Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Defining Features of MDSCs
2.1. Markers of Mouse MDSCs
2.2. Markers of Human MDSCs
3. Inflammation-Driven MDSC Expansion and Activation in IBD
3.1. MDSC Distribution in IBD Patients and Mouse Models of IBD
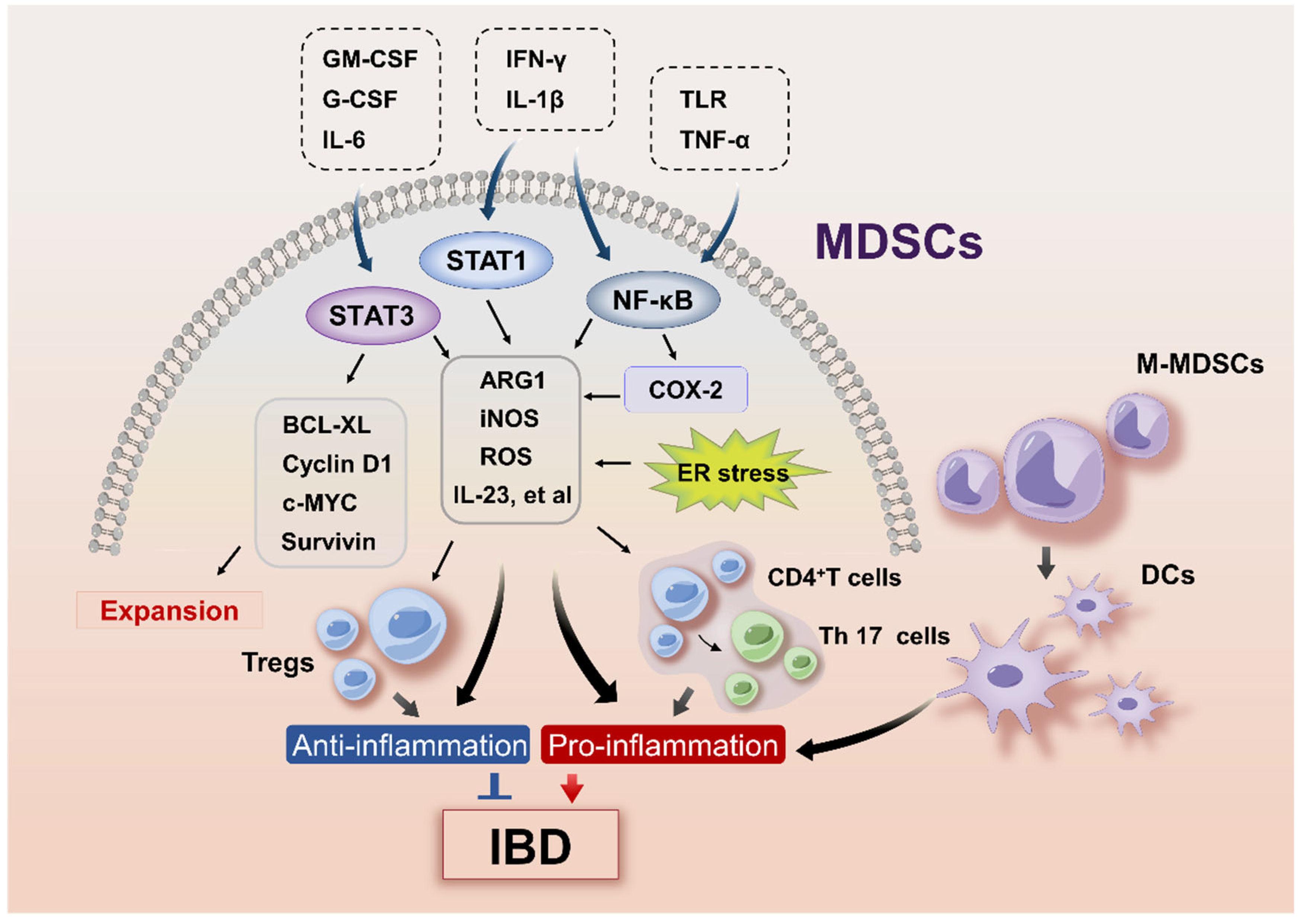
3.2. Signaling Pathways Regulating MDSC Expansion in IBD
3.3. Signaling Pathways Involved in MDSC Activation in IBD
3.4. Hormone-Driven MDSC Activity in IBD
4. MDSCs in IBD Pathogenesis
4.1. The Protective Role of MDSCs in IBD
4.2. The Proinflammatory Function of MDSCs in IBD
4.3. Plasticity of M-MDSCs in IBD
5. Effect of MDSCs During the Development of IBD-Associated Colorectal Cancer
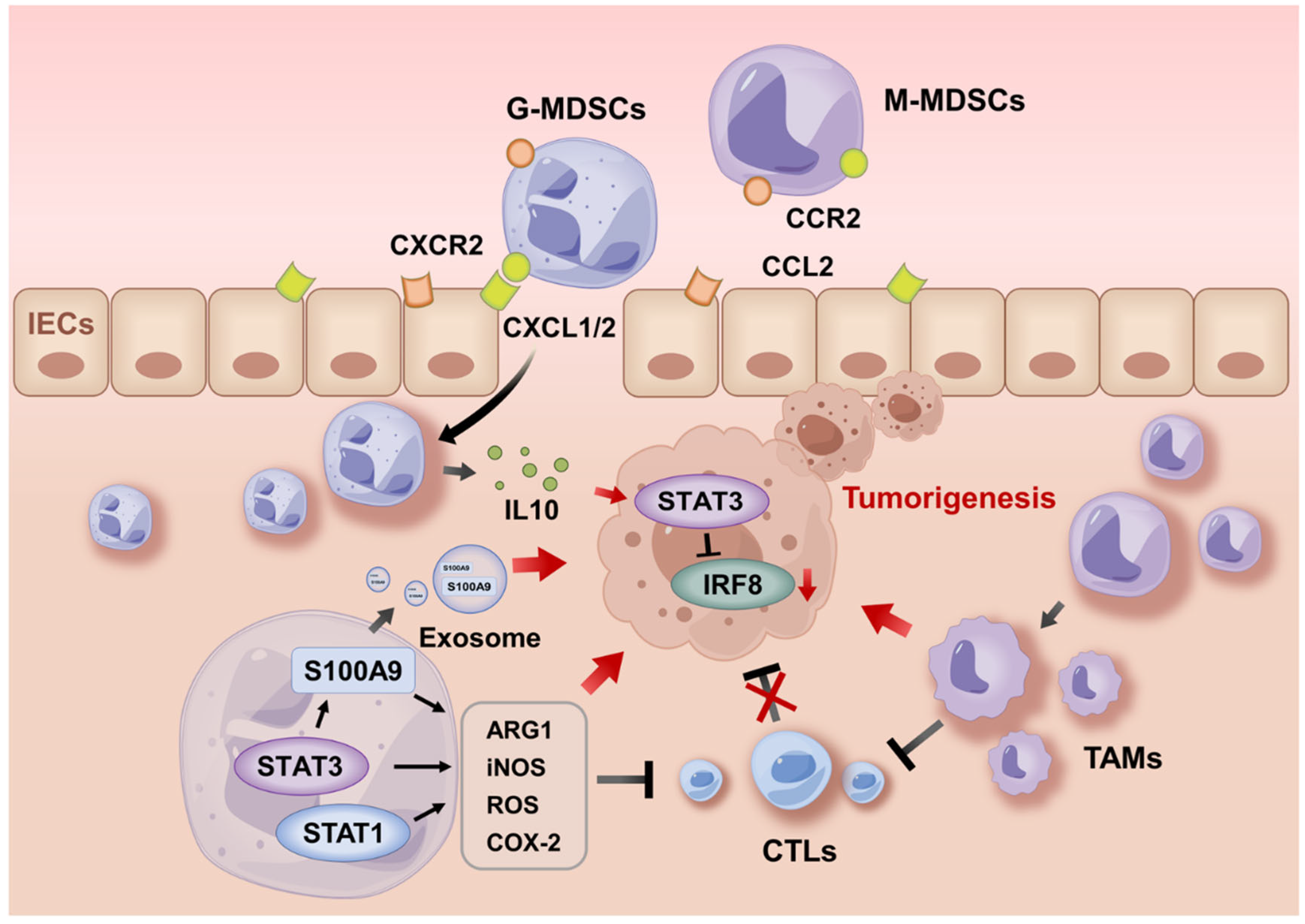
5.1. MDSC Recruitment During Tumorigenesis
5.2. Activities of MDSCs During Tumor Progression
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gabrilovich, D.I.; Bronte, V.; Chen, S.-H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Tesi, R.J. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol. Sci. 2019, 40, 4–7. [Google Scholar]
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W.Y.; et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review With Temporal Analyses. Clin. Gastroenterol. Hepatol. 2023, 21, 2211–2221. [Google Scholar]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar]
- Barreiro-de Acosta, M.; Molero, A.; Artime, E.; Díaz-Cerezo, S.; Lizán, L.; de Paz, H.D.; Martín-Arranz, M.D. Epidemiological, Clinical, Patient-Reported and Economic Burden of Inflammatory Bowel Disease (Ulcerative colitis and Crohn’s disease) in Spain: A Systematic Review. Adv. Ther. 2023, 40, 1975–2014. [Google Scholar]
- Fornaro, R.; Caratto, M.; Caratto, E.; Caristo, G.; Fornaro, F.; Giovinazzo, D.; Sticchi, C.; Casaccia, M.; Andorno, E. Colorectal Cancer in Patients With Inflammatory Bowel Disease: The Need for a Real Surveillance Program. Clin. Color. Cancer 2016, 15, 204–212. [Google Scholar]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar]
- Damuzzo, V.; Pinton, L.; Desantis, G.; Solito, S.; Marigo, I.; Bronte, V.; Mandruzzato, S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytom. B Clin. Cytom. 2015, 88, 77–91. [Google Scholar] [CrossRef]
- Yang, R.; Cai, Z.; Zhang, Y.; Yutzy, W.H.; Roby, K.F.; Roden, R.B.S. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006, 66, 6807–6815. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Kamada, N.; Seong, S.-Y.; Seo, S.-U. CD115- monocytic myeloid-derived suppressor cells are precursors of OLFM4high polymorphonuclear myeloid-derived suppressor cells. Commun. Biol. 2023, 6, 272. [Google Scholar] [CrossRef]
- Haile, L.A.; Gamrekelashvili, J.; Manns, M.P.; Korangy, F.; Greten, T.F. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 2010, 185, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Biermann, H.; Pietz, B.; Dreier, R.; Schmid, K.W.; Sorg, C.; Sunderkötter, C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J. Leukoc. Biol. 1999, 65, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ostanin, D.V.; Kurmaeva, E.; Furr, K.; Bao, R.; Hoffman, J.; Berney, S.; Grisham, M.B. Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. J. Immunol. 2012, 188, 1491–1502. [Google Scholar] [CrossRef]
- Cassetta, L.; Baekkevold, E.S.; Brandau, S.; Bujko, A.; Cassatella, M.A.; Dorhoi, A.; Krieg, C.; Lin, A.; Loré, K.; Marini, O.; et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. 2019, 68, 687–697. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [PubMed]
- Haile, L.A.; von Wasielewski, R.; Gamrekelashvili, J.; Krüger, C.; Bachmann, O.; Westendorf, A.M.; Buer, J.; Liblau, R.; Manns, M.P.; Korangy, F.; et al. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology 2008, 135, 871–881.e5. [Google Scholar]
