Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress
Abstract
1. Introduction
2. Results
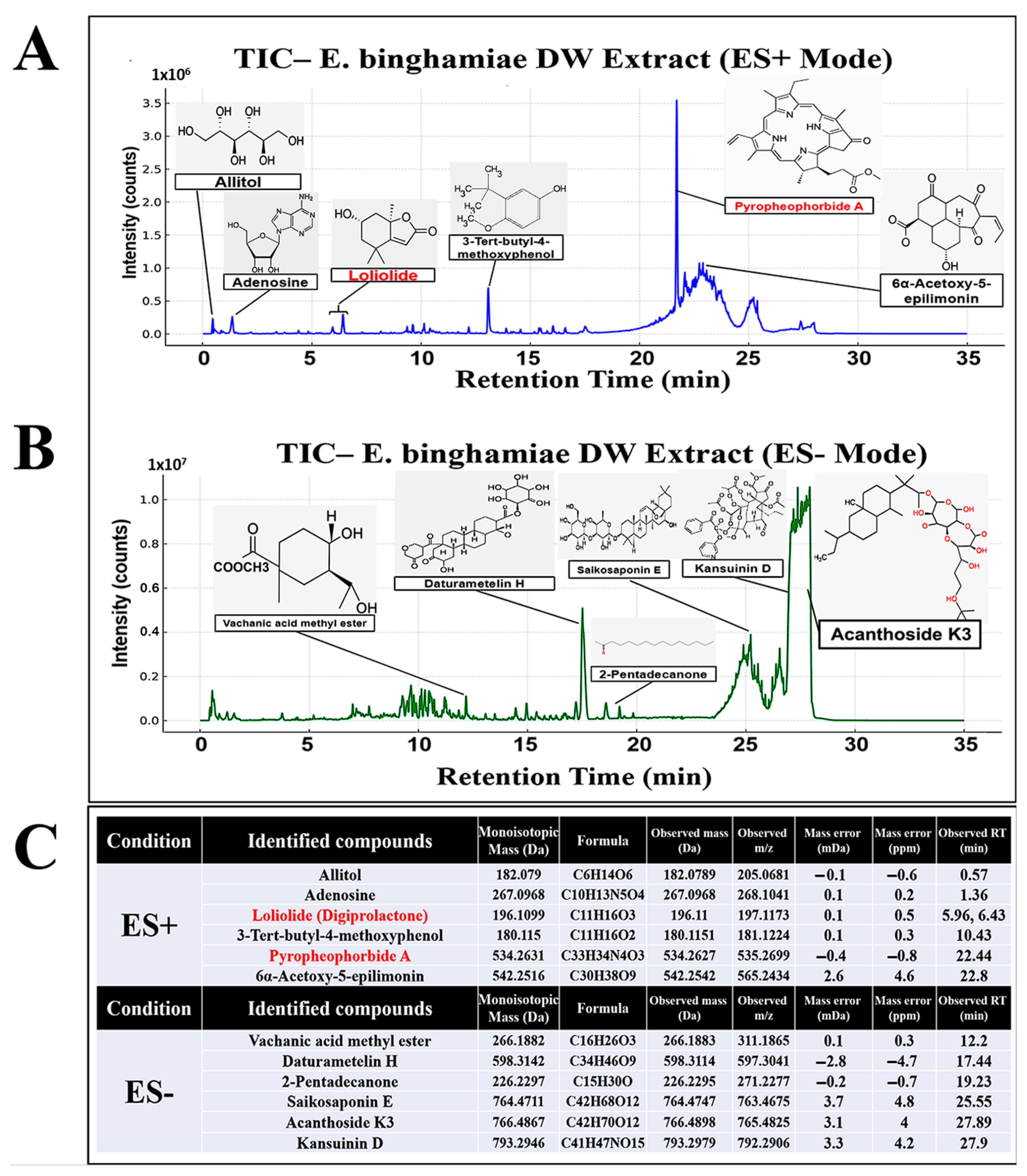
2.1. Tentative UHPLC-MS/MS Identification of Secondary Metabolites in EB-WE
2.2. Inhibition of Weight Gain by EB-WE in an Acute Obesity Model
2.3. Inhibition of Adipocyte Differentiation by EB-WE in 3T3-L1 Cells
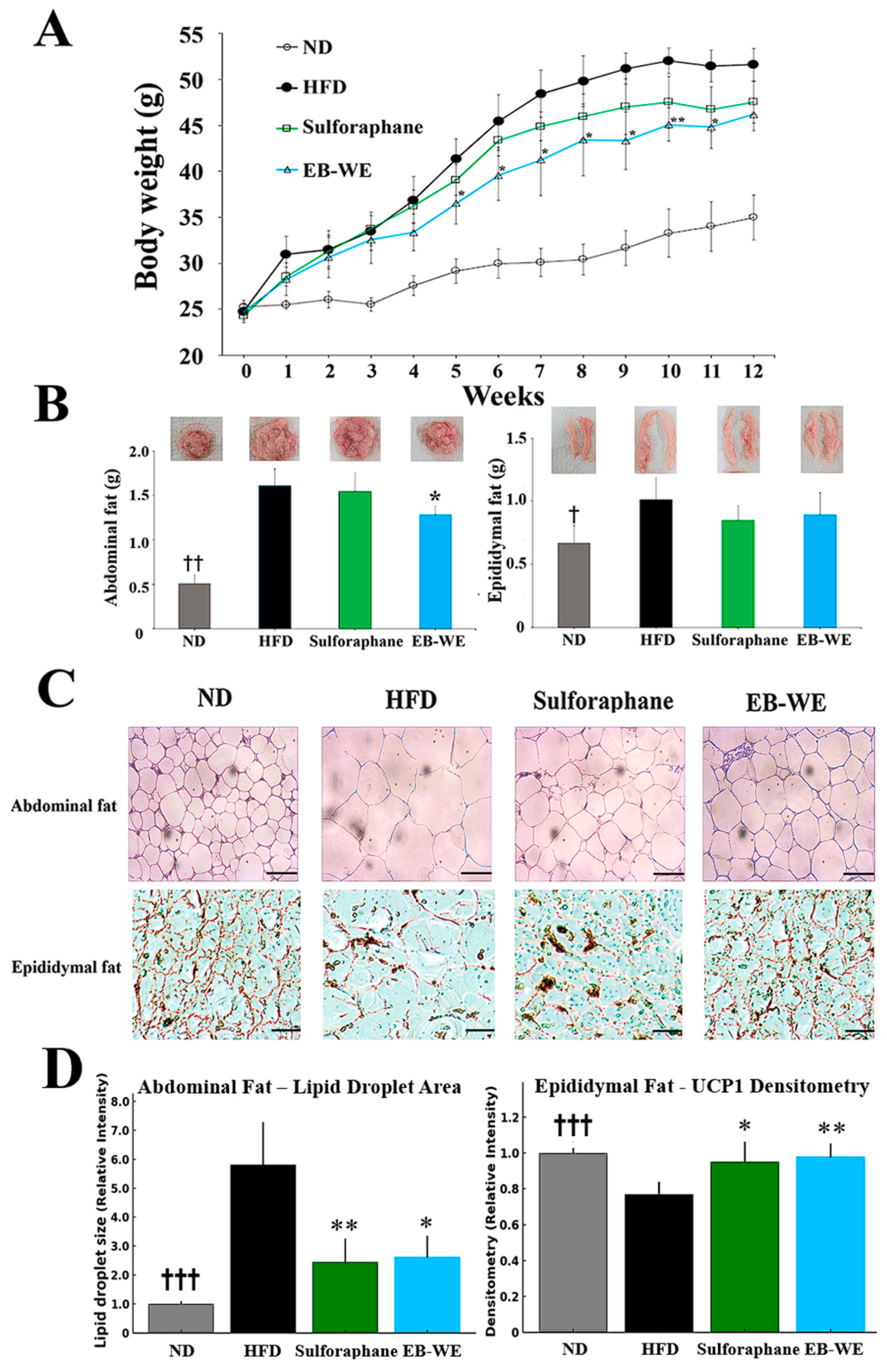
2.4. Inhibitory Effect of EB-WE on Weight Gain in the MASLD Model
2.5. Blood Profiles of Lipid-Related Indicators in the MASLD Model
2.6. Inhibition of Lipid Metabolism and Oxidative Stress by EB-WE in the Livers
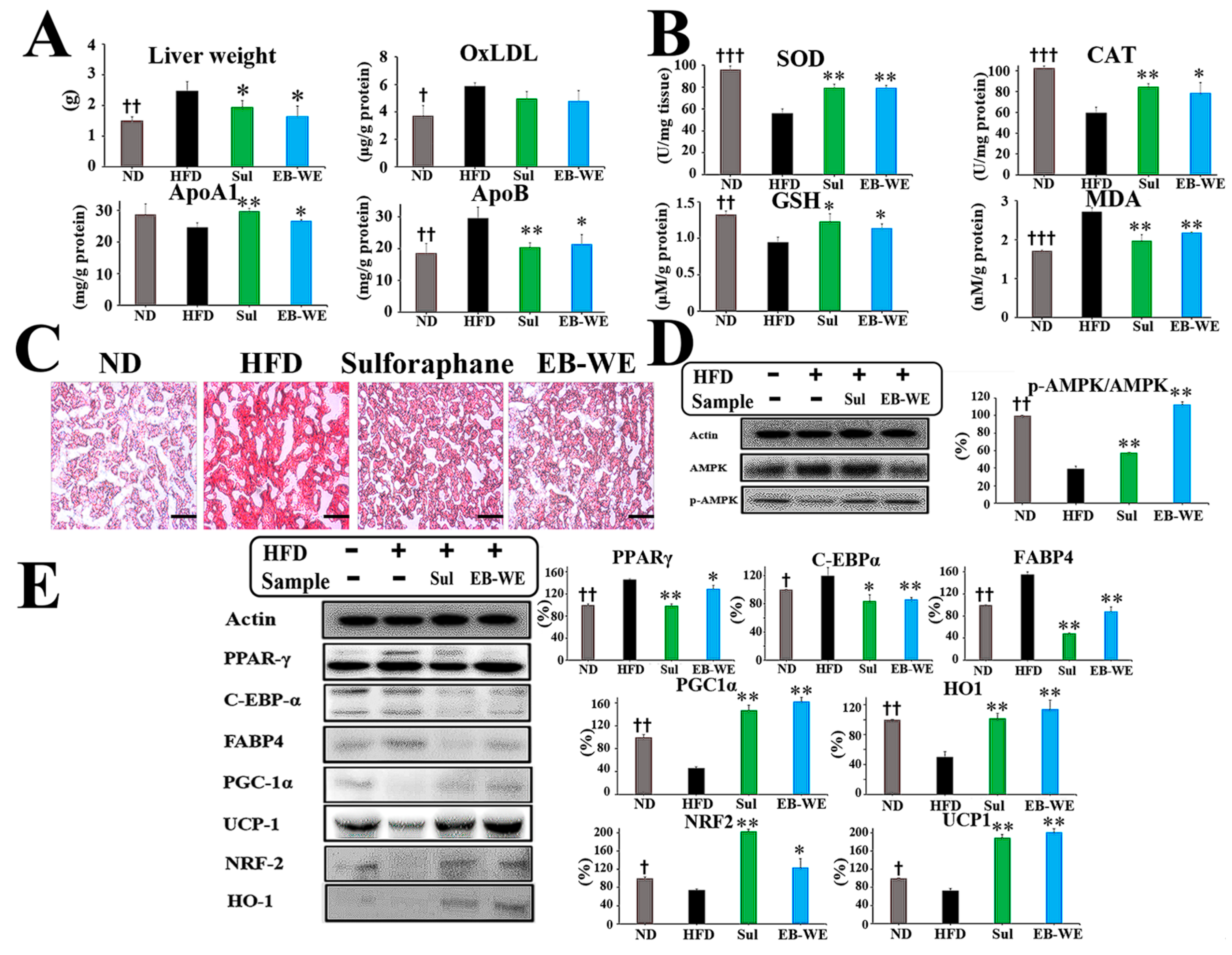
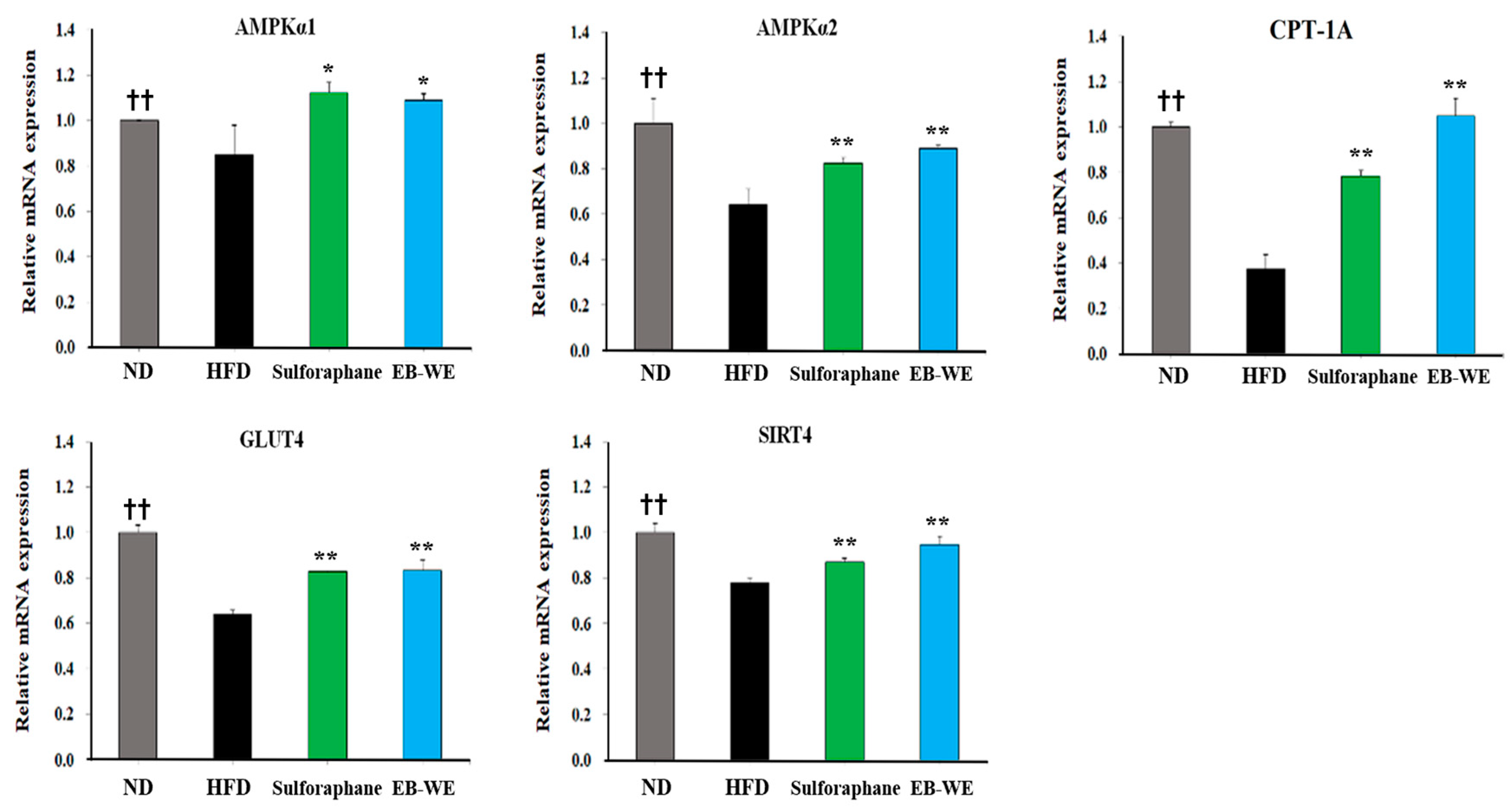
2.7. Regulation of mRNA Expression in Relation to Lipid Metabolism, Glucose Metabolism, and Oxidation in Liver Tissues
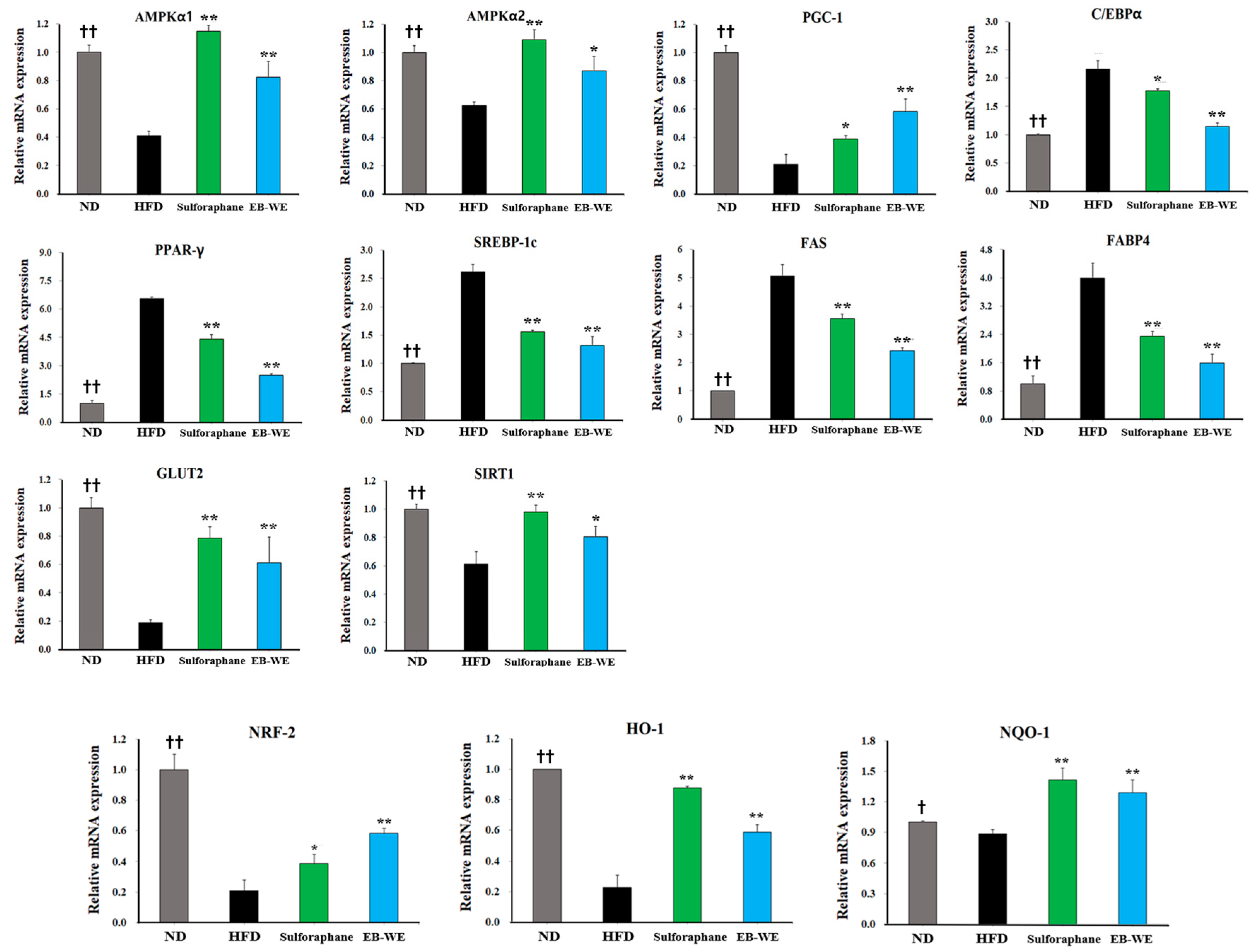
2.8. Regulation of mRNA Expression in Skeletal Muscle Tissues
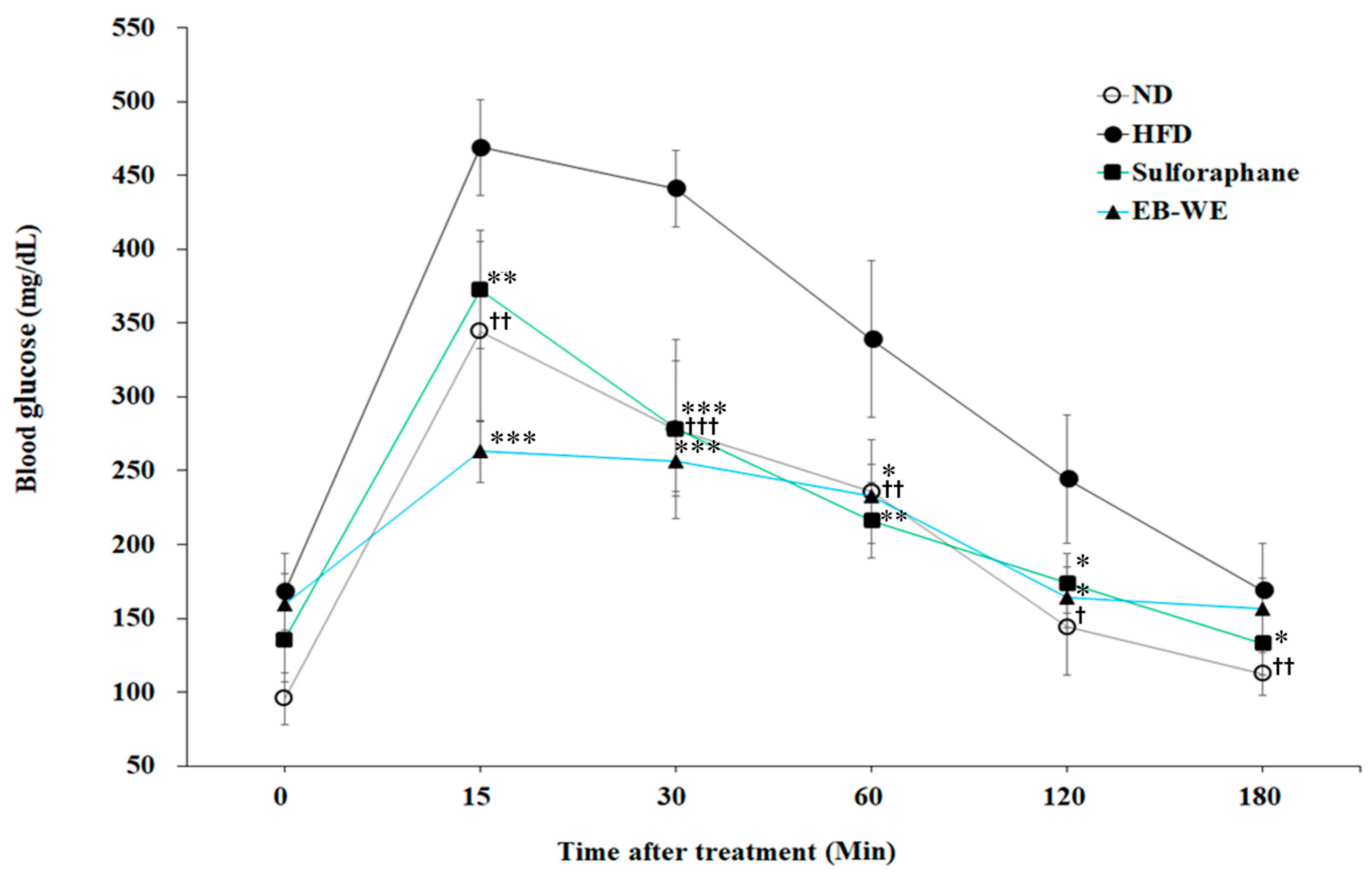
2.9. Improvement of Circulating Glucose Regulation by EB-WE in MASLD Model
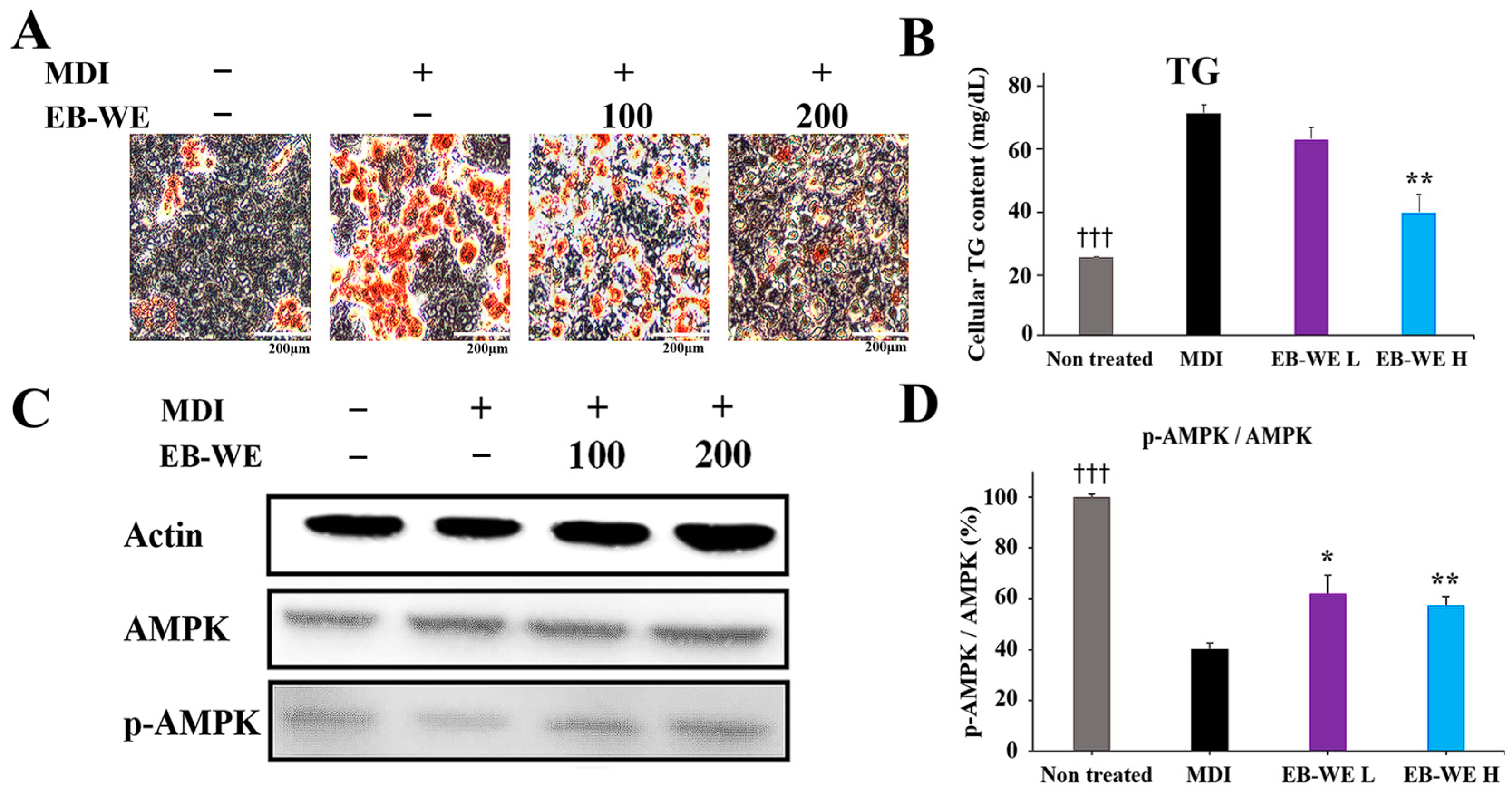
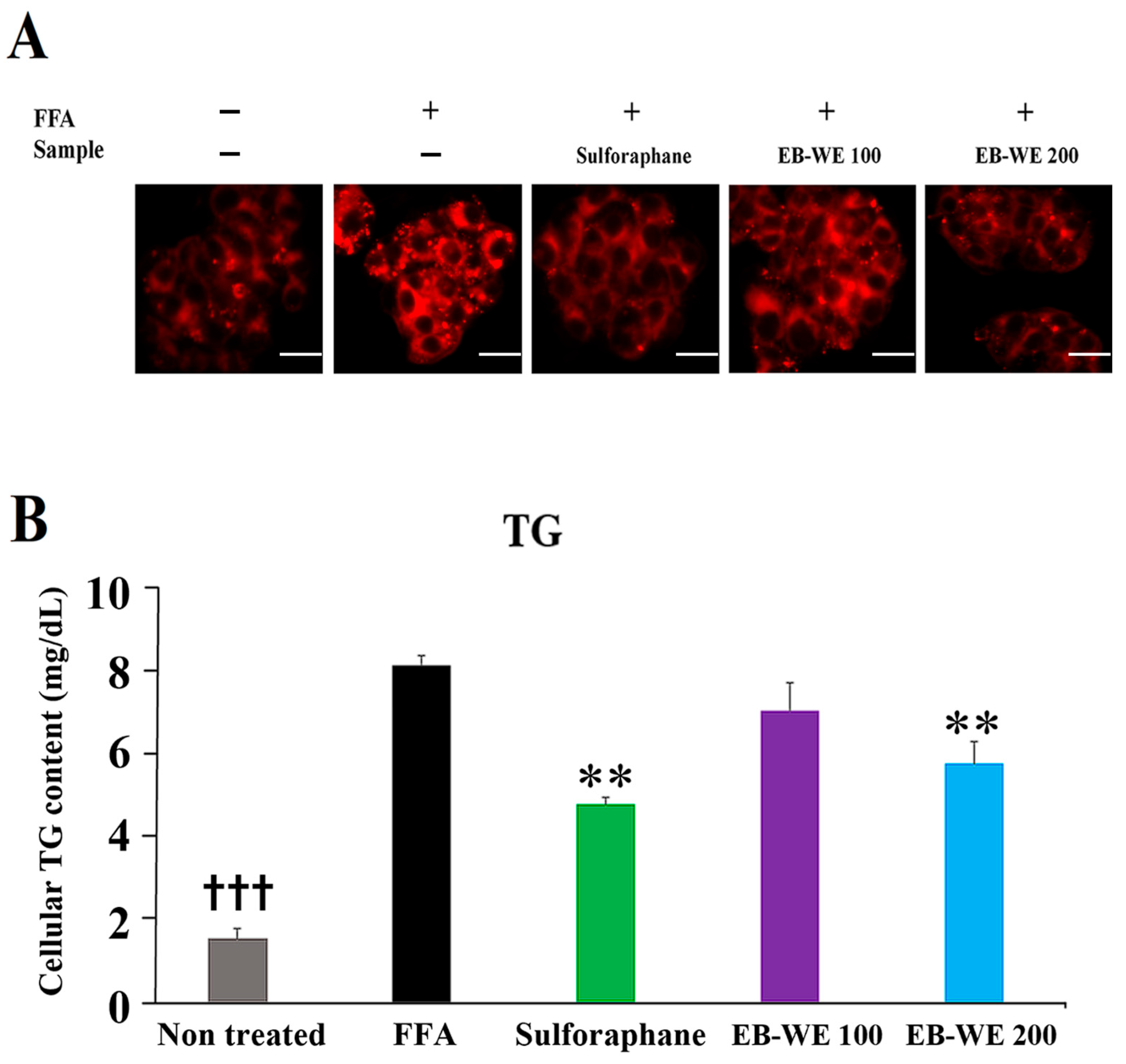
2.10. Inhibitory Effect of EB-WE on Lipid Accumulation in an In Vitro MASLD Model
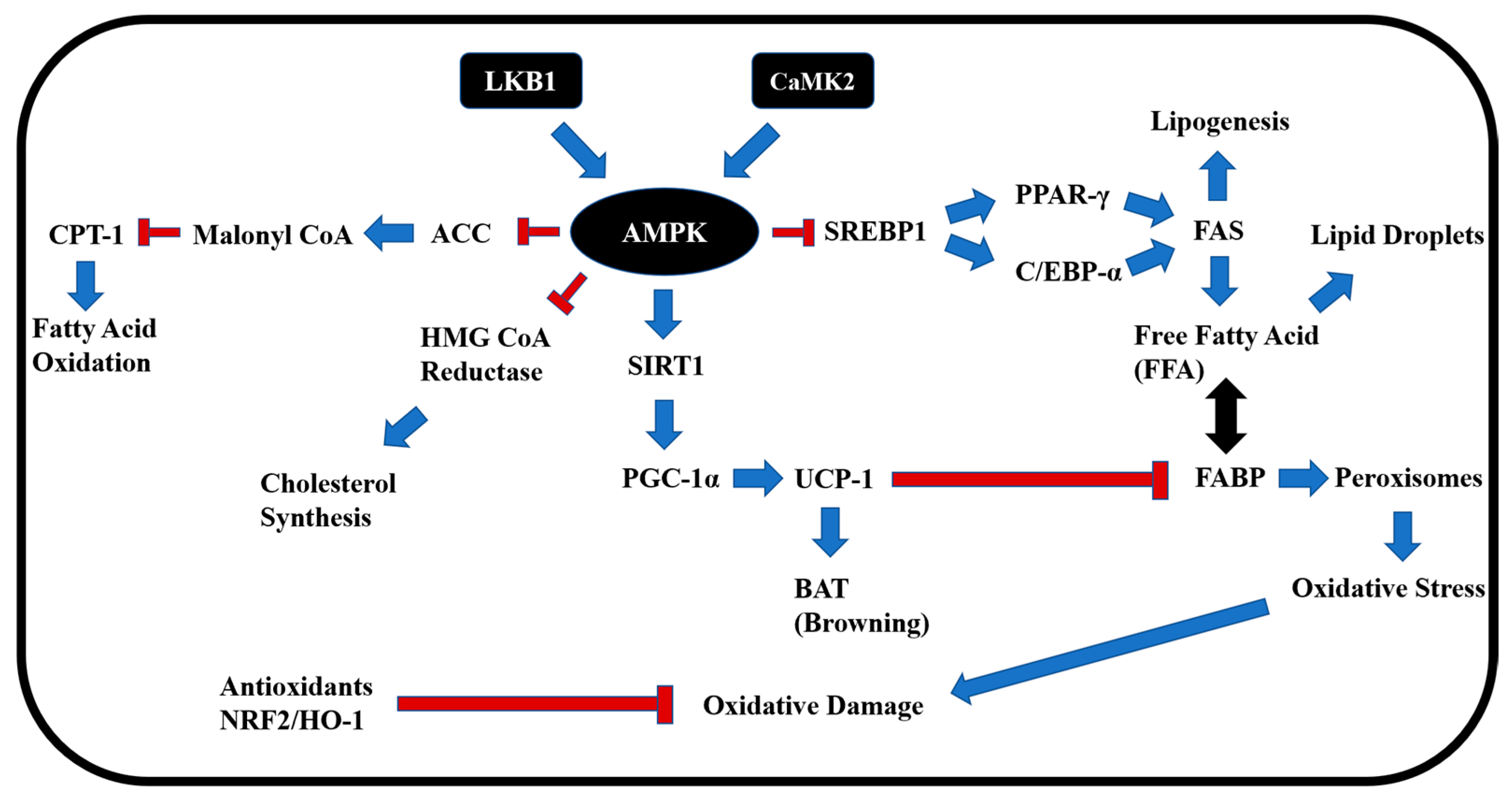
3. Discussion
3.1. Activation of AMPK and Suppression of Lipogenesis
3.2. Induction of Fat Browning and CPT-1-Mediated Fatty Acid Oxidation
3.3. Regulation of Glucose Metabolism via SIRT–GLUT Axis
3.4. Antioxidant Response via NRF2 and Redox Enzymes
3.5. Tentative Identification of Key Bioactive Compounds
3.6. Functional Relevance of Additional Tentative Compounds
4. Materials and Methods
4.1. Reagents
4.2. Preparation of EB-WE
4.3. UHPLC-MS/MS
4.4. Obesity Model in Mice
4.5. HFD-Induced MASLD Model and Oral Glucose Tolerance Test (OGTT)
4.6. Serological Analysis
4.7. Histological and Immunohistochemical Analysis
4.8. Western Blot
4.9. Real-Time PCR
4.10. Adipogenic Differentiation
4.11. In Vitro MASLD Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Abdelmalek, M.F. Current treatment options, including diet, exercise, and medications: The impact on histology. Clin. Liver Dis. 2023, 27, 397–412. [Google Scholar] [CrossRef]
- Le, P.; Payne, J.Y.; Zhang, L.; Deshpande, A.; Rothberg, M.B.; Alkhouri, N.; Herman, W.; Hernandez, A.V.; Schleicher, M.; Ye, W.; et al. Disease state transition probabilities across the spectrum of NAFLD: A systematic review and meta-analysis of paired biopsy or imaging studies. Clin. Gastroenterol. Hepatol. 2023, 21, 1154–1168. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J. Gastroenterol. 2016, 22, 4079–4090. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Lakshmi, T. A molecular insight into the role of antioxidants in nonalcoholic fatty liver diseases. Oxid. Med. Cell. Longev. 2022, 2022, 9233650. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Hellberg, K.; Chaix, A.; Wallace, M.; Herzig, S.; Badur, M.G.; Lin, T.; Shokhirev, M.N.; Pinto, A.F.M.; Ross, D.S.; et al. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 2019, 26, 192–208.e6. [Google Scholar] [CrossRef]
- Bence, K.K.; Birnbaum, M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101143. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, L.; Ying, H.; Sun, J.; Zhao, J.; Lu, Y.; Bian, Z.; Chen, J.; Fang, A.; Zhang, X.; et al. Multisystem health comorbidity networks of metabolic dysfunction-associated steatotic liver disease. Med 2024, 5, 1413–1423.e3. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown algae and their multiple applications as functional ingredient in food production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef] [PubMed]
- Rashed, Z.E.; Grasselli, E.; Khalifeh, H.; Canesi, L.; Demori, I. Brown-algae polysaccharides as active constituents against nonalcoholic fatty liver disease. Planta Med. 2022, 88, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Ko, J.C.; Ko, H.J.; Park, S.E.; Cha, H.K.; Choi, H.G. Seasonal variation in community structure of subtidal seaweeds in Jeju Island, Korea. Korean J. Fish. Aquat. Sci. 2013, 46, 607–618. [Google Scholar] [CrossRef]
- Kang, S.I.; Kim, M.H.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Park, J.G.; Ko, H.C.; Lee, N.H.; Chung, W.S.; Kim, S.J. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J. Nutr. Biochem. 2010, 21, 1251–1257. [Google Scholar] [CrossRef]
- Kang, J.S.; Choi, I.W.; Han, M.H.; Lee, D.S.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. The cytoprotective effect of Petalonia binghamiae methanol extract against oxidative stress in C2C12 myoblasts: Mediation by upregulation of heme oxygenase-1 and nuclear factor-erythroid 2 related factor 2. Mar. Drugs 2015, 13, 2666–2679. [Google Scholar] [CrossRef]
- Xin, X.; Li, J.; Wu, W.; Zhao, P.; Yang, Y.; Zhu, Y.; Ren, L.; Qin, C.; Yin, L. ROS-scavenging nanomedicine for “multiple crosstalk” modulation in non-alcoholic fatty liver disease. Biomater. Sci. 2023, 11, 3709–3725. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Song, J.; Zhang, C.; Li, M.; Mao, Y.; Liu, A.; Du, J. Role of apolipoprotein A1 in PPAR signaling pathway for nonalcoholic fatty liver disease. PPAR Res. 2022, 2022, 4709300. [Google Scholar] [CrossRef]
