Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure–Activity Relationship Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Insecticidal Action of EOs
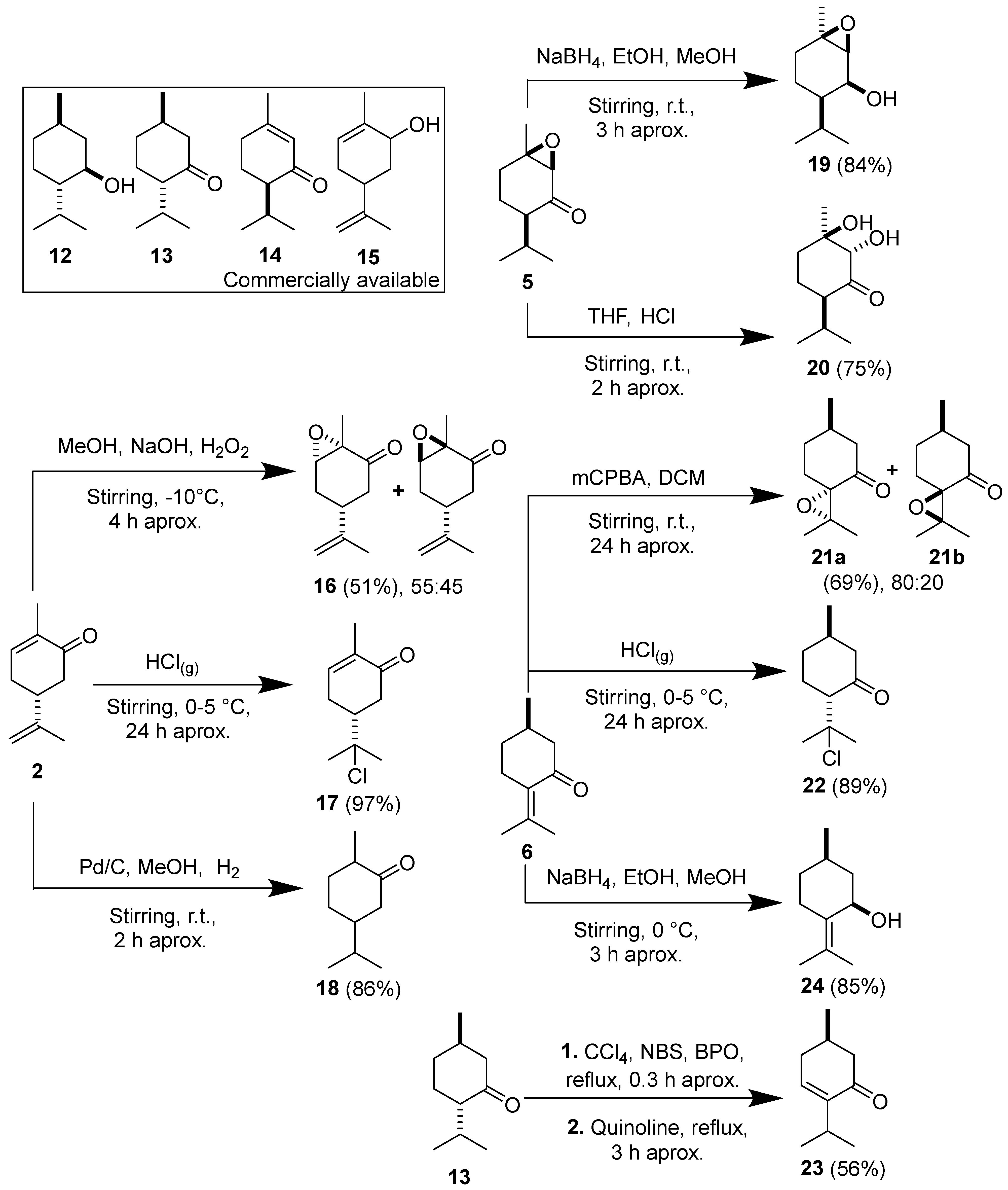
2.2. Determination of Insecticidal Action of Some Chemical Constituents Present in the EOs
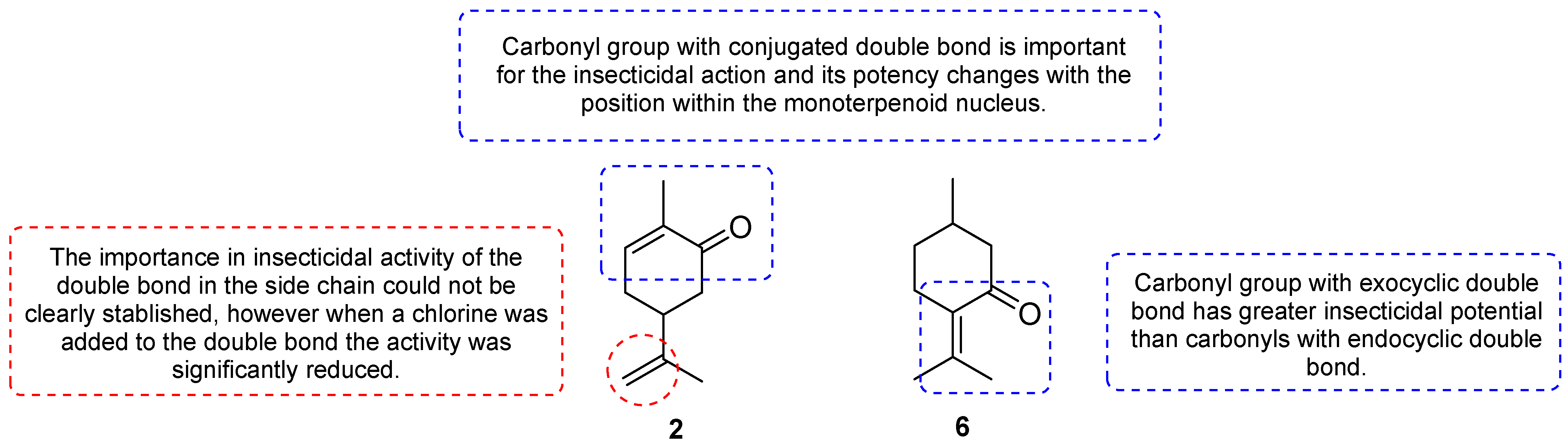
2.3. Preliminary Structure–Activity Relationship Study of Monoterpenoids Against S. zeamais and T. castaneum
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection of Plant Material and Extraction EOs
3.2.1. Plant Material
3.2.2. Extraction of EOs
3.3. Chemical Characterization of Essential Oils
3.3.1. Sample Preparation
3.3.2. Analysis by GC–MS
3.3.3. Tentative Identification of the Chemical Composition of EOs
3.4. Obtaining of the Major Chemical Constituents Present in the EOs (1–11)
3.5. Obtention of Chemical Compounds 12 to 24
3.6. Assessment of Insecticidal Activity of EOs and Compounds
3.6.1. Insect Rearing
3.6.2. Fumigant Toxicity Assay
3.6.3. Topical Contact Toxicity Assay
3.6.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| δ | Chemical shift |
| δC | Carbon shift |
| δH | Hydrogen shift |
| °C | Degree Celsius |
| 13C-NMR | Carbon Nuclear Magnetic Resonance |
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| approx | Approximately |
| APT | Attached Proton Test |
| BPO | Benzoyl peroxide |
| bs | broad singlet |
| CDCl3 | Deuterated chloroform |
| d | Doublet |
| DCM | Dichloromethane |
| dd | Double doublet |
| ddd | double double doublet |
| EI | Electron impact |
| EO | Essential oil |
| EOs | Essentials oils |
| EtOAc | Ethyl Acetate |
| eV | Electron volt |
| Exp | Experimental |
| Exp LRI | Experimental Linear Retention Index |
| F254 | Fluorescence indicator at a wavelength of 254 nm |
| FC | Flash Chromatography |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| GCMS-TQ | Gas Chromatography–Mass Spectrometry with Triple Quadrupole |
| Hz | Hertz |
| ICA | Instituto Colombiano Agropecuario |
| J | Coupling constant |
| L | Liter |
| LC50 | Median Lethal Concentration |
| LD50 | Median Dose Concentration |
| LRI | Linear retention index |
| m | Multiplet |
| M | Molar |
| m/z | Mass/charge |
| mCPBA | Metachloroperbenzoic acid |
| MeOH | Methanol |
| mg | Milligram |
| MHz | Megahertz |
| min | Minutes |
| mL | Milliliter |
| mmol | Millimole |
| NBS | N-bromosuccinimide |
| NMR | Nuclear Magnetic Resonance |
| NOESY | Nuclear Overhauser Enhancement Spectroscopy |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| Pd/C | Palladium supported on carbon |
| PEG | Polyethylene glycol |
| ppm | Parts per million |
| PTFE | Polytetrafluoroethylene |
| q | Quartet |
| r.t. | Room temperature |
| Ref | Reference |
| Ref LRI | Reference linear retention index |
| RH | Relative Humidity |
| Rt | Time retention |
| s | Singlet |
| t | Triplet |
| td | triple doublet |
| tdd | triple double doublet |
| THF | Tetrahydrofuran |
| TLC | Thin-layer chromatography |
| UV | Ultraviolet |
| μL | Microliter |
References
- Gashu, D.; Nalivata, P.C.; Amede, T.; Ander, E.L.; Bailey, E.H.; Botoman, L.; Chagumaira, C.; Gameda, S.; Haefele, S.M.; Hailu, K.; et al. The Nutritional Quality of Cereals Varies Geospatially in Ethiopia and Malawi. Nature 2021, 594, 71–76. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.A.; Wahba, T.F.; Shaarawy, N.; Moustafa, F.I.; Guedes, R.N.C.; Dewer, Y. Stored Grain Pest Prevalence and Insecticide Resistance in Egyptian Populations of the Red Flour Beetle Tribolium Castaneum (Herbst) and the Rice Weevil Sitophilus oryzae (L.). J. Stored. Prod. Res. 2020, 87, 101611. [Google Scholar] [CrossRef]
- Berhe, M.; Subramanyam, B.; Chichaybelu, M.; Demissie, G.; Abay, F.; Harvey, J. Post-Harvest Insect Pests and Their Management Practices for Major Food and Export Crops in East Africa: An Ethiopian Case Study. Insects 2022, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.S.; Pereira, E.J.G.; Cordeiro, E.M.G.; Braga, L.S.; Guedes, R.N.C. Insecticide Resistance, Mixture Potentiation and Fitness in Populations of the Maize Weevil (Sitophilus zeamais). Crop Prot. 2011, 30, 1655–1666. [Google Scholar] [CrossRef]
