Phenotypic Screening and Marker-Assisted Validation of Sources of Aphis craccivora Koch Resistance in Cowpea (Vigna unguiculata L.)
Abstract
1. Introduction
2. Results
2.1. Cowpea Adult Aphid Population Density Growth
2.2. Number of Nymphs Produced
2.3. The Number of Alates (Winged Aphids)
2.4. Aphid Damage and Plant Vigour Score
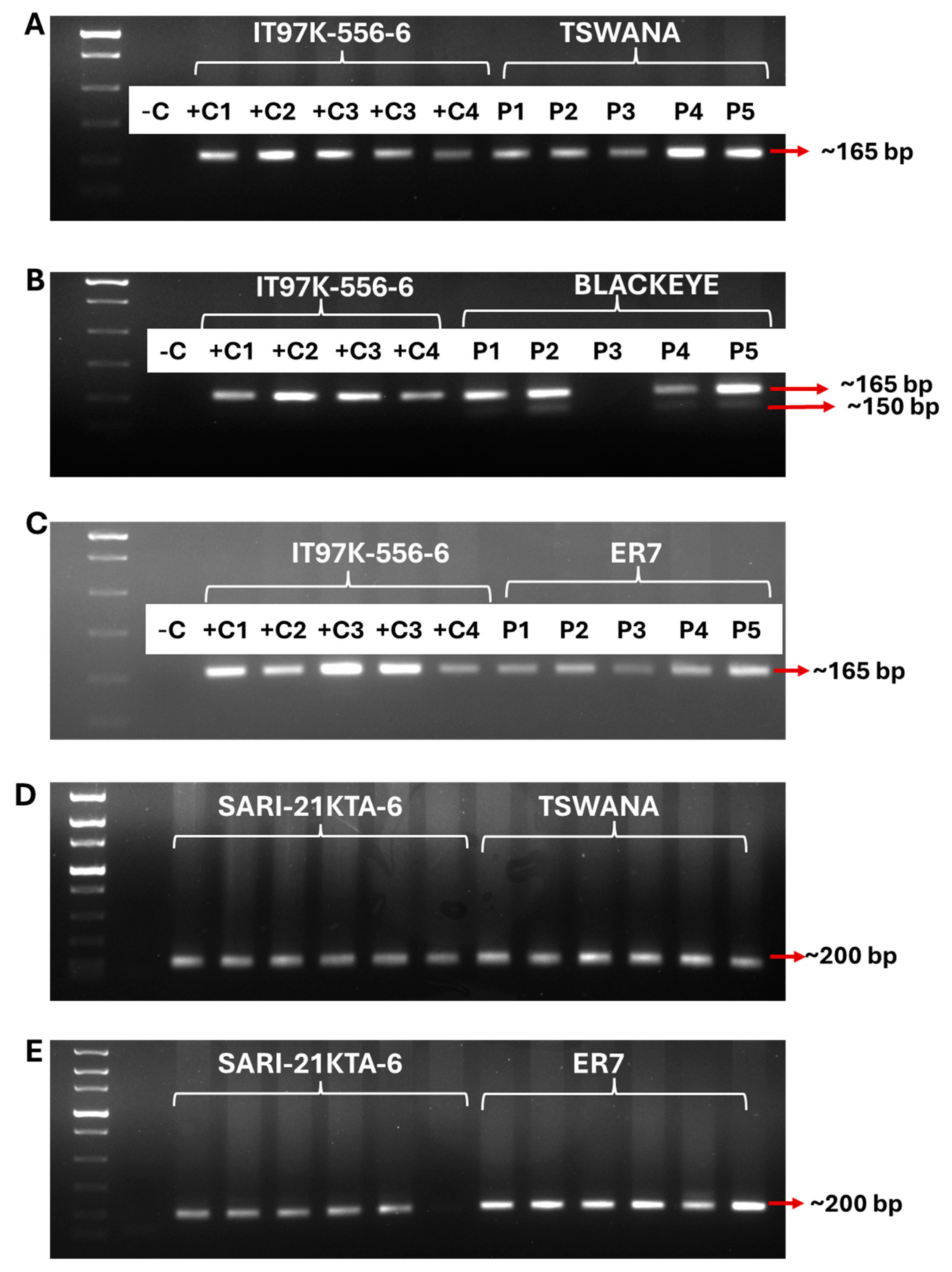
2.5. Genotypic Marker-Assisted Screening for Aphid Resistance
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Planting Materials and Experimental Design
4.3. Aphid Culture
4.4. Aphid Infestation and Plant Damage Severity Score
4.5. Marker-Assisted Polymorphic Tests
4.6. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Agbogidi, O.M. Response of six cultivars of cowpea (Vigna unguiculata (L.) Walp.) to spent engine oil. Afr. J. Food Sci. Technol. 2010, 1, 139–142. [Google Scholar]
- Statistics Botswana. Annual Agriculture Survey Report 2013; Statistics Botswana: Gaborone, Botswana, 2015; ISBN 9789996842856. [Google Scholar]
- Langyintuo, A.S.; Lowenberg-DeBoer, J.; Faye, M.; Lambert, D.; Ibro, G.; Moussa, B.; Kergna, A.; Kushwaha, S.; Musa, S.; Ntoukam, G. Cowpea supply and demand in West and Central Africa. Field Crops Res. 2003, 82, 215–231. [Google Scholar]
- Abebe, B.K.; Alemayehu, M.T. A Review of the nutritional use of cowpea (Vigna unguiculata L. Walp.) for human and animal diets. J. Agric. Food Res. 2022, 10, 100383. [Google Scholar] [CrossRef]
- Dube, E.; Fanadzo, M. Maximising yield benefits from dual-purpose cowpea. Food Secur. 2013, 5, 769–779. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Savala, E.N.; Chikoye, D.; Abaidoo, R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant Sci. 2017, 8, 646. [Google Scholar] [CrossRef]
- Ayalew, T.; Yoseph, T. Cowpea (Vigna unguiculata L. Walp.): A choice crop for sustainability during the climate change periods. J. Appl. Biol. Biotechnol. 2022, 10, 54–162. [Google Scholar] [CrossRef]
- Horn, L.N. Breeding Cowpea (Vigna unguiculata [L.] Walp.) for Improved Yield and Related Traits Using Gamma Irradiation. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2016. [Google Scholar]
- Motlhaodi, T. NPGRC Brief; Department of Agricultural Research: Gaborone, Botswana, 2020; Volume 21. [Google Scholar]
- Mulila Mitti, J.; Mba, C.; Lupupa, T. Southern African countries develop the national strategy for plant genetic resources for food and agriculture. Acta Hortic. 2020, 1267, 38. [Google Scholar] [CrossRef]
- Raina, A.; Laskar, R.A.; Tantray, Y.R.; Khursheed, S.; Wani, M.R.; Khan, S. Characterization of induced high yielding cowpea mutant lines using physiological, biochemical and molecular markers. Sci. Rep. 2020, 10, 3687. [Google Scholar] [CrossRef]
- Jackai, L.E.N.; Daoust, R.A. Insect pests of cowpeas. Annu. Rev. Entomol. 1986, 31, 95–119. [Google Scholar] [CrossRef]
