Sakuranetin, A Laxative Component from Peach Leaves and Its Intervention in Metabolism
Abstract
1. Introduction
2. Results
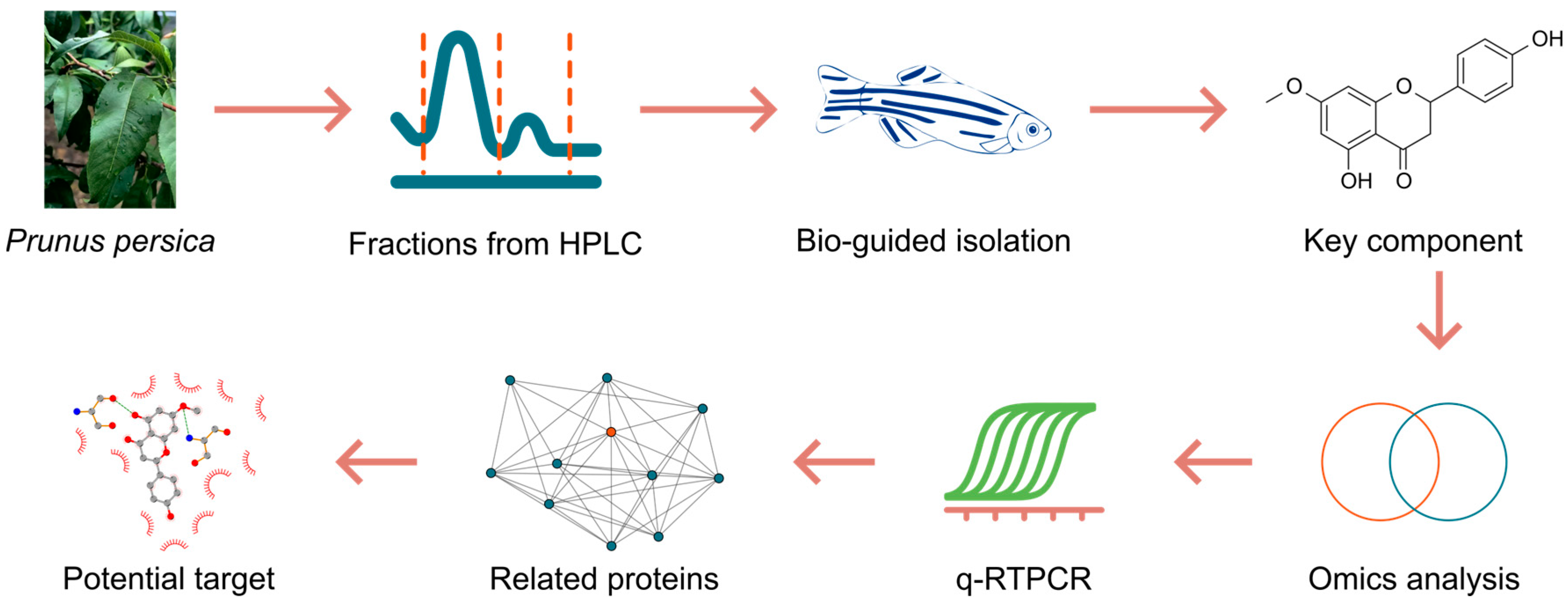
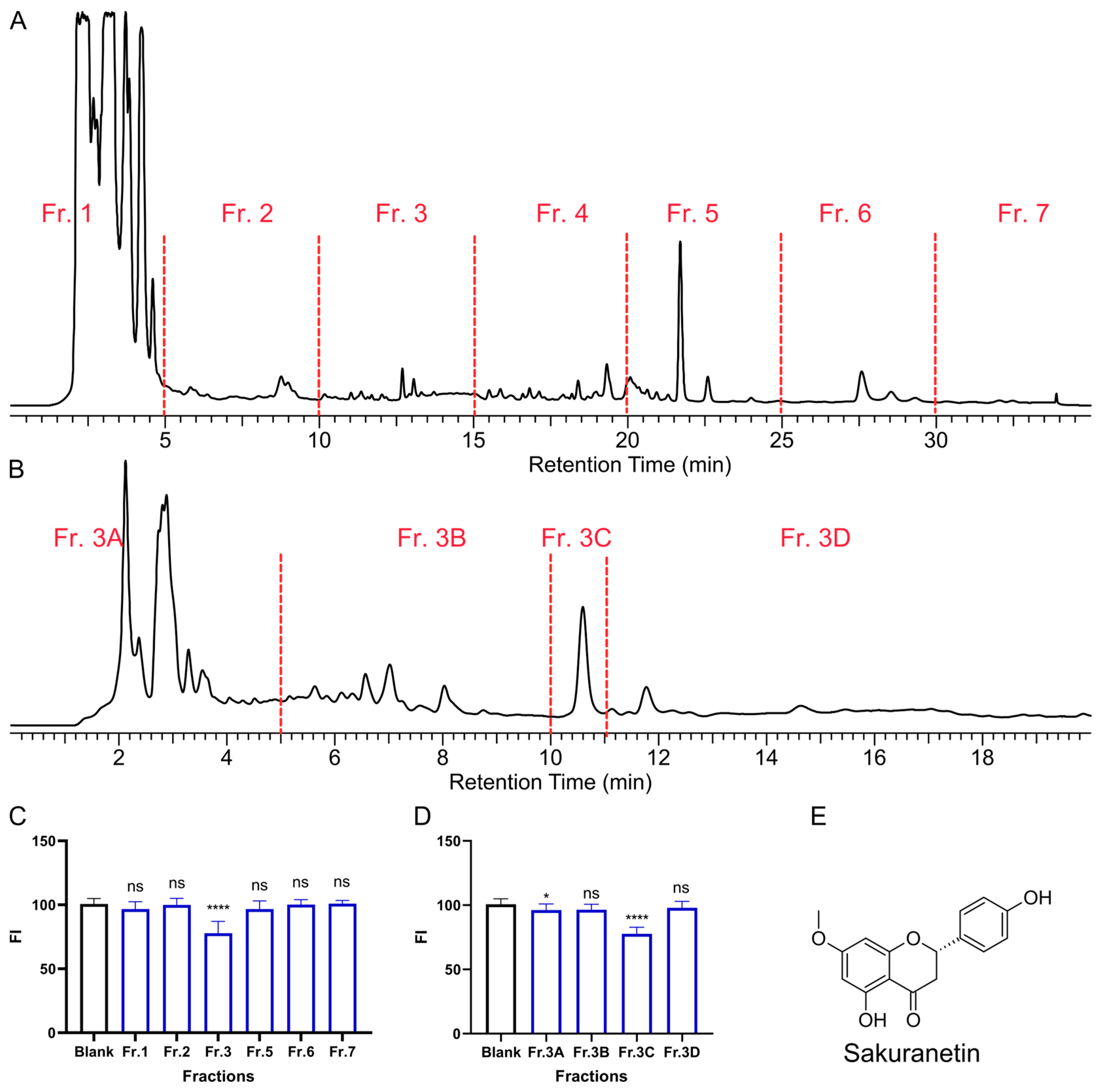
2.1. Bio-Guided Isolation of Laxative Component in P. persica Leaves
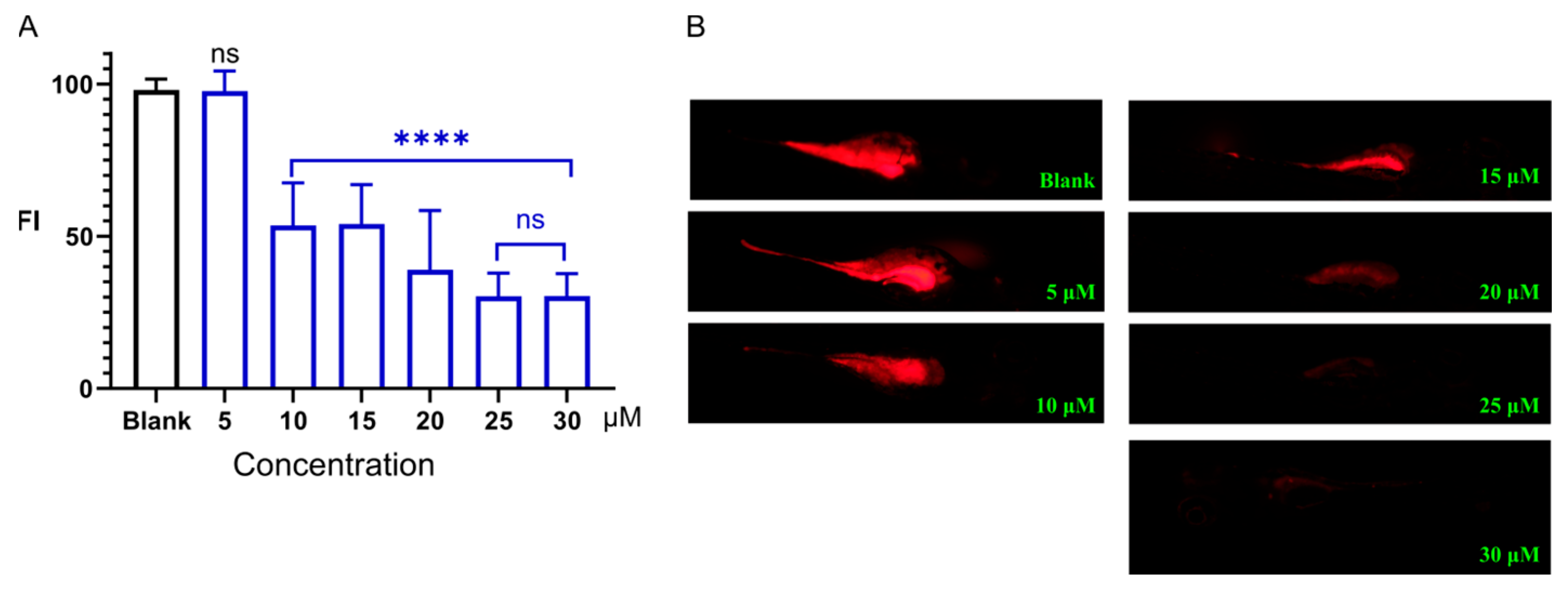
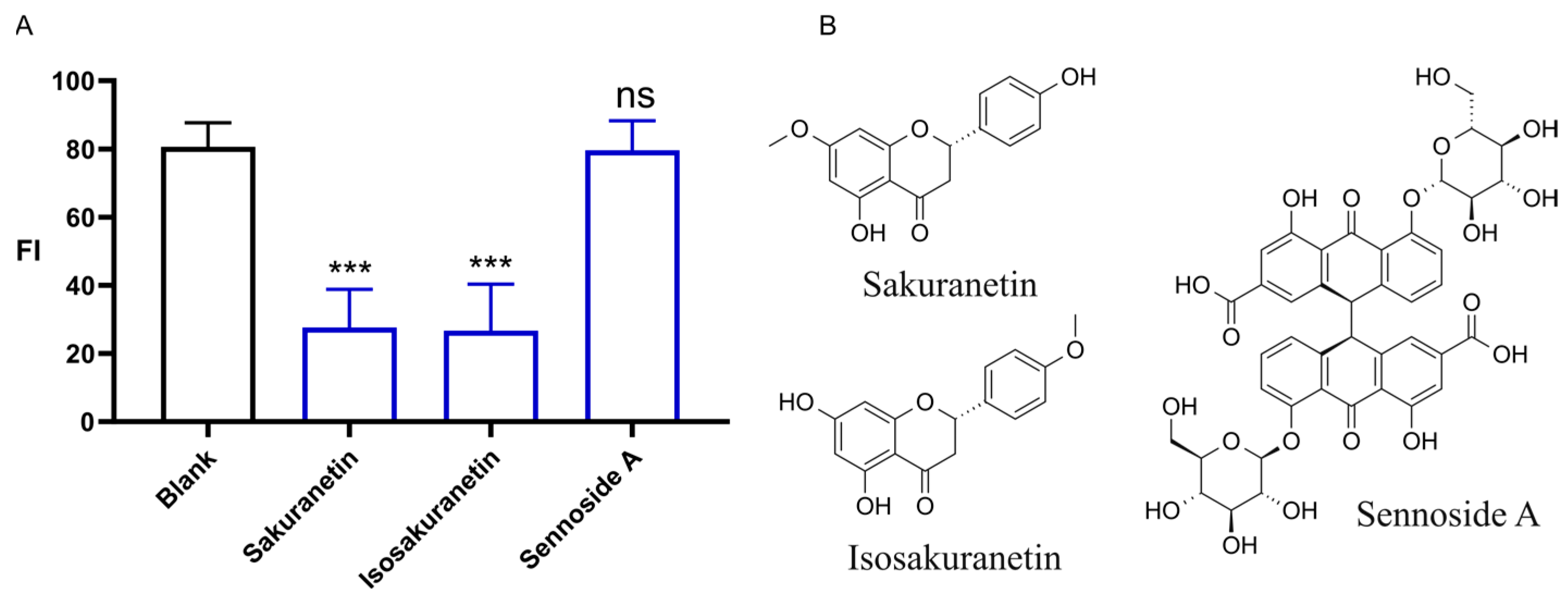
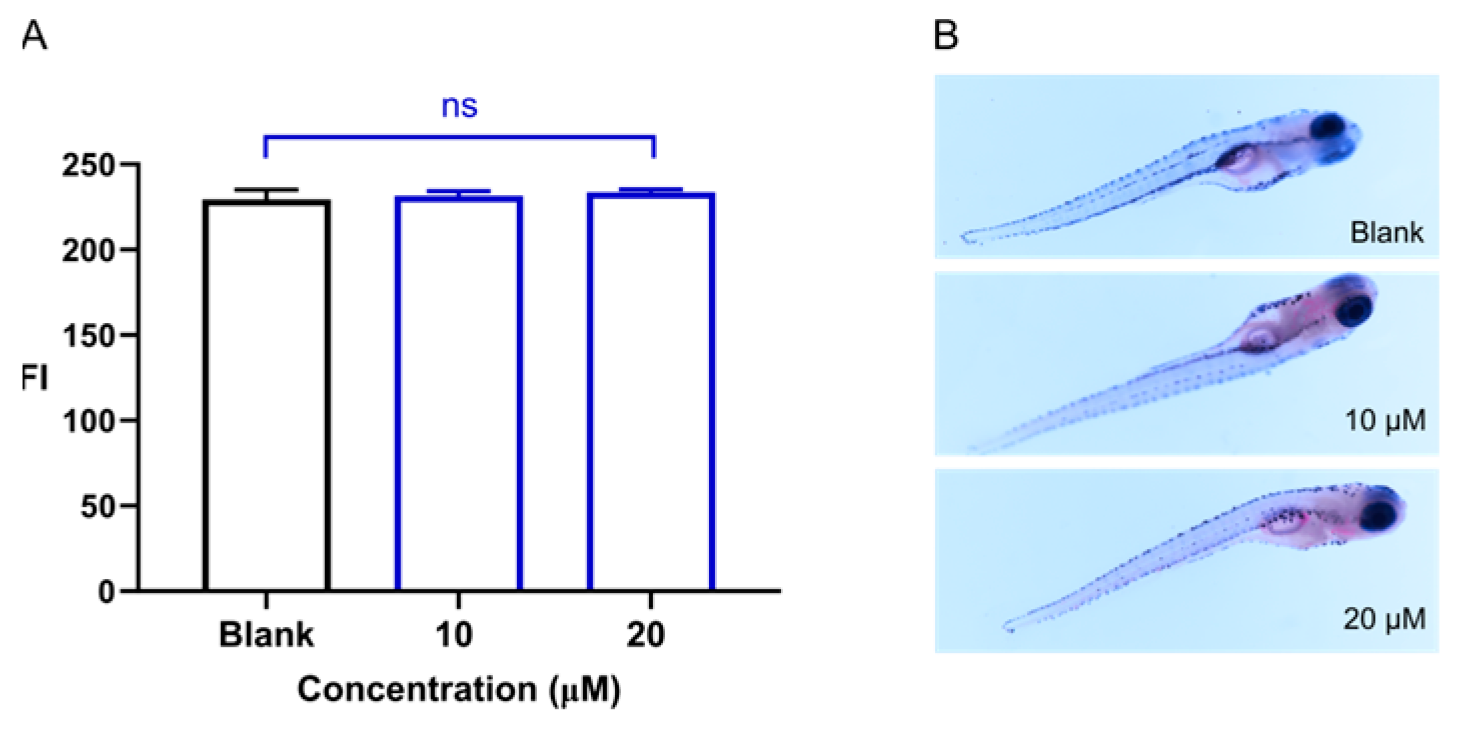
2.2. Laxative Activity of Sakuranetin
2.3. The Effects of Sakuranetin on Lipid Accumulation
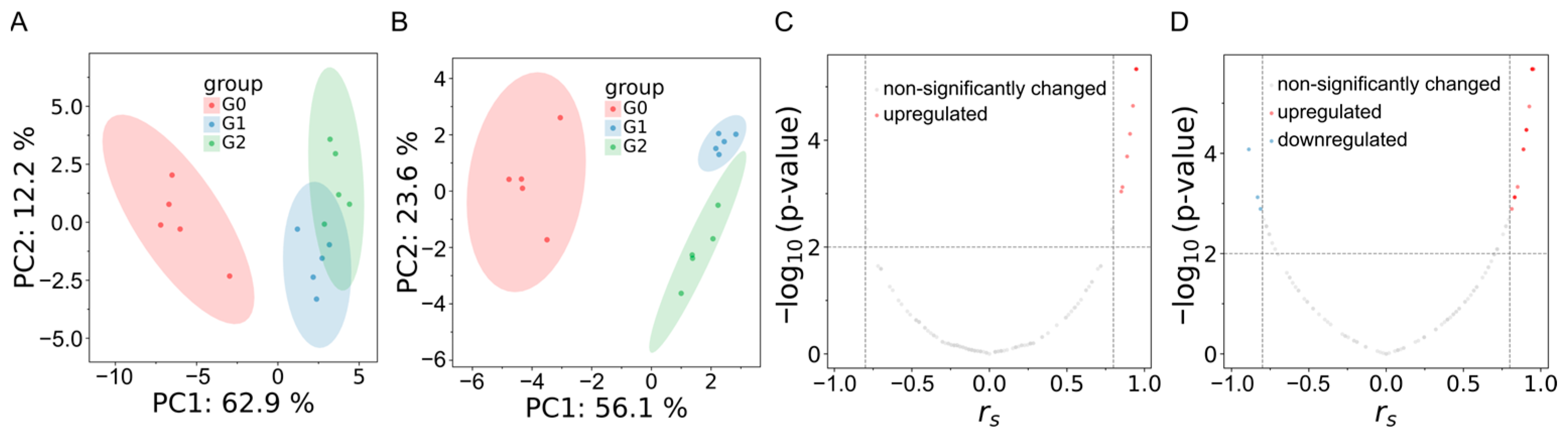
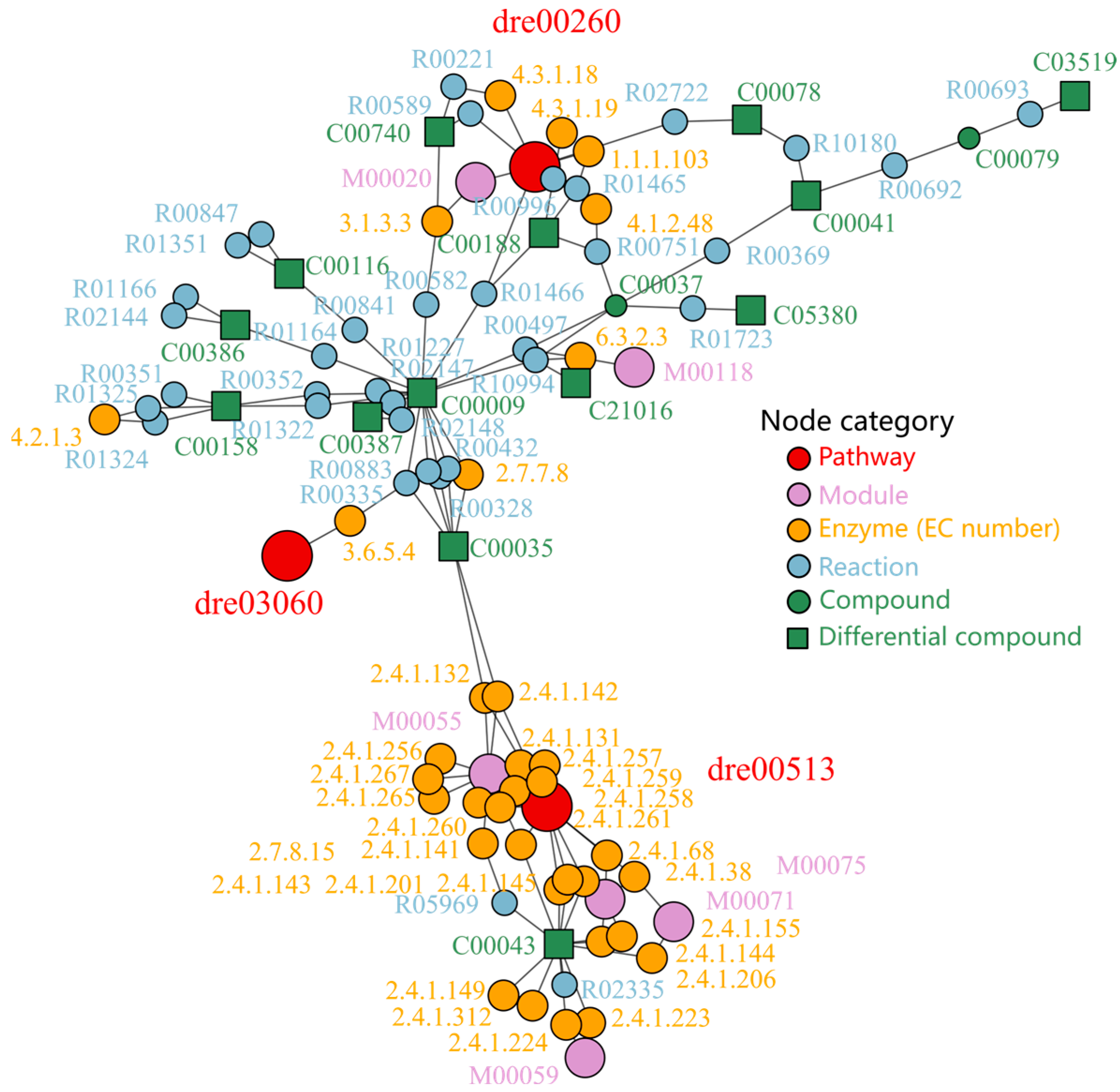
2.4. Intervention of Sakuranetin on Metabolome In Vivo
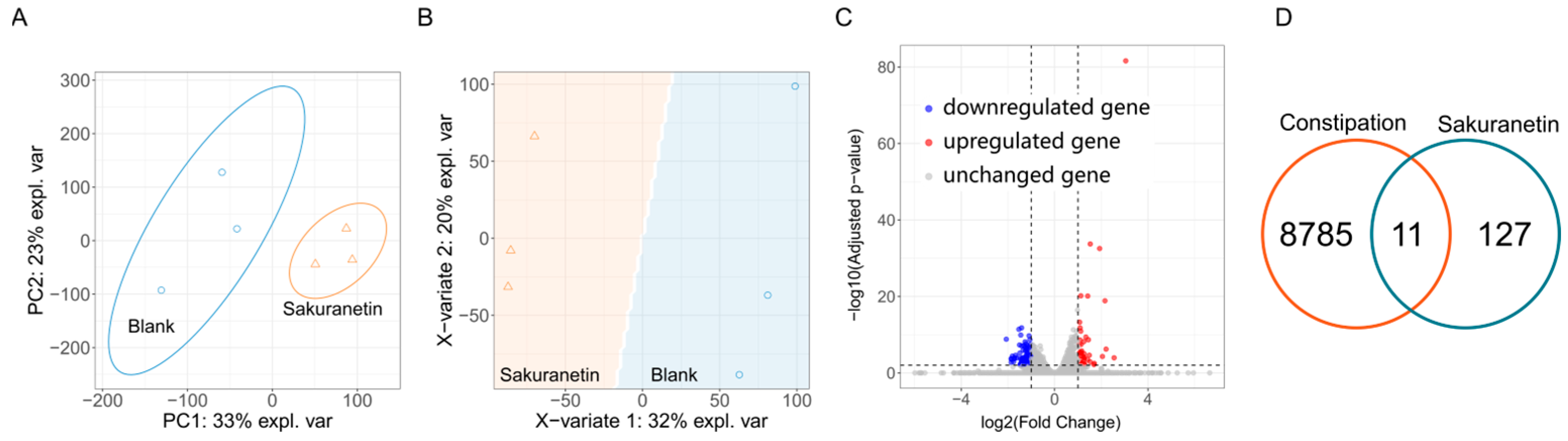
2.5. Transcriptomic Profiling Under Sakuranetin Intervention
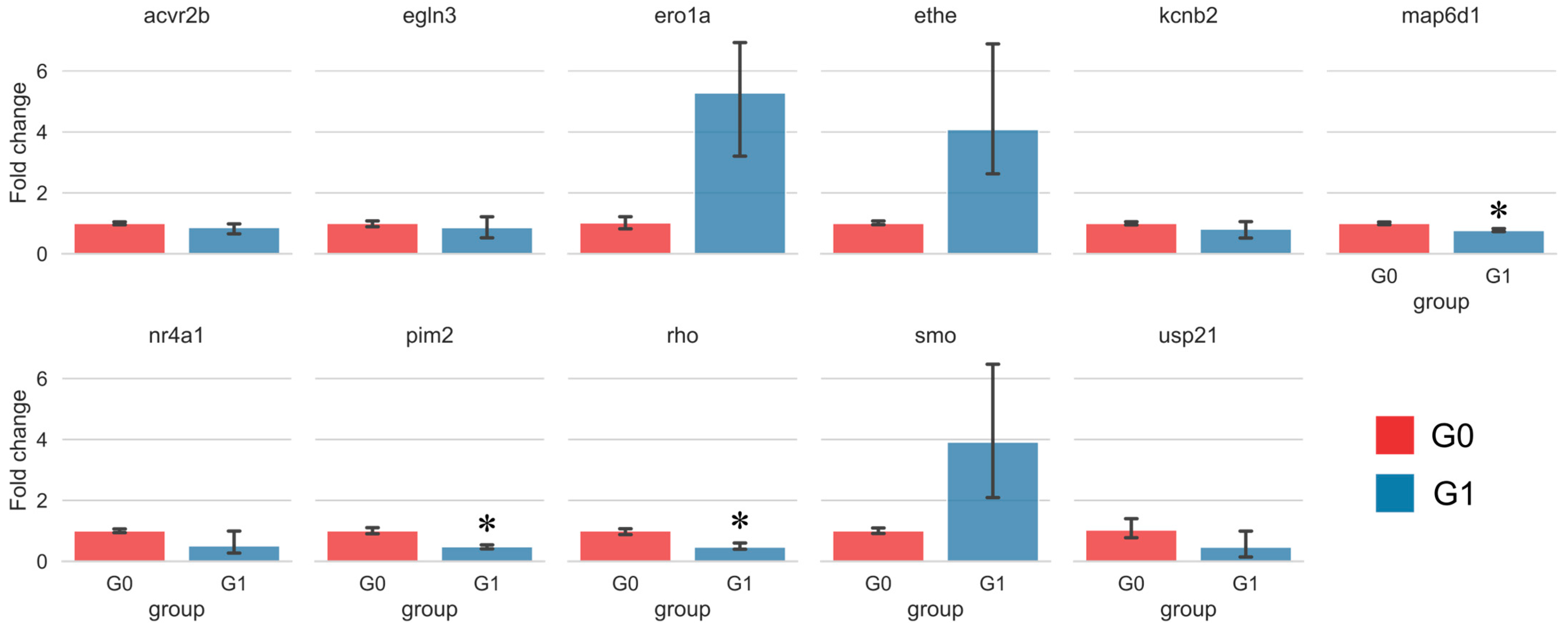
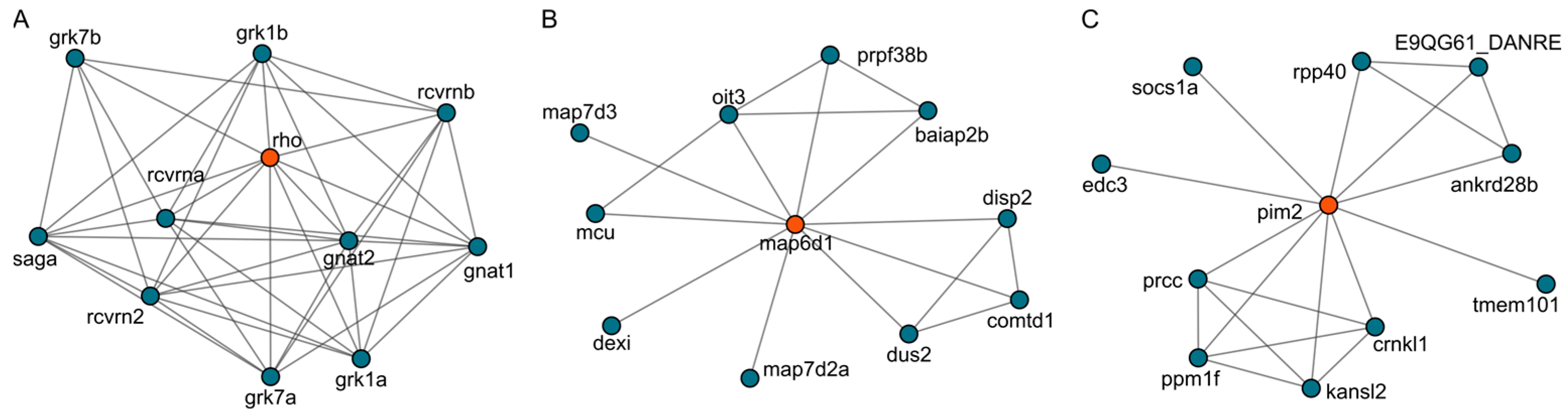
2.6. Target Analysis Based on Differentially Expressed Genes
3. Discussion
3.1. Sakuranetin Was a Newly Recognized Compound with Laxative Activity
3.2. Sakuranetin Modulated Metabolic Pathways Linked to Intestinal Inflammation
3.3. Sakuranetin Inhibited Rho Gene Level Related to Intestinal Inflammation
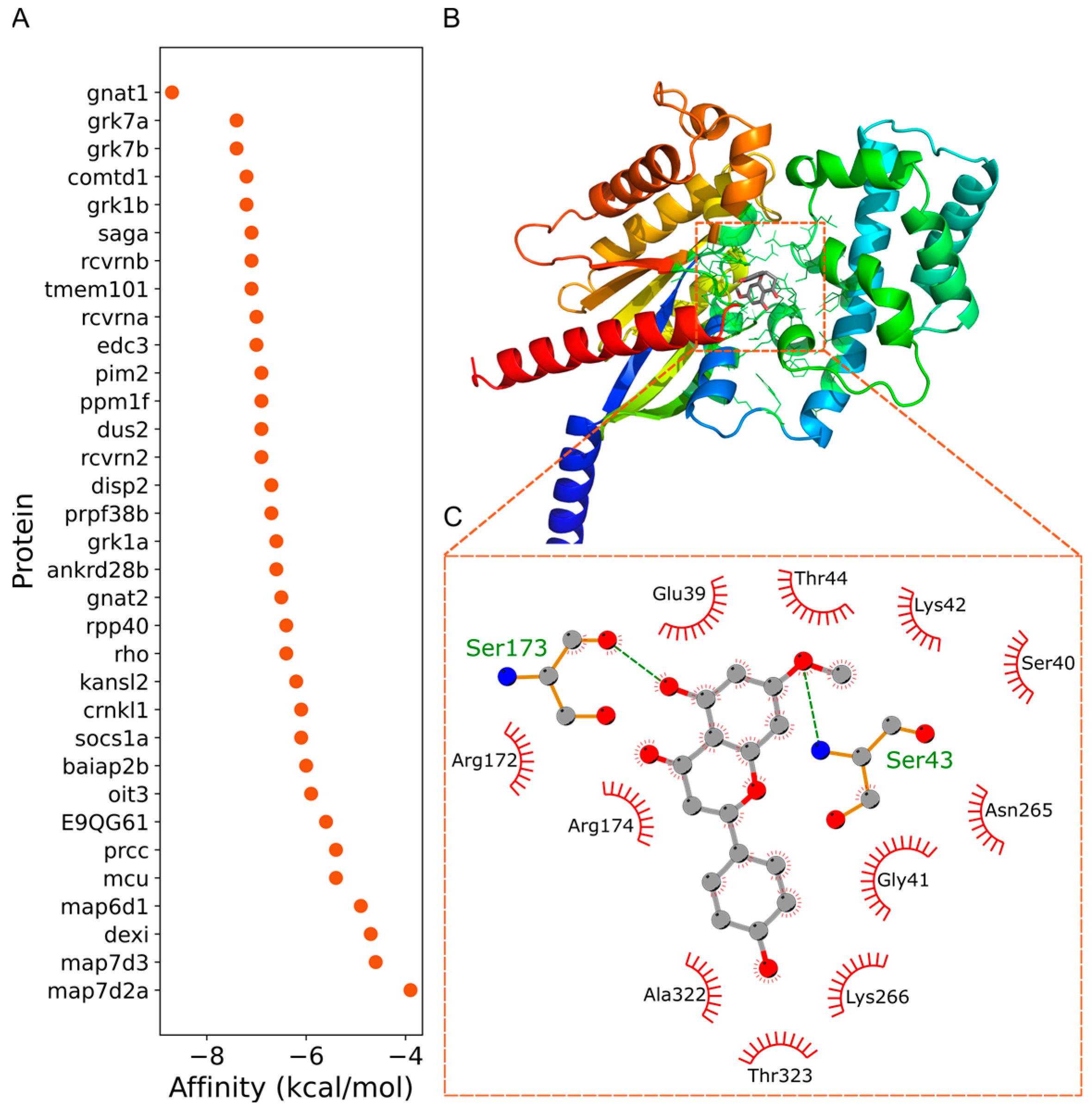
3.4. GNAT1 as a Putative Target of Sakuranetin’s Laxative Action
3.5. Hypothesis, Limitations, and Future Directions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Bio-Guided Isolation of Laxative Component from Peach Leaves
4.3. Laxative Activity
4.4. Assessment of Lipid Accumulation
4.5. Metabolomic Analysis
4.6. Transcriptomic Analysis
4.7. RT-qPCR
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Rao, S.S.C.; Brenner, D.M. Efficacy and Safety of Over-the-Counter Therapies for Chronic Constipation: An Updated Systematic Review. Am. J. Gastroenterol. 