inflammatory pathways and pro-inflammatory cytokines like TNF-α, IL-1, and IL-6, causing synovial hyperplasia and cartilage destruction. RA also impacts cardiovascular health, increasing the risk of CVDs due to systemic inflammation. The NLRP3 inflammasome is a key mediator in RA inflammation, and inhibiting it reduces disease severity. NRF2, a transcription factor, downregulates NLRP3 and protects joint cells from oxidative stress. Promising NRF2 activators like RTA 408, bardoxolone methyl, sulforaphane, curcumin, and EGCG are in development. | [83,90,91,92,93,94] |
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALR | Absent In Melanoma 2-Like Receptor |
| ARF | Acute Rheumatic Fever |
| AS | Ankylosing Spondylitis |
| CAD | Coronary Artery Disease |
| CVD | Cardiovascular Disease |
| CARD | Caspase Activation and Recruitment Domain |
| DAMP | Damage-Associated Molecular Patterns |
| EC | Endothelial Cell |
| FMF | Familial Mediterranean Fever |
| IFN | Interferon |
| IL | Interleukin |
| KD | Kawasaki Disease |
| miR | microRNA |
| MI | Myocardial Infarction |
| NLR | Nucleotide-Binding Domain-Like Receptor |
| NF | Nuclear Factor |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| NK | Natural Killer |
| oxLDL | Oxidized Low-Density Lipoprotein |
| PAMP | Pathogen-Associated Molecular Patterns |
| PRR | Pattern Recognition Receptor |
| ROS | Reactive Oxygen Species |
| RHD | Rheumatic Heart Disease |
| RA | Rheumatoid Arthritis |
| SLE | Systemic Lupus Erythematosus |
| TLR | Toll-Like Receptors |
References
- Mason, J.C.; Libby, P. Cardiovascular Disease in Patients with Chronic Inflammation: Mechanisms Underlying Premature Cardiovascular Events in Rheumatologic Conditions. Eur. Heart J. 2015, 36, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Garshick, M.; Liao, K.P.; Di Carli, M. Sore, Hot, and at Risk: The Emerging Specialty of Cardio-Rheumatology. J. Am. Heart Assoc. 2023, 12, e027846. [Google Scholar] [CrossRef]
- Yang, C.-A.; Chiang, B.-L. Inflammasomes and Childhood Autoimmune Diseases: A Review of Current Knowledge. Clin. Rev. Allergy Immunol. 2021, 61, 156–170. [Google Scholar] [CrossRef]
- Conrad, N.; Verbeke, G.; Molenberghs, G.; Goetschalckx, L.; Callender, T.; Cambridge, G.; Mason, J.C.; Rahimi, K.; McMurray, J.J.V.; Verbakel, J.Y. Autoimmune Diseases and Cardiovascular Risk: A Population-Based Study on 19 Autoimmune Diseases and 12 Cardiovascular Diseases in 22 Million Individuals in the UK. Lancet 2022, 400, 733–743. [Google Scholar] [CrossRef]
- Eder, L.; Harvey, P. Cardio-Rheumatology: It’s Time to Collaborate. Nat. Rev. Rheumatol. 2022, 18, 247–248. [Google Scholar] [CrossRef]
- Wiseman, S.J.; Ralston, S.H.; Wardlaw, J.M. Cerebrovascular Disease in Rheumatic Diseases: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 943–950. [Google Scholar] [CrossRef]
- Dong, C.; Fu, T.; Ji, J.; Li, Z.; Gu, Z. The Role of Interleukin-4 in Rheumatic Diseases. Clin. Exp. Pharmacol. Physiol. 2018, 45, 747–754. [Google Scholar] [CrossRef]
- Mohan, C.; Assassi, S. Biomarkers in Rheumatic Diseases: How Can They Facilitate Diagnosis and Assessment of Disease Activity? BMJ 2015, 351, h5079. [Google Scholar] [CrossRef]
- So, A.; Ives, A.; Joosten, L.A.B.; Busso, N. Targeting Inflammasomes in Rheumatic Diseases. Nat. Rev. Rheumatol. 2013, 9, 391–399. [Google Scholar] [CrossRef]
