Enhancing the Biosorption Capacity of Macrocystis pyrifera: Effects of Acid and Alkali Pretreatments on Recalcitrant Organic Pollutants Removal
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of M. pyrifera Biomass
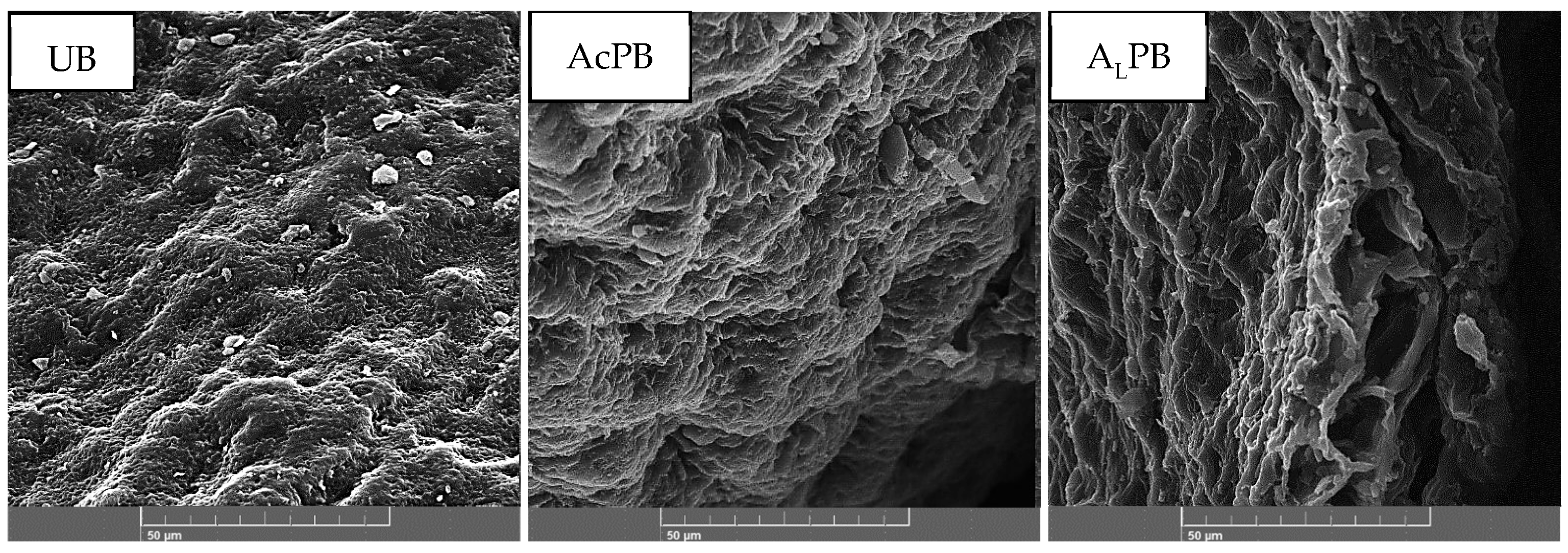
2.1.1. SEM and Textural Properties
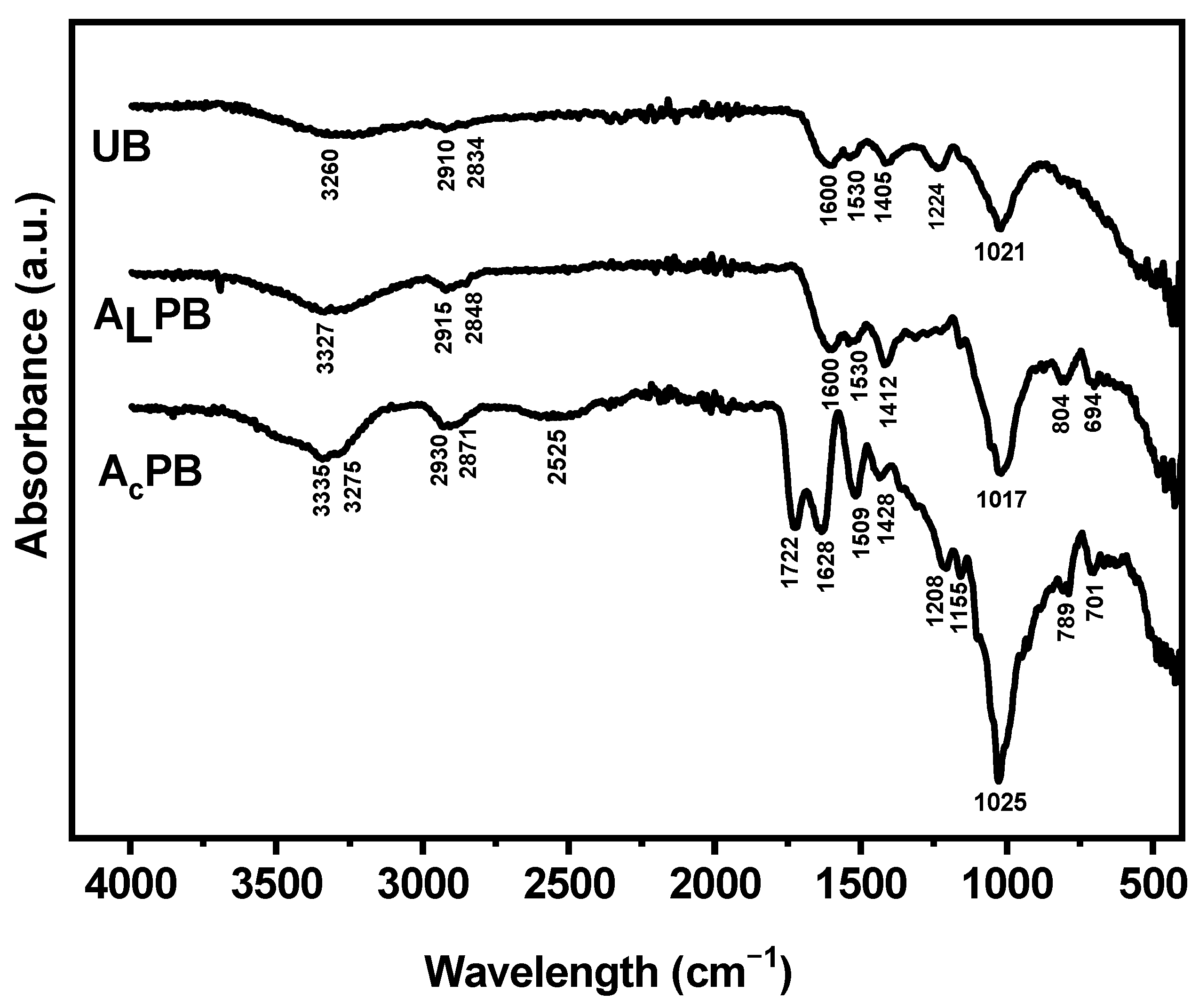
2.1.2. FTIR Characterization
2.1.3. Potentiometric Titrations
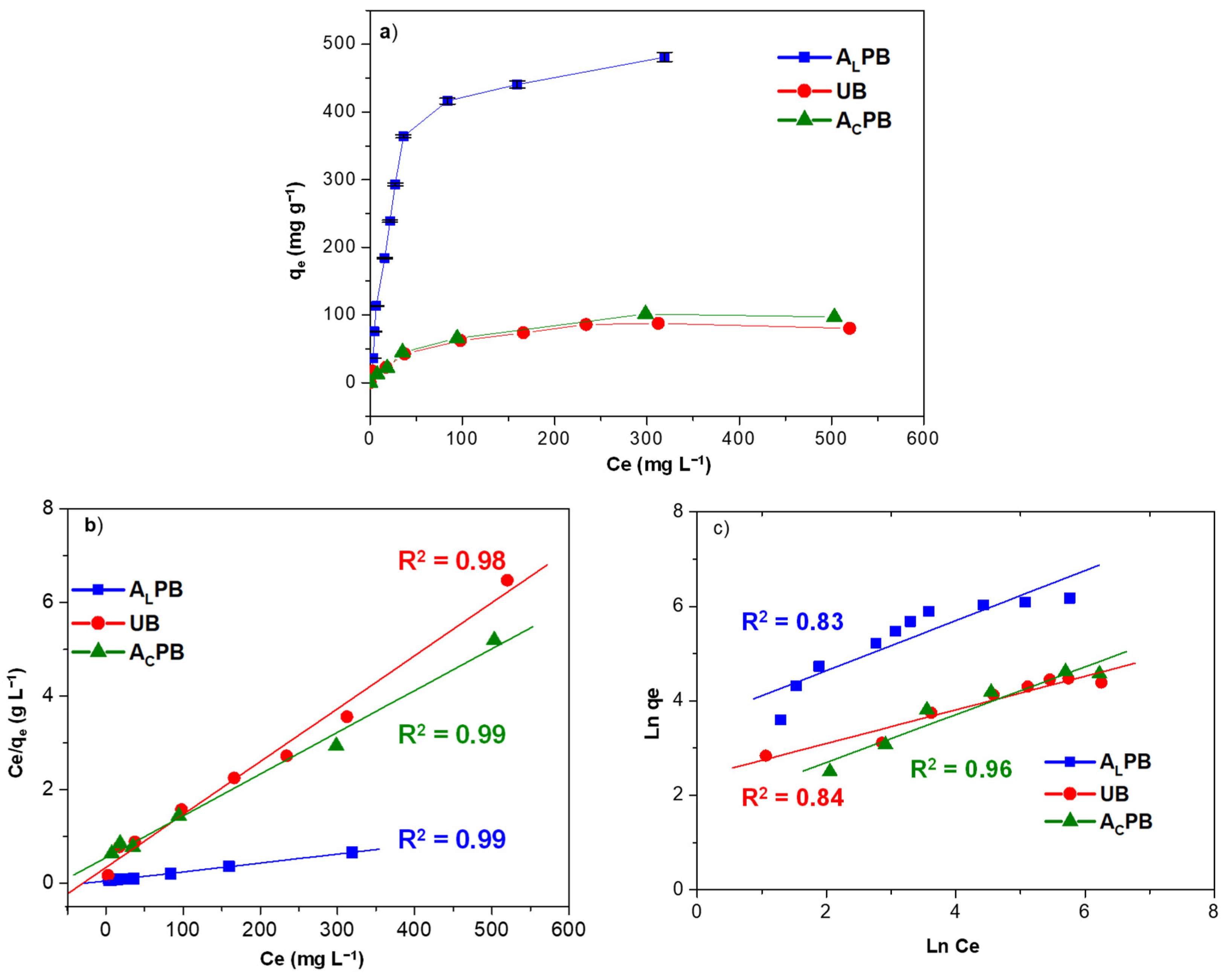