- Xi, Q.; Li, Y.; Dai, J.; Chen, W. High frequency of mononuclear myeloid-derived suppressor cells is associated with exacerbation of inflammatory bowel disease. Immunol. Investig. 2015, 44, 279–287. [Google Scholar]
- Xue, G.; Hua, L.; Liu, D.; Zhong, M.; Chen, Y.; Zhou, B.; Xie, Y.; Li, J. Tim-4 expressing monocytes as a novel indicator to assess disease activity and severity of ulcerative colitis. Life Sci. 2021, 269, 119077. [Google Scholar]
- Kontaki, E.; Boumpas, D.T.; Tzardi, M.; Mouzas, I.A.; Papadakis, K.A.; Verginis, P. Aberrant function of myeloid-derived suppressor cells (MDSCs) in experimental colitis and in inflammatory bowel disease (IBD) immune responses. Autoimmunity 2017, 50, 170–181. [Google Scholar] [PubMed]
- Agollah, G.D.; Wu, G.; Peng, H.-L.; Kwon, S. Dextran sulfate sodium-induced acute colitis impairs dermal lymphatic function in mice. World J. Gastroenterol. 2015, 21, 12767–12777. [Google Scholar] [CrossRef]
- Guan, Q.; Moreno, S.; Qing, G.; Weiss, C.R.; Lu, L.; Bernstein, C.N.; Warrington, R.J.; Ma, Y.; Peng, Z. The role and potential therapeutic application of myeloid-derived suppressor cells in TNBS-induced colitis. J. Leukoc. Biol. 2013, 94, 803–811. [Google Scholar] [CrossRef]
- Oh, S.Y.; Cho, K.-A.; Kang, J.L.; Kim, K.H.; Woo, S.-Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, P.; Feng, T.; Lu, K.; Wang, X.; Zhou, S.; Qiang, Y. Butyrate functions in concert with myeloid-derived suppressor cells recruited by CCR9 to alleviate DSS-induced murine colitis. Int. Immunopharmacol. 2021, 99, 108034. [Google Scholar]
- De Cicco, P.; Sanders, T.; Cirino, G.; Maloy, K.J.; Ianaro, A. Hydrogen Sulfide Reduces Myeloid-Derived Suppressor Cell-Mediated Inflammatory Response in a Model of Helicobacter hepaticus-Induced Colitis. Front. Immunol. 2018, 9, 499. [Google Scholar]
- Li, P.; Chen, Y.; Xiang, Y.; Guo, R.; Li, X.; Liu, J.; Zhou, Y.; Fu, X. 17β-estradiol promotes myeloid-derived suppressor cells functions and alleviates inflammatory bowel disease by activation of Stat3 and NF-κB signalings. J. Steroid Biochem. Mol. Biol. 2024, 242, 106540. [Google Scholar]
- Ma, Z.; Zhen, Y.; Hu, C.; Yi, H. Myeloid-Derived Suppressor Cell-Derived Arginase-1 Oppositely Modulates IL-17A and IL-17F Through the ESR/STAT3 Pathway During Colitis in Mice. Front. Immunol. 2020, 11, 687. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, S.; Wang, Z.; Huang, J.; Xu, L.; Tang, X.; Wan, Y.Y.; Li, Q.-J.; Symonds, A.L.J.; Long, H.; et al. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat. Commun. 2019, 10, 2427. [Google Scholar] [CrossRef]
- Kurmaeva, E.; Bhattacharya, D.; Goodman, W.; Omenetti, S.; Merendino, A.; Berney, S.; Pizarro, T.; Ostanin, D.V. Immunosuppressive monocytes: Possible homeostatic mechanism to restrain chronic intestinal inflammation. J. Leukoc. Biol. 2014, 96, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Watanabe, N.; Koda, Y.; Oya, Y.; Kaminuma, O.; Katayama, K.; Fan, Z.; Sakurai, F.; Kawabata, K.; Hiroi, T.; et al. Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: Implications of myeloid-derived suppressor cells and NO production. Int. Immunol. 2020, 32, 187–201. [Google Scholar] [CrossRef]
- Däbritz, J.; Judd, L.M.; Chalinor, H.V.; Menheniott, T.R.; Giraud, A.S. Altered gp130 signalling ameliorates experimental colitis via myeloid cell-specific STAT3 activation and myeloid-derived suppressor cells. Sci. Rep. 2016, 6, 20584. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef]