2021, 116, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kistemaker, K.R.J.; Sijani, F.; Brinkman, D.J.; de Graeff, A.; Burchell, G.L.; Steegers, M.A.H.; van Zuylen, L. Pharmacological Prevention and Treatment of Opioid-Induced Constipation in Cancer Patients: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2024, 125, 102704. [Google Scholar] [CrossRef]
- Dudi-Venkata, N.N.; Kroon, H.M.; Bedrikovetski, S.; Lewis, M.; Lawrence, M.J.; Hunter, R.A.; Moore, J.W.; Thomas, M.L.; Sammour, T. Impact of STIMUlant and Osmotic LAXatives (STIMULAX Trial) on Gastrointestinal Recovery after Colorectal Surgery: Randomized Clinical Trial. Br. J. Surg. 2021, 108, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, C.; Li, X.; Yuan, J.; Gao, X.; Li, B.; Zhao, Z.; Toh, S.; Yu, X.; Brayne, C.; et al. Association Between Regular Laxative Use and Incident Dementia in UK Biobank Participants. Neurology 2023, 100, e1702–e1711. [Google Scholar] [CrossRef]
- Goldman, P.N.; Cody, P.J. Severe Edema after Cessation of Laxative Abuse and Use of a Loop Diuretic: Case Report. Int. J. Eat. Disord. 2020, 53, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Shibuya, T.; Nagahara, A. Management of Chronic Constipation: A Comprehensive Review. Intern. Med. 2025, 64, 7–15. [Google Scholar] [CrossRef]
- Ihara, E.; Manabe, N.; Ohkubo, H.; Ogasawara, N.; Ogino, H.; Kakimoto, K.; Kanazawa, M.; Kawahara, H.; Kusano, C.; Kuribayashi, S.; et al. Evidence-Based Clinical Guidelines for Chronic Constipation 2023. Digestion 2024, 106, 62–89. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, H.; Chen, F.; Cai, P.; Qi, F. Considering Traditional Chinese Medicine as Adjunct Therapy in the Management of Chronic Constipation by Regulating Intestinal Flora. Biosci. Trends 2024, 18, 127–140. [Google Scholar] [CrossRef]
