Investigating Morphological and Physiological Responses to Stress in Begonia semperflorens
Abstract
1. Introduction
2. Results
2.1. Leaf Thickness, Fresh and Dry Weight of Four Wax Begonia Genotypes Under Shaded and Non-Shaded Conditions
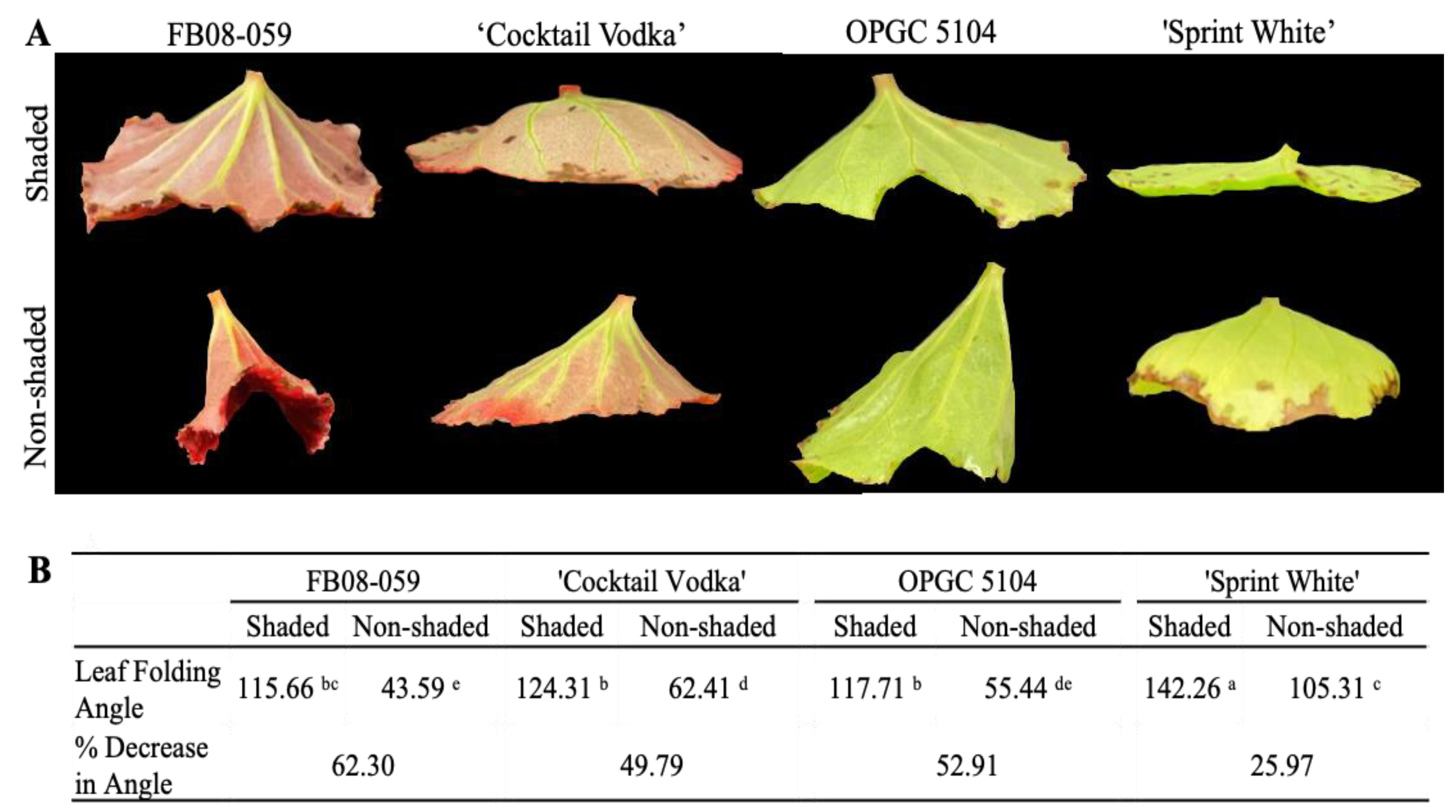
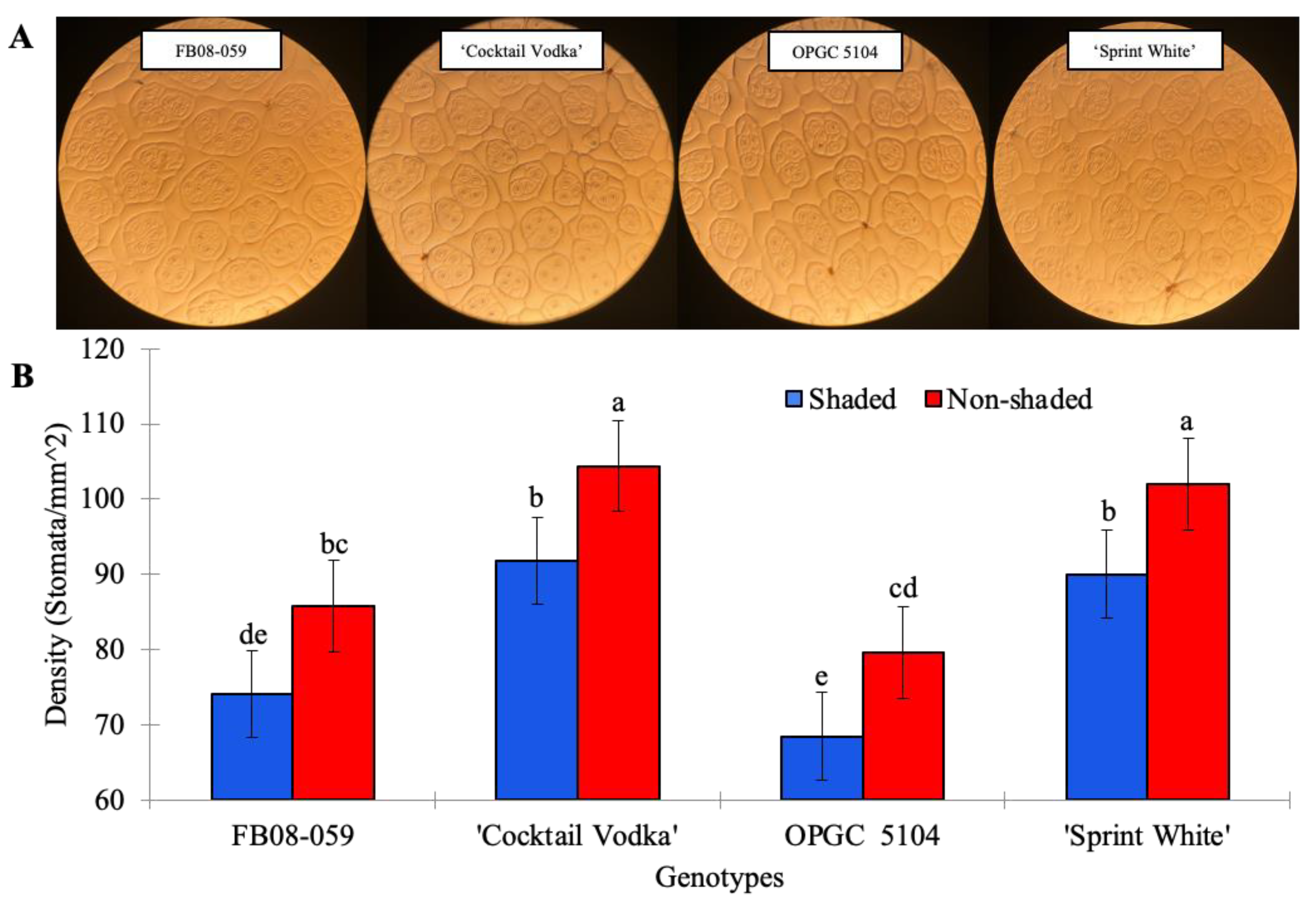
2.2. Leaf Folding and Stomatal Density of Four Wax Begonia Genotypes Under Shaded and Non-Shaded Conditions
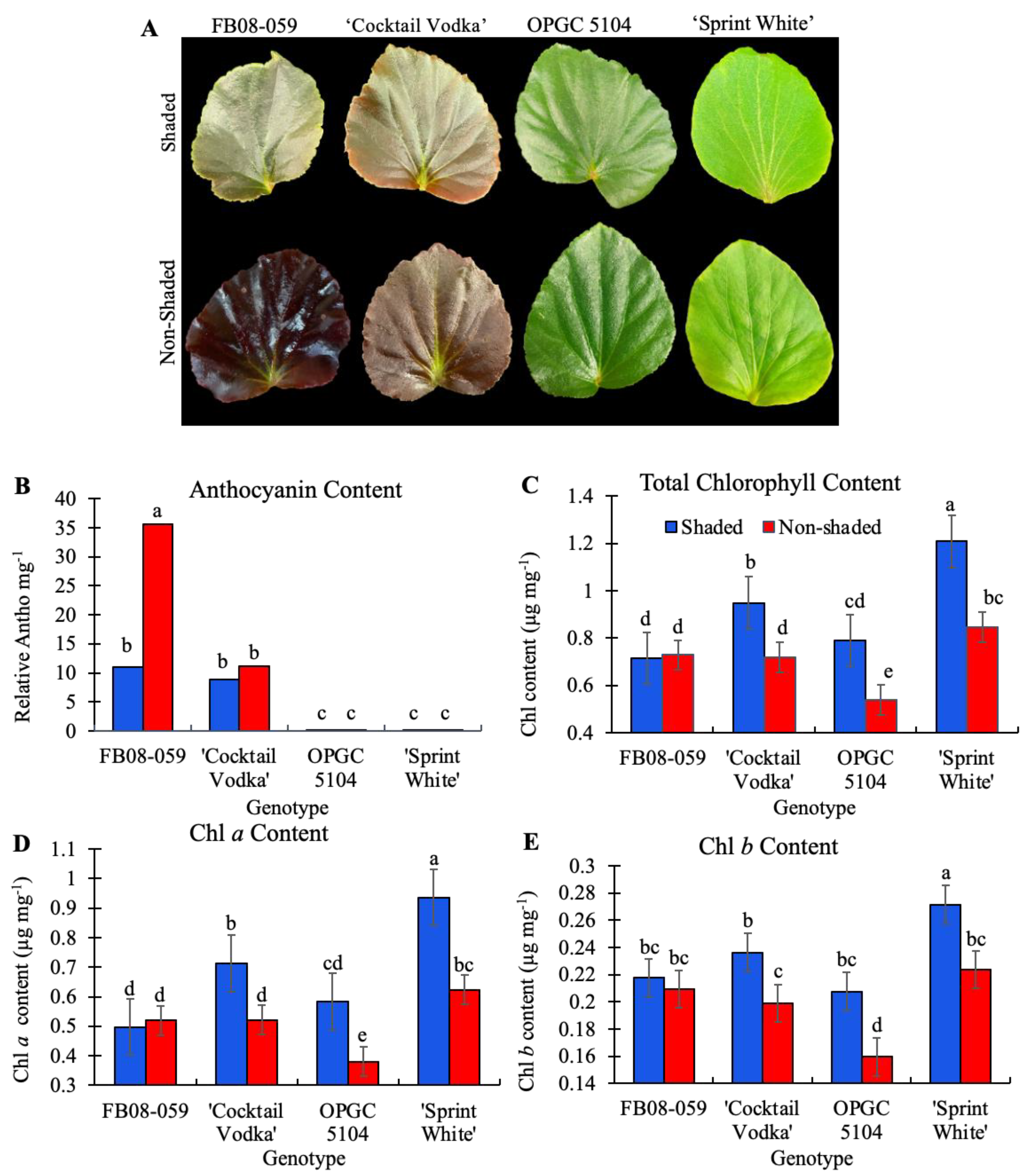
2.3. Photosynthetic Parameters, Chlorophyll and Anthocyanin Contents of Four Wax Begonia Genotypes Under Shaded and Non-Shaded Conditions
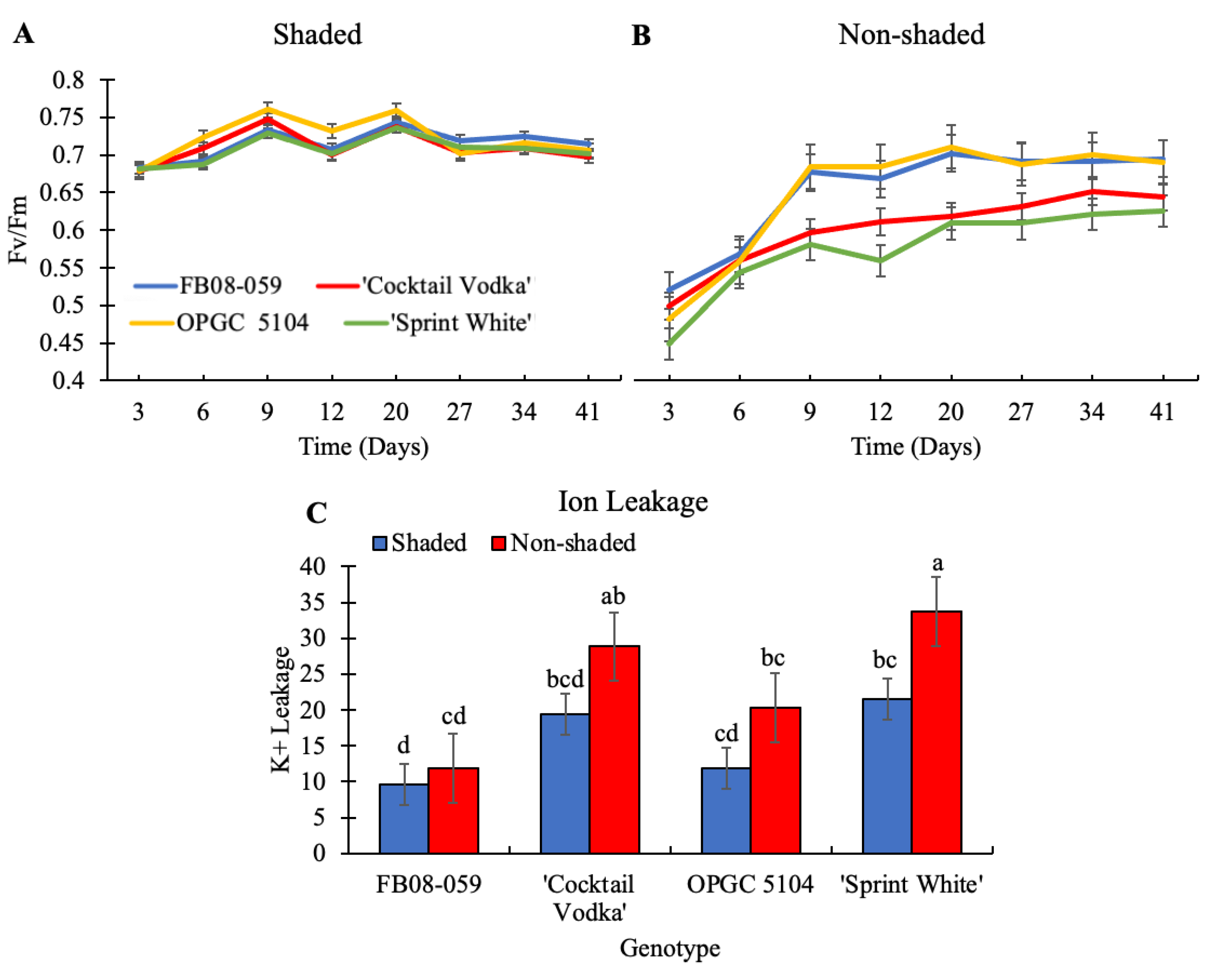
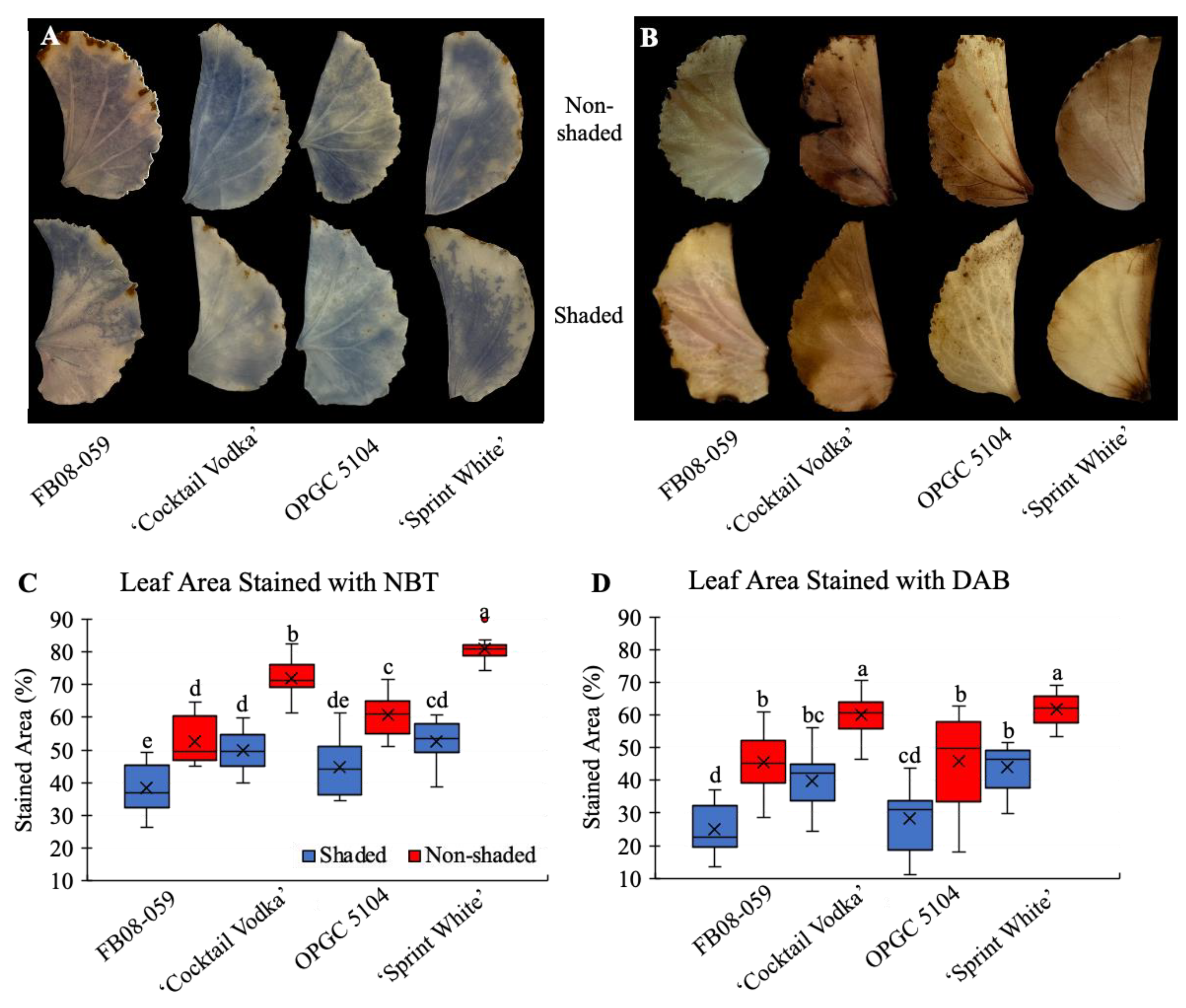
2.4. Photosynthetic Efficiency and Oxidative Stress Responses of Four Wax Begonia Genotypes Under Shaded and Non-Shaded Conditions
3. Discussion
3.1. Stomatal Density and Gas Exchange Regulation in Wax Begonia
3.2. Structural Adaptations: Leaf Folding and Cuticle Thickening in Wax Begonia
3.3. Anthocyanin Accumulation and Photoprotection in Wax Begonia
3.4. Oxidative Stress and Membrane Stability in Wax Begonia
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Morphological Measurements
4.3. Physiological Measurements
4.4. Measurements of Stress Responses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidner, C.; Neale, S.; Goodall-Copestake, W.; Kidner, C.A. The Evolution of Diversity in Begonia; Global Science Books: Bexhill-On-Sea, UK, 2014. [Google Scholar]
