Barrier-Strengthening Effects of Cannabidiol on Porcine Peyer’s Patches
Abstract
1. Introduction
2. Results
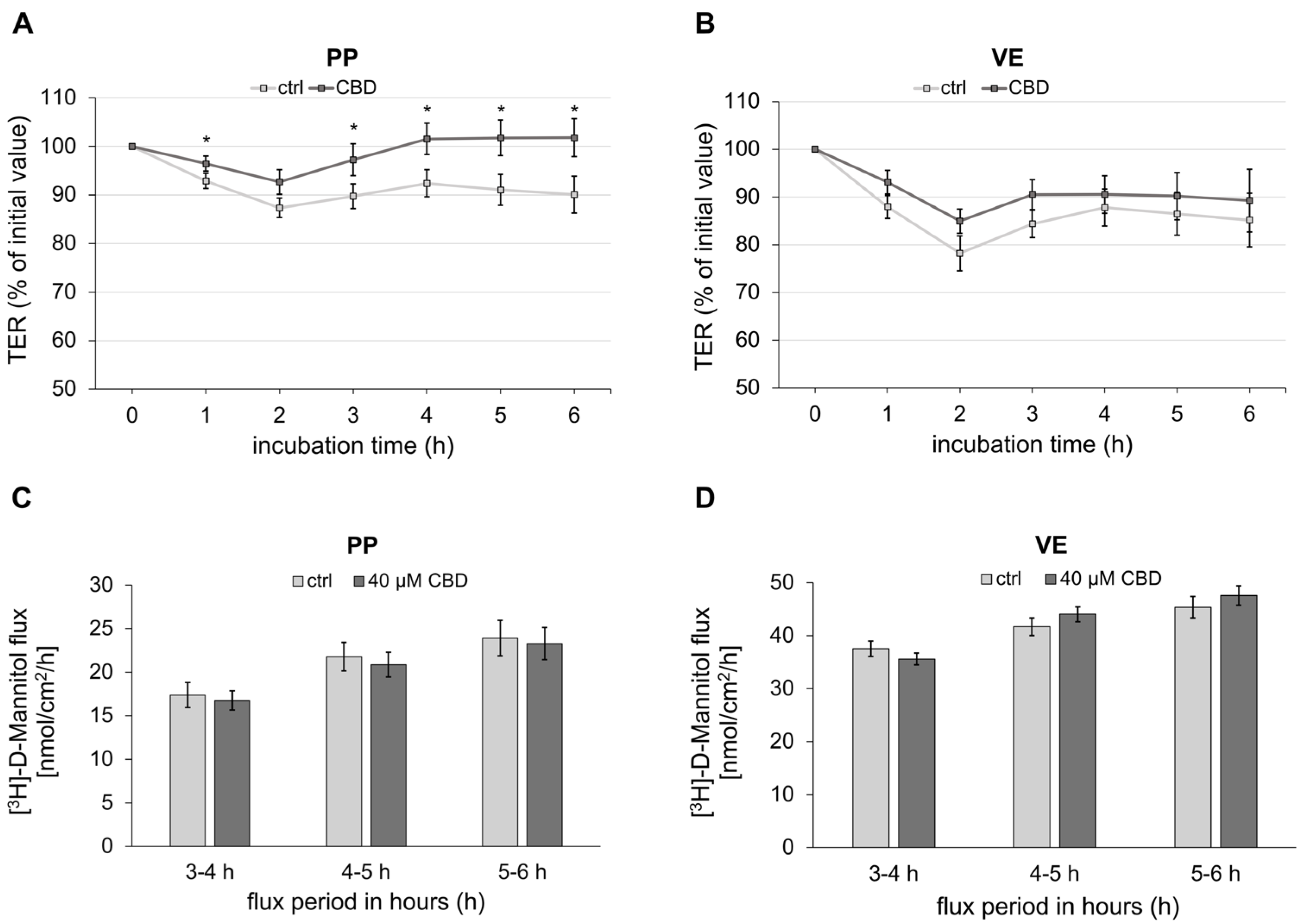
2.1. Impact of CBD on Transepithelial Barrier Function of Porcine Peyer’s Patch and Villus Epithelium
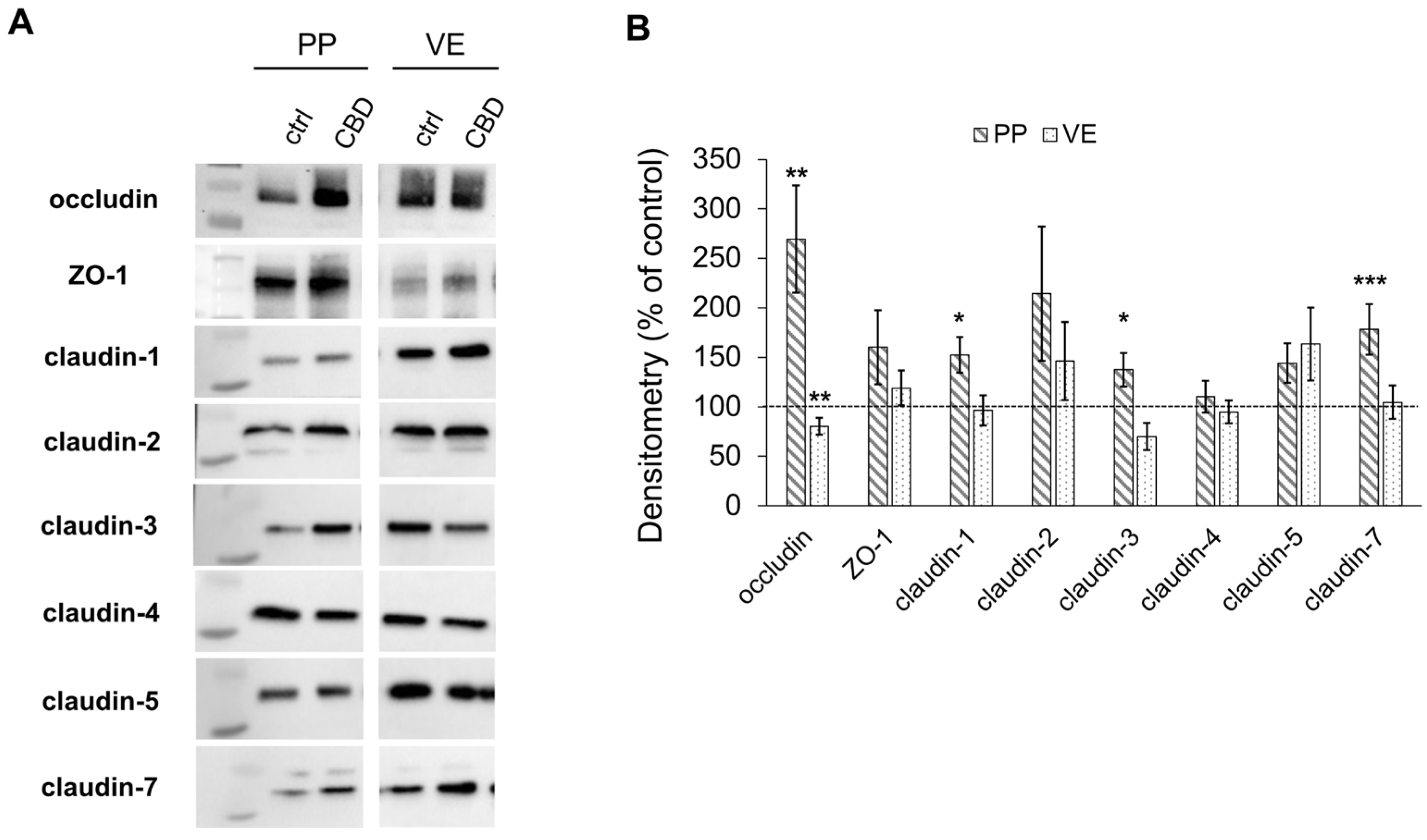
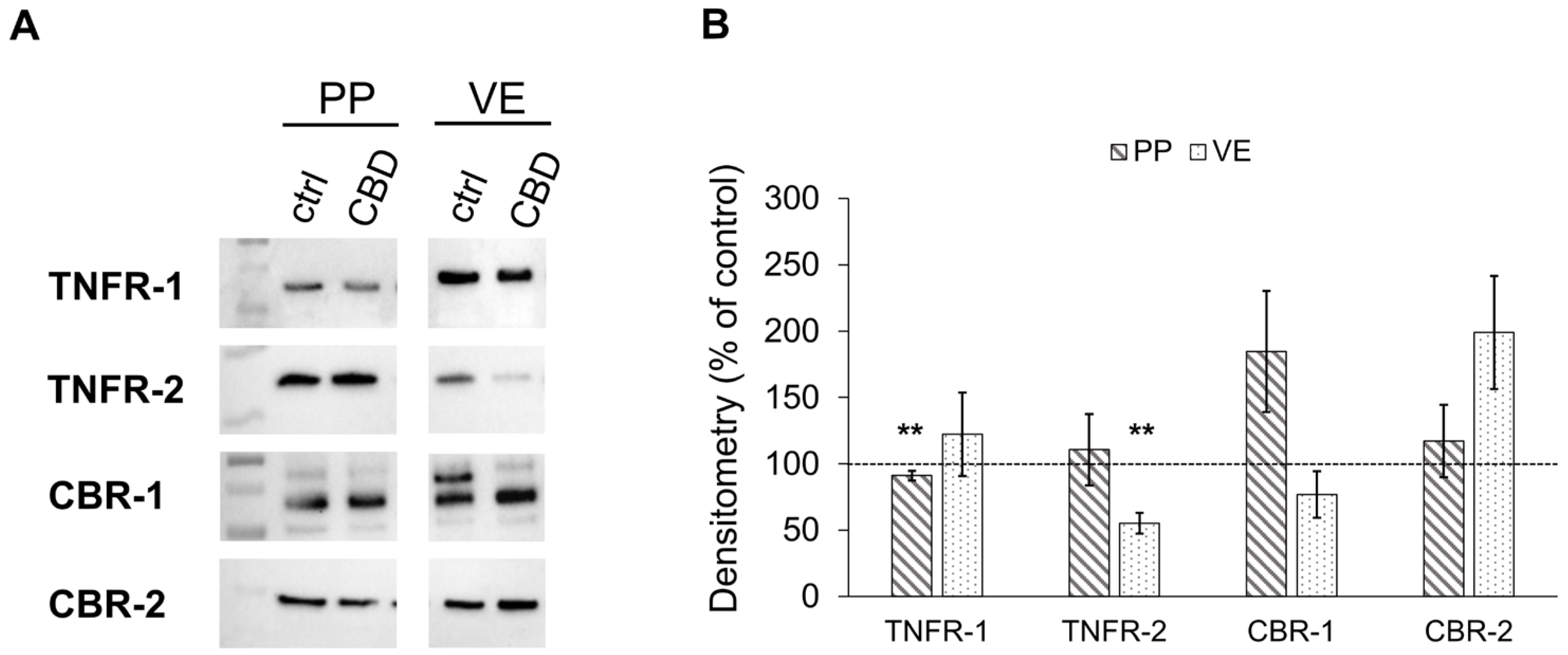
2.2. Immunoblotting
2.2.1. Effects on Tight Junction Proteins
2.2.2. Effects of CBD on the Expression of Cannabinoid and TNF Receptors
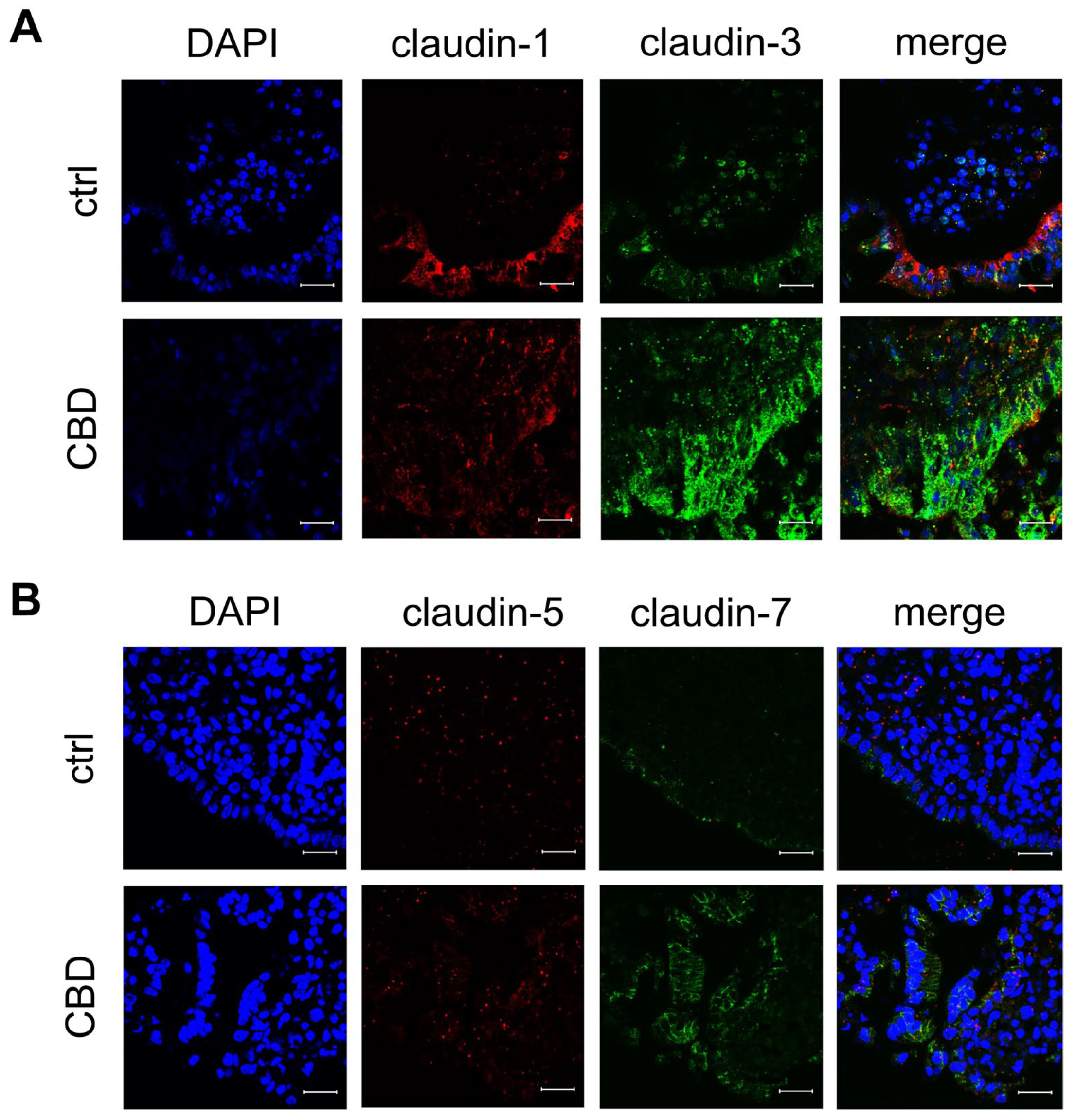
2.3. Confocal Laser-Scanning Immunofluorescence Microscopy
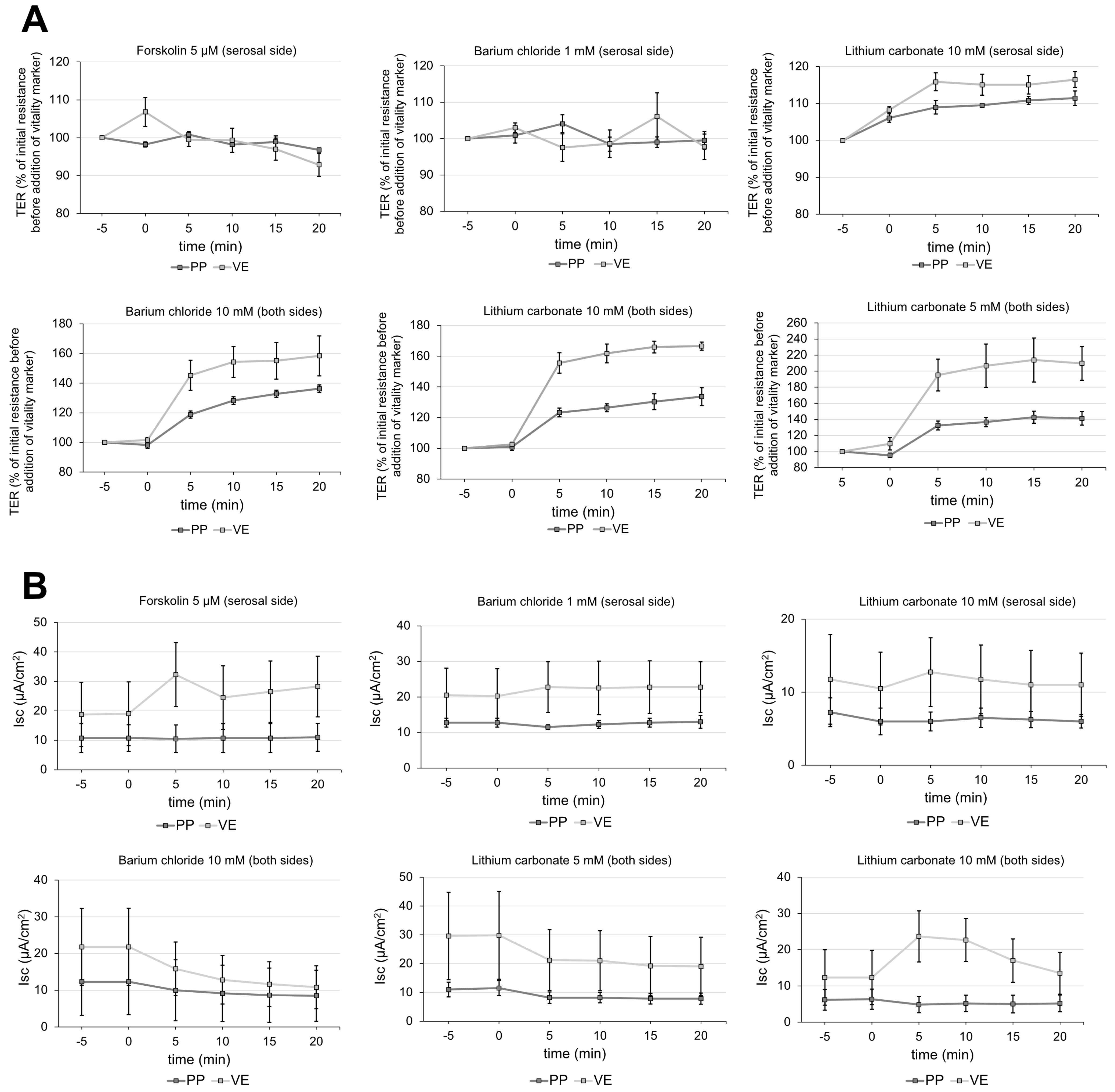
2.4. Vitality Test for Peyer’s Patch
3. Discussion
4. Materials and Methods
4.1. Tissue Preparation
4.2. Buffer
4.3. Chemicals
4.4. Ussing Chamber Experiment
4.5. Paracellular Flux Measurement
4.6. Protein Extraction and Immunoblots
4.6.1. Tight Junction Proteins
4.6.2. Cannabinoid and TNF Receptors
4.7. Immunohistochemistry
4.8. Vitality Test
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| CBR | Cannabinoid receptor |
| EMA | European Medicines Agency |
| FAE | follicle-associated epithelium |
| FDA | Food and Drug Administration |
| GALT | gut-associated lymphoid tissue |
| IBDs | Inflammatory Bowel Diseases |
| Isc | short-current circuit |
| LPS | lipopolysaccharide |
| PP | Peyer’s Patch |
| SEM | standard error of the mean |
| TER | Transepithelial resistance |
| TJ | Tight junction |
| TNFɑ | Tumor necrosis factor alpha |
| TNFR | Tumor necrosis factor–receptor |
| VE | villus epithelium |
| ZO-1 | Zonula occludens 1 |
References
- Langkamp-Henken, B.; Glezer, J.A.; Kudsk, K.A. Immunologic structure and function of the gastrointestinal tract. Nutr. Clin. Pract. 1992, 7, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Mantis, N.J.; Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001, 2, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Peyer, J.C. Exercitatio Anatomico-Medica de Glandulis Intestinorum, 1st ed.; Onophrius a Waldkirch: Schaffhausen, Switzerland, 1677. [Google Scholar]
- Mörbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Moghaddami, M.; Cummins, A.; Mayrhofer, G. Lymphocyte-filled villi: Comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 1998, 115, 1414–1425. [Google Scholar] [CrossRef]
- Pabst, R. The anatomical basis for the immune function of the gut. Anat. Embryol. 1987, 176, 135–144. [Google Scholar] [CrossRef]
- Owen, R.L. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer’s patches—A personal and historical perspective. Semin. Immunol. 1999, 11, 157–163. [Google Scholar] [CrossRef]
- Hamada, H.; Hiroi, T.; Nishiyama, Y.; Takahashi, H.; Masunaga, Y.; Hachimura, S.; Kaminogawa, S.; Takahashi-Iwanaga, H.; Iwanaga, T.; Kiyono, H.; et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 2002, 168, 57–64. [Google Scholar] [CrossRef]
- Bockman, D.E.; Cooper, M.D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am. J. Anat. 1973, 136, 455–477. [Google Scholar] [CrossRef]
- Mantis, N.J.; Cheung, M.C.; Chintalacharuvu, K.R.; Rey, J.; Corthesy, B.; Neutra, M.R. Selective adherence of IgA to murine Peyer’s patch M cells: Evidence for a novel IgA receptor. J. Immunol. 2002, 169, 1844–1851. [Google Scholar] [CrossRef]
- Neutra, M.R.; Frey, A.; Kraehenbuhl, J.P. Epithelial M cells: Gateways for mucosal infection and immunization. Cell 1996, 86, 345–348. [Google Scholar] [CrossRef]
- Radloff, J.; Falchuk, E.L.; Markov, A.G.; Amasheh, S. Molecular Characterization of Barrier Properties in Follicle-Associated Epithelium of Porcine Peyer’s Patches Reveals Major Sealing Function of Claudin-4. Front. Physiol. 2017, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol.-Gastrointest. Liver Physiol. 1981, 241, G275–G288. [Google Scholar] [CrossRef]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Ullrich, R.; Zeitz, M. Oral tolerance induction in humans. Exp. Mol. Pathol. 2012, 93, 449–454. [Google Scholar] [CrossRef]