- Sebayang, A.; Rohimatun; Salim; Rubiana, R.; Sipi, S.; Manwan, S.W.; Fattah, A.; Arrahman, A.; Yasin, M.; Saenong, M.S. Sitophilus zeamais (Motschulsky): The Primary Obstacles in the Maize Quality and Quantity. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012089. [Google Scholar] [CrossRef]
- Peschiutta, M.L.; Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Zygadlo, J.A.; Zunino, M.P. Fumigant Toxicity of Essential Oils against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae): A Systematic Review and Meta-Analysis. J. Pest Sci. 2021, 95, 1037–1056. [Google Scholar] [CrossRef]
- Phokwe, O.J.; Manganyi, M.C. Medicinal Plants as a Natural Greener Biocontrol Approach to “The Grain Destructor” Maize Weevil (Sitophilus zeamais) Motschulsky. Plants 2023, 12, 2505. [Google Scholar] [CrossRef]
- Ali, S.M.A.; Ammad, A.M.; Ahmad, S.K.; Bilal, I.M.; Abdullah, A.; Talal, I.; Zulfiqar, M.; Ali, S.M.A.; Ammad, A.M.; Ahmad, S.K.; et al. Effectiveness of Different Plant Extracts along with New Chemistry Insecticide against Tribolium castaneum. GSC Biol. Pharm. Sci. 2020, 13, 95–100. [Google Scholar] [CrossRef]
- Ahmad, F.; Iqbal, N.; Zaka, S.M.; Qureshi, M.K.; Saeed, Q.; Khan, K.A.; Ghramh, H.A.; Ansari, M.J.; Jaleel, W.; Aasim, M.; et al. Comparative Insecticidal Activity of Different Plant Materials from Six Common Plant Species against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What Can We Learn from Commercial Insecticides? Efficacy, Toxicity, Environmental Impacts, and Future Developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Grassi, E.; Cocozza di Montanara, A.; Guidi, L.; Sandulli, R.; Manachini, B.; Semprucci, F. Essential Oils and Their Applications in Agriculture and Agricultural Products: A Literature Analysis through VOSviewer. Biocatal. Agric. Biotechnol. 2022, 45, 102502. [Google Scholar] [CrossRef]
- Mssillou, I.; Saghrouchni, H.; Saber, M.; Zannou, A.J.; Balahbib, A.; Bouyahya, A.; Allali, A.; Lyoussi, B.; Derwich, E. Efficacy and Role of Essential Oils as Bio-Insecticide against the Pulse Beetle Callosobruchus maculatus (F.) in Post-Harvest Crops. Ind. Crops Prod. 2022, 189, 115786. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides Based on Plant Essential Oils: A Short Overview. Z. Naturforsch C. J. Biosci. 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Achimón, F.; Peschiutta, M.L.; Brito, V.D.; Beato, M.; Pizzolitto, R.P.; Zygadlo, J.A.; Zunino, M.P. Exploring Contact Toxicity of Essential Oils against Sitophilus zeamais through a Meta-Analysis Approach. Plants 2022, 11, 3070. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Abbasi, A.M.; Abid, M.; Mozūratis, R.; Alwahibi, M.S.; Elshikh, M.S. Pesticidal Potential of Some Wild Plant Essential Oils against Grain Pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab. J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Oviedo-Sarmiento, J.S.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Cuca Suárez, L.E.; Herrera Daza, E.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Fumigant Toxicity and Biochemical Effects of Selected Essential Oils toward the Red Flour Beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2021, 179, 104941. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Nagles Galeano, L.J.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Herrera Daza, E.; Suárez, L.E.C.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effects of Essential Oils from 24 Plant Species on Sitophilus zeamais Motsch (Coleoptera, Curculionidae). Insects 2021, 12, 532. [Google Scholar] [CrossRef]
- Chahal, K.; Monika; Kumar, A.; Bhardwaj, U.; Kaur, R. Chemistry and Biological Activities of Anethum graveolens L. (Dill) Essential Oil: A Review. J. Pharmacogn. Phytochem. 2017, 6, 295–306. [Google Scholar]