- Nass, K.J.; van den Berg, E.H.; Faber, K.N.; Schreuder, T.C.M.A.; Blokzijl, H.; Dullaart, R.P.F. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease: The lifelines cohort study. Metabolism 2017, 72, 37–46. [Google Scholar] [CrossRef]
- Choe, Y.G.; Jin, W.; Cho, Y.K.; Chung, W.G.; Kim, H.J.; Jeon, W.K.; Kim, B.I. Apolipoprotein B/AI ratio is independently associated with non-alcoholic fatty liver disease in nondiabetic subjects. J. Gastroenterol. Hepatol. 2013, 28, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Sazaki, I.; Sakurai, T.; Yamahata, A.; Mogi, S.; Inoue, N.; Ishida, K.; Kikkai, A.; Takeshita, H.; Sakurai, A.; Takahashi, Y.; et al. Oxidized low-density lipoproteins trigger hepatocellular oxidative stress with the formation of cholesteryl ester hydroperoxide-enriched lipid droplets. Int. J. Mol. Sci. 2023, 24, 4281. [Google Scholar] [CrossRef] [PubMed]
- Sabir, U.; Irfan, H.M.; Alamgeer; Ullah, A.; Althobaiti, Y.S.; Asim, M.H. Reduction of hepatic steatosis, oxidative stress, inflammation, ballooning and insulin resistance after therapy with safranal in NAFLD animal model: A new approach. J. Inflamm. Res. 2022, 15, 1293–1316. [Google Scholar] [CrossRef]
- Nguyen-Phuong, T.; Seo, S.; Cho, B.K.; Lee, J.H.; Jang, J.; Park, C.G. Determination of progressive stages of type 2 diabetes in a 45% high-fat diet-fed C57BL/6J mouse model is achieved by utilizing both fasting blood glucose levels and a 2-hour oral glucose tolerance test. PLoS ONE 2023, 18, e0293888. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Kircher, S.; Hermanns, H.M.; Geier, A. Animal models of NAFLD from a hepatologist’s point of view. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 943–953. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Hurley, R.L.; Anderson, K.A.; Franzone, J.M.; Kemp, B.E.; Means, A.R.; Witters, L.A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005, 280, 29060–29066. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef]
- Woods, A.; Williams, J.R.; Muckett, P.J.; Mayer, F.V.; Liljevald, M.; Bohlooly, Y.M.; Carling, D. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 2017, 18, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Bailey, D.; Wray, J. Peroxisome proliferator-activated receptors: A critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003, 71, 1–22. [Google Scholar] [CrossRef]