- Obopile, M. Economic threshold and injury levels for control of cowpea aphid, Aphis crassivora Linnaeus (Homoptera: Aphididae), on cowpea. Afr. Plant Prot. 2006, 12, 111–115. [Google Scholar]
- Togola, A.; Boukar, O.; Servent, A.; Chamarthi, S.; Tamò, M.; Fatokun, C. Identification of sources of resistance in cowpea mini core accessions to Aphis craccivora Koch (Homoptera: Aphididae) and their biochemical characterization. Euphytica 2020, 216, 88. [Google Scholar] [CrossRef] [PubMed]
- Boukar, O.; Togola, A.; Chamarthi, S.; Belko, N.; Ishikawa, H.; Suzuki, K.; Fatokun, C. Cowpea [Vigna unguiculata (L.) Walp.] Breeding. In Advances in Plant Breeding Strategies: Legumes; Springer International Publishing: Cham, Switzerland, 2019; Volume 7, pp. 201–243. ISBN 9783030234003. [Google Scholar]
- Mweke, A.; Akutse, K.S.; Ulrichs, C.; Fiaboe, K.K.M.; Maniania, N.K.; Ekesi, S. Integrated management of aphis craccivora in cowpea using intercropping and entomopathogenic fungi under field conditions. J. Fungi 2020, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; CABI: Wallingford, UK, 2007. [Google Scholar]
- Ibrahim, H.; Salam, A.; Abdel-Mogib, M.; El-nagar, M.; Salem, H.; Nada, M. Survey of entomopathogenic fungi naturally infecting cowpea aphid, Aphis craccivora. Koch. J. Plant Prot. Pathol. 2011, 2, 1063–1070. [Google Scholar] [CrossRef]
- Jayasinghe, W.H.; Akhter, M.S.; Nakahara, K.; Maruthi, M.N. Effect of aphid biology and morphology on plant virus transmission. Pest Manag. Sci. 2022, 78, 416–427. [Google Scholar] [CrossRef]
- Singh, R.; Singh, G. Aphids. In Polyphagous Pests of Crops; Springer: Singapore, 2021; pp. 105–182. ISBN 9789811580758. [Google Scholar]
- Zhang, R.J.; Chen, J.; Jiang, L.Y.; Qiao, G.X. The genes expression difference between winged and wingless bird cherry-oat Aphid rhopalosiphum Padi based on transcriptomic data. Sci. Rep. 2019, 9, 4754. [Google Scholar] [CrossRef]
- Ofuya, T.I. Control of the cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae), in cowpea, Vigna unguiculata (L.) Walp. Integr. Pest Manag. Rev. 1997, 2, 199–207. [Google Scholar] [CrossRef]
- Stout, M.J.; Bernaola, L.; Acevedo, F. Recent history and future trends in host-plant resistance. Ann. Entomol. Soc. Am. 2024, 117, 139–149. [Google Scholar] [CrossRef]
- Togola, A.; Boukar, O.; Belko, N.; Chamarthi, S.K.; Fatokun, C.; Tamo, M.; Oigiangbe, N. Host plant resistance to insect pests of cowpea (Vigna unguiculata L. Walp.): Achievements and future prospects. Euphytica 2017, 213, 239. [Google Scholar] [CrossRef]
- Dogimont, C.; Bendahmane, A.; Chovelon, V.; Boissot, N. Host plant resistance to aphids in cultivated crops: Genetic and molecular bases, and interactions with aphid populations. Comptes Rendus Biol. 2010, 333, 566–573. [Google Scholar] [CrossRef]