2.2. Methylene Blue Biosorption Capacity
Adsorption Model
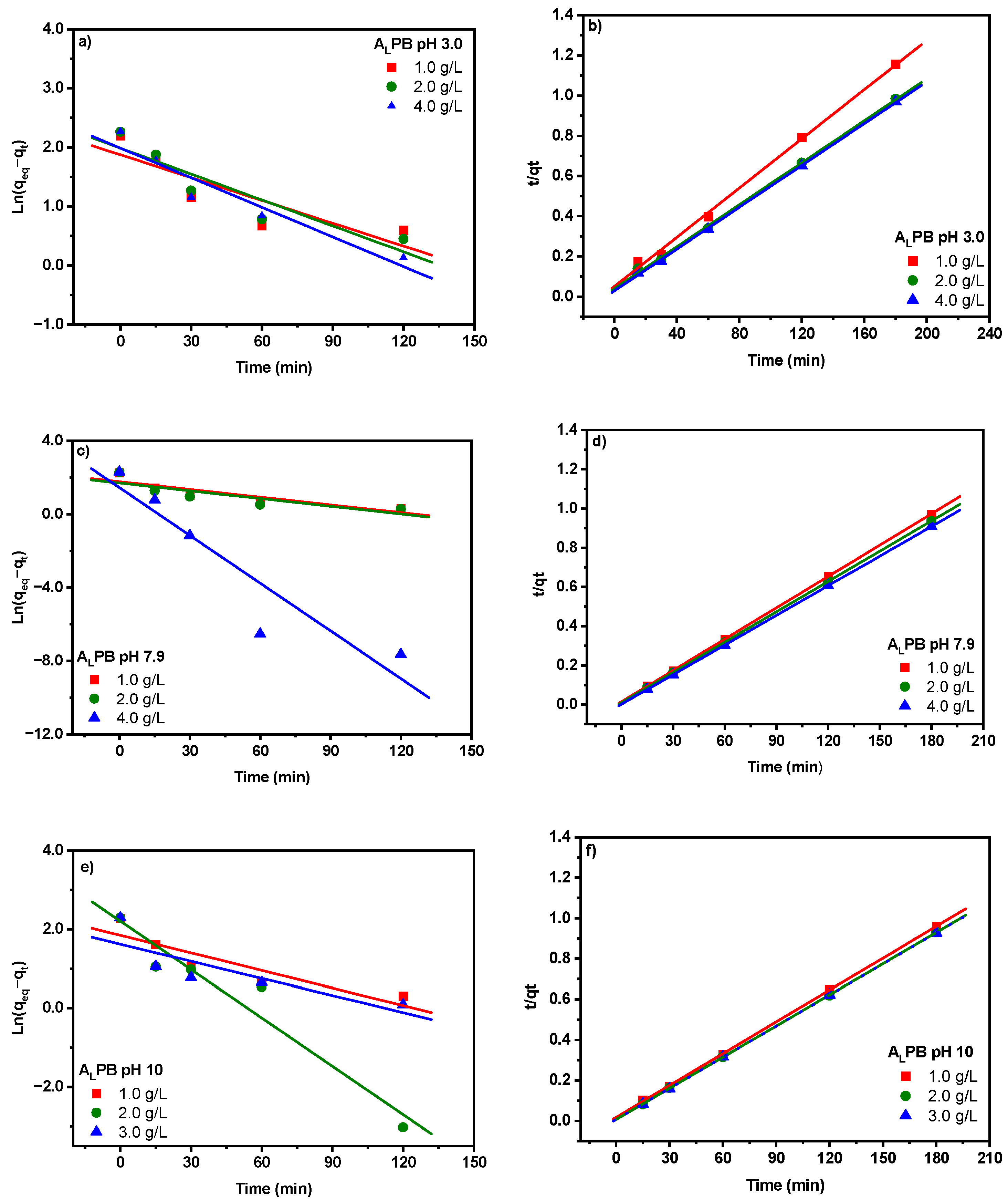
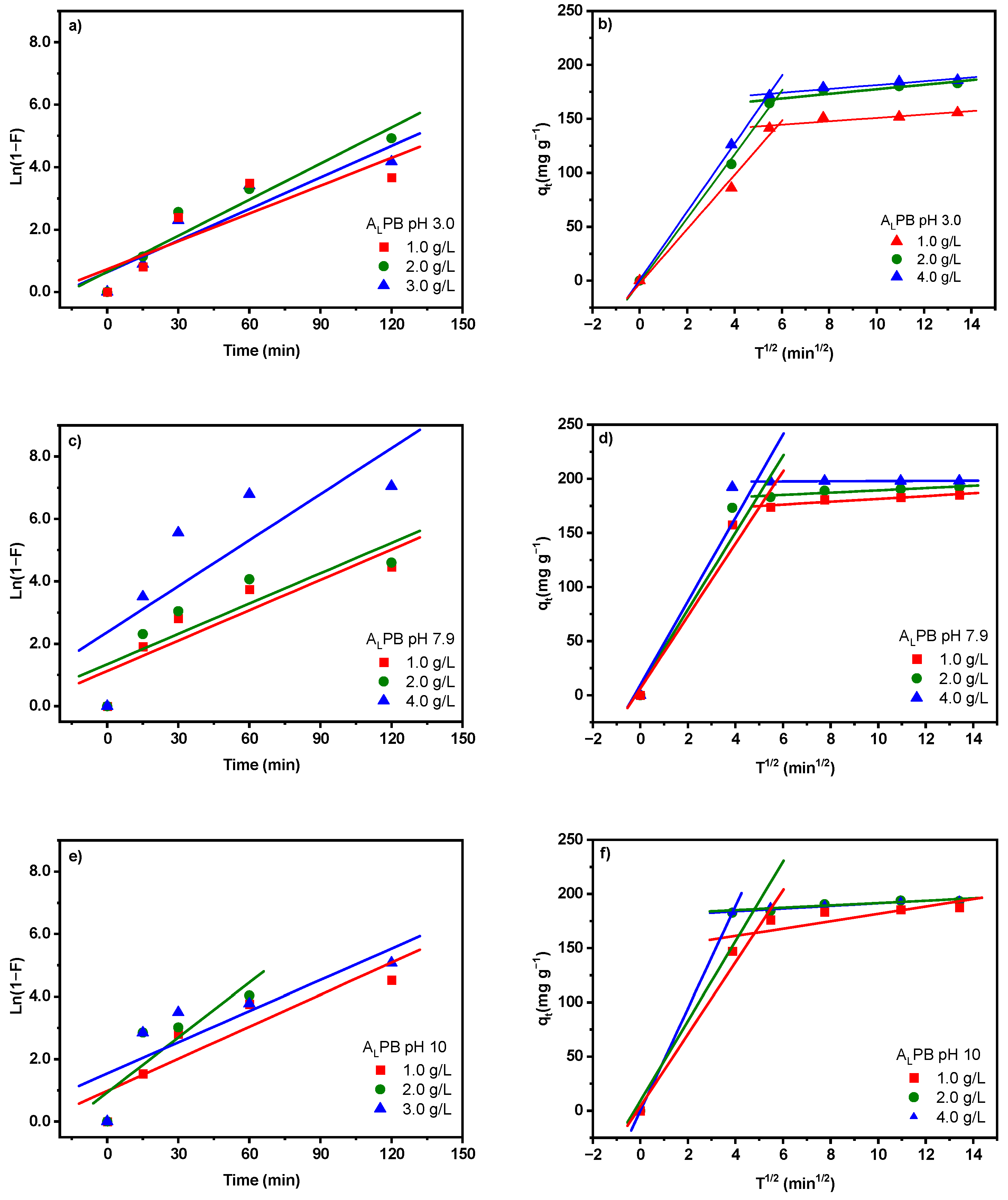
2.3. Adsorption Kinetic Study
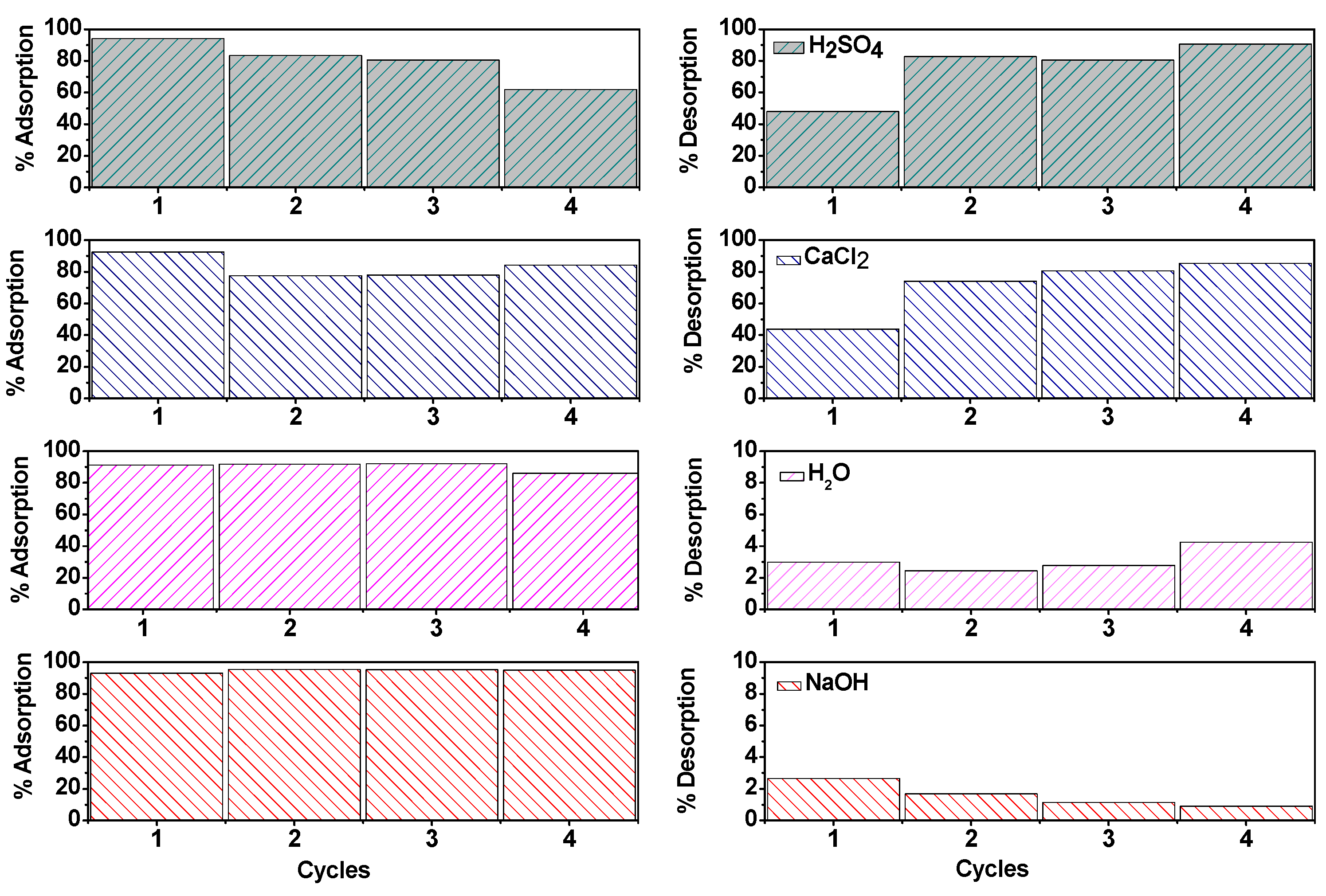
2.4. Desorption Study
2.5. Practical Application
3. Materials and Methods
3.1. Chemicals
3.2. Biosorbent Preparation
3.3. Biosorbent Characterization
3.4. Adsorption and Desorption Tests
3.4.1. Batch Adsorption Studies
3.4.2. Batch Desorption Studies and Reusability
3.4.3. Equilibrium Modeling
3.5. Sorption Kinetics Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging Contaminants as Global Environmental Hazards. A Bibliometric Analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Wei, L.; Su, Z.; Yue, Q.; Huang, X.; Wei, M.; Wang, J. Microplastics, Heavy Metals, Antibiotics, and Antibiotic Resistance Genes in Recirculating Aquaculture Systems. TrAC Trends Anal. Chem. 2024, 172, 117564. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging Environmental Contaminants: A Global Perspective on Policies and Regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef]
- United Nation. United Nations Secretary-General’s Plan: Water Action Decade 2018–2028. Available online: https://wateractiondecade.org/wp-content/uploads/2018/03/UN-SG-Action-Plan_Water-Action-Decade-web.pdf (accessed on 20 January 2025).
