1. Introduction
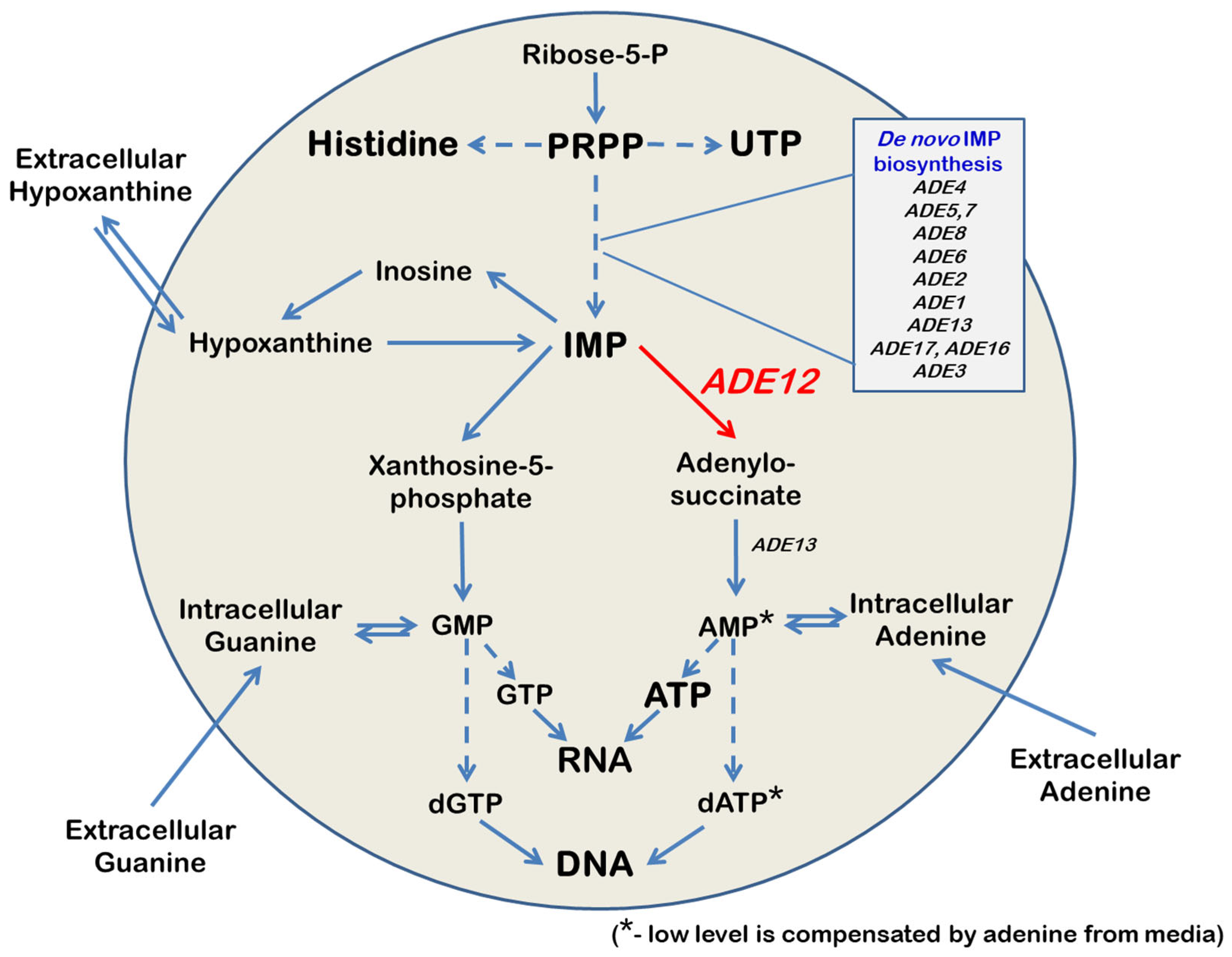
Adenylosuccinate synthetase (AdSS) is critical in cellular metabolism (
Figure 1). AdSS catalyzes the first committed step in the synthesis of adenosine 5′-monophosphate (AMP) [
1,
2,
3], a precursor of nucleic acids, and signaling and energy-transmitting molecules. AdSS utilizes inosine 5′-monophosphate (IMP) as a substrate to synthesize adenylosuccinate. In the second step, adenylosuccinate lyase cleaves adenylosuccinate to produce AMP [
1]. In addition to the de novo IMP biosynthesis [
4,
5], IMP can also be derived from extracellular purines through a branched salvage pathway [
6,
7]. Thus, IMP is a unique link between the salvage and de novo purine biosynthesis pathways (
Figure 1).