- Moonlight, P.W.; Richardson, J.E.; Tebbitt, M.C.; Thomas, D.C.; Hollands, R.; Peng, C.I.; Hughes, M. Continental-Scale Diversification Patterns in a Megadiverse Genus: The Biogeography of Neotropical Begonia. J. Biogeogr. 2015, 42, 1137–1149. [Google Scholar] [CrossRef]
- Moonlight, P.W.; Ardi, W.H.; Padilla, L.A.; Chung, K.F.; Fuller, D.; Girmansyah, D.; Hollands, R.; Jara-Muñoz, A.; Kiew, R.; Leong, W.C.; et al. Dividing and Conquering the Fastest-Growing Genus: Towards a Natural Sectional Classification of the Mega-Diverse Genus Begonia (Begoniaceae). Taxon 2018, 67, 267–323. [Google Scholar] [CrossRef]
- Ardi, W.H.; Thomas, D. Three new species of begonia from the outer islands of southeastern sulawesi. Edinb. J. Bot. 2023, 80, 1–17. [Google Scholar] [CrossRef]
- Hughes, M.; Girmansyah, D.; Ardi, W.H. Further Discoveries in the Ever-Expanding Genus Begonia (Begoniaceae): Fifteen New Species from Sumatra. Eur. J. Taxon. 2015, 2015, 1–140. [Google Scholar] [CrossRef][Green Version]
- Kang, J.-G.; Van Iersel, M.W. Managing Fertilization of Bedding Plants: A Comparison of Constant Fertilizer Concentrations versus Constant Leachate Electrical Conductivity. HortScience 2009, 44, 151–156. [Google Scholar] [CrossRef]
- Broschat, T.K.; Moore, K.A. Influence of Substrate and Fertilizer Analysis and Rate on Growth and Quality of Five Species of Bedding Plants. Horttechnology 2001, 11, 434–437. [Google Scholar] [CrossRef]