- Lechner, M.G.; Liebertz, D.J.; Epstein, A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010, 185, 2273–2284. [Google Scholar] [CrossRef]
- Fukuzawa, H.; Sawada, M.; Kayahara, T.; Morita-Fujisawa, Y.; Suzuki, K.; Seno, H.; Takaishi, S.; Chiba, T. Identification of GM-CSF in Paneth cells using single-cell RT-PCR. Biochem. Biophys. Res. Commun. 2003, 312, 897–902. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Cárcamo, C.V. Innate Lymphoid Cells in Intestinal Inflammation. Front. Immunol. 2017, 8, 1296. [Google Scholar]
- van Deventer, H.W.; Burgents, J.E.; Wu, Q.P.; Woodford, R.-M.T.; Brickey, W.J.; Allen, I.C.; McElvania-Tekippe, E.; Serody, J.S.; Ting, J.P.Y. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010, 70, 10161–10169. [Google Scholar] [PubMed]
- Chow, M.T.; Sceneay, J.; Paget, C.; Wong, C.S.F.; Duret, H.; Tschopp, J.; Möller, A.; Smyth, M.J. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012, 72, 5721–5732. [Google Scholar]
- Zhang, W.-J.; Chen, S.-J.; Zhou, S.-C.; Wu, S.-Z.; Wang, H. Inflammasomes and Fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal 2024, 17, eadh1641. [Google Scholar]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar]
- Bunt, S.K.; Clements, V.K.; Hanson, E.M.; Sinha, P.; Ostrand-Rosenberg, S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 2009, 85, 996–1004. [Google Scholar]
- Guo, Y.; Xiong, J.; Wang, J.; Wen, J.; Zhi, F. Inhibition of Rac family protein impairs colitis and colitis-associated cancer in mice. Am. J. Cancer Res. 2018, 8, 70–80. [Google Scholar]
- Kusmartsev, S.; Gabrilovich, D.I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 2005, 174, 4880–4891. [Google Scholar]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar]
- Sinha, P.; Clements, V.K.; Fulton, A.M.; Ostrand-Rosenberg, S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007, 67, 4507–4513. [Google Scholar] [PubMed]
- Talmadge, J.E. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 2007, 13 Pt 1, 5243–5248. [Google Scholar]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar]
- Mandula, J.K.; Rodriguez, P.C. Tumor-related stress regulates functional plasticity of MDSCs. Cell Immunol. 2021, 363, 104312. [Google Scholar]
- Brummer, C.; Singer, K.; Brand, A.; Bruss, C.; Renner, K.; Herr, W.; Pukrop, T.; Dorn, C.; Hellerbrand, C.; Matos, C.; et al. Sex-Dependent T Cell Dysregulation in Mice with Diet-Induced Obesity. Int. J. Mol. Sci. 2024, 25, 8234. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Genes and environment: How will our concepts on the pathophysiology of IBD develop in the future? Dig. Dis. 2010, 28, 395–405. [Google Scholar]
- Shah, S.C.; Khalili, H.; Chen, C.-Y.; Ahn, H.S.; Ng, S.C.; Burisch, J.; Colombel, J.-F. Sex-based differences in the incidence of inflammatory bowel diseases-pooled analysis of population-based studies from the Asia-Pacific region. Aliment. Pharmacol. Ther. 2019, 49, 904–911. [Google Scholar] [PubMed]
- Shah, S.C.; Khalili, H.; Gower-Rousseau, C.; Olen, O.; Benchimol, E.I.; Lynge, E.; Nielsen, K.R.; Brassard, P.; Vutcovici, M.; Bitton, A.; et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology 2018, 155, 1079–1089. [Google Scholar]
- Khalili, H.; Higuchi, L.M.; Ananthakrishnan, A.N.; Richter, J.M.; Feskanich, D.; Fuchs, C.S.; Chan, A.T. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013, 62, 1153–1159. [Google Scholar]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Hiller-Vallina, S.; Mondejar-Ruescas, L.; Caamaño-Moreno, M.; Cómitre-Mariano, B.; Alcivar-López, D.; Sepulveda, J.M.; Hernández-Laín, A.; Pérez-Núñez, Á.; Segura-Collar, B.; Gargini, R. Sexual-biased necroinflammation is revealed as a predictor of bevacizumab benefit in glioblastoma. Neuro Oncol. 2024, 26, 1213–1227. [Google Scholar] [CrossRef]