The purine biosynthesis pathway is highly conserved in all living organisms, and AdSS was found in bacteria, fungi, plants, and animals [
2,
3,
8,
9,
10]. While most organisms possess both de novo purine nucleotide biosynthesis and purine salvage pathways, certain pathogenic organisms, e.g.,
Helicobacter pylori [
11] and
Leishmania gonovani [
12], rely exclusively on the purine salvage pathway for purine nucleotide synthesis. The development of AdSS inhibitors is considered to be a promising therapeutic strategy in the fight against antibiotic-resistant strains of these parasites [
13,
14]. In humans, mutations in the
ADSSL1 gene, which encodes a muscle-specific isoform of AdSS, are associated with an ultra-rare myopathy. The ADSSL1 myopathy was first described by Park and colleagues in 2016 in Korean patients suffering from distal muscle weakness [
15]. Symptoms of the disease typically manifest during childhood and progress gradually with age. The affected patients experience distal muscle dysfunction and fatigue after mild physical exercise, which is later accompanied by facial muscle weakness. Cardiomyopathy and respiratory deficiency are also common in advanced disease stages, with death frequently caused by respiratory failure [
16,
17,
18]. There have been 124 genetically diagnosed cases by 2024, mainly in Asian populations, but it is estimated that there could be about 4500 undiagnosed ADSSL1 myopathy patients worldwide [
19].
Because of IMP’s central position in the purine metabolism pathway, it could be predicted that mutations in the genes encoding enzymes that control IMP utilization or biosynthesis will have pleotropic effects. This idea is undoubtedly true in the case of the
ADE12 gene encoding AdSS in yeast
Saccharomyces cerevisiae. Like most mutants in the de novo purine biosynthesis pathway, yeast
ade12 mutants are auxotrophic for adenine [
1,
20]. All auxotrophic
ade mutants, except
ade12, can grow on a medium containing hypoxanthine as the sole purine source and utilize it via the purine salvage pathway. Yeast cells can utilize external hypoxanthine and convert it to IMP; however, AMP biosynthesis requires AdSS activity. Therefore, adenine, but not hypoxanthine, can compensate for the growth defect of the
ade12 mutants on minimal media [
1,
20]. Additionally, yeast
ade12 mutants exhibit slow growth even on complete media, and spores harboring the
ade12 mutation demonstrate poor germination rates. It is possible that
ade12 deletion blocks the production of AMP and, subsequently, ATP, leading to energy depletion and the slowdown of all energy-consuming processes required for cell growth, including DNA synthesis. Mutations affecting the upstream steps of purine metabolism rescue these defects [
1,
20]. It is also known that the
ade12 mutants accumulate hypoxanthine and inosine and excrete them into the medium [
1]. Interestingly, in the food industry, IMP and GMP, combined with monosodium glutamate, increase the umami flavor. The performance of industrial strains of filamentous fungi
Ashbya gossypii, producing IMP and GMP for umami flavor, is improved by AdSS inhibition, which increases the excretion of IMP and GMP into the medium [
21]. In our previous experiments, we have found that
ade12 mutants are sensitive to mutagenic analogues of adenine (6-
N-hydroxylaminopurine—HAP and 2-amino-6-hydroxylaminopurine—AHA) [
22,
23]. We propose that the toxic effect of
ADE12 deletion and sensitivity to HAP and AHA are caused by the elevated levels of IMP, which can presumably be converted into ribo- and deoxyribonucleosidetriphosphates. The excess ITP and dITP can saturate Ham1p—pyrophosphatase, an essential enzyme for the destruction of the nucleoside triphosphates of hypoxanthine [
24,
25], and HAP in nucleotide pools, leading to the frequent incorporation of mutagenic dHAPTP and dAHATP into DNA during replication [
26,
27].
The main goal of this study is to elucidate the mechanisms by which an AdSS deficiency leads to a decrease in the growth rate and an increase in mutation rates in S. cerevisiae, thereby contributing to a deeper understanding of purine biosynthesis and its broader biological consequences.