- Jana, S.; Shekhawat, G. Anethum Graveolens: An Indian Traditional Medicinal Herb and Spice. Pharmacogn. Rev. 2010, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Blazquez, M.A. Chemical Composition of the Essential Oil of Anethum graveolens Aerial Parts. J.Essent. Oil-Bear. Plants. 2014, 17, 1219–1223. [Google Scholar] [CrossRef]
- Soltani, R.; Ktari, R.; Barhoumi, L.; Chouaibi, M.H. Chemical Composition and Bio-Insecticidal Activity of the Dill, Anethum graveolens, Essential Oils against the Red Flour, Tribolium castaneum. Tunis J. Plant. Prot. 2024, 19, 27–42. [Google Scholar] [CrossRef]
- Attique Babri, R.; Khokhar, I.; Mahmood, Z.; Mahmud, S. Chemical Composition And Insecticidal Activity Of The Essential Oil Of Anethum graveolens L. Sci. Int. 2012, 24, 453–455. [Google Scholar]
- Azuero Montej, J.D. Caracterización Química de Aceites Esenciales Comerciales y Evaluación de Su Capacidad Antialimentaria y Fumigante Contra Tribolium castaneum. Bachelor’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2019. [Google Scholar]
- Gleiser, R.M.; Bonino, M.A.; Zygadlo, J.A. Bioactividad De Aceites Esenciales De Minthostachys mollis Contra Mosquitos. Bol. Latinoam. Caribe Plantas Med. Aromat. 2007, 6, 350–351. [Google Scholar]
- Torrenegra-Alarcón, M.; Granados-Conde, C.; Durán-Lengua, M.; León-Méndez, G.; Yáñez-Rueda, X.; Martínez, C.; Pájaro-Castro, N. Composición Química y Actividad Antibacteriana Del Aceite Esencial de Minthostachys mollis. Orinoquia 2016, 20, 69–74. [Google Scholar] [CrossRef]
- Linares-Otoya, V. Consideraciones Para El Uso y Estudio de La “Muña” Peruana Minthostachys mollis (Benth.) Griseb y Minthostachys setosa (Briq.) Epling. Ethnobot. Res. Appl. 2020, 19, 1–9. [Google Scholar] [CrossRef][Green Version]
- Benites, J.; Guerrero-Castilla, A.; Salas, F.; Martinez, J.L.; Jara-Aguilar, R.; Venegas-Casanova, E.A.; Suarez-Rebaza, L.; Guerrero-Hurtado, J.; Calderon, P.B. Chemical Composition, in Vitro Cytotoxic and Antioxidant Activities of the Essential Oil of Peruvian Minthostachys mollis Griseb. Bol. Latinoam. Caribe Plantas Med. Aromát. 2018, 6, 566–574. [Google Scholar]
- Mora, F.D.; Araque, M.; Rojas, L.B.; Ramírez, R.; Silva, B.; Usubillaga, A. Chemical Composition and in Vitro Antibacterial Activity of the Essential Oil of Minthostachys mollis (Kunth) Griseb Vaught from the Venezuelan Andes. Nat. Prod. Commun. 2009, 4, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Ortiz-Sánchez, J.M.; Palomino-Pacheco, M.; Hilario-Vargas, H.J.; Herrera-Calderón, O.; Hilario-Vargas, J. Potential Toxicity of the Essential Oil from Minthostachys mollis: A Medicinal Plant Commonly Used in the Traditional Andean Medicine in Peru. J. Toxicol. 2019, 2019, 1987935. [Google Scholar] [CrossRef]
- Álvarez S., D.E.; Botina, J.J.A.; Ortiz, C.A.J.; Botina, J.L.L. Nematicide Evaluation of the Essential Oil from Tagetes zypaquirensis in the Control of the Nematode Meloidogyne Spp. Rev. Cienc. Agríc. 2016, 33, 22–33. [Google Scholar] [CrossRef]
- Benavente Mina, Q. Evaluación de la Actividad Biocida del Aceite Esencial de Hojas de Muña (Minthostachys mollis) en el Gorgojo de Maíz (Sitophilus zeamais). Bachelor’s Thesis, Universidad Nacional Micaela Bastidas de Apurímac, Abancay, Peru, 2018. [Google Scholar]
- Tucker, A.O.; Libbey, L.M. Essential Oil of Satureja viminea L. (Lamiaceae). J. Essent. Oil Res. 2000, 12, 283–284. [Google Scholar] [CrossRef]
- Vila, R.; Clcció, J.F.; Cañigueral, S. Essential Oil of Satureja viminea L. from Costa Rica. J. Essent. Oil Res. 2000, 12, 279–282. [Google Scholar] [CrossRef]
- Zapata-Maldonado, C.I.; Serrato Cruz, M.A.; Emmanuel, I.; Naranjo Puente, B. Chemical Compounds of Essential Oil of Tagetes Species of Ecuador. Ecorfan J. 2015, 1, 19–26. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–809. [Google Scholar]
- Pherobase The Pherobase: Database of Pheromones and Semiochemicals | The World Largest Database of Behavioural Modifying Chemicals. Available online: https://pherobase.com/ (accessed on 6 May 2024).