- Gerstner, M.; Heller, V.; Fechner, J.; Hermann, B.; Wang, L.; Lausen, J. Prmt6 represses the pro-adipogenic Ppar-gamma-C/ebp-alpha transcription factor loop. Sci. Rep. 2024, 14, 6656. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jang, H.J.; Muthamil, S.; Shin, U.C.; Lyu, J.H.; Kim, S.W.; Go, Y.; Park, S.H.; Lee, H.G.; Park, J.H. Novel insights into regulators and functional modulators of adipogenesis. Biomed. Pharmacother. 2024, 177, 117073. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.P.; Uldry, M.; Kajimura, S.; Arany, Z.; Spiegelman, B.M. Modulation of PGC-1 coactivator pathways in brown fat differentiation through LRP130. J. Biol. Chem. 2008, 283, 31960–31967. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhao, X.Y.; Lin, J.D. The brown fat secretome: Metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 2015, 26, 231–237. [Google Scholar] [CrossRef]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef]
- Moreno-Vedia, J.; Girona, J.; Ibarretxe, D.; Masana, L.; Rodríguez-Calvo, R. Unveiling the role of the fatty acid binding protein 4 in the metabolic-associated fatty liver disease. Biomedicines 2022, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, J.W.; Yang, M.S.; Park, C.; Kim, J.H.; Lim, C.W.; Kim, B. Beneficial effects of Korean red ginseng in the progression of non-alcoholic steatohepatitis via FABP4 modulation. Am. J. Chin. Med. 2018, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Sheng, W.; Gao, R.; Feng, J.; Huang, W.; Cui, L.; Liu, J.; Li, Y. Ethanolic extract of root from Arctium lappa L ameliorates obesity and hepatic steatosis in rats by regulating the AMPK/ACC/CPT-1 pathway. J. Food Biochem. 2022, 46, e14455. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Sirtuins and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 10084–10092. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chan, M.H.; Yang, Y.F.; Li, C.H.; Hsiao, M. Glucose transporter 4: Insulin response mastermind, glycolysis catalyst and treatment direction for cancer progression. Cancer Lett. 2023, 563, 216179. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Svobodová, G.; Horní, M.; Velecká, E.; Boušová, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2024, 99, 1–24. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretić, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered mitochondrial function in MASLD: Key features and promising therapeutic approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Ageing, metabolic dysfunction, and the therapeutic role of antioxidants. Subcell. Biochem. 2023, 103, 341–435. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, Y. Association between composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease: Result from NHANES, 2017–2020. Front. Nutr. 2024, 11, 1412516. [Google Scholar] [CrossRef]
