Carvacrol Protects IPEC-J2 Cells from Oxidative Stress by Suppressing Autophagy
Abstract
1. Introduction
2. Results
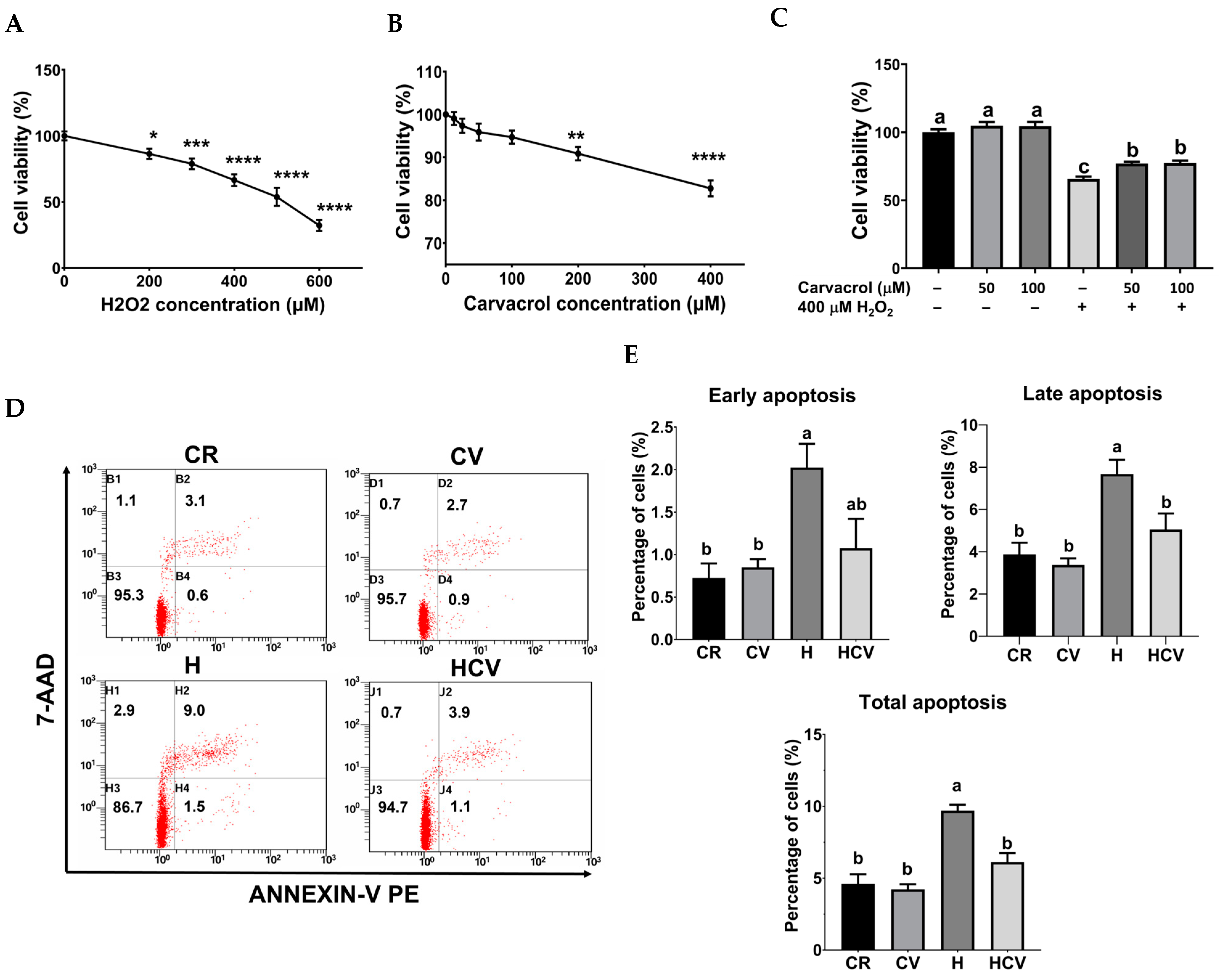
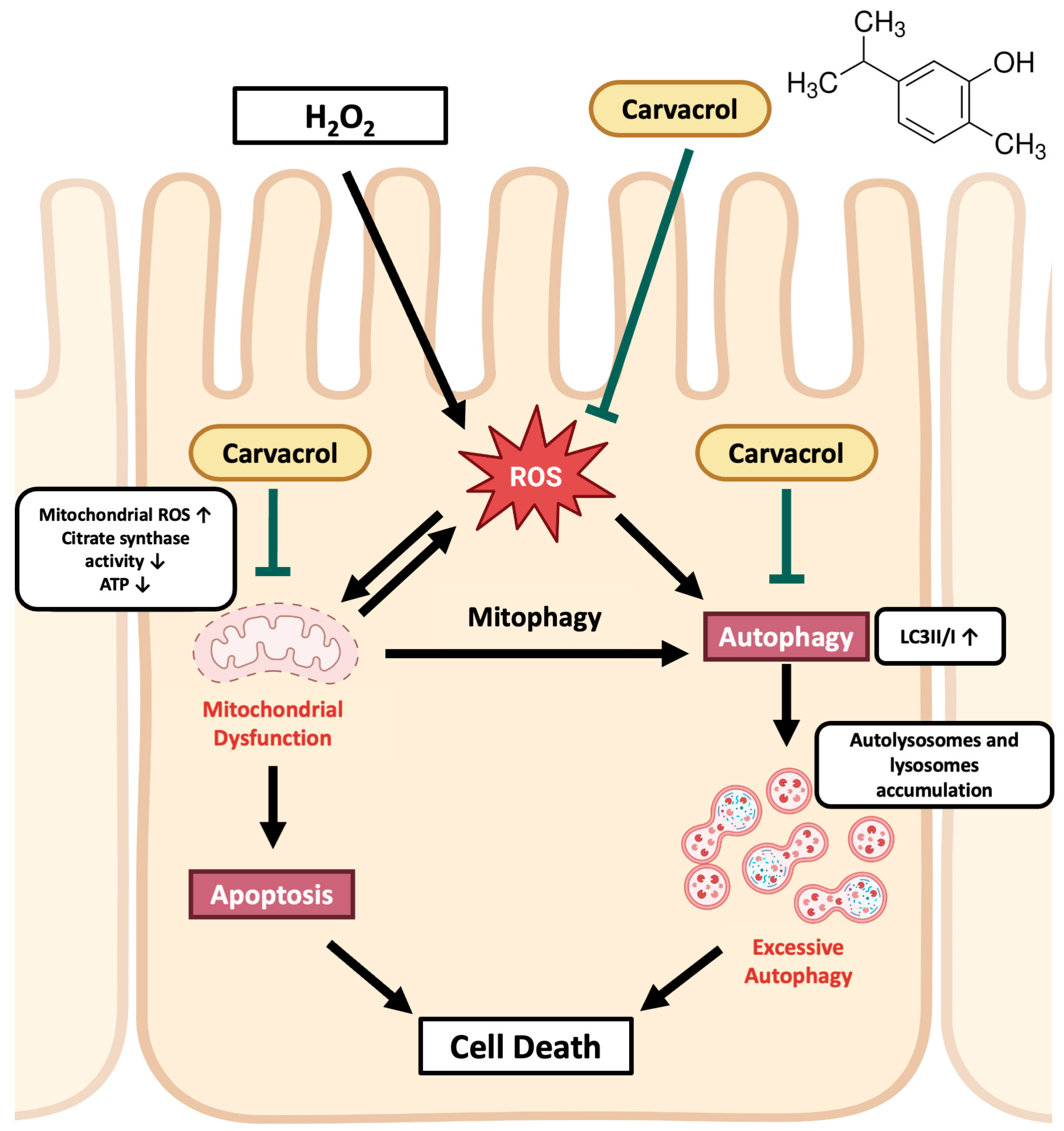
2.1. Carvacrol Reduced H2O2-Induced Apoptosis in IPEC-J2 Cells
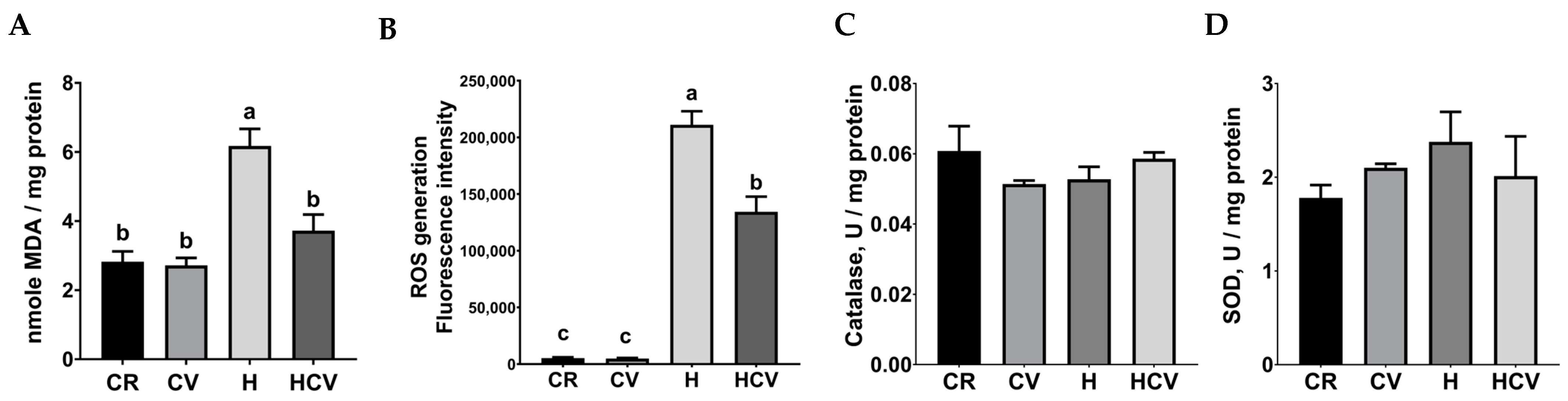
2.2. Carvacrol Relieved H2O2-Induced Oxidative Stress in IPEC-J2 Cells
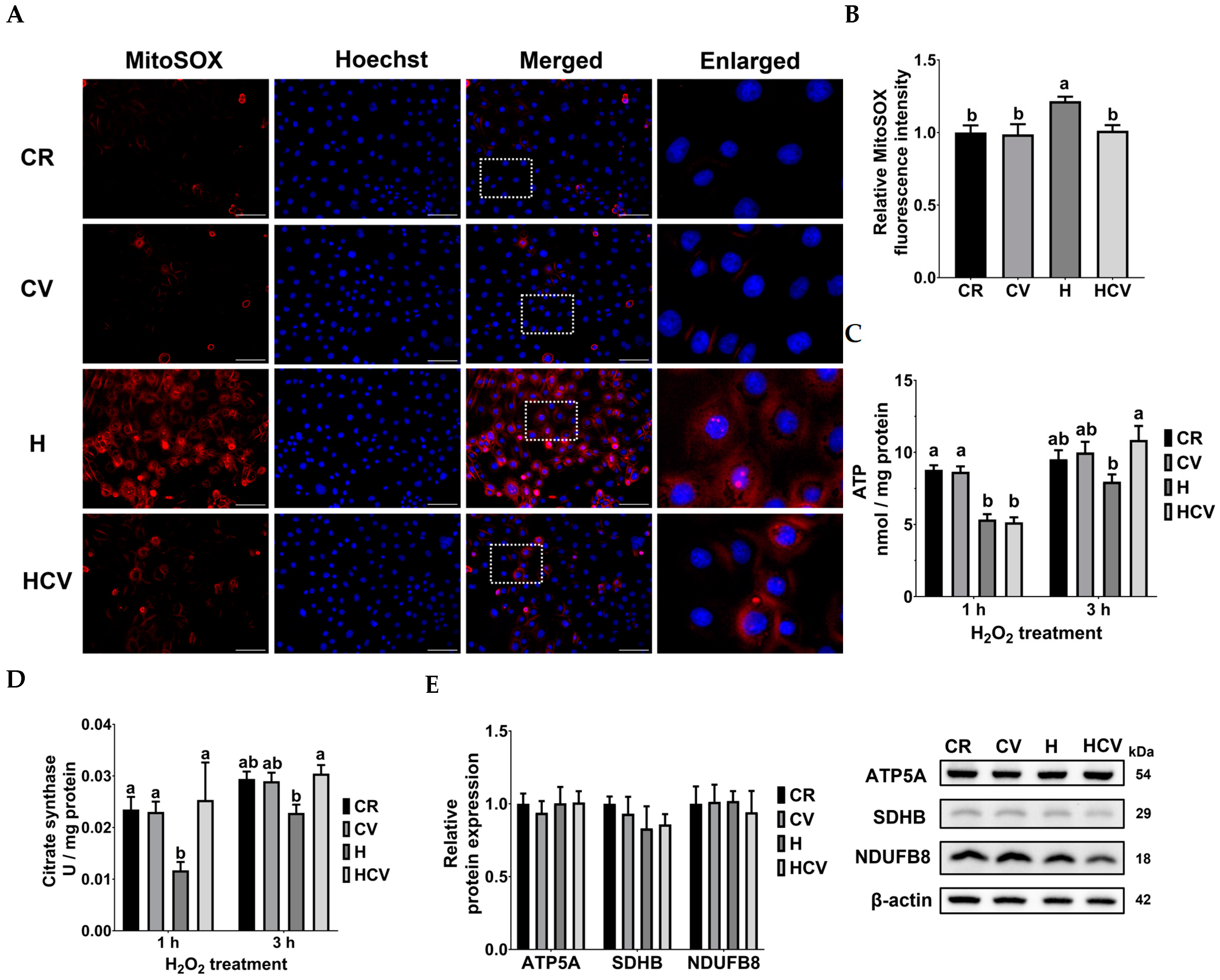
2.3. Carvacrol Ameliorated Mitochondrial Dysfunction in IPEC-J2 Cells Under Oxidative Stress
2.4. Carvacrol Inhibited Mitophagy in IPEC-J2 Cells Under Oxidative Stress
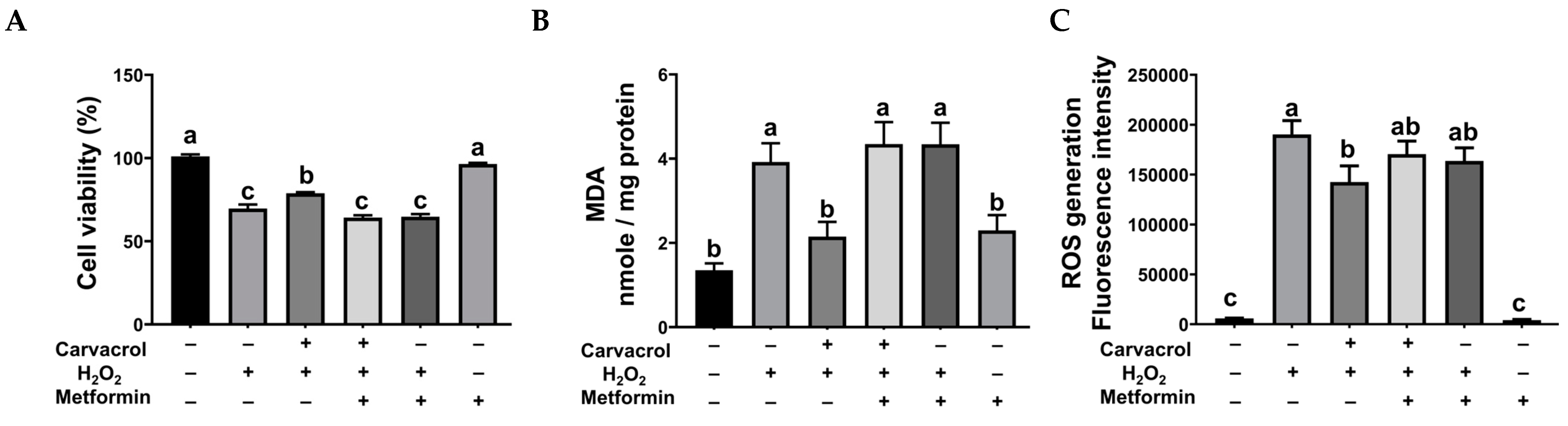
2.5. Autophagy Inhibition Was Necessary for Carvacrol’s Protection in IPEC-J2 Cells Under Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Malondialdehyde (MDA) Level
4.5. Intracellular ROS Generation
4.6. Activities of Catalase and Superoxide Dismutase (SOD)
4.7. Mitochondrial ROS Detection
4.8. ATP Production
4.9. Quantification of Citrate Synthase Activity
4.10. Western Blotting
4.11. Fluorescence Imaging of Acidic Vacuoles
4.12. Detection of Apoptotic and Necrotic Cells
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volk, N.; Lacy, B. Anatomy and physiology of the small bowel. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 1–13. [Google Scholar] [PubMed]
- Sui, G.Y.; Wang, F.; Lee, J.; Roh, Y.S. Mitochondrial control in inflammatory gastrointestinal diseases. Int. J. Mol. Sci. 2022, 23, 14890. [Google Scholar] [CrossRef] [PubMed]