- Davison, L.M.; Alberto, A.A.; Dand, H.A.; Keller, E.J.; Patt, M.; Khan, A.; Dvorina, N.; White, A.; Sakurai, N.; Liegl, L.N.; et al. S100a9 Protects Male Lupus-Prone NZBWF1 Mice From Disease Development. Front. Immunol. 2021, 12, 681503. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Luckey, D.; Luthra, H.; David, C.; Taneja, V. B cells influence sex specificity of arthritis via myeloid suppressors and chemokines in humanized mice. Clin. Immunol. 2017, 178, 10–19. [Google Scholar] [CrossRef]
- Köstlin-Gille, N.; Flaig, L.-A.; Ginzel, M.; Arand, J.; Poets, C.F.; Gille, C. Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex. Nutrients 2020, 12, 2571. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Bayik, D.; Zhou, Y.; Park, C.; Hong, C.; Vail, D.; Silver, D.J.; Lauko, A.; Roversi, G.; Watson, D.C.; Lo, A.; et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020, 10, 1210–1225. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Dolcetti, L.; Peranzoni, E.; Ugel, S.; Marigo, I.; Fernandez Gomez, A.; Mesa, C.; Geilich, M.; Winkels, G.; Traggiai, E.; Casati, A.; et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010, 40, 22–35. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, T.; Shao, S.; Shi, B.; Zhao, Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1004983. [Google Scholar] [CrossRef]
- Ferrer, G.; Jung, B.; Chiu, P.Y.; Aslam, R.; Palacios, F.; Mazzarello, A.N.; Vergani, S.; Bagnara, D.; Chen, S.-S.; Yancopoulos, S.; et al. Myeloid-derived suppressor cell subtypes differentially influence T-cell function, T-helper subset differentiation, and clinical course in CLL. Leukemia 2021, 35, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Bothwell, A.L.M. Functional Diversity of Myeloid-Derived Suppressor Cells: The Multitasking Hydra of Cancer. J. Immunol. 2019, 203, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- van der Touw, W.; Kang, K.; Luan, Y.; Ma, G.; Mai, S.; Qin, L.; Bian, G.; Zhang, R.; Mungamuri, S.K.; Hu, H.-M.; et al. Glatiramer Acetate Enhances Myeloid-Derived Suppressor Cell Function via Recognition of Paired Ig-like Receptor, B. J. Immunol. 2018, 201, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.-H.; Ye, M.B.; Lee, W.; Sim, K.-Y.; Kang, J.-A.; Kim, Y.-C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-β during Murine Colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef]
- Zhang, R.; Ito, S.; Nishio, N.; Cheng, Z.; Suzuki, H.; Isobe, K.I. Dextran sulphate sodium increases splenic Gr1+CD11b+ cells which accelerate recovery from colitis following intravenous transplantation. Clin. Exp. Immunol. 2011, 164, 417–427. [Google Scholar] [CrossRef]
- Nemoto, Y.; Kanai, T.; Tohda, S.; Totsuka, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Sakamoto, N.; Fukuda, T.; Miura, O.; et al. Negative feedback regulation of colitogenic CD4+ T cells by increased granulopoiesis. Inflamm. Bowel Dis. 2008, 14, 1491–1503. [Google Scholar] [CrossRef]
- Kühl, A.A.; Kakirman, H.; Janotta, M.; Dreher, S.; Cremer, P.; Pawlowski, N.N.; Loddenkemper, C.; Heimesaat, M.M.; Grollich, K.; Zeitz, M.; et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology 2007, 133, 1882–1892. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018, 9, 2499. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [PubMed]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar]
- Gallina, G.; Dolcetti, L.; Serafini, P.; De Santo, C.; Marigo, I.; Colombo, M.P.; Basso, G.; Brombacher, F.; Borrello, I.; Zanovello, P.; et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Investig. 2006, 116, 2777–2790. [Google Scholar]