3. Discussion
This study demonstrated that ade12 mutants lacking AdSS cannot grow in a standard YPD medium and possess a mutator phenotype. Both manifestations are suppressed by the addition of adenine to the growth medium or by the genetic blocking of de novo IMP biosynthesis. To elucidate the molecular mechanisms underlying the phenotypes of the ade12 mutation, we conducted genetic and biochemical analyses with a special focus on the nucleotide pools.
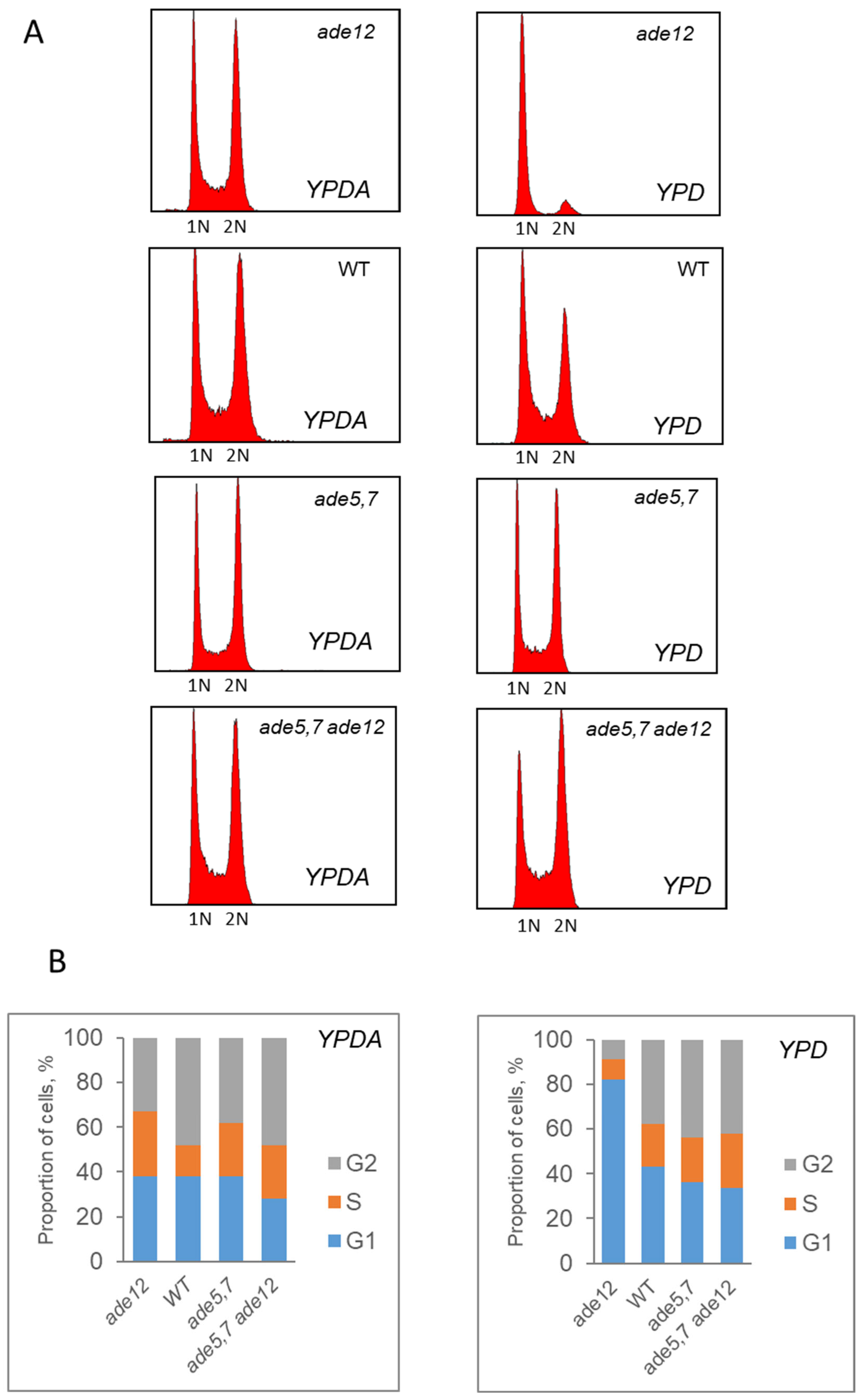
Flow cytometry data (
Figure 5), in agreement with the results of the culture growth experiments (
Figure 2), confirm that yeast cells bearing the
ade12 mutation cannot divide normally and do not progress through the cell cycle, stopping at the G1 phase. Similar phenotypes were also observed in the yeast strains with mutations in the other genes involved in AMP biosynthesis. For example, the
ade2-1 mutant (strain W303-1A) growing in a YPD medium without additional adenine supplementation had reduced cell division and budding rates, and a larger cell size [
33]. Similarly, the
ade8 mutants grown in the medium lacking adenine (SD-ade) demonstrated a two-fold decrease in the budding rate compared to the control (SD+ade), an increase in cell size, and a predominance of cells with 1n DNA content indicative of G1-phase arrest [
34].