- Zhou, K.; Shi, L.; Wang, Y.; Chen, S.; Zhang, J. Recent Advances of the NLRP3 Inflammasome in Central Nervous System Disorders. J. Immunol. Res. 2016, 2016, 9238290. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Thaiss, C.A.; Flavell, R.A. Inflammasomes and Metabolic Disease. Annu. Rev. Physiol. 2014, 76, 57–78. [Google Scholar] [CrossRef]
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and Autoimmune and Rheumatic Diseases: A Comprehensive Review. J. Autoimmun. 2019, 103, 102299. [Google Scholar] [CrossRef]
- Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Shah, B.; Bohra, M.; Singh, U.; Jagtap, P.; Baidya, F.; Kalia, K.; et al. The Role of Inflammasomes in Atherosclerosis and Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4234–4245. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, K.; Zhu, L. Emerging Roles of Inflammasomes in Cardiovascular Diseases. Front. Immunol. 2022, 13, 834289. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Leal, V.N.C.; Souza De Lima, D.; Reis, E.C.; Pontillo, A. Inflammasome Genetics and Complex Diseases: A Comprehensive Review. Eur. J. Hum. Genet. 2020, 28, 1307–1321. [Google Scholar] [CrossRef]
- Yi, Y.-S. Flavonoids: Nutraceuticals for Rheumatic Diseases via Targeting of Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 488. [Google Scholar] [CrossRef]
- De Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef]
- Molla, M.D.; Akalu, Y.; Geto, Z.; Dagnew, B.; Ayelign, B.; Shibabaw, T. Role of Caspase-1 in the Pathogenesis of Inflammatory-Associated Chronic Noncommunicable Diseases. J. Inflamm. Res. 2020, 13, 749–764. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Yang, G.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Inflammasomes and Their Roles in Arthritic Disease Pathogenesis. Front. Mol. Biosci. 2022, 9, 1027917. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, Z.; Yang, X.; Ji, N.; Wang, Y.; Shi, Q.; Li, M.; Zhang, J.; Zhu, Y. The NLRP3 Inflammasome: Multiple Activation Pathways and Its Role in Primary Cells during Ventricular Remodeling. J. Cell Physiol. 2021, 236, 5547–5563. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Winsor, N.; Krustev, C.; Bruce, J.; Philpott, D.J.; Girardin, S.E. Canonical and Noncanonical Inflammasomes in Intestinal Epithelial Cells. Cell Microbiol. 2019, 21, e13079. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The Inflammasome: A Caspase-1-Activation Platform That Regulates Immune Responses and Disease Pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Wu, J.; Dong, E.; Zhang, Y.; Xiao, H. The Role of the Inflammasome in Heart Failure. Front. Physiol. 2021, 12, 709703. [Google Scholar] [CrossRef]
- Takahashi, M. Cell-Specific Roles of NLRP3 Inflammasome in Myocardial Infarction. J. Cardiovasc. Pharmacol. 2019, 74, 188–193. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Kanneganti, T.-D. Mechanisms Governing Inflammasome Activation, Assembly and Pyroptosis Induction. Int. Immunol. 2017, 29, 201–210. [Google Scholar] [CrossRef]
- Akther, M.; Haque, M.E.; Park, J.; Kang, T.-B.; Lee, K.-H. NLRP3 Ubiquitination-A New Approach to Target NLRP3 Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 8780. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, L.; Dong, N.; Li, F. NLRP3 Inflammasome: The Rising Star in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 927061. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Que, X.; Zheng, S.; Song, Q.; Pei, H.; Zhang, P. Fantastic Voyage: The Journey of NLRP3 Inflammasome Activation. Genes. Dis. 2024, 11, 819–829. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 Inflammasome: Activation and Regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Burnstock, G. P2X Ion Channel Receptors and Inflammation. Purinergic Signal 2016, 12, 59–67. [Google Scholar] [CrossRef]