- Floriculture in 2022. Commercial Floriculture Survey. January 2023 (for 2022 Production). Available online: https://www.nass.usda.gov/Publications/Methodology_and_Data_Quality/Floriculture/07-2023/Floriculture_Questionnaire_CA_Jan2023.pdf (accessed on 14 February 2025).
- Christensen, J.H.; Christensen, O.B. A Summary of the PRUDENCE Model Projections of Changes in European Climate by the End of This Century. Clim. Change 2007, 81, 7–30. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Bourgault, R.; Matschi, S.; Vasquez, M.; Qiao, P.; Sonntag, A.; Charlebois, C.; Mohammadi, M.; Scanlon, M.J.; Smith, L.G.; Molina, I. Constructing Functional Cuticles: Analysis of Relationships between Cuticle Lipid Composition, Ultrastructure and Water Barrier Function in Developing Adult Maize Leaves. Ann. Bot. 2020, 125, 79–91. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an Ecological Context: The Remarkable Complexity of Thermal Energy Dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Nemali, K.S.; Van Iersel, M.W. Acclimation of Wax Begonia to Light Intensity: Changes in Photosynthesis, Respiration, and Chlorophyll Concentration. J. Am. Soc. Hortic. Sci. 2004, 129, 745–751. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. PHOTOPROTECTION REVISITED: Genetic and Molecular Approaches. Annu. Rev. Plant Biol. 2025, 50, 333–359. [Google Scholar] [CrossRef]
- Gould, K.S.; Neill, S.O.; Vogelmann, T.C. A Unified Explanation for Anthocyanins in Leaves? Adv. Bot. Res. 2002, 37, 167–192. [Google Scholar] [CrossRef]