- Haque, P.S.; Kapur, N.; Barrett, T.A.; Theiss, A.L. Mitochondrial function and gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 537–555. [Google Scholar] [CrossRef]
- Ho, G.T.; Theiss, A.L. Mitochondria and inflammatory bowel diseases: Toward a stratified therapeutic intervention. Annu. Rev. Physiol. 2022, 84, 435–459. [Google Scholar]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [PubMed]
- Kocot, A.M.; Wroblewska, B. Nutritional strategies for autophagy activation and health consequences of autophagy impairment. Nutrition 2022, 130–104, 111686. [Google Scholar]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut 2020, 69, 1928–1938. [Google Scholar]
- Alula, K.M.; Delgado-Deida, Y.; Callahan, R.; Till, A.; Underwood, L.; Thompson, W.E.; Souza, R.F. Inner mitochondrial membrane protein Prohibitin 1 mediates Nix-induced, Parkin-independent mitophagy. Sci. Rep. 2023, 13, 18. [Google Scholar]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M.D.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Zhao, B.C.; Wang, T.H.; Chen, J.; Qiu, B.H.; Xu, Y.R.; Zhang, Q.; Li, J.J.; Wang, C.J.; Nie, Q.F.; Li, J.L. Effects of dietary supplementation with a carvacrol-cinnamaldehyde-thymol blend on growth performance and intestinal health of nursery pigs. Porcine Health Manag. 2023, 9, 24. [Google Scholar] [CrossRef]
- Hsu, M.C.; Chen, C.Y. Carvacrol protects IPEC-J2 cells from oxidative stress via modulation of mitophagy and anti-oxidative effect. In Proceedings of the 20th Asian-Australasian Association of Animal Production Congress, Melbourne, Australia, 9–12 July 2024. [Google Scholar]