- Kelsall, B.L.; Strober, W. Peyer’s patch dendritic cells and the induction of mucosal immune responses. Res. Immunol. 1997, 148, 490–498. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- Hibi, T.; Ishibashi, T.; Ikenoue, Y.; Yoshihara, R.; Nihei, A.; Kobayashi, T. Ulcerative Colitis: Disease Burden, Impact on Daily Life, and Reluctance to Consult Medical Professionals: Results from a Japanese Internet Survey. Inflamm. Intest. Dis. 2020, 5, 27–35. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Silva, F.A.; Rodrigues, B.L.; Ayrizono, M.L.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef]
- Souza, R.F.; Caetano, M.A.F.; Magalhaes, H.I.R.; Castelucci, P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef]
- Parker, A.; Vaux, L.; Patterson, A.M.; Modasia, A.; Muraro, D.; Fletcher, A.G.; Byrne, H.M.; Maini, P.K.; Watson, A.J.M.; Pin, C. Elevated apoptosis impairs epithelial cell turnover and shortens villi in TNF-driven intestinal inflammation. Cell Death Dis. 2019, 10, 108. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020, 11, 70. [Google Scholar] [CrossRef]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.; Droessler, L.; Amasheh, S. Cannabidiol attenuates inflammatory impairment of intestinal cells expanding biomaterial-based therapeutic approaches. Mater. Today Bio 2023, 23, 100808. [Google Scholar] [CrossRef]
- Aswad, M.; Hamza, H.; Pechkovsky, A.; Zikrach, A.; Popov, T.; Zohar, Y.; Shahar, E.; Louria-Hayon, I. High-CBD Extract (CBD-X) Downregulates Cytokine Storm Systemically and Locally in Inflamed Lungs. Front. Immunol. 2022, 13, 875546. [Google Scholar] [CrossRef]