- Aghaei-Zarch, S.M.; Nia, A.H.S.; Nouri, M.; Mousavinasab, F.; Najafi, S.; Bagheri-Mohammadi, S.; Aghaei-Zarch, F.; Toolabi, A.; Rasoulzadeh, H.; Ghanavi, J.; et al. The Impact of Particulate Matters on Apoptosis in Various Organs: Mechanistic and Therapeutic Perspectives. Biomed. Pharmacother. 2023, 165, 115054. [Google Scholar] [CrossRef]
- Zhao, Z.; Zuo, X.; Han, C.; Zhang, Y.; Zhao, J.; Wang, Y.; Zhang, S.; Li, W. A Novel Purgative Mechanism of Multiflorin A Involves Changing Intestinal Glucose Absorption and Permeability. Phytomedicine 2023, 114, 154805. [Google Scholar] [CrossRef]
- Reis, V.H.D.O.T.; De Melo, V.X.; Da Silva, M.L.R.; Filho, P.S.L.; Portugal, L.C.; Sartoratto, A.; Rafacho, B.P.M.; Cazarin, C.B.B.; Cordeiro, L.M.C.; Dos Santos, E.F. Insoluble Dietary Fibers from Hancornia speciosa Alleviates Chronic Constipation on Experimental Loperamide-Induced Model. Int. J. Biol. Macromol. 2025, 306, 141215. [Google Scholar] [CrossRef]
- Deng, Z.; Fu, Z.; Yan, W.; Nie, K.; Ding, L.; Ma, D.; Huang, H.; Li, T.; Xie, J.; Fu, L. The Different Effects of Chinese Herb Solid Drink and Lactulose on Gut Microbiota in Rats with Slow Transit Constipation Induced by Compound Diphenoxylate. Food Res. Int. 2021, 143, 110273. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Gong, Z.-C.; Zhao, Q.; Xu, D.-Q.; Fu, R.-J.; Tang, Y.-P.; Chen, Y.-Y. Time-Dependent Laxative Effect of Sennoside A, the Core Functional Component of Rhubarb, Is Attributed to Gut Microbiota and Aquaporins. J. Ethnopharmacol. 2023, 311, 116431. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, C.-Z.; Sawadogo, W.R.; Bian, Z.-X.; Yuan, C.-S. Herbal Medicines for Constipation and Phytochemical Comparison of Active Components. Am. J. Chin. Med. 2022, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Li, Q.