- Hittalmani, S.; Parco, A.; Mew, T.V.; Zeigler, R.S.; Huang, N. Fine mapping and dna marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor. Appl. Genet. 2000, 100, 1121–1128. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Chatterjee, M.; Das, P.; Lahari, T.; Maji, A.; Mondal, N.; Pradhan, K.K.; Bhattacharyya, S. Molecular markers linked with bruchid resistance in Vigna radiata Var. Sublobata and their validation. J. Plant Biochem. Biotechnol. 2011, 20, 155–160. [Google Scholar] [CrossRef]
- Obopile, M.; Ositile, B. Life table and population parameters of cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae) on five cowpea Vigna unguiculata (L. Walp.) varieties. J. Pest Sci. 2010, 83, 9–14. [Google Scholar] [CrossRef]
- Munthali, D.C.; Lanamile, K. Evaluation of relative resistance of four cowpea (Vigna uniguiculata (L.) Walp.) varieties to the black eye aphid (Aphis craccivora) in Southeastern Botswana. Botsw. J. Agric. Appl. Sci. 2011, 7. [Google Scholar]
- Machacha, M.; Obopile, M.; Tshegofatso, A.B.N.; Tiroesele, B.; Gwafila, C.; Ramokapane, M. Demographic parameters of cowpea aphid Aphis craccivora (Homoptera: Aphididae) on different botswana cowpea landraces. Int. J. Trop. Insect Sci. 2012, 32, 189–193. [Google Scholar] [CrossRef]
- Kusi, F.; Obeng-Ofori, D.; Asante, S.; Padi, F. New sources of resistance in cowpea to the cowpea aphid (Aphis craccivora Koch) (Homoptera: Aphididae). J. Ghana Sci. Assoc. 2010, 12, 95–104. [Google Scholar] [CrossRef]
- Lattanzio, V.; Arpaia, S.; Cardinali, A.; Di Venere, D.; Linsalata, V. Role of endogenous flavonoids in resistance mechanism of vigna role of to aphids. J. Agric. Food Chem. 2000, 48, 5316–5320. [Google Scholar] [CrossRef] [PubMed]
- Huynh, B.L.; Ehlers, J.D.; Ndeve, A.; Wanamaker, S.; Lucas, M.R.; Close, T.J.; Roberts, P.A. Genetic mapping and legume synteny of aphid resistance in African cowpea (Vigna unguiculata L. Walp.) grown in California. Mol. Breed. 2015, 35, 36. [Google Scholar] [CrossRef] [PubMed]
- Kusi, F.; Padi, F.K.; Obeng-Ofori, D.; Asante, S.K.; Agyare, R.Y.; Sugri, I.; Timko, M.P.; Koebner, R.; Huynh, B.L.; Santos, J.R.P.; et al. A novel aphid resistance locus in cowpea identified by combining SSR and SNP markers. Plant Breed. 2018, 137, 203–209. [Google Scholar] [CrossRef]
- Ouedraogo, A.P.; Danquah, A.; Tignegre, J.B.; Paoda, L.S.; Batieno, J.B.; Asante, I.K.; Ouedraogo, J.T.; Ayertey, J.N.; Ofori, K. Determination of inheritance of aphid resistance in cowpea genotypes and identification of single sequence repeat markers linked to resistance genes. Legume Sci. 2022, 4, e127. [Google Scholar] [CrossRef]
- Seram, D.; Devi, Y.K. Insect resistance screening techniques and scoring for important horticultural crops. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 155–162. [Google Scholar]