- Gould, K.S.; Jay-Allemand, C.; Logan, B.A.; Baissac, Y.; Bidel, L.P.R. When Are Foliar Anthocyanins Useful to Plants? Re-Evaluation of the Photoprotection Hypothesis Using Arabidopsis Thaliana Mutants That Differ in Anthocyanin Accumulation. Environ. Exp. Bot. 2018, 154, 11–22. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Rahmati Ishka, M.; Julkowska, M. Tapping into the Plasticity of Plant Architecture for Increased Stress Resilience. F1000Res 2023, 12, 1257. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Casson, S.; Gray, J.E. Influence of Environmental Factors on Stomatal Development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of Leaf Photosynthetic Capacity through Increased Stomatal Density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Stomatal Control as a Driver of Plant Evolution. J. Exp. Bot. 2011, 62, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Pagay, V.; Zufferey, V.; Lakso, A.N. The Influence of Water Stress on Grapevine (Vitis vinifera L.) Shoots in a Cool, Humid Climate: Growth, Gas Exchange and Hydraulics. Funct. Plant Biol. 2016, 43, 827–837. [Google Scholar] [CrossRef]
- Hepworth, C.; Caine, R.S.; Harrison, E.L.; Sloan, J.; Gray, J.E. Stomatal Development: Focusing on the Grasses. Curr. Opin. Plant Biol. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Galmés, J.; Aranjuelo, I.; Medrano, H.; Flexas, J. Variation in Rubisco Content and Activity under Variable Climatic Factors. Photosynth. Res. 2013, 117, 73–90. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Abscisic Acid, Nitric Oxide and Stomatal Closure-Is Nitrate Reductase One of the Missing Links? Trends Plant Sci. 2003, 8, 20–26. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Roche, D. Stomatal Conductance Is Essential for Higher Yield Potential of C3 Crops. CRC Crit. Rev. Plant Sci. 2015, 34, 429–453. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2020, 9, 105. [Google Scholar] [CrossRef]
- Givnish, T.J. Comparative studies of leaf form: Assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol. 1987, 106, 131–160. [Google Scholar] [CrossRef]
- Vogel, S. Leaves in the Lowest and Highest Winds: Temperature, Force and Shape: Tansley Review. New Phytol. 2009, 183, 13–26. [Google Scholar] [CrossRef]
- O’toole, J.C.; Cruz, R.T. Response of Leaf Water Potential, Stomatal Resistance, and Leaf Rolling to Water Stress. Plant Physiol. 1980, 65, 428–432. [Google Scholar] [CrossRef]
- Shepherd, T.; Griffiths, D.W. The Effects of Stress on Plant Cuticular Waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Huerta-Espino, J.; Kehel, Z.; Autrique, E. Characterization of Heat-and Drought-Stress Tolerance in High-Yielding Spring Wheat. Crop Sci. 2015, 55, 1552–1562. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, L. Putative Megaenzyme DWA1 Plays Essential Roles in Drought Resistance by Regulating Stress-Induced Wax Deposition in Rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. BioMed Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Close, D.C.; Beaoce, C.L. The Ecophysiology of Foliar Anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in Metabolites of Purple Corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Blum, J.A.; Ma, F.; Wang, Y.; Borejsza-Wysocka, E.; Ma, F.; Cheng, L.; Li, P. Anthocyanin Accumulation Provides Protection against High Light Stress While Reducing Photosynthesis in Apple Leaves. Int. J. Mol. Sci. 2022, 23, 12616. [Google Scholar] [CrossRef]
- Zheng, X.T.; Yu, Z.C.; Tang, J.W.; Cai, M.L.; Chen, Y.L.; Yang, C.W.; Chow, W.S.; Peng, C.L. The Major Photoprotective Role of Anthocyanins in Leaves of Arabidopsis Thaliana under Long-Term High Light Treatment: Antioxidant or Light Attenuator? Photosynth. Res. 2021, 149, 25–40. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The Biosynthesis of Flavonoids Is Enhanced Similarly by UV Radiation and Root Zone Salinity in L. Vulgare Leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How Relevant Are Flavonoids as Antioxidants in Plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of Heat Stress Tolerance in Winter Wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Gashu, K.; Song, C.; Dubey, A.K.; Acuña, T.; Sagi, M.; Agam, N.; Bustan, A.; Fait, A. The Effect of Topo-Climate Variation on the Secondary Metabolism of Berries in White Grapevine Varieties (Vitis vinifera). Front. Plant Sci. 2022, 13, 847268. [Google Scholar] [CrossRef]
- Ginori, J.; Huo, H.; Wilson, S. Physiological Response of Wax Begonia to Heat and Light Stress. Int. Plant Propagator’s Soc. 2022, 72, 94–101. [Google Scholar]