- Wei, D.D.; Wang, H.C.; Yang, J.N.; Dai, Z.F.; Yang, R.L.; Meng, S.S.; Li, Y.Y.; Lin, X.H. Effects of O-1602 and CBD on TNBS-induced colonic disturbances. Neurogastroenterol. Motil. 2020, 32, e13756. [Google Scholar] [CrossRef]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Jamontt, J.M.; Molleman, A.; Pertwee, R.G.; Parsons, M.E. The effects of Δ9-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and motility disturbances in rat colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef]
- Borrelli, F.; Aviello, G.; Romano, B.; Orlando, P.; Capasso, R.; Maiello, F.; Guadagno, F.; Petrosino, S.; Capasso, F.; Di Marzo, V.; et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009, 87, 1111–1121. [Google Scholar] [CrossRef]
- Schicho, R.; Storr, M. Topical and Systemic Cannabidiol Improves Trinitrobenzene Sulfonic Acid Colitis in Mice. Pharmacology 2012, 89, 149–155. [Google Scholar] [CrossRef]
- Golub, V.; Reddy, D.S. Cannabidiol Therapy for Refractory Epilepsy and Seizure Disorders. Adv. Exp. Med. Biol. 2021, 1264, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Nabbout, R.; Thiele, E.A. The role of cannabinoids in epilepsy treatment: A critical review of efficacy results from clinical trials. Epileptic Disord. 2020, 22, S23–S28. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Pagano, E.; Lacroix, S.; Venneri, T.; Cristiano, C.; Calignano, A.; Parisi, O.A.; Izzo, A.A.; Di Marzo, V.; Borrelli, F. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front. Pharmacol. 2020, 11, 585096. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Zhao, B.; Tang, M.; Dong, H.; Zhang, L.; Lv, B.; Wei, L. Ex vivo and in situ approaches used to study intestinal absorption. J. Pharmacol. Toxicol. Methods 2013, 68, 208–216. [Google Scholar] [CrossRef]
- Ripken, D.; Hendricks, H.F.J. Porcine Ex Vivo Intestinal Segment Model. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Germany, 2015; pp. 255–262. [Google Scholar]