- Lima, H.; Jacobson, L.S.; Goldberg, M.F.; Chandran, K.; Diaz-Griffero, F.; Lisanti, M.P.; Brojatsch, J. Role of Lysosome Rupture in Controlling Nlrp3 Signaling and Necrotic Cell Death. Cell Cycle 2013, 12, 1868–1878. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, J.; Shen, Q.; Li, M.; Peng, Y. Mitochondrial Dysfunction Induces NLRP3 Inflammasome Activation during Cerebral Ischemia/Reperfusion Injury. J. Neuroinflamm. 2018, 15, 242. [Google Scholar] [CrossRef]
- Keshavarz-Bahaghighat, H.; Darwesh, A.M.; Sosnowski, D.K.; Seubert, J.M. Mitochondrial Dysfunction and Inflammaging in Heart Failure: Novel Roles of CYP-Derived Epoxylipids. Cells 2020, 9, 1565. [Google Scholar] [CrossRef]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Singh, A.; Patil, S.; Dhiman, R.; et al. Mitochondrial Dysfunction as a Driver of NLRP3 Inflammasome Activation and Its Modulation through Mitophagy for Potential Therapeutics. Int. J. Biochem. Cell Biol. 2021, 136, 106013. [Google Scholar] [CrossRef]
- Hamzeh, O.; Rabiei, F.; Shakeri, M.; Parsian, H.; Saadat, P.; Rostami-Mansoor, S. Mitochondrial Dysfunction and Inflammasome Activation in Neurodegenerative Diseases: Mechanisms and Therapeutic Implications. Mitochondrion 2023, 73, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kang, I. Advances in Disease Mechanisms and Translational Technologies: Clinicopathologic Significance of Inflammasome Activation in Autoimmune Diseases. Arthritis Rheumatol. 2020, 72, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 Priming in Inflammaging and Age Related Diseases. Cytokine Growth Factor. Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Xia, S.; Hollingsworth, L.R.; Wu, H. Mechanism and Regulation of Gasdermin-Mediated Cell Death. Cold Spring Harb. Perspect. Biol. 2020, 12, a036400. [Google Scholar] [CrossRef]
- Devant, P.; Kagan, J.C. Molecular Mechanisms of Gasdermin D Pore-Forming Activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Bürckstümmer, T.; Baumann, C.; Blüml, S.; Dixit, E.; Dürnberger, G.; Jahn, H.; Planyavsky, M.; Bilban, M.; Colinge, J.; Bennett, K.L.; et al. An Orthogonal Proteomic-Genomic Screen Identifies AIM2 as a Cytoplasmic DNA Sensor for the Inflammasome. Nat. Immunol. 2009, 10, 266–272. [Google Scholar] [CrossRef]
- Jin, T.; Curry, J.; Smith, P.; Jiang, J.; Xiao, T.S. Structure of the NLRP1 Caspase Recruitment Domain Suggests Potential Mechanisms for Its Association with Procaspase-1. Proteins 2013, 81, 1266–1270. [Google Scholar] [CrossRef]
- Isiadinso, I. RESPONSE: Collaboration Is the Key in Cardio-Rheumatology. J. Am. Coll. Cardiol. 2020, 75, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Singh, J.A.; Veras, M.M.S.; Tanjong Ghogomu, E.; Benkhalti Jandu, M.; Tugwell, P.; Wells, G.A. TNF-Alpha Inhibitors for Ankylosing Spondylitis. Cochrane Database Syst. Rev. 2015, 2015, CD005468. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Guilherme, L.; Ramasawmy, R.; Kalil, J. Rheumatic Fever and Rheumatic Heart Disease: Genetics and Pathogenesis. Scand. J. Immunol. 2007, 66, 199–207. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 Activator RTA-408 Attenuates Osteoclastogenesis by Inhibiting STING Dependent NF-Κb Signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galán, J.E.; Askenase, P.W.; et al. Critical Role for NALP3/CIAS1/Cryopyrin in Innate and Adaptive Immunity through Its Regulation of Caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Guilherme, L. Acute Rheumatic Fever. Lancet 2018, 392, 161–174. [Google Scholar] [CrossRef]