We hypothesized that the most probable biochemical cause of cell division failure in the
ade12 strain is a deficiency of the key molecules maintaining this process. These molecules are nucleic acid constituents (nucleotides, nucleosides, and their derivatives), proteins, and energy-transferring compounds, such as ATP and NADH. The
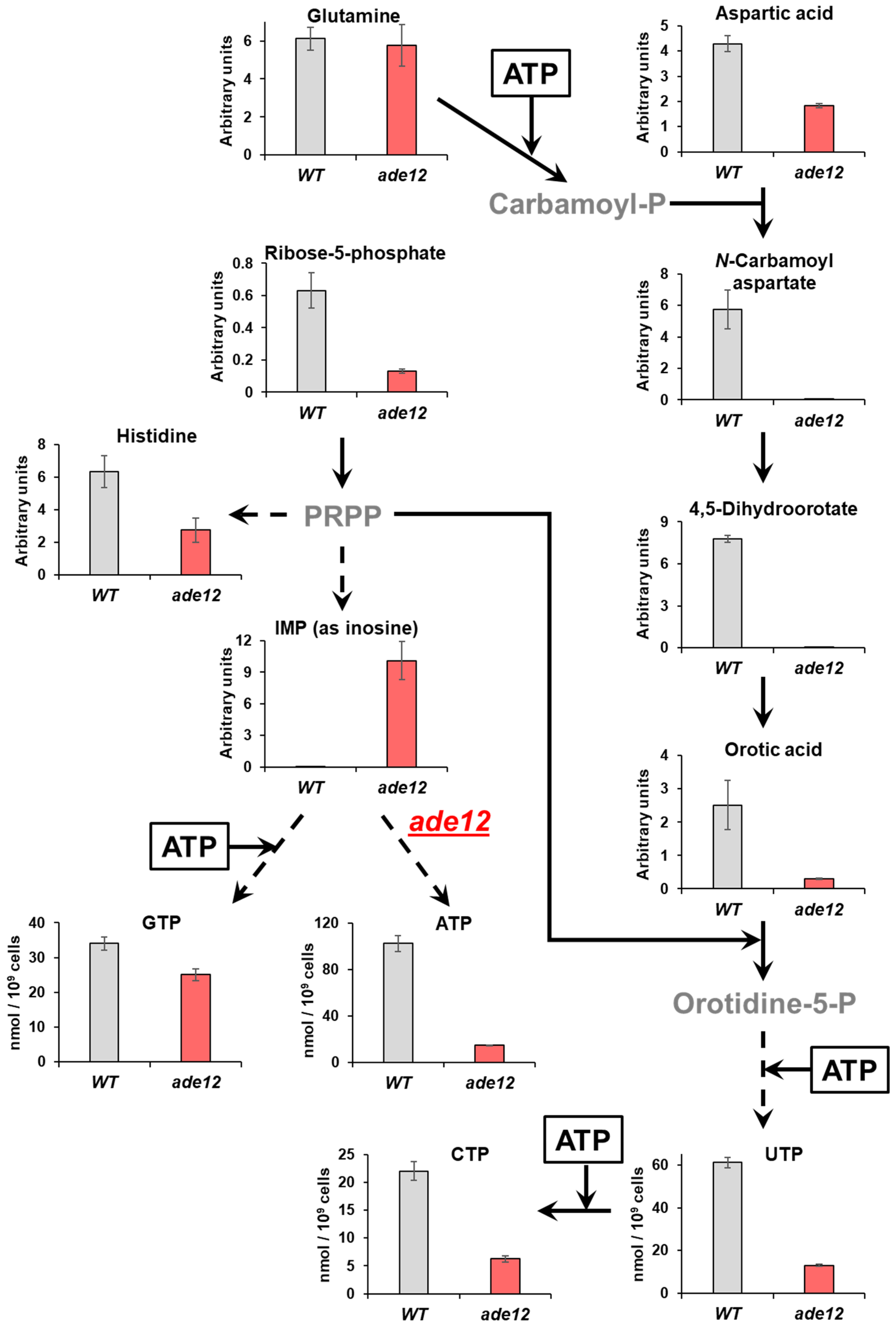
ADE12-encoded AdSS catalyzes one of the critical steps of purine nucleotide biosynthesis (