- Ermund, A.; Gustafsson, J.K.; Hansson, G.C.; Keita, Å.V. Mucus Properties and Goblet Cell Quantification in Mouse, Rat and Human Ileal Peyer’s Patches. PLoS ONE 2013, 8, e83688. [Google Scholar] [CrossRef]
- Droessler, L.; Cornelius, V.; Boehm, E.; Stein, L.; Brunner, N.; Amasheh, S. Barrier Perturbation in Porcine Peyer’s Patches by Tumor Necrosis Factor is Associated With a Dysregulation of Claudins. Front. Physiol. 2022, 13, 889552. [Google Scholar] [CrossRef]
- Radloff, J.; Cornelius, V.; Markov, A.G.; Amasheh, S. Caprate Modulates Intestinal Barrier Function in Porcine Peyer’s Patch Follicle-Associated Epithelium. Int. J. Mol. Sci. 2019, 20, 1418. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kamoi, R.; Iida, M. Pathogenesis of aphthoid ulcers in Crohn’s disease: Correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 1996, 38, 724–732. [Google Scholar] [CrossRef]
- Fujimura, Y.; Hosobe, M.; Kihara, T. Ultrastructural-Study of M-Cells from Colonic Lymphoid Nodules Obtained by Colonoscopic Biopsy. Digest. Dis. Sci. 1992, 37, 1089–1098. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Q.Y.; Wang, W.Z.; Chu, S.; Liu, X.X.; Liu, Y.J.; Tan, C.; Zhu, F.; Deng, S.J.; Dong, Y.L.; et al. Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice. Biomed. Pharmacother. 2021, 133, 110937. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Inai, T.; Kobayashi, J.; Shibata, Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 1999, 78, 849–855. [Google Scholar] [CrossRef]
- Awad, K.; Barmeyer, C.; Bojarski, C.; Nagel, O.; Lee, I.F.M.; Schweiger, M.R.; Schulzke, J.D.; Bücker, R. Epithelial Barrier Dysfunction in Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) via Downregulation of Claudin-1. Cells 2023, 12, 2846. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, B.; Thapa, I.; Talmon, G.A.; Salomon, J.; Ramer-Tait, A.E.; Bastola, D.K.; Dhawan, P.; Singh, A.B. Loss of claudin-3 expression increases colitis risk by promoting Gut Dysbiosis. Gut Microbes 2023, 15, 2282789. [Google Scholar] [CrossRef]