- Vahini, B.; Narenthiran, C.K.; Chandrasekar, K. Rheumatic Heart Disease in Indian Paediatrics: A Review. J. Pharm. Res. Int. 2021, 33, 27–33. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 Inflammasome Activation: The Convergence of Multiple Signalling Pathways on ROS Production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Eyal, S.; Weizman, A.; Toren, P.; Dor, Y.; Mester, R.; Rehavi, M. Chronic GnRH Agonist Administration Down-Regulates Platelet Serotonin Transporter in Women Undergoing Assisted Reproductive Treatment. Psychopharmacology 1996, 125, 141–145. [Google Scholar] [CrossRef]
- Toor, D.; Sharma, N. T Cell Subsets: An Integral Component in Pathogenesis of Rheumatic Heart Disease. Immunol. Res. 2018, 66, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Sikder, S.; Williams, N.L.; Sorenson, A.E.; Alim, M.A.; Vidgen, M.E.; Moreland, N.J.; Rush, C.M.; Simpson, R.S.; Govan, B.L.; Norton, R.E.; et al. Group G Streptococcus Induces an Autoimmune Carditis Mediated by Interleukin 17A and Interferon γ in the Lewis Rat Model of Rheumatic Heart Disease. J. Infect. Dis. 2018, 218, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Heithoff, D.M.; Aziz, P.V.; Sperandio, M.; Nizet, V.; Mahan, M.J.; Marth, J.D. Recurrent Infection Progressively Disables Host Protection against Intestinal Inflammation. Science 2017, 358, eaao5610. [Google Scholar] [CrossRef]
- Abdelmoula, B.; Bouayed Abdelmoula, N. Chronic Activation of Inflammasome Signaling Complexes and Enhancement of Behavioral Abnormalities. Eur. Psychiatr. 2023, 66, S333–S334. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial Dysfunction and Oxidative Stress Activate Inflammasomes: Impact on the Aging Process and Age-Related Diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef]
- Lindholm, D.E.; Whiteman, I.J.; Oliver, J.; Cheung, M.M.H.; Hope, S.A.; Brizard, C.P.; Horton, A.E.; Sheridan, B.; Hardy, M.; Osowicki, J.; et al. Acute Rheumatic Fever and Rheumatic Heart Disease in Children and Adolescents in Victoria, Australia. J. Paediatr. Child Health 2023, 59, 352–359. [Google Scholar] [CrossRef]
- Aguilera, M.; Darby, T.; Melgar, S. The Complex Role of Inflammasomes in the Pathogenesis of Inflammatory Bowel Diseases—Lessons Learned from Experimental Models. Cytokine Growth Factor. Rev. 2014, 25, 715–730. [Google Scholar] [CrossRef]
- Maltez, V.I.; Miao, E.A. Reassessing the Evolutionary Importance of Inflammasomes. J. Immunol. 2016, 196, 956–962. [Google Scholar] [CrossRef]
- LaRock, C.N.; Nizet, V. Inflammasome/IL-1β Responses to Streptococcal Pathogens. Front. Immunol. 2015, 6, 518. [Google Scholar] [CrossRef]
- Johnston, J.B.; Rahman, M.M.; McFadden, G. Strategies That Modulate Inflammasomes—Insights from Host–Pathogen Interactions. Semin. Immunopathol. 2007, 29, 261–274. [Google Scholar] [CrossRef]
- Bortolotti, P.; Faure, E.; Kipnis, E. Inflammasomes in Tissue Damages and Immune Disorders After Trauma. Front. Immunol. 2018, 9, 1900. [Google Scholar] [CrossRef]