- Zheng, W.; Song, H.; Luo, Z.; Wu, H.; Chen, L.; Wang, Y.; Cui, H.; Zhang, Y.; Wang, B.; Li, W.; et al. Acetylcholine ameliorates colitis by promoting IL-10 secretion of monocytic myeloid-derived suppressor cells through the nAChR/ERK pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2017762118. [Google Scholar] [PubMed]
- Xu, Y.; Yan, J.; Tao, Y.; Qian, X.; Zhang, C.; Yin, L.; Gu, P.; Liu, Y.; Pan, Y.; Tang, R.; et al. Pituitary hormone α-MSH promotes tumor-induced myelopoiesis and immunosuppression. Science 2022, 377, 1085–1091. [Google Scholar]
- Gravina, A.G.; Panarese, I.; Trotta, M.C.; D’Amico, M.; Pellegrino, R.; Ferraraccio, F.; Galdiero, M.; Alfano, R.; Grieco, P.; Federico, A. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity? World J. Gastroenterol. 2024, 30, 1132–1142. [Google Scholar]
- Cao, L.Y.; Chung, J.-S.; Teshima, T.; Feigenbaum, L.; Cruz, P.D.; Jacobe, H.T.; Chong, B.F.; Ariizumi, K. Myeloid-Derived Suppressor Cells in Psoriasis Are an Expanded Population Exhibiting Diverse T-Cell-Suppressor Mechanisms. J. Investig. Dermatol. 2016, 136, 1801–1810. [Google Scholar]
- Guo, C.; Hu, F.; Yi, H.; Feng, Z.; Li, C.; Shi, L.; Li, Y.; Liu, H.; Yu, X.; Wang, H.; et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann. Rheum. Dis. 2016, 75, 278–285. [Google Scholar]
- Wu, H.; Zhen, Y.; Ma, Z.; Li, H.; Yu, J.; Xu, Z.-G.; Wang, X.-Y.; Yi, H.; Yang, Y.-G. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med. 2016, 8, 331ra40. [Google Scholar]
- Ilkovitch, D.; Ferris, L.K. Myeloid-derived suppressor cells are elevated in patients with psoriasis and produce various molecules. Mol. Med. Rep. 2016, 14, 3935–3940. [Google Scholar] [PubMed]
- Soler, D.C.; McCormick, T.S. Expanding the List of Dysregulated Immunosuppressive Cells in Psoriasis. J. Investig. Dermatol. 2016, 136, 1749–1751. [Google Scholar] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [PubMed]
- Youn, J.-I.; Kumar, V.; Collazo, M.; Nefedova, Y.; Condamine, T.; Cheng, P.; Villagra, A.; Antonia, S.; McCaffrey, J.C.; Fishman, M.; et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat. Immunol. 2013, 14, 211–220. [Google Scholar]
- Varol, C.; Vallon-Eberhard, A.; Elinav, E.; Aychek, T.; Shapira, Y.; Luche, H.; Fehling, H.J.; Hardt, W.-D.; Shakhar, G.; Jung, S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 2009, 31, 502–512. [Google Scholar]
- Rivollier, A.; He, J.; Kole, A.; Valatas, V.; Kelsall, B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012, 209, 139–155. [Google Scholar]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar]
- Sun, H.; Tang, C.; Chung, S.-H.; Ye, X.-Q.; Makusheva, Y.; Han, W.; Kubo, M.; Shichino, S.; Ueha, S.; Matsushima, K.; et al. Blocking DCIR mitigates colitis and prevents colorectal tumors by enhancing the GM-CSF-STAT5 pathway. Cell Rep. 2022, 40, 111158. [Google Scholar]
- Harusato, A.; Viennois, E.; Etienne-Mesmin, L.; Matsuyama, S.; Abo, H.; Osuka, S.; Lukacs, N.W.; Naito, Y.; Itoh, Y.; Li, J.-D.; et al. Early-Life Microbiota Exposure Restricts Myeloid-Derived Suppressor Cell-Driven Colonic Tumorigenesis. Cancer Immunol. Res. 2019, 7, 544–551. [Google Scholar]
- Chun, E.; Lavoie, S.; Michaud, M.; Gallini, C.A.; Kim, J.; Soucy, G.; Odze, R.; Glickman, J.N.; Garrett, W.S. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015, 12, 244–257. [Google Scholar]
- Li, W.; Zhang, X.; Chen, Y.; Xie, Y.; Liu, J.; Feng, Q.; Wang, Y.; Yuan, W.; Ma, J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell 2016, 7, 130–140. [Google Scholar] [CrossRef]