- List of Compounds in the Essential Oil Components Library. Available online: https://diabloanalytical.com/ms-software/essentialoilcomponentsbygcms/list-of-compounds-in-the-essential-oil-components-database/ (accessed on 30 May 2024).
- Charles, D.J.; Simon, J.E.; Widrlechner, M.P. Characterization of Essential Oil of Dill (Anethum graveolens L.). J. Essent. Oil Res. 1995, 7, 11–20. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Tito, M.A.; Cartagena-Cutipa, R.; Flores-Valencia, E.; Collantes-Diaz, I. Chemical Composition and Antimicrobial Activity of Essential Oil from Minthostachys mollis against Oral Pathogens. Rev. Cubana Estomatol. 2021, 58, 1–7. [Google Scholar]
- Elechosa, M.A.; Molina, A.M.; Juárez, M.A.; van Baren, C.M.; Di Leo Lira, P.; Bandoni, A.L. Estudio Comparativo Del Aceite Esencial de Minthostachys mollis (KUNTH.) Griseb “Peperina” Obtenido de Colectas En 21 Poblaciones de Las Provincias de Tucuman, Cordoba, San Luis y Catamarca. Bol. Latinoam. Caribe Plantas Med. Aromat. 2007, 6, 244–245. [Google Scholar]
- Stashenko, E.E.; Gutiérrez-Avella, D.M.; Martínez, J.R.; Manrique-López, D.L. Análisis Por GC/FID y GC/MS de La Composición Química y Estudio de La Actividad Antioxidante de Los Metabolitos Secundarios Volátiles, Aislados Por Diferentes Técnicas, de Satureja viminea L. Cultivada En Colombia. Sci. Chromatogr. 2017, 9, 25–39. [Google Scholar] [CrossRef]
- Escobar, P.; Herrera, L.; Leal, M.; Duran, C.; Stashenko, E. Composición Química y Actividad Anti- Tripanosomal de Aceites Esenciales Obtenidos de Tagetes (Fam. Asteraceae), Recolectados En Colombia. Rev. Univ. Ind. Santander. Salud. 2009, 41, 280–286. [Google Scholar]
- Chaubey, M.K. Insecticidal Activities of Anethum graveolens L. and Illicium verum Hook. f. Essential Oils against Sitophilus zeamais Motschulsky. Rev. Cien. Agrí. 2021, 38, 38–49. [Google Scholar] [CrossRef]
- Perdomo Cedeño, L.F. Determinación del Potencial Insecticida y Repelente de Mezclas de Constituyentes Químicos Bioactivos Presentes en Aceites Esenciales para el Control de Tribolium castaneum. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2020. [Google Scholar]
- Nagles Galeano, L.J. Estudio de la Acción Insecticida de Constituyentes Químicos Presentes en Aceites Esenciales y su Efecto sobre Enzimas Desintoxicantes y de Función Motora para Sitophilus zeamais. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2021. [Google Scholar]
- Pizzolitto, R.P.; Herrera, J.M.; Zaio, Y.P.; Dambolena, J.S.; Zunino, M.P.; Gallucci, M.N.; Zygadlo, J.A. Bioactivities of Ketones Terpenes: Antifungal Effect on F. Verticillioides and Repellents to Control Insect Fungal Vector, S. Zeamais. Microorganisms 2015, 3, 851–865. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene Ketones as Natural Insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche 2016, 2016, 4595823. [Google Scholar] [CrossRef]
- Ma, G.; Xu, C.; Yang, S.; Zhu, Y.; Ye, S.; Qin, R.; Zeng, C.; Du, W.; Zhang, H.; Chen, J. The Trading of Space for Time under Weakly Activated Catalysis: Expeditious Synthesis of β-NH2 Alcohols via a Direct Ammonolysis of Epoxides with Ammonia. Green Chem. 2023, 25, 720–727. [Google Scholar] [CrossRef]
- Oguro, D.; Mori, N.; Takikawa, H.; Watanabe, H. A Novel Synthesis of (−)-Callicarpenal. Tetrahedron 2018, 74, 5745–5751. [Google Scholar] [CrossRef]
- Shull, B.K.; Koreeda, M. Halogen Effect on the Ring Opening of Pulegone Hydrohalides. J. Org. Chem. 1990, 55, 2249–2251. [Google Scholar]
- Chitiva-Chitiva, L.C.; Ladino-Vargas, C.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa. Molecules. 2021, 26, 3256. [Google Scholar] [CrossRef]