- Schreiner, I. Cowpea Aphid (Aphis craccivora Koch). In Agricultural Pests of the Pacific Cowpea Aphid (Aphis craccivora Koch) 2000; Agricultural Development in the American Pacific (AGAP): Washington, DC, USA, 2020; ISBN 1-931435-09-X. [Google Scholar]
- Souleymane, A.; Ova, M.E.A.; Fatokun, C.A.; Alabi, O.Y. Screening for resistance to cowpea aphid (Aphis craccivora Koch) in wild and cultivated cowpea (Vigna unguiculata L. Walp.) accessions. Int. J. Sci. Environ. 2013, 2, 611–621. [Google Scholar]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kityo, R.; Okdoi, J.B.; Ozimati, A.; Dramadri, I.O.; Agaba, R.; Ongom, P.O.; Nampala, P.; Edema, R.; Karungi, J.; Gibson, P.; et al. New sources and stability of resistance to aphids in cowpea. Afr. Crop Sci. J. 2021, 29, 209–228. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Zavala, D.; Moraga, F.; Herrera-Vásquez, A.; Blanco-Herrera, F. Oligogalacturonides enhance resistance against aphids through pattern-triggered immunity and activation of salicylic acid signaling. Int. J. Mol. Sci. 2022, 23, 9753. [Google Scholar] [CrossRef]
- Kusi, F.; Nboyine, J.A.; Attamah, P.; Awuku, J.F.; Sugri, I.; Zakaria, M.; Lamini, S.; Mensah, G.; Larweh, V.; Owusu, R.K.; et al. Stability of sources of resistance to cowpea aphid (Aphis craccivora Koch, Hemiptera: Aphididae) across major cowpea production zones in Ghana. Hindawi Int. J. Agron. 2020, 2020, 8869334. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.; Singh Pathania, V.; Thakur, S. Effect of plant nutrition in insect pest management: A Review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- Bansal, R.; Mian, M.A.R.; Michel, A. Characterizing Resistance to soybean aphid (Hemiptera: Aphididae): Antibiosis and antixenosis assessment. J. Econ. Entomol. 2021, 114, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- MacWilliams, J.R.; Chesnais, Q.; Nabity, P.; Mauck, K.; Kaloshian, I. Cowpea aphid resistance in cowpea line CB77 functions primarily through antibiosis and eliminates phytotoxic symptoms of aphid feeding. J. Pest Sci. 2023, 96, 539–553. [Google Scholar] [CrossRef]
- Martin, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef]
- Stec, K.; Kordan, B.; Sergiel, I.; Biesaga, M.; Mroczek, J.; Bocianowski, J.; Gabryś, B. Antixenosis in Glycine max (L.) Merr against Acyrthosiphon pisum (Harris). Sci. Rep. 2021, 11, 15289. [Google Scholar] [CrossRef]
- Hu, Z.; Zhong, X.; Zhang, H.; Luo, X.; Wang, Y.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, X.; An, H.; et al. GhMYB18 confers Aphis Gossypii Glover resistance through regulating the synthesis of salicylic acid and flavonoids in cotton plants. Plant Cell Rep. 2023, 42, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.G.; Palmer, N.A.; Donze-Reiner, T.; Scully, E.D.; Seravalli, J.; Amundsen, K.; Twigg, P.; Louis, J.; Bradshaw, J.D.; Heng-Moss, T.M.; et al. Aphid-responsive defense networks in hybrid switchgrass. Front. Plant Sci. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Feng, J.L.; Zhang, J.; Yang, J.; Zou, L.P.; Fang, T.T.; Xu, H.L.; Cai, Q.N. Exogenous salicylic acid improves resistance of aphid-susceptible wheat to the grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae). Bull. Entomol. Res. 2021, 111, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, S.; Huang, Y. Molecular interactions between plants and aphids: Recent advances and future perspectives. Insects 2024, 15, 935. [Google Scholar] [CrossRef]
- Sombie, P.A.E.D.; Ouedraogo, I.; Tignegré, J.B.D.L.S.; Hilou, A.; Ouedraogo, T.J.; Kiendrébéogo, M. Genotypic variation of mineral elements and phytate levels of thirty cowpeas (Vigna unguiculata L. Walp.) varieties cultivated in Burkina Faso. J. Food Chem. Nutr. 2018, 6, 13–20. [Google Scholar] [CrossRef]
- Omoigui, L.O.; Ugba, S.M.; Bello, L.L.; Timlo, M.P.; Gowda, B.S.; Motagi, B.N. SSR markers linked with Alectra vogelii resistance in cowpea Vigna unguiculata (L.) Walp] Omoigui. In Proceedings of the 5th International Conference on Next Generation Genomics & Integreted Breeding Crop Improvement, Patancheru, India, 18–20 February 2015; p. 158. [Google Scholar]
- Ouédraogo, A.P.; Batieno, B.J.; Traore, F.; Tignegre, J.-B.; Huynh, B.; Roberts, P.A.; Close, T.; Ouédraogo, J.T. Screening of cowpea (Vigna unguiculata (L.) Walp.) lines for resistance to three aphids (Aphis craccivora Koch) strains in Burkina Faso. Afr. J. Agric. Res. 2018, 13, 1487–1495. [Google Scholar] [CrossRef][Green Version]
- Pholo-Tait, M.; Le Maitre, N.C.; Lloyd, J.R.; Kossmann, J.; Hills, P.N.; Schulze, W.X.; Alseekh, S.; Valentine, A.J. Proteome profiling of lumichrome-treated arabidopsis thaliana suggests that various regulatory mechanisms mediate enhanced photosynthesis and plant growth. S. Afr. J. Bot. 2024, 172, 515–528. [Google Scholar] [CrossRef]
- Yates-Stewart, A.D.; Daron, J.; Wijeratne, S.; Shahid, S.; Edgington, H.A.; Slotkin, R.K.; Michel, A. Soybean aphids adapted to host-plant resistance by down regulating putative effectors and up regulating transposable elements. Insect Biochem. Mol. Biol. 2020, 121, 103363. [Google Scholar] [CrossRef]
- Attamah, P.; Kusi, F.; Kena, A.W.; Awuku, F.J.; Lamini, S.; Mensah, G.; Zackaria, M.; Owusu, E.Y.; Akromah, R. Pyramiding aphid resistance genes into the elite cowpea variety, Zaayura, using marker-assisted backcrossing. Heliyon 2024, 10, e31976. [Google Scholar] [CrossRef]
- Omoigui, L.O.; Ekeuro, G.C.; Kamara, A.Y.; Bello, L.L.; Timko, M.P.; Ogunwolu, G.O. New sources of aphids [Aphis craccivora (Koch)] resistance in cowpea germplasm using phenotypic and molecular marker approaches. Euphytica 2017, 213, 178. [Google Scholar] [CrossRef]



| Days After Infestation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | 7 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 | 25 | 27 |