- Li, Z.-W.; Sun, B.; Gong, T.; Guo, S.; Zhang, J.; Wang, J.; Sugawara, A.; Jiang, M.; Yan, J.; Gurary, A.; et al. GNAI1 and GNAI3 Reduce Colitis-Associated Tumorigenesis in Mice by Blocking IL6 Signaling and Down-regulating Expression of GNAI2. Gastroenterology 2019, 156, 2297–2312. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Q.; Hou, L.; Wang, Y.; Liu, Z. MDSCs are involved in the protumorigenic potentials of GM-CSF in colitis-associated cancer. Int. J. Immunopathol. Pharmacol. 2017, 30, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.-H.; Cho, J.; Ahn, J.-H.; Kwon, B.-E.; Kweon, M.-N.; Seo, S.-U.; Yoon, B.-I.; Chang, S.-Y.; Ko, H.-J. Plasmacytoid dendritic cells regulate colitis-associated tumorigenesis by controlling myeloid-derived suppressor cell infiltration. Cancer Lett. 2020, 493, 102–112. [Google Scholar] [CrossRef]
- Delgado-Ramirez, Y.; Baltazar-Perez, I.; Martinez, Y.; Callejas, B.E.; Medina-Andrade, I.; Olguín, J.E.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Terrazas, L.I.; Leon-Cabrera, S. STAT1 Is Required for Decreasing Accumulation of Granulocytic Cells via IL-17 during Initial Steps of Colitis-Associated Cancer. Int. J. Mol. Sci. 2021, 22, 7695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Xing, Z.; Lv, S.; Huang, L.; Liu, J.; Ye, S.; Li, X.; Chen, M.; Zuo, S.; et al. OLFM4 deficiency delays the progression of colitis to colorectal cancer by abrogating PMN-MDSCs recruitment. Oncogene 2022, 41, 3131–3150. [Google Scholar] [CrossRef]
- Wang, T.; Fan, C.; Yao, A.; Xu, X.; Zheng, G.; You, Y.; Jiang, C.; Zhao, X.; Hou, Y.; Hung, M.-C.; et al. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity 2018, 49, 504–514.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, C.; Wang, W.; Hui, Y.; Zhang, R.; Qiao, L.; Dai, Y. Embelin impairs the accumulation and activation of MDSCs in colitis-associated tumorigenesis. Oncoimmunology 2018, 7, e1498437. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Klement, J.D.; Lu, C.; Redd, P.S.; Xiao, W.; Yang, D.; Browning, D.D.; Savage, N.M.; Buckhaults, P.J.; Morse, H.C.; et al. Myeloid-Derived Suppressor Cells Produce IL-10 to Elicit DNMT3b-Dependent IRF8 Silencing to Promote Colitis-Associated Colon Tumorigenesis. Cell Rep. 2018, 25, 3036–3046.e6. [Google Scholar] [CrossRef]
- Xun, J.; Zhou, S.; Lv, Z.; Wang, B.; Luo, H.; Zhang, L.; Yang, L.; Zhang, A.; Wu, X.; Wang, Z.; et al. Dioscin modulates macrophages polarization and MDSCs differentiation to inhibit tumorigenesis of colitis-associated colorectal cancer. Int. Immunopharmacol. 2023, 117, 109839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zhang, Z.; Bian, D.; Shao, K.; Wang, S.; Ding, Y. G-MDSC-derived exosomes mediate the differentiation of M-MDSC into M2 macrophages promoting colitis-to-cancer transition. J. Immunother. Cancer 2023, 11, e006166. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-García, L.; Ruiz-Malagon, A.J.; Huertas, F.; Rodríguez-Sojo, M.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Becerra, P.; Mirón, B.; Morón, R.; Rodríguez-Nogales, A.; et al. Administration of intestinal mesenchymal stromal cells reduces colitis-associated cancer in C57BL/6J mice modulating the immune response and gut dysbiosis. Pharmacol. Res. 2023, 195, 106891. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Huycke, M.M. Commensal bacteria drive endogenous transformation and tumour stem cell marker expression through a bystander effect. Gut 2015, 64, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef]