- Yang, Z.; Song, S.; Li, L.; Yuan, Z.; Li, Y. Association between the composite dietary antioxidant index and metabolic dysfunction-associated steatotic liver disease in adults: A cross-sectional study from NHANES 2017–2020. Sci. Rep. 2024, 14, 13801. [Google Scholar] [CrossRef]
- Li, N.; Hao, L.; Li, S.; Deng, J.; Yu, F.; Zhang, J.; Nie, A.; Hu, X. The NRF-2/HO-1 signaling pathway: A promising therapeutic target for metabolic dysfunction-associated steatotic liver disease. J. Inflamm. Res. 2024, 17, 8061–8083. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Tukaj, Z. Biochemiczne i fizjologiczne aspekty rozkładu barwników chlorofilowych [biochemical and physiological aspects of chlorophyll breakdown]. Postepy Biochem. 2019, 65, 128–134. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal monitoring of volatiles and antioxidant activity of brown alga Cladostephus spongiosus. Mar. Drugs 2023, 21, 415. [Google Scholar] [CrossRef]
- Hong, C.O.; Nam, M.H.; Oh, J.S.; Lee, J.W.; Kim, C.T.; Park, K.W.; Lee, D.H.; Lee, K.W. Pheophorbide a from Capsosiphon fulvescens inhibits advanced glycation end products mediated endothelial dysfunction. Planta Med. 2016, 82, 46–57. [Google Scholar] [CrossRef]
- Samsuzzaman, M.; Lee, J.H.; Moon, H.; Lee, J.; Lee, H.; Lim, Y.; Park, M.G.; Kim, H.; Kim, S.Y. Identification of a potent NAFLD drug candidate for controlling T2DM-mediated inflammation and secondary damage in vitro and in vivo. Front. Pharmacol. 2022, 13, 943879. [Google Scholar] [CrossRef]
- Klimenko, A.; Huber, R.; Marcourt, L.; Tabakaev, D.; Koval, A.; Dautov, S.S.; Dautova, T.N.; Wolfender, J.-L.; Thew, R.; Kho-timchenko, Y.; et al. Shallow- and deep-water Ophiura species produce a panel of chlorin compounds with potent photo-dynamic anticancer activities. Antioxidants 2023, 12, 386. [Google Scholar] [CrossRef]
- Zhou, Q.; Liang, W.; Wan, J.; Wang, M. Spinach (Spinacia oleracea) microgreen prevents the formation of advanced glycation end products in model systems and breads. Curr. Res. Food Sci. 2023, 6, 100490. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.-C.; Lee, K.-W.; Kang, S.-M.; Lee, W.-W.; Jeon, Y.-J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Je, J.G.; Fu, X.; Huang, C.; Ahn, G.; Oh, J.Y.; Sanjeewa, K.K.A.; Xu, J.; Gao, X.; et al. In vitro and in vivo photoprotective effects of (-)-loliode isolated from the brown seaweed, Sargassum horneri. Molecules 2021, 26, 6898. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, E.; Kim, S.; Kim, D.S.; Kim, J.H.; Chang, S.; Choi, J.S.; Park, K.J.; Roh, K.B.; Lee, J.; et al. Oxidative stress-protective and anti-melanogenic effects of loliolide and ethanol extract from fresh water green algae, Prasiola japonica. Int. J. Mol. Sci. 2018, 19, 2825. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.S.; Fernando, I.P.S.; Ahn, G. (−)-Loliolide isolated from Sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Je, J.-G.; Hwang, J.; Sanjeewa, K.K.A.; Lee, D.-S.; Song, K.-M.; Choi, Y.-S.; Kang, M.-C.; Jeon, Y.-J. Lipid inhibitory effect of (?)-loliolide isolated from Sargassum horneri in 3T3-L1 adipocytes: Inhibitory mechanism of adipose-specific proteins. Mar. Drugs 2021, 19, 96. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, H.S.; Lee, D.S.; Kim, A.; Je, J.G.; Seo, M.J.; Jee, Y.H.; Jeon, Y.J.; Kim, S.Y.; et al. (−)-Loliolide isolated from Sargassum horneri suppressed oxidative stress and inflammation by activating Nrf2/HO-1 signaling in IFN-γ/TNF-α-stimulated HaCaT keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Lestari, O.A.; Palupi, N.S.; Setiyono, A.; Kusnandar, F.; Yuliana, N.D. In vitro antioxidant potential and phytochemical profiling of Melastoma malabathricum leaf water extract. Food Sci. Technol. 2022, 42, e92021. [Google Scholar] [CrossRef]
- John, A.; Raza, H. Alterations in inflammatory cytokines and redox homeostasis in LPS-induced pancreatic beta-cell toxicity and mitochondrial stress: Protection by azadirachtin. Front. Cell Dev. Biol. 2022, 10, 867608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hesse, M. The spermine alkaloids of Chaenorhinum minus. Planta Med. 1988, 54, 430–433. [Google Scholar] [CrossRef]
- Jacome-Sosa, M.; Vacca, C.; Mangat, R.; Diane, A.; Nelson, R.C.; Reaney, M.J.; Shen, J.; Curtis, J.M.; Vine, D.F.; Field, C.J.; et al. Vaccenic acid suppresses intestinal inflammation by increasing anandamide and related N-acylethanolamines in the JCR:LA-cp rat. J. Lipid Res. 2016, 57, 638–649. [Google Scholar] [CrossRef]
- Máñez, S.; Recio, M.C.; Gil, I.; Gómez, C.; Giner, R.M.; Waterman, P.G.; Ríos, J.L. A glycosyl analogue of diacylglycerol and other antiinflammatory constituents from Inula viscosa. J. Nat. Prod. 1999, 62, 601–604. [Google Scholar] [CrossRef]
- Chen, C.S.; Pan, B.Y.; Tsai, P.H.; Chen, F.Y.; Yang, W.C.; Shen, M.Y. Kansuinine A ameliorates atherosclerosis and human aortic endothelial cell apoptosis by inhibiting reactive oxygen species production and suppressing IKKβ/IκBα/NF-κB signaling. Int. J. Mol. Sci. 2021, 22, 10309. [Google Scholar] [CrossRef]
- Yang, B.Y.; Guo, R.; Li, T.; Wu, J.J.; Zhang, J.; Liu, Y.; Wang, Q.H.; Kuang, H.X. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids 2014, 87, 26–34. [Google Scholar] [CrossRef]