- Aristatile, B.; Al-Numair, K.S.; Al-Assaf, A.H.; Veeramani, C.; Pugalendi, K.V. Protective effect of carvacrol on oxidative stress and cellular DNA damage induced by UVB irradiation in human peripheral lymphocytes. J. Biochem. Mol. Toxicol. 2015, 29, 497–507. [Google Scholar] [CrossRef]
- Chenet, A.L.; Duarte, A.R.; de Almeida, F.J.S.; Andrade, C.M.B.; de Oliveira, M.R. Carvacrol depends on heme oxygenase-1 (HO-1) to exert antioxidant, anti-inflammatory, and mitochondria-related protection in the human neuroblastoma SH-SY5Y cells line exposed to hydrogen peroxide. Neurochem. Res. 2019, 44, 884–896. [Google Scholar] [CrossRef]
- Simsek, H.; Gür, C.; Kücükler, S.; Ileritürk, M.; Akaras, N.; Öz, M.; Kandemir, F.M. Carvacrol reduces mercuric chloride-induced testicular toxicity by regulating oxidative stress, inflammation, apoptosis, autophagy, and histopathological changes. Biol. Trace Elem. Res. 2023, 202, 4605–4617. [Google Scholar]
- Wang, Y.; Yang, Z.; Zhou, Y.; Tan, J.; Sun, H.; Sun, D.; Mu, Y.; Peng, J.; Wei, H. Effects of different amino acid levels and a carvacrol-thymol blend on growth performance and intestinal health of weaned pigs. J. Anim. Sci. Biotechnol. 2022, 13, 22. [Google Scholar]
- Hsu, J.E. Effects of Essential Oil Mixtures on Fecal Odor and Plasma Healthy Biomarkers of Pigs. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2020. [Google Scholar] [CrossRef]