Figure 1). Therefore, it is logical to assume that the mutant cells may encounter an overall reduction and imbalance of the nucleotide pools. Our findings indicate that, in
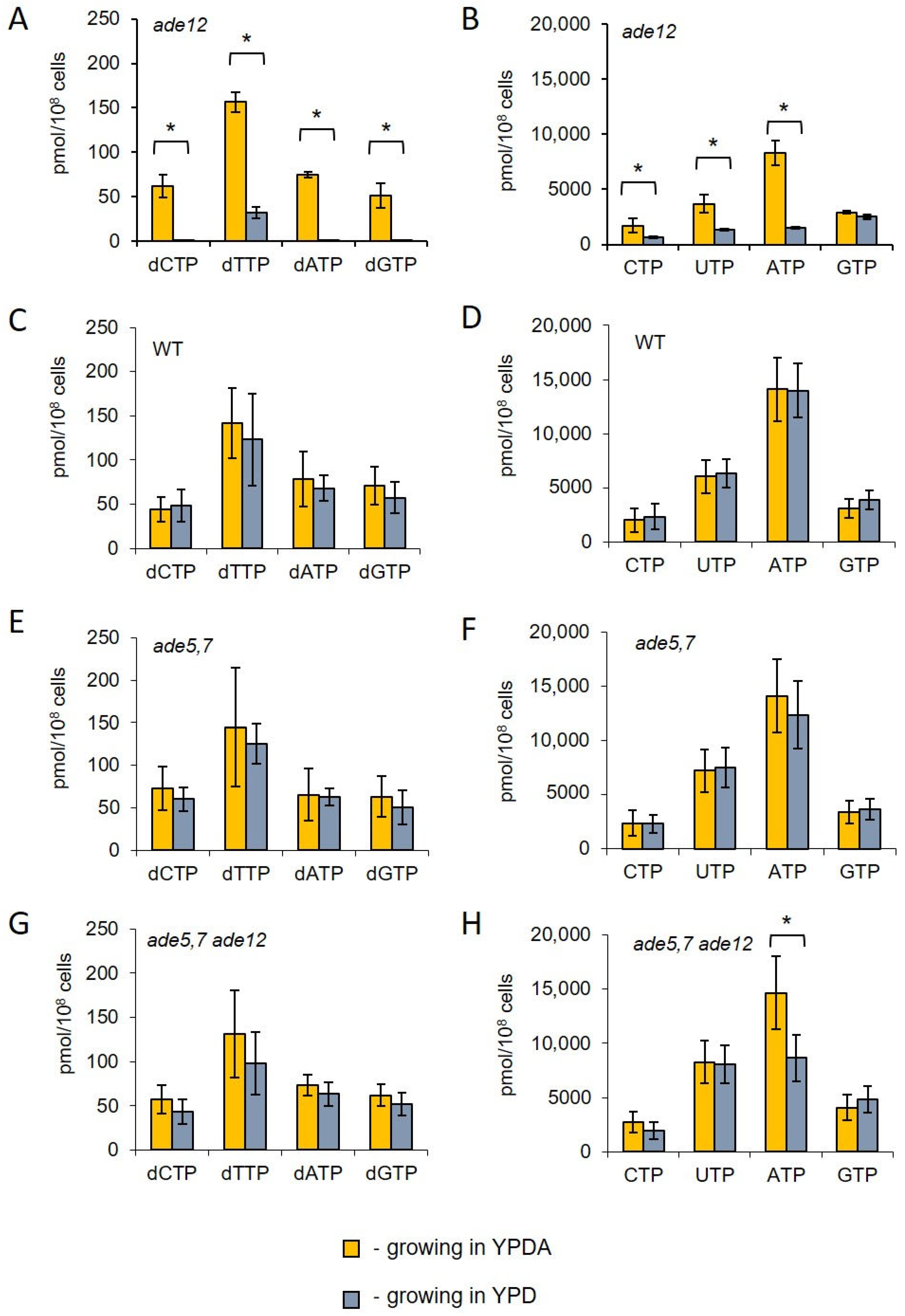
ade12 cells, NTP levels significantly decline when adenine is limited in the medium, and it is accompanied by an even more dramatic decrease in dNTP levels (
Figure 4). The activity of the ribonucleotide reductase responsible for producing dNTPs is regulated by the NTP/dNTP ratio [
35]. Consequently, as NTP levels decrease, dNTP production is also diminished, rendering DNA replication impossible.