- Hara, T.; Nakashima, Y.; Sakai, Y.; Nishio, H.; Motomura, Y.; Yamasaki, S. Kawasaki Disease: A Matter of Innate Immunity. Clin. Exp. Immunol. 2016, 186, 134–143. [Google Scholar] [CrossRef]
- Principi, N.; Rigante, D.; Esposito, S. The Role of Infection in Kawasaki Syndrome. J. Infect. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Burns, J.C. Kawasaki Disease. J. Am. Coll. Cardiol. 2016, 67, 1738–1749. [Google Scholar] [CrossRef]
- Shahi, A.; Afzali, S.; Firoozi, Z.; Mohaghegh, P.; Moravej, A.; Hosseinipour, A.; Bahmanyar, M.; Mansoori, Y. Potential Roles of NLRP3 Inflammasome in the Pathogenesis of Kawasaki Disease. J. Cell Physiol. 2023, 238, 513–532. [Google Scholar] [CrossRef]
- Christ, A.; Latz, E. Deciphering How NLRP3 Incites the Stromal Response in Kawasaki Vasculitis. Circ. Res. 2021, 129, 840–842. [Google Scholar] [CrossRef]
- Anzai, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Karasawa, T.; Komada, T.; Nakamura, J.; Nagi-Miura, N.; Ohno, N.; Takeishi, Y.; et al. Crucial Role of NLRP3 Inflammasome in a Murine Model of Kawasaki Disease. J. Mol. Cell. Cardiol. 2020, 138, 185–196. [Google Scholar] [CrossRef]
- Gupta, A. Role of Inflammasomes in Kawasaki Disease. Indian J. Pediatr. 2023, 90, 5–6. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H.; et al. Endothelial Cell Pyroptosis Plays an Important Role in Kawasaki Disease via HMGB1/RAGE/Cathespin B Signaling Pathway and NLRP3 Inflammasome Activation. Cell Death Dis. 2019, 10, 778. [Google Scholar] [CrossRef]
- Burridge, P.W.; Diecke, S.; Matsa, E.; Sharma, A.; Wu, H.; Wu, J.C. Modeling Cardiovascular Diseases with Patient-Specific Human Pluripotent Stem Cell-Derived Cardiomyocytes. Methods Mol. Biol. 2016, 1353, 119–130. [Google Scholar] [CrossRef]
- Lamata, P.; Casero, R.; Carapella, V.; Niederer, S.A.; Bishop, M.J.; Schneider, J.E.; Kohl, P.; Grau, V. Images as Drivers of Progress in Cardiac Computational Modelling. Prog. Progress. Biophys. Mol. Biol. 2014, 115, 198–212. [Google Scholar] [CrossRef]
- Nandi, D.; Farid, N.S.S.; Karuppiah, H.A.R.; Kulkarni, A. Imaging Approaches to Monitor Inflammasome Activation. J. Mol. Biol. 2022, 434, 167251. [Google Scholar] [CrossRef]
- Manda, G.; Milanesi, E.; Genc, S.; Niculite, C.M.; Neagoe, I.V.; Tastan, B.; Dragnea, E.M.; Cuadrado, A. Pros and Cons of NRF2 Activation as Adjunctive Therapy in Rheumatoid Arthritis. Free Radic. Biol. Med. 2022, 190, 179–201. [Google Scholar] [CrossRef]
- Ranganathan, V.; Gracey, E.; Brown, M.A.; Inman, R.D.; Haroon, N. Pathogenesis of Ankylosing Spondylitis—Recent Advances and Future Directions. Nat. Rev. Rheumatol. 2017, 13, 359–367. [Google Scholar] [CrossRef]
- Gao, X.; Enten, G.A.; DeSantis, A.J.; Volkman, B.F.; Gaponenko, V.; Majetschak, M. Characterization of Heteromeric Complexes between Chemokine (C-X-C Motif) Receptor 4 and A1-Adrenergic Receptors Utilizing Intermolecular Bioluminescence Resonance Energy Transfer Assays. Biochem. Biophys. Res. Commun. 2020, 528, 368–375. [Google Scholar] [CrossRef]
- Jahid, M.; Khan, K.U.; Ahmed, R.S. Overview of Rheumatoid Arthritis and Scientific Understanding of the Disease. Mediterr. J. Rheumatol. 2023, 34, 284–291. [Google Scholar] [CrossRef]
- Jonsson, A.H. Synovial Tissue Insights into Heterogeneity of Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2024, 26, 81–88. [Google Scholar] [CrossRef]