- Li, B.F.; Zhang, X.L.; Zhang, Q.Z.; Zheng, T.H.; Li, Q.H.; Yang, S.W.; Shao, J.Y.; Guan, W.T.; Zhang, S.H. Nutritional strategies to reduce intestinal cell apoptosis by alleviating oxidative stress. Nutr. Rev. 2024, 83, e518–e532. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhao, J.; Han, Z.; Tang, F. Protective effects of Semen Crotonis Pulveratum on trinitrobenzene sulphonic acid-induced colitis in rats and H2O2-induced intestinal cell apoptosis. Int. J. Mol. Med. 2015, 35, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.Y.; Fan, Y.T.; Yu, Y.; Loor, J.J.; Elsabagh, M.; Peng, A. L-arginine alleviates hydrogen peroxide-induced oxidative damage in ovine intestinal epithelial cells by regulating apoptosis, mitochondrial function, and autophagy. J. Nutr. 2021, 151, 1038–1046. [Google Scholar] [CrossRef]
- Zhu, L.H.; Xu, J.X.; Zhu, S.W.; Cai, X.; Yang, S.F.; Chen, X.L.; Guo, Q. Gene expression profiling analysis reveals weaning-induced cell cycle arrest and apoptosis in the small intestine of pigs. J. Anim. Sci. 2014, 92, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Liu, J.L.; Ma, Y.F.; Wei, Y.S.; Liu, J.X.; Wang, H.F. Impairment of intestinal barrier function induced by early weaning autophagy and apoptosis associated with gut microbiome and metabolites. Front. Immunol. 2021, 12, 804870. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.H.; Liang, Z.N.; Yi, J.N.; Chen, X.J.; Li, R.F.; Wu, Y.; Wu, J.; Sun, Z.L. Protective effect of koumine, an alkaloid from Gelsemium Sempervirens, on injury induced by H2O2 in IPEC-J2 cells. Int. J. Mol. Sci. 2019, 20, 754. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhang, H.; Ji, S.L.; Jia, P.L.; Chen, Y.P.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef]