- Paquette, L.A.; Doehner, R.F. Synthesis of Optically Active Triazolinediones and Examination of Their Utility for Inducing Asymmetry in Diels-Alder Cycloaddition Reaction. J. Org. Chem. 1980, 45, 5105–5113. [Google Scholar] [CrossRef]
- Tymann, D.; Bednarzick, U.; Iovkova-Berends, L.; Hiersemann, M. Progress toward the Total Synthesis of Gukulenin A: Photochemically Triggered Two-Carbon Ring Expansion Key to α-Tropolonic Ether Synthesis. Org. Lett. 2018, 20, 4072–4076. [Google Scholar] [PubMed]
- Plummer, C.M.; Gericke, R.; Kraft, P.; Raynor, A.; Froese, J.; Hudlický, T.; Rook, T.J.; Jones, O.A.H.; Hügel, H.M. Synthesis of Saturated Benzodioxepinone Analogues: Insight into the Importance of the Aromatic Ring Binding Motif for Marine Odorants. European J. Org. Chem. 2015, 2015, 486–495. [Google Scholar] [CrossRef]
- Maestro, A.; Nagy, B.S.; Ötvös, S.B.; Kappe, C.O. A Telescoped Continuous Flow Enantioselective Process for Accessing Intermediates of 1-Aryl-1,3-Diols as Chiral Building Blocks. J. Org. Chem. 2023, 88, 15523–15529. [Google Scholar]
- Olivier, A.; Müller, D.S. Hydrochlorination of Alkenes with Hydrochloric Acid. Org. Process Res. Dev. 2024, 28, 305–309. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.; Choi, W.J.; Seo, H.; Kim, P.; Kim, G.J.; Kim, Y.W.; Shin, J. Thermoset Elastomers Derived from Carvomenthide. Biomacromolecules 2015, 16, 246–256. [Google Scholar]
- Su, S.; Lin, C.; Zhai, H. Divergent Total Syntheses of (−)-Daphnezomines A and B and (+)-Dapholdhamine B. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303402. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Emsen, B.; Kordali, S. İnsecticidal Effects of Monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar] [CrossRef]
- Aggarvval, K.K.; Tripathi, A.K.; Ahmad, A.; Frajapati, V.; Verma, N.; Kumar, S. Toxicity of L-Menthol and Its Derivatives against Four Storage Insects. Int. J. Trop. Insect Sci. 2001, 21, 229–235. [Google Scholar] [CrossRef]
- Saad, M.M.G.; El-Deeb, D.A.; Abdelgaleil, S.A.M. Insecticidal Potential and Repellent and Biochemical Effects of Phenylpropenes and Monoterpenes on the Red Flour Beetle, Tribolium castaneum Herbst. Environ. Sci. and Pollut. Res. Int. 2019, 26, 6801–6810. [Google Scholar]
- Bossou, A.D.; Ahoussi, E.; Ruysbergh, E.; Adams, A.; Smagghe, G.; De Kimpe, N.; Avlessi, F.; Sohounhloue, D.C.K.; Mangelinckx, S. Characterization of Volatile Compounds from Three Cymbopogon Species and Eucalyptus Citriodora from Benin and Their Insecticidal Activities against Tribolium castaneum. Ind. Crops Prod. 2015, 76, 306–317. [Google Scholar] [CrossRef]
- Rice, P.J.; Coats, J.R. Insecticidal Properties of Several Monoterpenoids to the House Fly (Diptera: Muscidae), Red Flour Beetle (Coleoptera: Tenebrionidae), and Southern Corn Rootworm (Coleoptera: Chrysomelidae). J Econ. Entomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Zunino, M.P.; Massuh, Y.; Pizzollito, R.P.; Dambolena, J.S.; Gañan, N.A.; Zygadlo, J.A. Fumigant Toxicity of Five Essential Oils Rich in Ketones against Sitophilus zeamais (Motschulsky). Agriscientia. 2014, 31, 35–41. [Google Scholar] [CrossRef]
- Santos, S.R.L.; Melo, M.A.; Cardoso, A.V.; Santos, R.L.C.; de Sousa, D.P.; Cavalcanti, S.C.H. Structure–Activity Relationships of Larvicidal Monoterpenes and Derivatives against Aedes aegypti Linn. Chemosphere. 2011, 84, 150–153. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; González-Coloma, A.; Andrés, M.F.; Vidali, V.P.; Polissiou, M.G.; Santana-Méridas, O. Biocidal Compounds from Mentha Sp. Essential Oils and Their Structure–Activity Relationships. Chem. Biodivers. 2017, 14, e1600270. [Google Scholar] [CrossRef]