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Sillanpää, M.; Bhatnagar, A. A Comparative Study of Methylene Blue Biosorption Using Different Modified Brown, Red and Green Macroalgae—Effect of Pretreatment. Chem. Eng. J. 2017, 307, 435–446. [Google Scholar] [CrossRef]
- Aravindhan, R.; Rao, J.R.; Nair, B.U. Application of a Chemically Modified Green Macro Alga as a Biosorbent for Phenol Removal. J. Environ. Manag. 2009, 90, 1877–1883. [Google Scholar] [CrossRef]
- Lezana, N.; Fernández-Vidal, F.; Berríos, C.; Garrido-Ramírez, E. Electrochemical and Photo-Electrochemical Processes of Methylene Blue Oxidation by Ti/TiO2 Electrodes Modified with Fe-Allophane. J. Chil. Chem. Soc. 2017, 62, 3529–3534. [Google Scholar] [CrossRef]
- Castro, J.; Fernández, F.; Olivares, F.; Berríos, C.; Garrido-Ramírez, E.; Blanco, E.; Escalona, N.; Aspée, A.; Barrías, P.; Ureta-Zañartu, M.S. Electrodes Based on Zeolites Modified with Cobalt and/or Molybdenum for Pesticide Degradation: Part II—2,4,6-Trichlorophenol Degradation. J. Solid State Electrochem. 2021, 25, 117–131. [Google Scholar] [CrossRef]
- Castro-Rojas, J.; Jofré-Dupre, P.; Escalona, N.; Blanco, E.; Ureta-Zañartug, M.S.; Mora, M.L.; Garrido-Ramírez, E. Atrazine Degradation through a Heterogeneous Dual-Effect Process Using Fe-TiO2-Allophane Catalysts under Sunlight. Heliyon 2024, 10, e32894. [Google Scholar] [CrossRef]
- Castro-Rojas, J.; Rao, M.; Berruti, I.; Mora, M.L.; Garrido-Ramírez, E.; Polo-Lopéz, M.I. Assessment of Solar Photocatalytic Wastewater Disinfection and Microcontaminants Removal by Modified-Allophane Nanoclays Based on TiO2, Fe and ZnO at Laboratory and Pilot Scale. Chem. Eng. J. 2025, 503, 157894. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Marco, J.F.; Escalona, N.; Ureta-Zañartu, M.S. Preparation and Characterization of Bimetallic Fe-Cu Allophane Nanoclays and Their Activity in the Phenol Oxidation by Heterogeneous Electro-Fenton Reaction. Microporous Mesoporous Mater. 2016, 225, 303–311. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and Me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Kousha, M.; Jokar, M.; Koutahzadeh, N.; Guibal, E. Acidic Dye Biosorption onto Marine Brown Macroalgae: Isotherms, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2012, 204–205, 225–234. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hammud, H.H.; Fayoumi, L.; Fayoumi, L.; Holail, H. Biosorption Studies of Methylene Blue by Mediterranean Algae Carolina and Its Chemically Modified Forms. Linear and Nonlinear Models’ Prediction Based on Statistical Error Calculation. Int. J. Chem. 2011, 3, 147–163. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro Algae Gracilaria verrucosa as a Biosorbent: A Study of Sorption Mechanisms. Algal Res. 2012, 1, 194–204. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Bernardelli, C.; Viera, M.; Donati, E.; Guibal, E. Zinc and Cadmium Biosorption by Untreated and Calcium-Treated Macrocystis pyrifera in a Batch System. Bioresour. Technol. 2012, 116, 195–203. [Google Scholar] [CrossRef]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; Sastre de Vicente, M.E. Removal of Methylene Blue from Aqueous Solutions Using as Biosorbent Sargassum muticum: An Invasive Macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef]
- Luo, F.; Liu, Y.; Li, X.; Xuan, Z.; Ma, J. Biosorption of Lead Ion by Chemically-Modified Biomass of Marine Brown Algae Laminaria japonica. Chemosphere 2006, 64, 1122–1127. [Google Scholar] [CrossRef]
- García, F.E.; Plaza-Cazón, J.; Montesinos, V.N.; Donati, E.R.; Litter, M.I. Combined Strategy for Removal of Reactive Black 5 by Biomass Sorption on Macrocystis pyrifera and Zerovalent Iron Nanoparticles. J. Environ. Manag. 2018, 207, 70–79. [Google Scholar] [CrossRef]
- Schiel, D.R.; Foster, M.S. The Structure, Function, and Abiotic Requirements of Giant Kelp. In The Biology and Ecology of Giant Kelp Forests; University of California Press: Berkeley, CA, USA, 2015; pp. 23–40. ISBN 9780520278868. [Google Scholar]
- Cazón, J.P.H.; Benítez, L.; Donati, E.; Viera, M. Biosorption of Chromium(III) by Two Brown Algae Macrocystis pyrifera and Undaria pinnatifida: Equilibrium and Kinetic Study. Eng. Life Sci. 2012, 12, 95–103. [Google Scholar] [CrossRef]