The metabolomics data provide further insights into the
ade12-dependent changes in cell biochemistry. Our results showed that
ade12 mutation leads not only to an imbalance of the nucleotide pools, but also to a general impairment of nitrogen exchange in the yeast cells. A decrease in the content of many amino acids (including both proteinogenic ones and those involved in nucleotide metabolism) and the accumulation of their precursors (such as α-ketoglutaric acid) imply a general inhibition of amino acid and, consequently, protein biosynthesis. Most of the nitrogen released due to this inhibition in the mutant cells is invested into forming IMP constituents and derivatives (inosine, methylinosine, and hypoxanthine) (
Table 5). The accumulation of inosine and hypoxanthine in the yeast cells bearing the
ade12 mutation was seen earlier [
1]. Notably, the author, similar to us, believed that this inosine was derived from IMP and, thus, was a manifest of IMP accumulation. In WT cells, the excessive accumulation of IMP is prevented by the feedback inhibition of purine biosynthesis by ATP and ADP [
20,
36]. However, this regulation fails in the
ade12 mutants lacking extracellular adenine [
20].
Both the metabolite profiling and analysis of the nucleotide pools showed that the cells bearing a mutation in the purine biosynthesis pathway also exhibited considerable impairment in pyrimidine nucleotide biosynthesis. Thus, one of the most striking features of the metabolite profiles of
ade12 cells, along with inosine accumulation, was the three-orders-of-magnitude decrease in the content of 4,5-dihydroorotic acid, compared to the WT strain (
Table 5,
Figure 6). The purine and pyrimidine biosynthesis pathways are metabolically and energetically interconnected (
Figure 6) [
36], and we suggest that the main cause of the pyrimidine synthesis impairment in the
ade12 strain may be energy limitation. Indeed, the dramatic decrease in ATP content and a general decline in the amounts of sugar phosphates and nicotinamide (a NADH constituent) indicate that the
ade12 cells encounter severe energy deprivation (
Figure 4 and
Figure 6,
Table 5). A similar drop in ATP and ADP content was shown earlier in the
ade8 mutants growing under adenine limitation [
34]. We can see a decrease in the contents of the key intermediates of de novo pyrimidine biosynthesis at the next step after the first ATP-dependent reaction (formation of
N-carbamoyl aspartate from glutamine via carbamoyl phosphate) (
Figure 6). We could not detect carbamoyl phosphate in our samples, but its formation is likely suppressed in the
ade12 cells by an ATP shortage. This effect may be one of the reasons why glutamine is among the few amino acids whose content did not decline in the mutant cells. One more reason for the UTP and CTP decrease in the
ade12 strain may be the deficit of 5-phosphoribosyl pyrophosphate (PRPP), an intermediate enabling the metabolic crosstalk between the purine, pyrimidine, and histidine biosynthesis pathways (
Figure 1) [
36]. In the mutant cells, the main flux of PRPP is shifted towards the formation of IMP on account of the inhibition of the other pathway branches, supporting pyrimidine and histidine synthesis (
Figure 6).