- Weyers, J.D.B.; Johansen, L.G. Accurate estimation of stomatal aperture from silicone rubber impressions. New Phytol. 1985, 101, 109–115. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Anthocyanin Extraction Method and Sample Preparation Affect Anthocyanin Yield of Strawberries. Nat. Prod. Commun. 2022, 17, 1934578X221099970. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E. Plant Cold Acclimation Methods and Protocols Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2020. [Google Scholar]


| FB08-059 | “Cocktail Vodka” | OPGC 5104 | “Sprint White” | |||||
|---|---|---|---|---|---|---|---|---|
| Shaded | Non-Shaded | Shaded | Non-Shaded | Shaded | Non-Shaded | Shaded | Non-Shaded | |
| Total Leaf Thickness | 5.35 ± 0.09 b | 6.04 ± 0.08 a | 4.36 ± 0.20 d | 4.68 ± 0.12 cd | 4.59 ± 0.09 d | 5.22 ± 0.10 bc | 3.15 ± 0.15 e | 3.46 ± 0.08 e |
| Cuticle Thickness | 2.89 ± 0.06 b | 3.63 ± 0.10 a | 1.86 ± 0.09 d | 2.16 ± 0.05 cd | 2.18 ± 0.06 c | 2.61 ± 0.06 b | 1.3 ± 0.06 e | 1.51 ± 0.05 e |
| Mesophyll Thickness | 2.47 ± 0.05 | 2.41 ± 0.11 | 2.50 ± 0.13 | 2.53 ± 0.09 | 2.41 ± 0.07 | 2.6 ± 0.11 | 1.85 ± 0.11 | 1.95 ± 0.08 |
| Cuticle/Total Leaf Ratio | 0.54 ± 0.01 b | 0.61 ± 0.02 a | 0.43 ± 0.01 de | 0.46 ± 0.01 cde | 0.48 ± 0.01 cd | 0.50 ± 0.01 bc | 0.42 ± 0.01 e | 0.43 ± 0.02 de |
| Shoot FW (g) | 356.87 ± 20.85 a | 177.09 ± 16.60 cd | 180.53 ± 9.55 cd | 109.91 ± 9.74 e | 284.11 ± 11.78 b | 135.61 ± 7.09 cde | 194.68 ± 15.82 c | 119.08 ± 13.95 de |