| Tswana | 5.3 ± 0.6 a | 4 ± 0.0 bc | 47.5 ± 11.5 abc | 93.3 ± 19.7 bc | 57.7 ± 36.3 b | 119 ± 100.8 a | 118 ± 58.1 a | 101.7 ± 51.0 a | 94.8 ± 69.8 a | 40.8 ± 26.6 a | 37.5 ± 16.7 a |
| Blackeye | 4.7 ± 0.6 a | 4.5 ± 0.5 bc | 31.5 ± 23.3 cd | 115.7 ± 58.7 abc | 179.8 ± 89.0 b | 269.5 ± 168.1 a | 288.7 ± 245.4 a | 102.8 ± 4.6 a | 34.8 ± 24.4 a | 23.3 ± 15.9 a | 27.5 ± 19.3 a |
| ER7 | 5.8 ± 3.8 a | 4.3 ± 1.2 bc | 13.5 ± 11.2 d | 44.8 ± 32.3 c | 100.3 ± 57.5 b | 140.5 ± 101.2 a | 137.2 ± 41.9 a | 127.3 ± 78.2 a | 54.7 ± 35.5 a | 33.7 ± 34.8 a | 28.7 ± 29.7 a |
| B013-F | 4.7 ± 0.8 a | 5.2 ± 1.3 b | 38.2 ± 14.2 bcd | 101.7 ± 47.2 abc | 177.5 ± 132.4 ab | 316.2 ± 148.2 a | 129.2 ± 108.0 a | 55.5 ± 9.9 a | 52.0 ± 31.1 a | 20.2 ± 5.5 a | 20.3 ± 16.4 a |
| B261-B | 4.8 ± 0.3 a | 3.5 ± 0.0 bc | 70.2 ± 20.7 a | 147.2 ± 36.3 ab | 251.7 ± 29.1 ab | 364.0 ± 205.8 a | 210.2 ± 108.0 a | 125.0 ± 34.6 a | 32.8 ± 5.5 a | 37.7 ± 11.3 a | 79.3 ± 26.5 a |
| B301 | 5.0 ± 0.0 a | 5 ± 0.0 bc | 64.8 ± 16.6 ab | 93.3 ± 38.8 bc | 173.5 ± 77.0 b | 206.5 ± 102.4 a | 136.3 ± 50.5 a | 73.8 ± 23.4 a | 28.3 ± 9.1 a | 37.8 ± 5.5 a | 76.4 ± 1.9 a |
| B339 | 4.2 ± 0.6 a | 4 ± 0.87 bc | 41.7 ± 13.9 abcd | 121.8 ± 58.8 abc | 156.5 ± 109.6 b | 139.5 ± 87.3 a | 128.2 ± 54.0 a | 39.2 ± 29.2 a | 37.7 ± 5.5 a | 30.8 ± 5.3 a | 41.8 ± 29.6 a |
| B359 | 4.8 ± 0.3 a | 3.2 ± 1.0 c | 31.7 ± 6.0 cd | 80 ± 19.1 bc | 177.2 ± 87.2 ab | 248.2 ± 221.0 a | 187 ± 197.7 a | 101.3 ± 60.9 a | 66.2 ± 51.1 a | 49.8 ± 39.3 a | 51 ± 42.4 a |
| SARC 1-57-2 | 4.8 ± 1.2 a | 3.8 ± 1.5 bc | 40.7 ± 21.5 abcd | 189 ± 95.0 a | 198.3 ± 100.6 ab | 195.2 ± 53.8 a | 124.2 ± 31.6 a | 53.8 ± 12.4 a | 83.8 ± 12.4 a | 38.5 ± 0.0 a | 25.5 ± 0.0 a |
| SARI-2KTA-6 | 4.5 ± 1.3 a | 7 ± 2.6 a | 26.3 ± 8.1 cd | 109.3 ± 44.6 abc | 244 ± 56.6 ab | 305 ± 134.3 a | 184.2 ± 72.4 a | 107 ± 30.0 a | 68.5 ± 54.9 a | 34.5 ± 21.9 a | 46.5 ± 19.1 a |
| IT97K-556-6 | 4.3 ± 1.2 a | 4.8 ± 0.3 bc | 49.3 ± 13.6 abc | 192.2 ± 37.4 a | 255.3 ± 165.4 ab | 366.5 ± 213.1 a | 324.8 ± 170.8 a | 55.0 ± 37.7 a | 112.7 ± 47.6 a | 33.5 ± 2.1 a | 21.0 ± 21.2 a |
| KVX295-2-124-99 | 4.8 ± 0.3 a | 4.8 ± 0.3 bc | 32.3 ± 17.9 cd | 100.3 ± 32.5 abc | 377.8 ± 143.2 a | 343.8 ± 277.9 a | 241.7 ± 188.0 a | 145.7 ± 115.8 a | 72.5 ± 45.9 a | 48.3 ± 45.9 a | 44.7 ± 47.1 a |
| p-value | 0.95 ns | 0.05 * | 0.01 ** | 0.05 * | 0.09 ns | 0.56 ns | 0.60 ns | 0.42 ns | 0.32 ns | 0.96 ns | 0.25 ns |
| F-value | 0.38 | 2.96 | 2.96 | 2.3 | 1.89 | 0.89 | 0.85 | 1.09 | 1.25 | 0.34 | 1.34 |
| CV% | 25.7 | 22.21 | 39.2 | 42.1 | 52.47 | 65.96 | 71.1 | 60.02 | 66.57 | 66.57 | 64.15 |
| Days After Infestation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | 7 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 | 25 | 27 |
| Tswana | 39.5 ± 27.8 abc | 94.5 ± 38.6 abc | 137.8 ± 11.5 ab | 240.2 ± 75.7 ab | 210.2 ± 151.6 c | 248.5 ± 136.0 b | 312.3 ± 40.0 b | 329.0 ± 320.1 b | 267.7 ± 189.7 b | 325.2 ± 150.5 a | 386.8 ± 67.7 abc |