- Wang, K.; Li, T.Y.; Xu, C.; Ding, Y.H.; Li, W.J.; Ding, L. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via the promotion of epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019, 508, 797–804. [Google Scholar] [CrossRef]
- Xu, C.; Wang, K.; Ding, Y.H.; Li, W.J.; Ding, L. Claudin-7 gene knockout causes destruction of intestinal structure and animal death in mice. World J. Gastroenterol. 2019, 25, 584–599. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. CSH Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, G323–G341. [Google Scholar] [CrossRef]
- Cheng, C.T.; Hsiao, J.C.; Hoffmann, A.; Tu, H.L. TNFR1 mediates heterogeneity in single-cell NF-κB activation. iScience 2024, 27, 109486. [Google Scholar] [CrossRef] [PubMed]
- Magen, I.; Avraham, Y.; Ackerman, Z.; Vorobiev, L.; Mechoulam, R.; Berry, E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010, 159, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, S.; Bigdeli, M.R. Correlation Between Cannabidiol-Induced Reduction of Infarct Volume and Inflammatory Factors Expression in Ischemic Stroke Model. Basic. Clin. Neurosci. 2017, 8, 139–146. [Google Scholar] [CrossRef]
- Piguet, P.F.; Vesin, C.; Guo, J.; Donati, Y.; Barazzone, C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur. J. Immunol. 1998, 28, 3499–3505. [Google Scholar] [CrossRef]
- Grabinger, T.; Bode, K.J.; Demgenski, J.; Seitz, C.; Delgado, M.E.; Kostadinova, F.; Reinhold, C.; Etemadi, N.; Wilhelm, S.; Schweinlin, M.; et al. Inhibitor of Apoptosis Protein-1 Regulates Tumor Necrosis Factor-Mediated Destruction of Intestinal Epithelial Cells. Gastroenterology 2017, 152, 867–879. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G.; et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef]