- Gwyn, K.; Sinicrope, F.A. Chemoprevention of colorectal cancer. Am. J. Gastroenterol. 2002, 97, 13–21. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Solomon, S.D.; Kim, K.; Tang, J.; Rosenstein, R.B.; Wittes, J.; Corle, D.; et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006, 355, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Zhou, H.J.; Ji, W.; Min, W. Mitochondrial Redox Signaling and Tumor Progression. Cancers 2016, 8, 40. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [PubMed]
- Ke, Z.; Wang, C.; Wu, T.; Wang, W.; Yang, Y.; Dai, Y. PAR2 deficiency enhances myeloid cell-mediated immunosuppression and promotes colitis-associated tumorigenesis. Cancer Lett. 2020, 469, 437–446. [Google Scholar] [PubMed]
- Condamine, T.; Kumar, V.; Ramachandran, I.R.; Youn, J.-I.; Celis, E.; Finnberg, N.; El-Deiry, W.S.; Winograd, R.; Vonderheide, R.H.; English, N.R.; et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Investig. 2014, 124, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.T.; Sierra, R.A.; Raber, P.L.; Al-Khami, A.A.; Trillo-Tinoco, J.; Zarreii, P.; Ochoa, A.C.; Cui, Y.; Del Valle, L.; Rodriguez, P.C. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 2014, 41, 389–401. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1. [Google Scholar]
- He, A.; Pu, Y.; Jia, C.; Wu, M.; He, H.; Xia, Y. The Influence of Exercise on Cancer Risk, the Tumor Microenvironment and the Treatment of Cancer. Sports Med. 2024, 54, 1371–1397. [Google Scholar] [CrossRef]
- Buzaglo, G.B.B.; Telles, G.D.; Araújo, R.B.; Junior, G.D.S.; Ruberti, O.M.; Ferreira, M.L.V.; Derchain, S.F.M.; Vechin, F.C.; Conceição, M.S. The Therapeutic Potential of Physical Exercise in Cancer: The Role of Chemokines. Int. J. Mol. Sci. 2024, 25, 13740. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Safarian, B.; Esparham, A.; Mirzaei, M.; Esmaeilzadeh, M.; Boskabady, M.H. The modulatory effects of exercise on lipopolysaccharide-induced lung inflammation and injury: A systemic review. Life Sci. 2022, 293, 120306. [Google Scholar] [CrossRef]
- Cui, B.; Guo, X.; Zhou, W.; Zhang, X.; He, K.; Bai, T.; Lin, D.; Wei-Zhang, S.; Zhao, Y.; Liu, S.; et al. Exercise alleviates neovascular age-related macular degeneration by inhibiting AIM2 inflammasome in myeloid cells. Metabolism 2023, 144, 155584. [Google Scholar] [CrossRef]
- Poh, T.W.; Madsen, C.S.; Gorman, J.E.; Marler, R.J.; Leighton, J.A.; Cohen, P.A.; Gendler, S.J. Downregulation of hematopoietic MUC1 during experimental colitis increases tumor-promoting myeloid-derived suppressor cells. Clin. Cancer Res. 2013, 19, 5039–5052. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, J.M.; Crist, S.A.; Elzey, B.D.; Cimen, C.; Ratliff, T.L. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur. J. Immunol. 2011, 41, 749–759. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Deng, Y.; Zheng, Y.; Wang, S. Role of myeloid-derived suppressor cells in the promotion and immunotherapy of colitis-associated cancer. J. Immunother. Cancer 2020, 8, e000609. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, W.; Song, J.; Shen, Z.; Cui, D. The paradoxical role of MDSCs in inflammatory bowel diseases: From bench to bedside. Front. Immunol. 2022, 13, 1021634. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Hibi, T. Improving IBD outcomes in the era of many treatment options. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Saegusa, J.; Matsuki, F.; Akashi, K.; Kageyama, G.; Morinobu, A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates arthritis in SKG mice. Arthritis Rheumatol. 2015, 67, 893–902. [Google Scholar] [CrossRef]
- Sendo, S.; Saegusa, J.; Yamada, H.; Nishimura, K.; Morinobu, A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res. Ther. 2019, 21, 184. [Google Scholar] [CrossRef]