- Prieto, J.A.; Patiño, O.J.; Delgado, W.A.; Moreno, J.P.; Cuca, L.E. Chemical Composition, Insecticidal, and Antifungal Activities of Fruit Essential Oils of Three Colombian Zanthoxylum Species. Chil. J. Agric. Res. 2011, 71, 73–82. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C. Natural P-Menthene Monoterpenes: Synthesis of the Enantiomeric Forms of Wine Lactone, Epi-Wine Lactone, Dill Ether, and Epi-Dill Ether Starting from a Common Intermediate. Helv. Chim. Acta. 2004, 87, 2100–2109. [Google Scholar] [CrossRef]
- Bensel, N.; Höhn, J.; Marschall, H.; Weyerstahl, P. γ-Lactone aus α, β-ungesättigten Ketonen durch P-O-Olefinierung. Chem. Ver. 1979, 112, 2256–2277. [Google Scholar] [CrossRef]
- Engel, W. In Vivo Studies on the Metabolism of the Monoterpene Pulegone in Humans Using the Metabolism of Ingestion-Correlated Amounts (MICA) Approach: Explanation for the Toxicity Differences between (S)-(−)- and (R)-(+)-Pulegone. J. Agric. Food Chem. 2003, 51, 6589–6597. [Google Scholar] [CrossRef]
- Kumar, R.; Maurya, S.K. Synthesis of γ-Butyrolactone Derivatives from Dihydrotagetone and Evaluation of Their Antidiabetic Activity. ChemistrySelect. 2022, 7, e202203064. [Google Scholar] [CrossRef]
- Kon, Y.; Hachiya, H.; Ono, Y.; Matsumoto, T.; Sato, K. An Effective Synthesis of Acid-Sensitive via Oxidation of Terpenes and Styrenes Using Hydrogen Peroxide under Organic Solvent-Free Conditions. Synthesis 2011, 2011, 1092–1098. [Google Scholar] [CrossRef]
- Yadav, V.K.; Babu, K.G. A Remarkably Efficient Markovnikov Hydrochlorination of Olefins and Transformation of Nitriles into Imidates by Use of AcCl and an Alcohol. Eur. J. Org. Chem. 2005, 2005, 452–456. [Google Scholar] [CrossRef]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget Action of Xanthones from Garcinia Mangostana against α-Amylase, α-Glucosidase and Pancreatic Lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef]
- Mang, H.; Gross, J.; Lara, M.; Goessler, C.; Schoemaker, H.E.; Guebitz, G.M.; Kroutil, W. Biocatalytic Single-Step Alkene Cleavage from Aryl Alkenes: An Enzymatic Equivalent to Reductive Ozonization. Angew. Chem. Int. Ed. Engl. 2006, 45, 5201–5203. [Google Scholar] [CrossRef]
- Baragliu, A.; Grandolini, G.; Rossi, C.; Casinovi, C.G. Synthesis of 1,2,3-Trihydroxy-p-Menthanes. Tetrahedron 1980, 36, 645–649. [Google Scholar] [CrossRef]
- Liu, Z.L.; Goh, S.H.; Ho, S.H. Screening of Chinese Medicinal Herbs for Bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored. Prod. Res. 2007, 43, 290–296. [Google Scholar] [CrossRef]
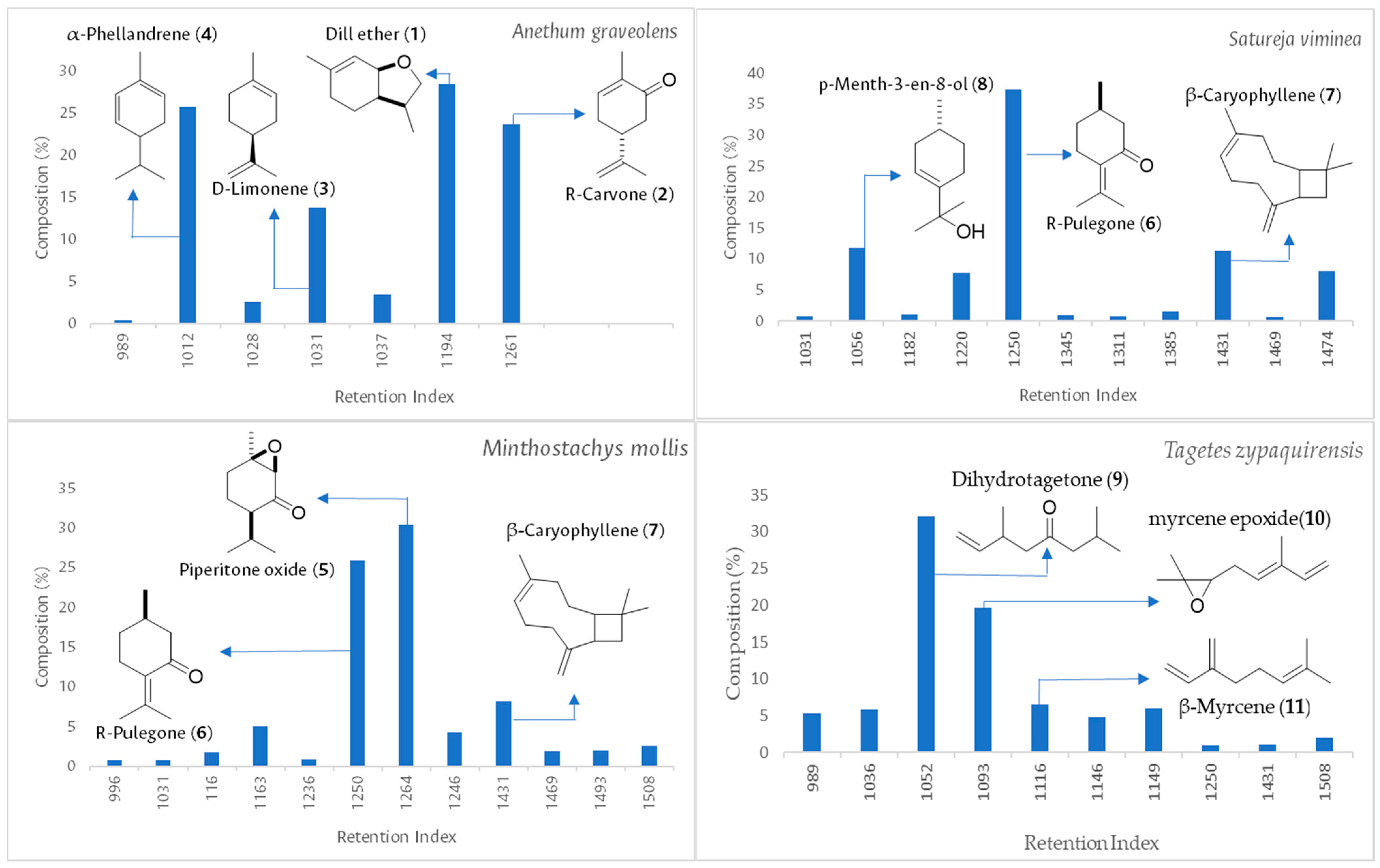
| Compound | DB-5MS | HP-INNOWax | % Relative | |||||
|---|---|---|---|---|---|---|---|---|
| Exp LRI | Ref LRI | Exp LRI | Ref LRI | A.G | M.M | S.V | T.Z | |
| α-Pinene | 937 | 932–939 | 1028 | 1019–1030 | 0.25 | 0.18 | ||
| Isopropyl tiglate | 966 | 959–976 | - | - | 0.20 | 0.21 | 0.27 | |
| Sabinene | 979 | 960–980 | - | - | 0.13 | 0.16 | 0.35 | |
| β-Pinene | 983 | 980–990 | - | - | 0.29 | 0.3 | ||
| β-Myrcene | 989 | 986–994 | 1147 | 1145–1187 | 0.40 | 5.3 | ||
| 3-Octanol | 996 | 991–995 | 1383 | 1368–1400 | 0.77 | |||
| α-Phellandrene | 1012 | 1005–1032 | - | - | 25.78 | |||
| o-Cymene | 1028 | 1026–1036 | - | - | 2.51 | |||
| Limonene | 1031 | 1031–1039 | - | - | 13.77 | 0.75 | 0.8 | 0.2 |
| (E)-β-Ocimene | 1036 | 1037–1043 | 1270 | 1242–1270 | 5.83 | |||
| β-Phellandrene | 1037 | 1031–1053 | 1238 | 1189–1241 | 3.46 | |||
| Eucalyptol | 1038 | 1031–1039 | - | - | 0.49 | |||