| Shoot DW (g) | 64.31 ± 1.13 a | 33.86 ± 1.96 c | 52.14 ± 0.49 b | 25.07 ± 1.39 d | 61.02 ± 0.92 a | 31.48 ± 2.01 c | 53.71 ± 0.57 b | 28.70 ± 1.30 cd |
| Water Weight (g) | 292.56 ± 19.77 a | 143.23 ± 15.29 c | 128.39 ± 9.40 c | 84.84 ± 10.20 c | 223.1 ± 10.99 b | 104.13 ± 5.98 c | 140.97 ± 15.37 c | 90.38 ± 13.41 c |
| Root DW (g) | 3.98 ± 0.43 | 1.83 ± 0.19 | 2.50 ± 0.49 | 1.08 ± 0.13 | 3.23 ± 0.34 | 1.56 ± 0.10 | 2.8 ± 0.27 | 1.34 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginori, J.; Nguyen, C.D.; Wilson, S.; Deng, Z.; Huo, H. Investigating Morphological and Physiological Responses to Stress in Begonia semperflorens. Int. J. Mol. Sci. 2025, 26, 3514. https://doi.org/10.3390/ijms26083514
Ginori J, Nguyen CD, Wilson S, Deng Z, Huo H. Investigating Morphological and Physiological Responses to Stress in Begonia semperflorens. International Journal of Molecular Sciences. 2025; 26(8):3514. https://doi.org/10.3390/ijms26083514
Chicago/Turabian StyleGinori, Julian, Chi D. Nguyen, Sandra Wilson, Zhanao Deng, and Heqiang Huo. 2025. "Investigating Morphological and Physiological Responses to Stress in Begonia semperflorens" International Journal of Molecular Sciences 26, no. 8: 3514. https://doi.org/10.3390/ijms26083514
APA StyleGinori, J., Nguyen, C. D., Wilson, S., Deng, Z., & Huo, H. (2025). Investigating Morphological and Physiological Responses to Stress in Begonia semperflorens. International Journal of Molecular Sciences, 26(8), 3514. https://doi.org/10.3390/ijms26083514


_Kim.png)