- Plaza, J.; Viera, M.; Donati, E.; Guibal, E. Biosorption of Mercury by Macrocystis pyrifera and Undaria pinnatifida: Influence of Zinc, Cadmium and Nickel. J. Environ. Sci. 2011, 23, 1778–1786. [Google Scholar] [CrossRef]
- Cid, H.; Ortiz, C.; Pizarro, J.; Moreno-Piraján, J.C. Effect of Copper (Ii) Biosorption over Light Metal Cation Desorption in the Surface of Macrocystis pyrifera Biomass. J. Environ. Chem. Eng. 2020, 8, 103729. [Google Scholar] [CrossRef]
- Araya, M.; Rivas, J.; Sepúlveda, G.; Espinoza-González, C.; Lira, S.; Meynard, A.; Blanco, E.; Escalona, N.; Ginocchio, R.; Garrido-Ramírez, E.; et al. Applied Sciences Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera. Appl. Sci. 2021, 11, 9233. [Google Scholar] [CrossRef]
- Flores-Chaparro, C.E.; Ruiz, L.F.C.; de la Torre, M.C.A.; Huerta-Diaz, M.A.; Rangel-Mendez, J.R. Biosorption Removal of Benzene and Toluene by Three Dried Macroalgae at Different Ionic Strength and Temperatures: Algae Biochemical Composition and Kinetics. J. Environ. Manag. 2017, 193, 126–135. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C. Mechanisms of Alizarin Red S and Methylene Blue Biosorption onto Olive Stone By-Product: Isotherm Study in Single and Binary Systems. J. Environ. Manag. 2015, 164, 86–93. [Google Scholar] [CrossRef]
- Abdallah, R.; Taha, S. Biosorption of Methylene Blue from Aqueous Solution by Nonviable Aspergillus fumigatus. Chem. Eng. J. 2012, 195–196, 69–76. [Google Scholar] [CrossRef]
- Koyuncu, H.; Kul, A.R. Biosorption Study for Removal of Methylene Blue Dye from Aqueous Solution Using a Novel Activated Carbon Obtained from Nonliving Lichen (Pseudevernia furfuracea (L.) Zopf.). Surf. Interfaces 2020, 19, 100527. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Viera, M.; Sala, S.; Donati, E. Biochemical Characterization of Macrocystis pyrifera and Undaria pinnatifida (Phaeophyceae) in Relation to Their Potentiality as Biosorbents. Phycologia 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Jiang, D.; Lu, W.; Ling, Q.; Zhong, S.; Chen, H.; Barati, B.; Hu, X.; Gong, X.; et al. Insights into Simultaneous Efficient Removal of Cationic and Anionic Dyes by Nitrogen-Rich Seaweed Carbon Adsorbent. Process Saf. Environ. Prot. 2024, 184, 38–49. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Yuan, Y.; Jing, C.; Cao, J.; Wang, Y.; Zhang, L.; Zhang, C.; Li, Y. Purification and Characterization of a Fucoidan from the Brown Algae Macrocystis pyrifera and the Activity of Enhancing Salt-Stress Tolerance of Wheat Seedlings. Int. J. Biol. Macromol. 2021, 180, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Tunali, S.; Kiran, I. Botrytis cinerea as a New Fungal Biosorbent for Removal of Pb(II) from Aqueous Solutions. Biochem. Eng. J. 2005, 25, 227–235. [Google Scholar] [CrossRef]
- El Atouani, S.; Belattmania, Z.; Reani, A.; Tahiri, S.; Aarfane, A.; Bentiss, F.; Jama, C.; Zrid, R.; Sabour, B. Brown Seaweed Sargassum muticum as Low-Cost Biosorbent of Methylene Blue. Int. J. Environ. Res. 2019, 13, 131–142. [Google Scholar] [CrossRef]
- Ashkenazy, R.; Gottlieb, L.; Yannai, S. Characterization of Acetone-Washed Yeast Biomass Functional Groups Involved in Lead Biosorption. Biotechnol. Bioeng. 1997, 55, 1–10. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar Prepared from Co-Pyrolysis of Municipal Sewage Sludge and Tea Waste for the Adsorption of Methylene Blue from Aqueous Solutions: Kinetics, Isotherm, Thermodynamic and Mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Beratto-Ramos, A.; Agurto-Muñoz, C.; Pablo Vargas-Montalba, J.; Castillo, R.d.P. Fourier-Transform Infrared Imaging and Multivariate Analysis for Direct Identification of Principal Polysaccharides in Brown Seaweeds. Carbohydr. Polym. 2020, 230, 115561. [Google Scholar] [CrossRef]