| Dihydrotagetone | 1052 | 1047–1082 | 1285 | 1268–1319 | 0.13 | 35.39 | ||
| p-Menth-3-en-8-ol | 1056 | 1147–1149 | 1613 | 1600–1621 | 11.83 | |||
| p-Mentha-3,8-diene | 1075 | 1070–1076 | - | - | 0.3 | |||
| Ipsenone | 1085 | 1083–1086 | 1440 | 1444 | 1.26 | |||
| 6,7-Epoxymyrcene | 1093 | 1092–1096 | 1399 | 1398–1415 | 19.64 | |||
| Linalool | 1100 | 1096–1101 | 1580 | 1557–1581 | 0.12 | 0.33 | 0.29 | |
| 4-t-Pentylcyclohexene | 1116 | 1100–1128 | 1724 | 1720 | 6.49 | |||
| 3-Octanyl acetate | 1116 | 1102–1123 | 1438 | 1424–1490 | 1.79 | |||
| (Z)-Epoxyocimene | 1128 | 1120–1132 | - | - | 0.4 | |||
| (E)-Tagetone | 1146 | 1144–1149 | 1500 | 1501–1522 | 4.74 | |||
| (Z)-Tagetone | 1149 | 1147–1152 | 1501 | 1500–1517 | 6.00 | |||
| Menthone | 1163 | 1148–1164 | - | - | 5.10 | 0.23 | ||
| (Z)-Isocitral | 1169 | 1164–1184 | - | - | 0.18 | |||
| Cis-Linalool oxide | 1172 | 1170–1174 | - | - | 0.37 | |||
| Isopulegone | 1182 | 1157–1179 | 1590 | 1582–1597 | 1.13 | |||
| Dill ether | 1194 | 1183–1194 | 1489 | 1484–1529 | 28.56 | |||
| γ-Terpineol | 1201 | 1195–1199 | 1685 | 1684–1695 | 0.44 | |||
| trans-Pulegol | 1220 | 1214–1221 | - | - | 7.79 | |||
| Perilla ketone | 1236 | 1230–1248 | - | - | 0.88 | |||
| β-Citral | 1241 | 1240–1242 | - | - | 0.41 | |||
| Piperitone | 1246 | 1243–1247 | - | - | 4.63 | |||
| Pulegone | 1250 | 1209–1237 | 1665 | 1661–1665 | 25.91 | 37.4 | 0.97 | |
| Carvone | 1261 | 1242–1272 | - | - | 23.67 | |||
| Piperitone oxide | 1264 | 1230–1251 | 1712 | 1700–1722 | 31.70 | |||
| Isopulegyl acetate | 1267 | 1277–1309 | 1695 | 1581–1608 | 2.00 | |||
| (E)-Citral | 1269 | 1267–1270 | 2039 | 1721–1737 | 0.62 | |||
| Carvacrol | 1291 | 1286–1299 | 2217 | 2215–2219 | 0.43 | |||
| Dihydrocarveol acetate | 1311 | 1307–1344 * | - | - | 0.81 | |||
| Myrtenyl acetate | 1327 | 1326–1332 | - | - | 0.21 | |||
| Piperitenone | 1345 | 1340–1349 | 1710 | 1705–1739 | 2.03 | 0.95 | ||
| α-Copaene | 1385 | 1372–1389 | 1504 | 1488–1520 | 0.19 | 1.51 | ||
| β-Bourbonene | 1396 | 1387–1401 | - | - | 0.50 | |||
| β-Caryophyllene | 1431 | 1418–1449 | 1627 | 1594–1657 | 8.17 | 11.33 | 1.09 | |
| α-Humulene | 1469 | 1446–1464 | 1691 | 1660–1710 | 1.87 | 0.73 | 0.47 | |
| Alloaromadendrene | 1474 | 1458–1478 | 1634 | 1616–1662 | 8.14 | 0.18 | ||
| Germacrene D | 1493 | 1485–1519 | 1722 | 1716–1724 | 2.07 | 2.87 | 0.40 | |
| Bicyclogermacrene | 1508 | 1494–1517 | 1747 | 1736–1738 | 2.57 | 2.34 | 1.97 | |
| δ-Cadinene | 1531 | 1523–1531 | 1769 | 1764–1772 | 2.76 | 0.25 | ||
| 14-Hydroxycaryophyllene | 1662 | 1660–1667 | - | - | 0.29 | |||
| (E)-14-Hydroxy-9-epicaryophyllene | 1668 | 1660–1670 | 1969 | 1966–1989 | 1.21 | |||
| Not identified | 1724 | - | - | - | 1.55 | |||
| Monoterpenoids | 98.15 | 76.14 | 61.61 | 82.43 | ||||
| Sesquiterpenoids | 0.00 | 17.66 | 32.72 | 4.36 | ||||
| Total | 98.15 | 93.8 | 94.33 | 86.79 | ||||
| EOs | T. castaneum | S. zeamais | ||
|---|---|---|---|---|
| Fumigant LC50 (µL/ L Air) | Contact LD50 (µg/Insect) | Fumigant LC50 (µL/L Air) | Contact LD50 (µg/Insect) | |
| T. zypaquirensis | 23.1 (12.7–33.3) | 66.6 (49.8–87.7) | 104.4 (74.4–143.2) | 91.1 (71.7–111.4) |
| S. viminea | 6.4 (3.9–9.7) | 16.4 (10.9–24.9) | 20.6 (12.2–31.9) | 24.6 (12.8–38.3) |
| M. mollis | 4.8 (3.3–6.8) | 6.5 (4.6–9.3) | 7.0 (6.1–8.0) | 15.8 (9.3–24.2) |
| A. graveolens | 15.5 (13.1–18.3) | 86.1 (75.1–99.3) | 40.1 (35.4–46.1) | 140.3 (124.5–160.6) |
| Dichlorvos | 2.1 (1.5–3.8) | N/A | 1.0 (0.1–1.9) | N/A |
| Cypermethrin | N/A | 1.0 (0.1–2.0) | N/A | 10.5 (0.1–20.0) |