-D.; Feng, Y.; Liu, J.; Wang, E.-K.; Zhong, L.; Sun, Q.-L.; Yuan, J.-Y. Animal Models of Cathartic Colon. World J. Clin. Cases 2021, 9, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Fu, S.; Ren, B.; Ma, L.; Sun, D. Based on Network Pharmacology and Gut Microbiota Analysis to Investigate the Mechanism of the Laxative Effect of Pterostilbene on Loperamide-Induced Slow Transit Constipation in Mice. Front. Pharmacol. 2022, 13, 913420. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Rehman, N.U.; Azhar, I.; Palla, A. Unraveling the Multi-Faceted Role of Rosmarinus officinalis L. (Rosemary) and Diosmetin in Managing Gut Motility. J. Ethnopharmacol. 2024, 332, 118395. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, S.-Y.; Zhang, Y.; Li, C.-Q. Human Prokinetic Drugs Promote Gastrointestinal Motility in Zebrafish. Neurogastroenterol. Motil. 2014, 26, 589–595. [Google Scholar] [CrossRef]
- Ge, L.; Xie, Q.; Wei, X.; Li, Y.; Shen, W.; Hu, Y.; Yao, J.; Wang, S.; Du, X.; Zeng, X. Five Undescribed Plant-Derived Bisphenols from Artemisia capillaris Aerial Parts: Structure Elucidation, Anti-Hepatoma Activities and Plausible Biogenetic Pathway. Arab. J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bae, K. Anti-Inflammatory Activity of Flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Gao, X. Nitroreductase from Enterococcus faecalis Catalyzes the Metabolic Activation of Sennoside A in the Colon via a Unique CC Reductive Cleavage. Int. J. Biol. Macromol. 2025, 319, 145153. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Sarigun, A.; Uyar, B.; Franke, V.; Akalin, A. PocketVina Enables Scalable and Highly Accurate Physically Valid Docking through Multi-Pocket Conditioning. arXiv 2025. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Li, H.; Bai, X.; Hu, T.; Xue, X.; An, J.; Zhang, Y.; Dong, X. Alteration of Serum Metabolites in Women of Reproductive Age with Chronic Constipation. Med. Sci. Monit. 2022, 28, e934117. [Google Scholar] [CrossRef]