| Blackeye | 32.2 ± 17.9 bcd | 104.5 ± 56.9 abc | 89.3 ± 35.2 b | 347.7 ± 207.6 ab | 767.2 ± 486.3 abc | 914.7 ± 564.6 ab | 1150.8 ± 883.4 ab | 1056.5 ± 241.1 a | 1016.5 ± 407.3 ab | 538.8 ± 54.8 a | 650.3 ± 464.9 a |
| ER7 | 8.0 ± 7.0 d | 57.8 ± 39.1 c | 121.5 ± 60.0 ab | 135.0 ± 42.7 b | 333.2 ± 198.5 bc | 543.8 ± 351.2 ab | 649.5 ± 494.0 b | 655.7 ± 356.2 ab | 309.7 ± 223.0 b | 374.0 ± 288.2 a | 137.7 ± 139.3 bc |
| B013-F | 31.3 ± 9.8 bcd | 102.3 ± 35.1 abc | 116.3 ± 22.9 ab | 486.8 ± 452.6 ab | 609.7 ± 620.6 abc | 981.5 ± 807.8 ab | 587.2 ± 645.8 b | 431.5 ± 75.7 ab | 233.3 ± 138.9 b | 160.3 ± 24.5 a | 94.2 ± 62.6 c |
| B261-B | 58.3 ± 20.0 ab | 85 ± 28.5 abc | 217.2 ± 127.9 a | 696.3 ± 284.7 a | 1059 ± 545.1 ab | 1672.3 ± 750.3 a | 1071 ± 409.0 ab | 721.7 ± 168.9 ab | 281.2 ± 26.8 b | 321.3 ± 121.8 a | 184.2 ± 87.4 abc |
| B301 | 64.2 ± 16.0 a | 149.3 ± 10.5 a | 170.8 ± 29.2 ab | 333 ± 64.0 ab | 727.7 ± 237.9 abc | 755.2 ± 348.4 ab | 621.3 ± 486.8 b | 481.2 ± 277.1 ab | 223.2 ± 66.8 b | 273.7 ± 59.1 a | 285 ± 24.8 abc |
| B339 | 23.7 ± 9.1 cd | 100.8 ± 26.4 abc | 183.8 ± 75.5 ab | 350.7 ± 180 ab | 487.5 ± 283.3 abc | 665 ± 383.2 ab | 854.8 ± 481.0 ab | 751.5 ± 354.3 ab | 771.5 ± 348.6 ab | 393.8 ± 347.5 a | 173.2 ± 120.9 bc |
| B359 | 32.0 ± 9.5 bcd | 78.7 ± 28.0 bc | 95.3 ± 25.0 b | 416.2 ± 226.9 ab | 571.5 ± 347.4 abc | 1672.3 ± 750.3 a | 498.5 ± 447.5 b | 435.3 ± 301.3 ab | 250.8 ± 128.0 b | 390.7 ± 261.6 a | 501 ± 343.7 abc |
| SARC 1-57-2 | 28.7 ± 18.6 bcd | 104.8 ± 36.8 abc | 159.2 ± 21.3 ab | 407.0 ± 229.9 ab | 484.3 ± 211.9 abc | 1055.2 ± 231.0 ab | 867.0 ± 254.6 ab | 361.3 ± 111.4 b | 375.8 ± 25.1 b | 481.0 ± 0.0 a | 322.0 ± 0.0 abc |
| SARI-2KTA-6 | 28.8 ± 7.3 bcd | 132.8 ± 17.5 ab | 104.3 ± 17.2 b | 482.7 ± 235.3 ab | 924.0 ± 148.6 abc | 1184.3 ± 87.7 ab | 1097.2 ± 331.4 ab | 576.3 ± 85.8 ab | 648.5 ± 384.7 ab | 411.7 ± 209.4 a | 608.5 ± 130.8 ab |
| IT97K-556-6 | 38.0 ± 7.7 abcd | 139.0 ± 18.5 ab | 84.8 ± 67.9 b | 551.0 ± 224.1 ab | 1163.7 ± 622.7 a | 1636.3 ± 881.5 a | 1810.3 ± 778.3 a | 720.0 ± 550.3 ab | 1573.5 ± 977.3 a | 629.5 ± 75.7 a | 330.5 ± 410.8 abc |
| KVX295-2-124-99 | 29.5 ± 20.8 bcd | 82.2 ± 32.8 bc | 116.0 ± 42.3 ab | 539.8 ± 431.4 ab | 886.3 ± 323.4 abc | 947.5 ± 700.8 ab | 1021.3 ± 743.4 ab | 683.5 ± 421.5 ab | 1127.2 ± 814.0 ab | 688.5 ± 878.7 a | 557.8 ± 504.5 abc |
| p-value | 0.03 * | 0.1 ns | 0.09 ns | 0.50 ns | 0.20 ns | 0.16 ns | 0.22 ns | 0.38 ns | 0.03 * | 0.83 ns | 0.09 ns |
| F-value | 2.59 | 1.86 | 1.88 | 0.97 | 1.55 | 1.8 | 1.45 | 1.15 | 2.61 | 0.57 | 2.04 |
| CV% | 46.26 | 32.9 | 39.4 | 63.42 | 58.85 | 57.57 | 64.73 | 52.43 | 78 | 79.91 | 62.1 |
| Days After Infestation | |||||
|---|---|---|---|---|---|
| Genotypes | 19 | 21 | 23 | 25 | 27 |