The other conspicuous feature of the metabolite profiles of the
ade12 cells is an enhanced accumulation of storage carbohydrates (trehalose, glycerol, and mannitol) compared to the WT strain (
Table 5). Such an increase in carbon investment into metabolically relatively inert sugars and polyols is typical for non-dividing cells [
37]. It may be explained by the impairment of nitrogen exchange and the inhibition of the biosynthesis of N-containing compounds (nucleic acids, proteins, and their monomers) in the
ade12 mutants with cell division arrest. Similar effects were seen for yeast cells under various stress conditions. Trehalose, the major soluble carbohydrate in yeast cells, not only serves as a storage compound but also plays a protective role under stress conditions [
37]. Trehalose helps to maintain cellular integrity under heat, cold, desiccation, dehydration, and oxidation stresses primarily by preventing protein denaturation. Numerous studies, initiated by the pioneering work of Singer and Lindquist [
38], have demonstrated that trehalose interacts with proteins and functions as a chemical chaperone, recovering protein aggregation and maintaining proteins in a partially folded state to facilitate their refolding by chaperones. Additionally, trehalose has been shown to prevent the amyloid aggregation of huntingtin [
39] and amyloid beta [
40]. Glycerol also plays a protective role in yeast: cells of
S. cerevisiae rapidly accumulate glycerol when exposed to a highly osmolar medium to counteract dehydration [
41,
42]. We may conclude that the adenine limitation in
ade12 mutant leads to the activation of the protective mechanisms that typically serve to safeguard cells from adverse environmental conditions. According to the literature, mutations in the other
ADE genes (
ade2 and
ade8) also induce trehalose accumulation in the yeast cells, which is accompanied by a decrease in protein and nucleic acid content [
33,
34]. A similar phenotype has also been documented in yeast cells under the limitations of other compounds, such as uracil and leucine [
43]. Our data on the changes in the carbohydrate composition in
ade12 cells are consistent with the findings from other studies indicating that the majority of yeast genes involved in purine biosynthesis (
ADE13,
ADE1,
ADE2,
ADE4,
ADE5,7,
ADE6,
ADE8, and
ADE16) are involved in the metabolism of carbon sources [
44,
45]. It has been shown that
ade13 mutants cannot grow in a complete medium containing glucose, but can grow in medium containing glycerol or ethanol as the sole carbon source. Additional mutations (
ade1,
ade2,
ade4, and
ade5,7) occurring at earlier stages of purine biosynthesis alleviate the conditional lethality associated with the
ade13 mutation [
44]. Mutations in the
ADE2 gene result in the inability of yeast cells to utilize hypoxanthine as a purine source in a glycerol medium. Mutations in
ade4,
ade5,
ade8,
ade6, and
ade7 suppress this phenotype of the
ade2 mutation [
45]. It has been shown that the expression of the
ADE16 gene, but not
ADE17, depends on the type of carbon source in the medium. Specifically, the amount of Ade16p protein is higher in cells grown on non-fermentable carbon sources than in cells grown in the glucose medium [
46]. Thus, the
ADE genes, including
ADE12, either directly regulate glycolysis or are involved in shaping cellular responses to the type of carbon source in the medium. The effect is explained by the need to coordinate the cell division with the nucleotide synthesis rates required for DNA replication and other cellular processes, depending on the quality of the carbon sources present in the environment [
44]. The pivotal role of AdSS in coordinating purine and carbohydrate metabolism was supported by findings from a metabolite screening of endogenous factors that mediate glucose-induced insulin secretion in the rat insulinoma cell line 832/13. Glucose stimulation resulted in insulin secretion, decreased IMP levels, and increased adenylosuccinate, a product of AdSS [
47].
Biochemical studies of the
ade12 cells grown in the presence of extra adenine or with the additional
ade5,7 mutation enabling the block of de novo IMP biosynthesis showed that both these factors effectively mitigate the most conspicuous
ade12-dependent metabolic disorders, such as an IMP accumulation and 4,5-dihydroorotate decrease, as well as nucleotide pool imbalance (
Table 6 and
Table S4,
Figure 4). Though the double
ade5,7 ade12 mutants still have a reduced ATP content when grown without the adenine addition, the difference is much smaller than that for the single
ade12 mutants (
Figure 4). The mitigating effect of adenine supplementation may be explained by the restoration of the cell AMP pool due to the salvage pathway consuming extracellular adenine (
Figure 1) [
48,
49,
50]. The mechanism of the ameliorating influence of the
ade5,7 mutation is more complex, as this mutation itself considerably affects yeast cell metabolism (
Figure S4). The metabolomic data imply that the main target of the
ade12-dependent changes occurring on the background of the
ade5,7 mutation is again the nitrogen exchange, as double mutants specifically accumulate multiple nitrogen-rich metabolites, such as polyamines, arginine, and histidine (
Table S4). However, this branch of our research goes beyond the scope of this paper and deserves a separate, more in-depth study, which is an ongoing plan in our laboratory.