- Święch, E.; Tuśnio, A.; Taciak, M.; Barszcz, M. Modulation of Mucin Secretion in the Gut of Young Pigs by Dietary Threonine and Non-Essential Amino Acid Levels. Animals 2022, 12, 270. [Google Scholar] [CrossRef]
- Gaifem, J.; Gonçalves, L.G.; Dinis-Oliveira, R.J.; Cunha, C.; Carvalho, A.; Torrado, E.; Rodrigues, F.; Saraiva, M.; Castro, A.G.; Silvestre, R. L-Threonine Supplementation During Colitis Onset Delays Disease Recovery. Front. Physiol. 2018, 9, 01247. [Google Scholar] [CrossRef]
- Kang, J.; Guo, X.; Peng, H.; Deng, Y.; Lai, J.; Tang, L.; Aoieong, C.; Tou, T.; Tsai, T.; Liu, X. Metabolic Implications of Amino Acid Metabolites in Chronic Kidney Disease Progression: A Metabolomics Analysis Using OPLS-DA and MBRole2.0 Database. Int. Urol. Nephrol. 2024, 56, 1173–1184. [Google Scholar] [CrossRef]
- Yang, D.-X.; Yang, M.-J.; Yin, Y.; Kou, T.-S.; Peng, L.-T.; Chen, Z.-G.; Zheng, J.; Peng, B. Serine Metabolism Tunes Immune Responses To Promote Oreochromis Niloticus Survival upon Edwardsiella Tarda Infection. mSystems 2021, 6, e0042621. [Google Scholar] [CrossRef]
- Asakawa, T.; Onizawa, M.; Saito, C.; Hikichi, R.; Yamada, D.; Minamidate, A.; Mochimaru, T.; Asahara, S.; Kido, Y.; Oshima, S.; et al. Oral Administration of D-Serine Prevents the Onset and Progression of Colitis in Mice. J. Gastroenterol. 2021, 56, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, X.; Niu, P.; Yang, X.; Lu, Y. Incremental Effects of Eurotium Cristatum Fermentation of Soybean on Its Nutrients, Flavor Profile and Laxative Regulation in Experimental Constipated Rats. Food Funct. 