- Cornelius, V.; Droessler, L.; Amasheh, S. Quercetin Improves Barrier Properties in Porcine Small Intestine but Not in Peyer’s Patches. Int. J. Mol. Sci. 2024, 25, 1530. [Google Scholar] [CrossRef]
- Westerhout, J.; Wortelboer, H.; Verhoeckx, K. Ussing Chamber. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Germany, 2015; pp. 263–273. [Google Scholar]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sisk, L.M.; Rapuano, K.M.; Conley, M.I.; Greene, A.S.; Horien, C.; Rosenberg, M.D.; Scheinost, D.; Constable, R.T.; Glatt, C.E.; Casey, B.J.; et al. Genetic variation in endocannabinoid signaling is associated with differential network-level functional connectivity in youth. J. Neurosci. Res. 2022, 100, 731–743. [Google Scholar] [CrossRef] [PubMed]
- McNamara, B.; Winter, D.C.; Cuffe, J.E.; O’Sullivan, G.C.; Harvey, B.J. Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J. Physiol. 1999, 519, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rohaim, A.; Gong, L.; Li, J.; Rui, H.; Blachowicz, L.; Roux, B. Barium blockade of the KcsA channel in open and closed conformation datasets. Data Brief 2020, 32, 106135. [Google Scholar] [CrossRef]
- Cho, Y.W. Lithium-induced inhibition of Na-K ATPase and Ca ATPase activities in rat brain synaptosome. J. Korean Med. Sci. 1995, 10, 7–13. [Google Scholar] [CrossRef]
- Severson, E.A.; Kwon, M.; Hilgarth, R.S.; Parkos, C.A.; Nusrat, A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem. Biophys. Res. Commun. 2010, 397, 592–597. [Google Scholar] [CrossRef]
- Dolman, D.E.; Edmonds, C.J. The effect of lithium on the transport of sodium, potassium and chloride by the colon of normal and sodium-depleted rats. J. Physiol. 1976, 259, 771–783. [Google Scholar] [CrossRef]