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, Y.; Zhang, X.Y.; Lu, Y.P.; Chen, H.X. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H.K. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxid. Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef]
- Shah, S.; Pushpa Tryphena, K.; Singh, G.; Kulkarni, A.; Pinjala, P.; Kumar Khatri, D. Neuroprotective role of carvacrol via Nrf2/HO-1/NLRP3 axis in rotenone-induced PD mice model. Brain Res. 2024, 1836, 148954. [Google Scholar]
- Arkali, G.; Aksakal, M.; Kaya, S.Ö. Protective effects of carvacrol against diabetes-induced reproductive damage in male rats: Modulation of Nrf2/HO-1 signalling pathway and inhibition of Nf-kB-mediated testicular apoptosis and inflammation. Andrologia 2021, 53, e13899. [Google Scholar] [PubMed]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssière, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [PubMed]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar]
- Liao, S.M.; Tang, S.G.; Chang, M.N.; Qi, M.; Li, J.J.; Tan, B.; Gao, Q.; Zhang, S.; Li, X.Z.; Yin, Y.L.; et al. Chloroquine downregulation of intestinal autophagy to alleviate biological stress in early-weaned piglets. Animals 2020, 10, 290. [Google Scholar] [CrossRef]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182. [Google Scholar]
- Spalletta, S.; Flati, V.; Toniato, E.; Di Gregorio, J.; Marino, A.; Pierdomenico, L.; Marchisio, M.; D’Orazi, G.; Cacciatore, I.; Robuffo, I. Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression. PLoS ONE 2018, 13, e0206894. [Google Scholar]