2025, 16, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Chang, Y.B.; Kim, S.M.; Han, K.; Sim, W.; Hong, K.-B.; Suh, H.J.; Han, S.H. Impact of the Probiotic Bacillus Coagulans on Loperamide-Induced Delayed Bowel Movement in Sprague–Dawley Rats. Food Funct. 2025, 16, 720–730. [Google Scholar] [CrossRef]
- Duan, T.; Wang, X.; Dong, X.; Wang, C.; Wang, L.; Yang, X.; Li, T. Broccoli-Derived Exosome-like Nanoparticles Alleviate Loperamide-Induced Constipation, in Correlation with Regulation on Gut Microbiota and Tryptophan Metabolism. J. Agric. Food Chem. 2023, 71, 16568–16580. [Google Scholar] [CrossRef]
- Pradhan, R.; Ngo, P.A.; Martínez-Sánchez, L.d.C.; Neurath, M.F.; López-Posadas, R. Rho GTPases as Key Molecular Players within Intestinal Mucosa and GI Diseases. Cells 2021, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pei, G.; Sun, X.; Xiao, Y.; Miao, C.; Zhou, L.; Wang, B.; Yang, L.; Yu, M.; Zhang, Z.-S.; et al. RhoB Affects Colitis through Modulating Cell Signaling and Intestinal Microbiome. Microbiome 2022, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Qiao, P.; Fu, R.; Wang, Y.; Lu, J.; Ling, X.; Liu, L.; Sun, Y.; Ren, C.; Yu, Z. Phosphorylation of PFKFB4 by PIM2 Promotes Anaerobic Glycolysis and Cell Proliferation in Endometriosis. Cell Death Dis. 2022, 13, 790. [Google Scholar] [CrossRef]
- Xu, X.; Xu, P.; Shen, G.; Peng, X.; Liu, Z.; Chen, C.; Yu, W.; Su, Z.; Lin, J.; Zheng, G.; et al. Targeting Macrophage Polarization by Inhibiting Pim2 Alleviates Inflammatory Arthritis via Metabolic Reprogramming. Cell. Mol. Immunol. 2025, 22, 418–436. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Akdel, M.; Pires, D.E.V.; Pardo, E.P.; Jänes, J.; Zalevsky, A.O.; Mészáros, B.; Bryant, P.; Good, L.L.; Laskowski, R.A.; Pozzati, G.; et al. A Structural Biology Community Assessment of AlphaFold2 Applications. Nat. Struct. Mol. Biol. 2022, 29, 1056–1067. [Google Scholar] [CrossRef]