| Compound | S. zeamais LC50 and LD50 (Confidence Intervals 95%) | T. castaneum LC50 and LD50 (Confidence Intervals 95%) | ||
|---|---|---|---|---|
| µL/L Air | µg/Insect | µL/L air | µg/Insect | |
| 1 | 88.8 (81.9–95.0) | 66.7 (54.0–87.7) | 13.5 (12.1–13.0) | 9.7 (8.2–18.0) |
| 2 | 42.4 (28.7–63.3) | 16.3 (14.4–18.2) | 4.3 (1.3–7.6) | 4.8 (2.9–6.7) |
| 3 | 91.8 (87.0–99.7) | Not active | 11.3 (9.0–14.1) | 73.1 (58.8–87.4) |
| 4 | 92.0 (84.9–107.8) | Not active | 24.0 (14.2–31.1) | 27.0 (12.7–44.8) |
| 5 | 14.5 (12.9–16.1) | 24.6 (14.4–37.4) | 4.8 (3.4–6.7) | 5.9 (5.1–6.8) |
| 6 | 3.0 (3.3–2.7) | 14.9 (12.1–18.7) | 2.2 (2.0–2.4) | 13.1 (10.3–15.9) |
| 7 | Not active | |||
| 8 | Not active | 75.6 (52.1–120.9) | Not active | 78.6 (52.9–123.2) |
| 9 | 180.4 (161.1–208.2) | Not active | 42.1 (38.3–46.6) | 88.1 (65.1–120.3) |
| 10 | 37.1 (32.5–42.3) | 50.8 (43.6–59.0) | 16.9 (9.9–23.4) | 49.0 (37.2–61.7) |
| 11 | 104.0 (88.6–122.9) | Not active | 75.1 (57.9–111.0) | 105.2 (86.3–131.8) |
| Dichlorvos | 2.1 (1.5–3.8) | N/A | 1.04 (0.1–1.9) | N/A |
| Cypermethrin | N/A | 1.0 (0.1–2.0) | N/A | 10.5 (0.1–20.0) |
| Compound | S. zeamais LC50 and LD50 (Confidence Intervals 95%) | T. castaneum LC50 and LD50 (Confidence Intervals 95%) | ||
|---|---|---|---|---|
| µL/L Air | µg/Insect | µL/L Air | µg/Insect | |
| 2 | 42.4 (28.7–63.3) | 16.3 (14.4–18.2) | 4.3 (1.3–7.6) | 4.8 (2.9–6.7) |
| 3 | 91.8 (87.0–99.7) | Not active | 11.3 (9.0–14.1) | 73.1 (58.8–87.4) |
| 5 | 14.5 (12.9–16.1) | 24.6 (14.4–37.4) | 4.8 (3.4–6.7) | 5.9 (5.1–6.8) |
| 6 | 3.0 (3.3–2.7) | 14.9 (12.1–18.7) | 2.2 (2.0–2.4) | 13.1 (10.3–15.9) |
| 12 | Not active | |||
| 13 | 25.4 (22.0–29.4) | 38.7 (26.6–57.6) | 2.7 (1.9–3.5) | 16.3 (12.0–23.2) |
| 14 | Not active | 14.2 (8.7–19.0) | 2.8 (1.6–3.9) | 5.5 (4.7–7.1) |
| 15 | Not active | 44.0 (35.4–55.5) | No active | 21.1 (13.4–29.7) |
| 16 | Not active | 38.8 (32.1–45.4) | 25.4 (20.8–31.0) | 19.8 (17.0–21.7) |
| 17 | Not active | 69.1 (47.3–105.5) | Not active | 85.5 (70.9–107.4) |
| 18 | Not active | 28.3 (26.4–31.3) | Not active | 1.0 (0.7–3.9) |
| 19 | Not active | 109.7 (67.3–158.7) | Not active | 67.3 (11.5–38.5) |
| 20 | 41.8 (34.6–51.2) | 24.5 (17.6–31.4) | 4.5 (3.5–5.5) | 1.9 (1.0–4.9) |
| 21 | Not active | 263.0 (153.0–308.7) | 1.1 (0.1–3.7) | 80.0 (60.0–90.0) |
| 22 | 42.2 (36.6–49.2) | 30.2 (14.6–50.7) | 35.2 (27.3–44.0) | 16.6 (13.6–20.5) |
| 23 | 92.9 (74.4–124.0) | 45.1 (36.2–56.8) | 1.4 (0.1–3.9) | 17.6 (9.8–29.4) |
| 24 | Not active | |||
| Dichlorvos | 2.1 (1.5–3.8) | N/A | 1.04 (0.1–1.9) | N/A |
| Cypermethrin | N/A | 1.0 (0.1–2.0) | N/A | 10.5 (0.1–20.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Quitian, A.G.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure–Activity Relationship Study. Int. J. Mol. Sci. 2025, 26, 3407. https://doi.org/10.3390/ijms26073407
Sierra-Quitian AG, Prieto-Rodríguez JA, Patiño-Ladino OJ. Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure–Activity Relationship Study. International Journal of Molecular Sciences. 2025; 26(7):3407. https://doi.org/10.3390/ijms26073407
Chicago/Turabian StyleSierra-Quitian, Andrés G., Juliet A. Prieto-Rodríguez, and Oscar J. Patiño-Ladino. 2025. "Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure–Activity Relationship Study" International Journal of Molecular Sciences 26, no. 7: 3407. https://doi.org/10.3390/ijms26073407
APA StyleSierra-Quitian, A. G., Prieto-Rodríguez, J. A., & Patiño-Ladino, O. J. (2025). Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure–Activity Relationship Study. International Journal of Molecular Sciences, 26(7), 3407. https://doi.org/10.3390/ijms26073407