- Shaheen, H.S.; Oran, S.A.; Hudaib, M.; Bustanji, Y.K.; Althaher, A.R. An investigation of antiproliferative and antioxidant properties of crude extracts from Sedum nicaeense All. (Crassulaceae). Pak. J. Pharm. Sci. 2024, 37, 1259–1270. [Google Scholar]
- Wen, W.; Wu, P.; Zhang, Y.; Chen, Z.; Sun, J.; Chen, H. Comprehensive analysis of NAFLD and the therapeutic target identified. Front. Cell Dev. Biol. 2021, 9, 704704. [Google Scholar] [CrossRef]
- Matsuo, T.; Higaki, S.; Inai, R.; Takata, G.; Mochizuki, S.; Yoshihara, A.; Akimitsu, K. Effects of dietary allitol and D-allulose on body fat accumulation and cecal short-chain fatty acid production in rats fed a high-fat diet. J. Oleo Sci. 2024, 73, 1329–1337. [Google Scholar] [CrossRef]
- Higaki, S.; Inai, R.; Matsuo, T. Effects of dietary allitol on body fat accumulation in rats. J. Nutr. Sci. Vitaminol. 2022, 68, 348–352. [Google Scholar] [CrossRef]
- Song, Y.; Lai, M.; Liao, Z.; Zhang, Z.; Zhu, G.; Yang, M.; Ai, Z.; Zheng, Q.; Su, D. Saikosaponin antidepressant mechanism: Improving the sphingolipid metabolism in the cortex via apolipoprotein E and triggering neurovascular coupling. Phytomedicine 2024, 132, 155829. [Google Scholar] [CrossRef] [PubMed]
- Manalo-Cabalinan, R.A.M.; Dela Torre, G.L.T.; Atienza, A.A.; Arollado, E.C. Carica papaya flower extracts possess antioxidant and 5α-reductase inhibitory activities. Acta Med. Philipp. 2024, 58, 83–92. [Google Scholar] [CrossRef]
- He, X.; Zhou, C.; Shang, R.; Wang, X. Acanthoside B attenuates NLRP3-mediated pyroptosis and ulcerative colitis through inhibition of tAGE/RAGE pathway. Allergol. Immunopathol. 2025, 53, 112–122. [Google Scholar] [CrossRef]
- Kim, M.J.; Wang, H.S.; Lee, M.W. Anti-inflammatory effects of fermented bark of Acanthopanax sessiliflorus and its isolated compounds on lipopolysaccharide-treated RAW 264.7 macrophage cells. Evid. Based Complement. Alternat. Med. 2020, 2020, 6749425. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Kweon, M.H.; Park, S.Y.; Kim, J.S.; Kim, D.H.; Ganesan, P.; Choi, D.K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef]
- Lee, S.; Hong, E.; Kim, Y.; Kim, H.; Kim, S.; Lee, C.; Park, J.; Kim, J.; Kim, H.; Kim, I.; et al. Mychonastes sp. 247 induces apoptosis of A549 human lung cancer cells by promoting STRA6-mediated reactive oxygen species production. Nat. Prod. Commun. 2023, 18, 7. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.S.; Lee, G.Y.; Han, S.Y.; Kim, D.S.; Lee, B.H.; Yoo, Y.C. Endarachne binghamiae extract ameliorates inflammatory responses in macrophages through regulation of MAPK, NF-kB and PI3K/AKT pathways, and prevents acute lung injury in mice. Life 2025, 15, 88. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Yang, Y.; Li, J.; Sun, X.; Zhang, Y.; Liu, R.; Chen, F.; Li, X. Special correlation between diet and MASLD: Positive or negative? Cell Biosci. 2025, 15, 44. [Google Scholar] [CrossRef]