- Park, E.; Bathon, J. Cardiovascular Complications of Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2024, 36, 209–216. [Google Scholar] [CrossRef]
- Son, C.-N.; Bang, S.-Y.; Kim, J.H.; Choi, C.-B.; Kim, T.-H.; Jun, J.-B. Caspase-1 Level in Synovial Fluid Is High in Patients with Spondyloarthropathy but Not in Patients with Gout. J. Korean Med. Sci. 2013, 28, 1289–1292. [Google Scholar] [CrossRef]
- Tastan, B.; Arioz, B.I.; Genc, S. Targeting NLRP3 Inflammasome with Nrf2 Inducers in Central Nervous System Disorders. Front. Immunol. 2022, 13, 865772. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Liu, W.; Lv, X.; Wang, B.; Hu, B.; Shao, Z. Bardoxolone Methyl Breaks the Vicious Cycle between M1 Macrophages and Senescent Nucleus Pulposus Cells through the Nrf2/STING/NF-κB Pathway. Int. Immunopharmacol. 2024, 127, 111262. [Google Scholar] [CrossRef]
- Rajabi, S.; Darroudi, M.; Naseri, K.; Farkhondeh, T.; Samarghandian, S. Protective Effects of Curcumin and Its Analogues via the Nrf2 Pathway in Metabolic Syndrome. Curr. Med. Chem. 2023, 31, 3966–3976. [Google Scholar] [CrossRef]
- Ribeiro, M.; Alvarenga, L.; Coutinho-Wolino, K.S.; Nakao, L.S.; Cardozo, L.F.; Mafra, D. Sulforaphane Upregulates the mRNA Expression of NRF2 and NQO1 in Non-Dialysis Patients with Chronic Kidney Disease. Free Radic. Biol. Med. 2024, 221, 181–187. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Y.; Chen, D.; Reddy, M.B. The Protection of EGCG Against 6-OHDA-Induced Oxidative Damage by Regulating PPARγ and Nrf2/HO-1 Signaling. Nutr. Metab. Insights 2024, 17, 11786388241253436. [Google Scholar] [CrossRef]
- Chae, J.J.; Komarow, H.D.; Cheng, J.; Wood, G.; Raben, N.; Liu, P.P.; Kastner, D.L. Targeted Disruption of Pyrin, the FMF Protein, Causes Heightened Sensitivity to Endotoxin and a Defect in Macrophage Apoptosis. Mol. Cell 2003, 11, 591–604. [Google Scholar] [CrossRef]
- Ozen, S.; Bilginer, Y. A Clinical Guide to Autoinflammatory Diseases: Familial Mediterranean Fever and next-of-Kin. Nat. Rev. Rheumatol. 2014, 10, 135–147. [Google Scholar] [CrossRef]
- Arvind, B.; Ramakrishnan, S. Rheumatic Fever and Rheumatic Heart Disease in Children. Indian J. Pediatr. 2020, 87, 305–311. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, F.; Abdulla, M.; Retnowati, F.D.; Al-Khawaga, H.; Alhiraky, H.; Al-Harbi, K.M.; Al-Haidose, A.; Maayah, Z.H.; Abdallah, A.M. Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly. Int. J. Mol. Sci. 2025, 26, 3520. https://doi.org/10.3390/ijms26083520
Issa F, Abdulla M, Retnowati FD, Al-Khawaga H, Alhiraky H, Al-Harbi KM, Al-Haidose A, Maayah ZH, Abdallah AM. Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly. International Journal of Molecular Sciences. 2025; 26(8):3520. https://doi.org/10.3390/ijms26083520
Chicago/Turabian StyleIssa, Farah, Marah Abdulla, Faizah D. Retnowati, Huda Al-Khawaga, Hanin Alhiraky, Khalid M. Al-Harbi, Amal Al-Haidose, Zaid H. Maayah, and Atiyeh M. Abdallah. 2025. "Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly" International Journal of Molecular Sciences 26, no. 8: 3520. https://doi.org/10.3390/ijms26083520
APA StyleIssa, F., Abdulla, M., Retnowati, F. D., Al-Khawaga, H., Alhiraky, H., Al-Harbi, K. M., Al-Haidose, A., Maayah, Z. H., & Abdallah, A. M. (2025). Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly. International Journal of Molecular Sciences, 26(8), 3520. https://doi.org/10.3390/ijms26083520