| Tswana | 57.8 ± 54.5 a | 41.3 ± 12.2 b | 48.7 ± 23.2 b | 45.5 ± 30.5 a | 54.0 ± 30.2 bc |
| Blackeye | 117.0 ± 31.8 a | 260.5 ± 171.8 a | 219.3 ± 20.9 a | 116.3 ± 39.2 a | 174 ± 116.0 abc |
| ER7 | 22.2 ± 12.2 a | 67.3 ± 38.6 b | 131 ± 126.3 ab | 68.7 ± 70.9 a | 57.7 ± 64.3 bc |
| B013-F | 44.5 ± 36.3 a | 66.5 ± 12.7 b | 131.3 ± 132.6 ab | 44.5 ± 23.0 a | 26.0 ± 20.8 c |
| B261-B | 94.2 ± 53.5 a | 62 ± 13.5 b | 53.5 ± 4.8 b | 92.2 ± 16.8 a | 75.8 ± 10.3 bc |
| B301 | 125.8 ± 67.2 a | 90.2 ± 35.3 b | 148 ± 92.2 ab | 101.5 ± 79.0 a | 96 ± 22.6 bc |
| B339 | 97.5 ± 67.5 a | 101 ± 29.1 b | 99.3 ± 24.4 ab | 55.75 ± 9.5 a | 53 ± 20.1 bc |
| B359 | 66.7 ± 55.4 a | 91.5 ± 44.0 b | 83.7 ± 78.9 ab | 83 ± 68.2 a | 33 ± 31.4 c |
| SARC 1-57-2 | 111.5 ± 103.0 a | 157.5 ± 60.1 ab | 173.8 ± 87.3 ab | 134 ± 0.0 a | 63 ± 0.0 bc |
| SARI-2KTA-6 | 92 ± 45.0 a | 143.7 ± 72.4 ab | 190 ± 29.5 ab | 107.33 ± 39.9 a | 272.8 ± 6.0 a |
| IT97K-556-6 | 113.2 ± 40.6 a | 171.7 ± 108.7 ab | 169.7 ± 48.0 ab | 112 ± 10.0 a | 91.5 ± 113.8 bc |
| KVX295-2-124-99 | 76 ± 86.7 a | 117.5 ± 80.0 b | 131 ± 96.8 ab | 81.2 ± 68.0 a | 189.2 ± 177.3 ab |
| p-value | 0.57 ns | 0.08 ns | 0.27 ns | 0.48 ns | 0.03 * |
| F-value | 0.89 | 2.06 | 1.35 | 0.67 | 2.66 |
| CV% | 69.17 | 59.79 | 59.37 | 62.15 | 73.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaonosi, G.G.; Lekgari, L.; Mosupiemang, M.; Sehularo, M.N.; Tshegofatso, A.B.; Tiroesele, B.; Motlhaodi, T.; Ngwako, S.; Pholo-Tait, M. Phenotypic Screening and Marker-Assisted Validation of Sources of Aphis craccivora Koch Resistance in Cowpea (Vigna unguiculata L.). Int. J. Mol. Sci. 2025, 26, 4406. https://doi.org/10.3390/ijms26094406
Gaonosi GG, Lekgari L, Mosupiemang M, Sehularo MN, Tshegofatso AB, Tiroesele B, Motlhaodi T, Ngwako S, Pholo-Tait M. Phenotypic Screening and Marker-Assisted Validation of Sources of Aphis craccivora Koch Resistance in Cowpea (Vigna unguiculata L.). International Journal of Molecular Sciences. 2025; 26(9):4406. https://doi.org/10.3390/ijms26094406
Chicago/Turabian StyleGaonosi, Galalea Gillian, Lekgari Lekgari, Marang Mosupiemang, Metseyabeng Nametso Sehularo, Aobakwe Boisy Tshegofatso, Bamphithi Tiroesele, Tiny Motlhaodi, Samodimo Ngwako, and Motlalepula Pholo-Tait. 2025. "Phenotypic Screening and Marker-Assisted Validation of Sources of Aphis craccivora Koch Resistance in Cowpea (Vigna unguiculata L.)" International Journal of Molecular Sciences 26, no. 9: 4406. https://doi.org/10.3390/ijms26094406
APA StyleGaonosi, G. G., Lekgari, L., Mosupiemang, M., Sehularo, M. N., Tshegofatso, A. B., Tiroesele, B., Motlhaodi, T., Ngwako, S., & Pholo-Tait, M. (2025). Phenotypic Screening and Marker-Assisted Validation of Sources of Aphis craccivora Koch Resistance in Cowpea (Vigna unguiculata L.). International Journal of Molecular Sciences, 26(9), 4406. https://doi.org/10.3390/ijms26094406