- Ahmady-Asbchin, S.; Andrès, Y.; Gérente, C.; Cloirec, P. Le Biosorption of Cu(II) from Aqueous Solution by Fucus serratus: Surface Characterization and Sorption Mechanisms. Bioresour. Technol. 2008, 99, 6150–6155. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of Lead, Copper, Cadmium, Zinc, and Nickel by Marine Algal Biomass: Characterization of Biosorptive Capacity and Investigation of Mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Mao, J.; Won, S.W.; Choi, S.B.; Lee, M.W.; Yun, Y.S. Surface Modification of the Corynebacterium glutamicum Biomass to Increase Carboxyl Binding Site for Basic Dye Molecules. Biochem. Eng. J. 2009, 46, 1–6. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Arruda, M.A.Z. Biosorption of Heavy Metals Using Rice Milling By-Products. Characterisation and Application for Removal of Metals from Aqueous Effluents. Chemosphere 2004, 54, 987–995. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Esmaeli, A.R.; Jokar, M.; Khataee, A.R. Optimization of Acid Blue 25 Removal from Aqueous Solutions by Raw, Esterified and Protonated Jania adhaerens Biomass. Int. Biodeterior. Biodegrad. 2012, 69, 97–105. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene Blue Adsorption by Algal Biomass Based Materials: Biosorbents Characterization and Process Behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef]
- Sarici-Ozdemir, C. Adsorption and Desorption Kinetics Behaviour of Methylene Blue Onto Activated Carbon. Physicochem. Probl. Miner. Process. 2012, 48, 441–454. [Google Scholar]
- Suteu, D.; Zaharia, C.; Badeanu, M. Kinetic Modeling of Dye Sorption from Aqueous Solutions onto Apple Seed Powder. Cellul. Chem. Technol. 2016, 50, 1085–1091. [Google Scholar]
- El-Sayed, H.E.M.; El-Sayed, M.M.H. Assessment of Food Processing and Pharmaceutical Industrial Wastes as Potential Biosorbents: A Review. BioMed Res. Int. 2014, 2014, 146769. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative Approaches to Sustainable Wastewater Treatment: A Comprehensive Exploration of Conventional and Emerging Technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N.S. Treatment of Wastewater Using Seaweed: A Review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef]
- Sukmana, H.; Bellahsen, N.; Pantoja, F.; Hodur, C. Adsorption and Coagulation in Wastewater Treatment—Review. Prog. Agric. Eng. Sci. 2021, 17, 49–68. [Google Scholar] [CrossRef]
- Mazur, L.P.; Cechinel, M.A.P.; de Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Brown Marine Macroalgae as Natural Cation Exchangers for Toxic Metal Removal from Industrial Wastewaters: A Review. J. Environ. Manag. 2018, 223, 215–253. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is Biosorption Suitable for Decontamination of Metal-Bearing Wastewaters? A Critical Review on the State-of-the-Art of Biosorption Processes and Future Directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Modeling of Single and Competitive Metal Adsorption onto a Natural Polysaccharide. Environ. Sci. Technol. 2002, 36, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331. [Google Scholar]
- Hameed, B.H.; Hakimi, H. Utilization of Durian (Durio zibethinus Murray) Peel as Low Cost Sorbent for the Removal of Acid Dye from Aqueous Solutions. Biochem. Eng. J. 2008, 39, 338–343. [Google Scholar] [CrossRef]
- Sanzana, S.; Abreu, N.J.; Levío-Raimán, M.; Proal-Nájera, J.; Osorio, A.; Maza, S.; Daniele, L.; Castro-Rojas, J.; Soto, V.; González, C.; et al. Enhancing Manganese Sorption: Batch and Fixed-Bed Column Studies on Activated Zeolite. Environ. Technol. Innov. 2024, 33, 103495. [Google Scholar] [CrossRef]
- Javadian, H.; Ahmadi, M.; Ghiasvand, M.; Kahrizi, S.; Katal, R. Removal of Cr(VI) by Modified Brown Algae Sargassum bevanom from Aqueous Solution and Industrial Wastewater. J. Taiwan Inst. Chem. Eng. 2013, 44, 977–989. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solutions. J. Sanit. Eng. Div. 1963, 89, 31–39. [Google Scholar] [CrossRef]
- Khan, T.A.; Mukhlif, A.A.; Khan, E.A. Uptake of Cu2+ and Zn2+ from Simulated Wastewater Using Muskmelon Peel Biochar: Isotherm and Kinetic Studies. Egypt. J. Basic Appl. Sci. 2017, 4, 236–248. [Google Scholar] [CrossRef]
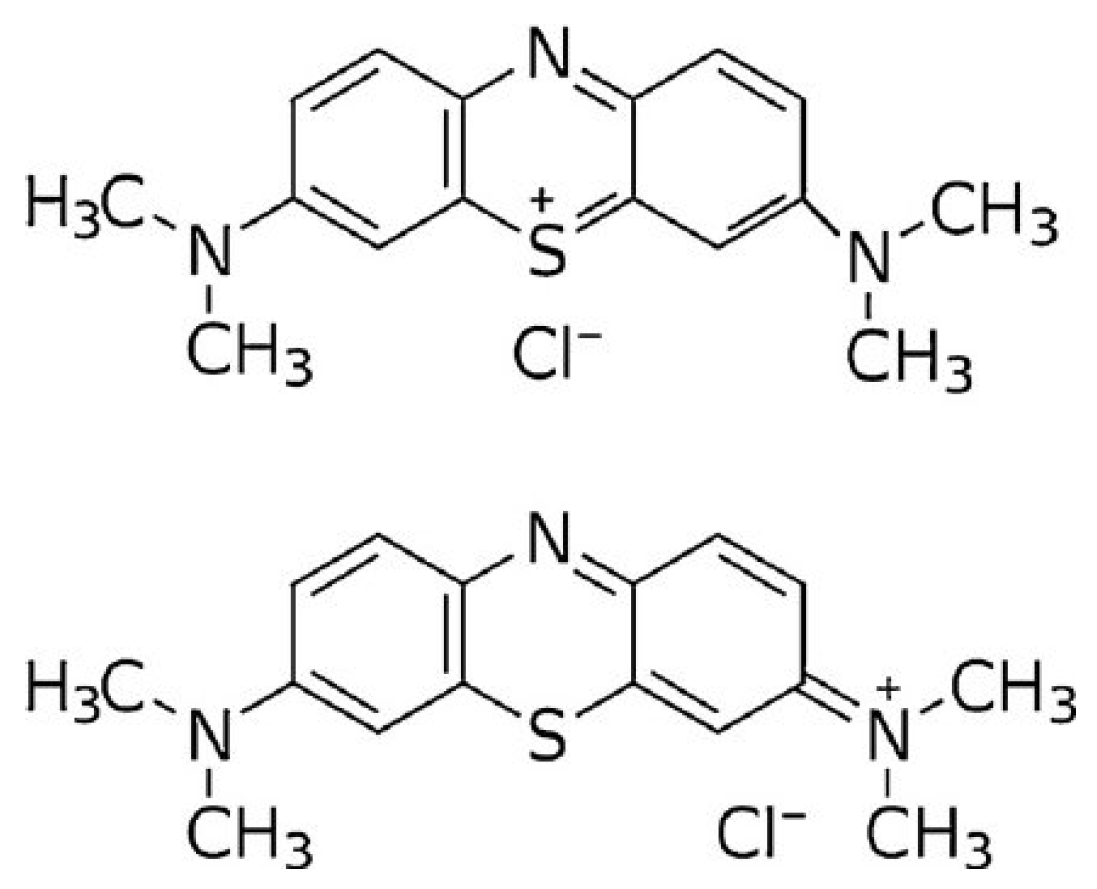
| Molecular Characteristics | |
|---|---|
| Chemical formula | C16H18ClN3S |
| Molecular weight (g mol−1) | 319.9 |
| Solubility in water (g L−1) | 50 |
| pKa | 3.8 |
| Biomass | Specific Surface Area m2 g−1 | Pore Volume cm3 g−1 | Pore Diameter nm |
|---|---|---|---|
| UB | 0.5 | 0.0021 | 114 |
| ACPB | 0.3 | 0.0012 | 87 |
| ALPB | 0.8 | 0.0065 | 86 |
| Biomass | PZC | Intrinsic pKa | Acid Groups [26] |
|---|---|---|---|
| UB | 7.1 | pKa1 = 5.64 | Carboxylic |
| pKa2 = 8.20 | Amine | ||
| pKa3 = 9.33 | Amino | ||
| ACPB | 5.1 | pKa1 = 4.24 | Carboxylic |
| pKa2 = 6.38 | Phosphonate | ||
| pKa3 = 9.02 | Amino | ||
| ALPB | 5.4 | pKa1 = 5.82 | Carboxylic |