- Costa, C.M.; de Carvalho, N.M.; de Oliveira, D.L.; Madureira, A.R. A Critical Review on In Vitro and Ex Vivo Models of the Intestinal Epithelium of Humans and Monogastric Animals. Gastrointest. Disord. 2024, 6, 337–358. [Google Scholar] [CrossRef]

| Substance | Molecular Formula | M g·mol−1 | cn mmol·L−1 | cm g·L−1 |
|---|---|---|---|---|
| Sodium chloride | NaCl | 58.44 | 119 | 6.95 |
| Sodium bicarbonate | NaHCO3 | 84.01 | 25 | 2.10 |
| Sodium dihydrogen phosphate | NaH2PO4 · H2O | 137.99 | 0.6 | 0.08 |
| Di-sodium hydrogen phosphate | Na2HPO4 · 2 H2O | 177.99 | 2.4 | 0.43 |
| Potassium chloride | KCl | 74.56 | 5 | 0.37 |
| HEPES | C8H18N2O4S | 238.30 | 10 | 2.38 |
| Glucose | C6H12O6·H2O | 198.18 | 10 | 1.98 |
| Calcium chloride | CaCl2 ·2 H2O | 147.02 | 1.2 | 0.18 |
| Magnesium chloride | MgCl2 · 6 H2O | 203.30 | 1.2 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehm, E.; Droessler, L.; Vollstaedt, M.-L.; Stein, L.; Amasheh, S. Barrier-Strengthening Effects of Cannabidiol on Porcine Peyer’s Patches. Int. J. Mol. Sci. 2025, 26, 3360. https://doi.org/10.3390/ijms26073360
Boehm E, Droessler L, Vollstaedt M-L, Stein L, Amasheh S. Barrier-Strengthening Effects of Cannabidiol on Porcine Peyer’s Patches. International Journal of Molecular Sciences. 2025; 26(7):3360. https://doi.org/10.3390/ijms26073360
Chicago/Turabian StyleBoehm, Elisa, Linda Droessler, Marie-Luise Vollstaedt, Laura Stein, and Salah Amasheh. 2025. "Barrier-Strengthening Effects of Cannabidiol on Porcine Peyer’s Patches" International Journal of Molecular Sciences 26, no. 7: 3360. https://doi.org/10.3390/ijms26073360
APA StyleBoehm, E., Droessler, L., Vollstaedt, M.-L., Stein, L., & Amasheh, S. (2025). Barrier-Strengthening Effects of Cannabidiol on Porcine Peyer’s Patches. International Journal of Molecular Sciences, 26(7), 3360. https://doi.org/10.3390/ijms26073360