- Devi, S.S.; Yadav, R.; Arya, R. Altered Actin Dynamics in Cell Migration of GNE Mutant Cells. Front. Cell Dev. Biol. 2021, 9, 603742. [Google Scholar] [CrossRef]
- Brünje, A.; Füßl, M.; Eirich, J.; Boyer, J.-B.; Heinkow, P.; Neumann, U.; Konert, M.; Ivanauskaite, A.; Seidel, J.; Ozawa, S.-I.; et al. The Plastidial Protein Acetyltransferase GNAT1 Forms a Complex With GNAT2, yet Their Interaction Is Dispensable for State Transitions. Mol. Cell. Proteomics 2024, 23, 100850. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhu, Z.-J.; Ren, S.-P.; Deng, Y.-C.; Xu, J.-Y.; Zhang, S.-M.; Gao, J.-M.; Zhang, Q. Metabolomic Navigated Citrus Waste Repurposing to Restore Amino Acids Disorder in Neural Lesion. Food Chem. 2022, 387, 132933. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Yan, L.; Zhang, Z.; Wei, J.; Li, L.; Zhang, Q. Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS. Int. J. Mol. Sci. 2022, 23, 10385. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Guo, D.; Huang, W.; Ren, H.; Zhang, Q. Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin. Int. J. Mol. Sci. 2024, 25, 8985. [Google Scholar] [CrossRef]
- systemsomicslab GitHub—Systemsomicslab/MsdialWorkbench: Universal Workbench Incorporating Msdial, Msfinder, and Mrmprobs. Available online: https://github.com/systemsomicslab/MsdialWorkbench (accessed on 20 June 2025).
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological Systems Database as a Model of the Real World. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Wang, F.; Allen, D.; Tian, S.; Oler, E.; Gautam, V.; Greiner, R.; Metz, T.O.; Wishart, D.S. CFM-ID 4.0—A Web Server for Accurate MS-Based Metabolite Identification. Nucleic Acids Res. 2022, 50, W165–W174. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R Package for ’omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Picart-Armada, S.; Fernández-Albert, F.; Vinaixa, M.; Yanes, O.; Perera-Lluna, A. FELLA: An R Package to Enrich Metabolomics Data. BMC Bioinform. 2018, 19, 538. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger/Pymol-Open-Source 2025. Schrödinger, LLC. PyMOL Molecular Graphics System (Open-Source Edition), Version 3.2; 2025. Available online: https://github.com/schrodinger/pymol-open-source (accessed on 2 July 2025).
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Song, Y.; Jiang, H.; Qi, C.; Zhang, X.; Wang, D.; Li, L.; Zhang, Q. Sakuranetin, A Laxative Component from Peach Leaves and Its Intervention in Metabolism. Int. J. Mol. Sci. 2025, 26, 8112. https://doi.org/10.3390/ijms26178112
Wang P, Song Y, Jiang H, Qi C, Zhang X, Wang D, Li L, Zhang Q. Sakuranetin, A Laxative Component from Peach Leaves and Its Intervention in Metabolism. International Journal of Molecular Sciences. 2025; 26(17):8112. https://doi.org/10.3390/ijms26178112
Chicago/Turabian StyleWang, Ping, Yi Song, Haixin Jiang, Chenyuan Qi, Xubo Zhang, Disheng Wang, Luqi Li, and Qiang Zhang. 2025. "Sakuranetin, A Laxative Component from Peach Leaves and Its Intervention in Metabolism" International Journal of Molecular Sciences 26, no. 17: 8112. https://doi.org/10.3390/ijms26178112
APA StyleWang, P., Song, Y., Jiang, H., Qi, C., Zhang, X., Wang, D., Li, L., & Zhang, Q. (2025). Sakuranetin, A Laxative Component from Peach Leaves and Its Intervention in Metabolism. International Journal of Molecular Sciences, 26(17), 8112. https://doi.org/10.3390/ijms26178112