| pKa2 = 10.82 | Phenolic, sulfhydryl | ||
| pKa3 = 11.1 | sulfhydryl |
| Biomass | Langmuir Constants | Freundlich Constants | Adsorption Energy 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q0 mg g−1 | K1 | R2 | RL | nF | KF | R2 | E kJ mol−1 | R2 | |
| UB | 333 | 0.032 | 0.98 | 0.050 | 1.561 | 14.450 | 0.84 | 8.45 | 0.93 |
| ACPB | 189 | 0.021 | 0.99 | 0.074 | 0.527 | 74.896 | 0.96 | 9.13 | 0.97 |
| ALPB | 526 | 0.036 | 0.99 | 0.034 | 1.906 | 36.778 | 0.83 | 10.00 | 0.88 |
| Adsorbent | Biosorbent Dose (g/L) | pH | Contact Time (min) | Q0 (mg g−1) | References |
|---|---|---|---|---|---|
| Untreated M. pyrifera | 1.00 | 7.9 | 120 | 333 | This study |
| Acid-pretreated M. pyrifera | 1.00 | 7.9 | 189 | This study | |
| Alkali-pretreated M. pyrifera | 1.00 | 7.9 | 526 | This study | |
| Nizamuddinia zanardinii | 0.24 | 6.5 | 180 | 142.08 | [5] |
| Alkali Nizamuddinia zanardinii | 0.24 | 6.5 | 99.69 | [5] | |
| Untreated Gracilaria parvispora | 0.24 | 6.5 | 87.78 | [5] | |
| Alkali Gracilaria parvispora | 0.24 | 6.5 | 110.64 | [5] | |
| Untreated Ulva fasciata | 0.24 | 6.5 | 143.97 | [5] | |
| Alkali Ulva fasciata | 0.24 | 6.5 | 149.28 | [5] | |
| Algae gelidium | NR | 6.0 | 180 | 171.00 | [44] |
| Sargassum muticum raw biomass | 0.20 | 7.0 | 120 | 142.87 | [35] |
| Biochar from municipal sludge and tea waste | 10.0 | 7.0 | 1440 | 12.57 | [37] |
| Biomass | pH 3.0 | pH 7.9 | pH 11 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALPB Dosage (g L−1) | ALPB Dosage (g L−1) | ALPB Dosage (g L−1) | |||||||
| 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | 4.0 | |
| Pseudo first order model | |||||||||
| qexp (mg g−1) | 156 | 92 | 47 | 185 | 96 | 50 | 188 | 97 | 49 |
| q (mg g−1) | 75 | 48 | 24 | 60 | 25 | 5 | 70 | 27 | 10 |
| k1 (min−1) | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.05 | 0.03 | 0.04 | 0.03 |
| R2 | 0.75 | 0.86 | 0.92 | 0.78 | 0.73 | 0.64 | 0.81 | 0.84 | 0.70 |
| Pseudo second order | |||||||||
| q (mg g−1) | 164 | 96 | 48 | 189 | 97 | 50 | 192 | 97 | 49 |
| K2 (g mg−1 min−1) | 0.0007 | 0.0014 | 0.0039 | 0.0021 | 0.0057 | 0.0680 | 0.0015 | 0.0079 | 0.0159 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 0.99 |
| External mass transfer (Liquid film diffusion) | |||||||||
| Kfd | 0.0297 | 0.0337 | 0.0385 | 0.0324 | 0.0324 | 0.0491 | 0.0342 | 0.0586 | 0.0332 |
| R2 | 0.752 | 0.857 | 0.915 | 0.783 | 0.726 | 0.637 | 0.812 | 0.753 | 0.7032 |
| Intra-particle diffusion | |||||||||
| kdif (mg g−1 min−1/2) | 25.14 | 14.82 | 7.89 | 33.41 | 17.76 | 9.64 | 33.20 | 18.07 | 9.194 |
| R2 | 0.99 | 0.99 | 0.99 | 0.96 | 0.94 | 0.93 | 0.98 | 0.92 | 0.93 |
| kdif (mg g−1 min−1/2) | 1.572 | 1.063 | 0.488 | 1.297 | 0.525 | 0.0211 | 1.334 | 0.558 | 0.196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varas, M.; Castro-Rojas, J.; Contreras-Porcia, L.; Ureta-Zañartu, M.S.; Blanco, E.; Escalona, N.; Muñoz, E.; Garrido-Ramírez, E. Enhancing the Biosorption Capacity of Macrocystis pyrifera: Effects of Acid and Alkali Pretreatments on Recalcitrant Organic Pollutants Removal. Int. J. Mol. Sci. 2025, 26, 3307. https://doi.org/10.3390/ijms26073307
Varas M, Castro-Rojas J, Contreras-Porcia L, Ureta-Zañartu MS, Blanco E, Escalona N, Muñoz E, Garrido-Ramírez E. Enhancing the Biosorption Capacity of Macrocystis pyrifera: Effects of Acid and Alkali Pretreatments on Recalcitrant Organic Pollutants Removal. International Journal of Molecular Sciences. 2025; 26(7):3307. https://doi.org/10.3390/ijms26073307
Chicago/Turabian StyleVaras, Magdalena, Jorge Castro-Rojas, Loretto Contreras-Porcia, María Soledad Ureta-Zañartu, Elodie Blanco, Néstor Escalona, Edmundo Muñoz, and Elizabeth Garrido-Ramírez. 2025. "Enhancing the Biosorption Capacity of Macrocystis pyrifera: Effects of Acid and Alkali Pretreatments on Recalcitrant Organic Pollutants Removal" International Journal of Molecular Sciences 26, no. 7: 3307. https://doi.org/10.3390/ijms26073307
APA StyleVaras, M., Castro-Rojas, J., Contreras-Porcia, L., Ureta-Zañartu, M. S., Blanco, E., Escalona, N., Muñoz, E., & Garrido-Ramírez, E. (2025). Enhancing the Biosorption Capacity of Macrocystis pyrifera: Effects of Acid and Alkali Pretreatments on Recalcitrant Organic Pollutants Removal. International Journal of Molecular Sciences, 26(7), 3307. https://doi.org/10.3390/ijms26073307