- Lang, P.; Hasselwander, S.; Li, H.; Xia, N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep. 2019, 9, 19556. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Teng, W. Sulforaphane attenuates nonalcoholic fatty liver disease by inhibiting hepatic steatosis and apoptosis. Nutrients 2022, 14, 76. [Google Scholar] [CrossRef]

| Profiles | ND | HFD | Fenofibrate | EB-WE 1 mg | EB-WE 2 mg |
|---|---|---|---|---|---|
| TG (mg/dL) | 107 ± 5 † | 139 ± 22 | 123 ± 5 * | 127 ± 15 | 105 ± 6 * |
| T-CHO (mg/dL) | 129 ± 15 †† | 275 ± 33 | 222 ± 23 | 201 ± 32 * | 185 ± 13 * |
| HDL (mg/dL) | 82 ± 6 †† | 110 ± 0 | 107 ± 4 | 110 ± 0 | 101 ± 6 * |
| LDL (mg/dL) | 21 ± 19 ††† | 137 ± 35 | 91 ± 26 | 73 ± 33 * | 71 ± 19 * |
| Profiles | ND | HFD | Sulforaphane | EB-WE |
|---|---|---|---|---|
| TG (mg/dL) | 103.4 ± 11.4 †† | 177.6 ± 16.8 | 136.3 ± 13.8 * | 117.6 ± 12.8 ** |
| T-CHO (mg/dL) | 117 ± 13.0 ††† | 212.4 ± 10.7 | 190.2 ± 9.7 * | 176.3 ± 28.7 * |
| HDL (mg/dL) | 100.9 ± 8.0 † | 110 ± 0 | 107.5 ± 3.9 | 109 ± 2.2 |
| LDL (mg/dL) | 10.5 ± 6.6 †† | 66.6 ± 10.5 | 55.1 ± 9.5 | 51.7 ± 8.4 |
| Adiponectin (ug/mL) | 31.3 ± 1.0 †† | 12 ± 0.2 | 21.8 ± 1.6 ** | 21.4 ± 0.2 ** |
| ApoA1 (ng/mL) | 47.5 ± 2.6 ††† | 29.9 ± 1.3 | 36.6 ± 3.3 * | 38.3 ± 4.0 ** |
| ApoB (ng/mL) | 24.1 ± 0.5 ††† | 39 ± 0.4 | 31.7 ± 0.9 ** | 31.1 ± 1.3 ** |
| Glucose (mg/dL) | 95.7 ± 18.0 ††† | 205 ± 15.0 | 135 ± 21.5 ** | 142 ± 17.0 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-S.; Lee, S.-H.; Kim, S.-Y.; Lee, G.-Y.; Han, S.-Y.; Lee, B.-H.; Yoo, Y.-C. Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 5103. https://doi.org/10.3390/ijms26115103
Lee S-S, Lee S-H, Kim S-Y, Lee G-Y, Han S-Y, Lee B-H, Yoo Y-C. Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(11):5103. https://doi.org/10.3390/ijms26115103
Chicago/Turabian StyleLee, Sang-Seop, Sang-Hoon Lee, So-Yeon Kim, Ga-Young Lee, Seung-Yun Han, Bong-Ho Lee, and Yung-Choon Yoo. 2025. "Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress" International Journal of Molecular Sciences 26, no. 11: 5103. https://doi.org/10.3390/ijms26115103
APA StyleLee, S.-S., Lee, S.-H., Kim, S.-Y., Lee, G.-Y., Han, S.-Y., Lee, B.-H., & Yoo, Y.-C. (2025). Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress. International Journal of Molecular Sciences, 26(11), 5103. https://doi.org/10.3390/ijms26115103