- Sachen, K.L.; Hammaker, D.; Sarabia, I.; Stoveken, B.; Hartman, J.; Leppard, K.L.; Manieri, N.A.; Bao, P.; Greving, C.; Lacy, E.R.; et al. Guselkumab binding to CD64+ IL-23–producing myeloid cells enhances potency for neutralizing IL-23 signaling. Front. Immunol. 2025, 16, 1532852. [Google Scholar] [CrossRef]
| Mice | Sex | Colitis Model | Colitis Stage | Distribution and Frequency | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | SP | MLN | LP | PP | BM | BaM | Int. | Col. | Ileum | IELs | |||||
| Balb/c | F | Intrarectal injection of 2–3% TNBS | Acute | - | Inc. | Inc. | - | - | - | - | - | - | - | - | [25] |
| C57BL/6 | F | 4% DSS in drinking water for 7 days | Acute | - | Inc. | - | Inc. | Inc. | - | Dec. | - | - | - | - | [28] |
| 2% DSS in drinking water for 5 days, drinking water for 5 days, 3 cycles | Chronic | - | Inc. | - | ns | Inc. | - | ns | - | - | - | - | |||
| Intrarectal injection of 2% TNBS | Acute | - | Inc. | Inc. | Inc. | - | Inc. | - | - | - | - | ||||
| Balb/c | - | DSS (5%, 7%, 7%) to the drinking water on days 0, 15, and 27, respectively, for 5 days | - | - | ns | ns | - | - | - | - | - | - | - | - | [22] |
| Adoptive transfer of CD8+Tcells isolated from CL4-TCR mice into VILLIN-HA mice | Acute | - | Inc. | - | - | - | - | - | Inc. | - | - | - | |||
| SCID mice transferred with WT CD4+CD45RBhigh T cells | Chronic | - | Inc. | - | - | - | - | - | - | - | - | - | |||
| Balb/c | M | 5% DSS induced in drinking water for 22 days | Chronic | Inc. | Inc. | - | - | - | Inc. | - | - | - | - | - | [23] |
| Balb/c | F | Intrarectally administered TNBS (1.5 mg and 1.8 mg) at a 1-week interval. | Acute | - | Inc. | - | Inc. | - | - | - | - | - | - | - | [27] |
| C57BL/6 | 4% DSS induced in drinking water for 5 days | Acute | - | ns | - | Inc. | - | - | - | - | - | - | - | ||
| C57BL/6 | M | 2% DSS in drinking water for 7 days | Acute | - | Inc. | - | - | - | Inc. | - | - | Inc. | - | - | [29] |
| - | - | Rag2−/− mice infected with Helicobacter hepaticus | Chronic | - | Inc. | - | Inc. | - | - | - | - | - | - | - | [30] |
| C57BL/6 | F | 4% 2,4-Dinitrobenzenesulfonic acid (DNB) enema for 5 days | Chronic | Inc. | Inc. | Inc. | Inc. | - | Inc. | - | - | - | - | - | [31] |
| C57BL/6 | F | 3.5% DSS in drinking water for 9 days | Acute | Inc. | ns | - | - | - | - | - | - | - | - | - | [32] |
| C57BL/6 | F | 2.5% DSS in drinking water for 7 days | Acute | Inc. | - | - | Inc. | - | Inc. | - | - | - | - | - | [33] |
| C57BL/6 | - | Rag2−/− mice transferred with WT CD4+CD45RBhigh T cells | Chronic | Inc. | - | Inc. | Inc. | - | - | - | - | - | - | - | [34] |
| - | 3%DSS drinking in water for 7 days | Acute | - | Inc. | - | - | - | - | - | - | Inc. | - | - | ||
| F, M | TnfΔARE mice | Chronic | - | Inc. | - | - | - | - | - | - | - | Inc. | - | ||
| C57BL/6 | - | Il10−/− mice | Chronic | ns | ns | Inc. | Inc. | - | ns | - | - | - | - | - | [35] |
| C57BL/6 | - | 3% DSS in drinking water for 7 days | Acute | - | ns | ns | Inc. | - | - | - | - | Inc. | - | Inc. | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Cao, S. Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 3291. https://doi.org/10.3390/ijms26073291
Zhu Y, Cao S. Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2025; 26(7):3291. https://doi.org/10.3390/ijms26073291
Chicago/Turabian StyleZhu, Yangzhuangzhuang, and Siyan Cao. 2025. "Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease" International Journal of Molecular Sciences 26, no. 7: 3291. https://doi.org/10.3390/ijms26073291
APA StyleZhu, Y., & Cao, S. (2025). Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease. International Journal of Molecular Sciences, 26(7), 3291. https://doi.org/10.3390/ijms26073291