Another question we addressed in the current research is the mechanism of the mutator phenotype of the
ade12 mutants. It is important to note that, in this study, we have shown, for the first time, that spontaneous mutagenesis is moderately increased in the strains bearing the
ade12 mutation; meanwhile, earlier, the
ade12 strain was also reported to have an increased mutation frequency induced by the mutagenic hypoxanthine analogues HAP and AHA [
22,
23]. Below, we discuss three non-contradictory explanations for the spontaneous mutator phenotype in the
ade12 strains.
First is the direct role of
ADE12 in maintaining the accuracy of genome replication.
S. cerevisiae AdSS specifically binds to ss-DNA containing autonomous replication sequences (ARSs) [
51]. This binding inhibits AdSS enzymatic activity and occurs only if AdSS is phosphorylated. However, the activity is specific for AdSS from
S. cerevisiae, while AdSS from
E. coli and
Dictiostelium discoideum showed only 1% of the activity found in the yeast homologue. Thus, AdSS may regulate genome replication. If this is actually the case, then, in the absence of AdSS, the accuracy of replication and repair closely related to the stages of the cell cycle may decrease and lead to an increased level of spontaneous mutagenesis. There are several arguments against this hypothesis. Thus, ss-DNA binding activity was proven only in vitro, and it is unclear how the results correspond to AdSS properties in living cells. AdSS is located in the cytoplasm [
52], thus making its DNA-binding activity irrelevant. Our findings do not support this mechanism. We have shown that elevated mutagenesis is suppressed in the
ade12 mutant in YPDA. Moreover, the overexpression of the
ADE12 gene does not affect the spontaneous mutation frequency. These results suggest that the level of spontaneous mutagenesis does not depend on the physical presence of AdSS in the origins of replication, but rather on AdSS’s role in purine metabolism.
A second assumption would be that the elevation of IMP in the cytoplasm of
ade12 mutants may promote the synthesis of dITP, which can then be incorporated into DNA, potentially leading to increased mutagenesis. However, the experimental data do not support this hypothesis, as the levels of inosine incorporation into DNA were reported to remain unchanged in the AdSS-deficient strains of both yeast and bacteria [
53]. Moreover, it was shown in
E. coli that dITP incorporation into DNA is non-mutagenic due to inosine’s strong pairing preference for cytosine [
54]. This may be the case for yeast as well, as
S. cerevisiae lacks inosine glycosylase due to the absence of a requirement to protect DNA from inosine incorporation.
A third idea is the indirect role of the missing AdSS in the increased mutation rate in the
ade12 strain. We have shown that the mutagenic effect observed in the
ade12 strain entirely depends on Pol ζ’s activity. Therefore, it appears that elevated IMP levels do not directly contribute to increased mutagenesis. In the
ade12 mutants grown in the YPD medium, dNTP pools and the ATP content are insufficient to maintain replication, resulting in cell cycle arrest. Under conditions of replication stalling, specialized DNA polymerase is more likely to be recruited for replication, similar to what occurs when yeast cells are incubated in a medium with hydroxyurea or during defective-replisome-induced mutagenesis (DRIM) [
31]. This notion is supported by evidence that Pol ζ, unlike replicative polymerases, is active at low dNTP levels [
55]. Interestingly, it was shown before that adenine starvation elevates the mutation rate in the yeast strains lacking the proofreading activity of DNA polymerase δ (
pol3-01) [
56]. Thus, through various mechanisms involving different DNA polymerases, adenine starvation in yeast can lead to an increased frequency of mutagenesis. The connections we have identified between nucleotide biosynthesis and elevated Pol ζ-dependent mutagenesis open up prospects for the discovery and development of nucleotide-analogue anticancer drugs [
57].
We conclude that the low viability of ade12 mutants in the standard YPD or synthetic medium low in adenine results from the impaired cellular energy metabolism, leading to a cascading dysregulation of key metabolic processes—the metabolism of nucleotides, carbohydrates, and amino acids. These metabolic perturbations may account for the cell division arrest observed in the ade12 yeast strains. The mutagenic effect observed in ade12 mutants appears to be a consequence of the slowed replication due to the insufficient energy, nucleotides, and proteins. Under these conditions, genomic DNA becomes more accessible to error-prone Pol ζ, increasing the likelihood of mutations. ADSSL1 myopathy in humans presents a similar challenge regarding energy metabolism, where ATP deficiency is a key issue. The parallels between the metabolic dysregulation observed in ade12 yeast mutants and human myopathies highlight the importance of understanding connections between energy metabolism and biosynthetic pathways in both yeast and higher organisms for the potential identification of therapeutic targets.