- Arruri, V.K.; Gundu, C.; Kalvala, A.K.; Sherkhane, B.; Khatri, D.K.; Singh, S.B. Carvacrol abates NLRP3 inflammasome activation by augmenting Keap1/Nrf-2/p62 directed autophagy and mitochondrial quality control in neuropathic pain. Nutr. Neurosci. 2022, 25, 1731–1746. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.L.; Wu, Z.; Shang, J.; Xie, Z.X.; Chen, C.R.; Zhang, C.N. The effects of metformin on autophagy. Biomed. Pharmacother. 2021, 137, 111286. [Google Scholar] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-C.; Wang, H.-T.; Chen, C.-Y. Carvacrol Protects IPEC-J2 Cells from Oxidative Stress by Suppressing Autophagy. Int. J. Mol. Sci. 2025, 26, 3495. https://doi.org/10.3390/ijms26083495
Hsu M-C, Wang H-T, Chen C-Y. Carvacrol Protects IPEC-J2 Cells from Oxidative Stress by Suppressing Autophagy. International Journal of Molecular Sciences. 2025; 26(8):3495. https://doi.org/10.3390/ijms26083495
Chicago/Turabian StyleHsu, Ming-Chun, Han-Tsung Wang, and Ching-Yi Chen. 2025. "Carvacrol Protects IPEC-J2 Cells from Oxidative Stress by Suppressing Autophagy" International Journal of Molecular Sciences 26, no. 8: 3495. https://doi.org/10.3390/ijms26083495
APA StyleHsu, M.-C., Wang, H.-T., & Chen, C.-Y. (2025). Carvacrol Protects IPEC-J2 Cells from Oxidative Stress by Suppressing Autophagy. International Journal of Molecular Sciences, 26(8), 3495. https://doi.org/10.3390/ijms26083495





