Structural and Dynamic Properties of Flame-Retardant Phosphorylated-Polycarbonate/Polycarbonate Blends
Abstract
1. Introduction
2. Results and Discussion
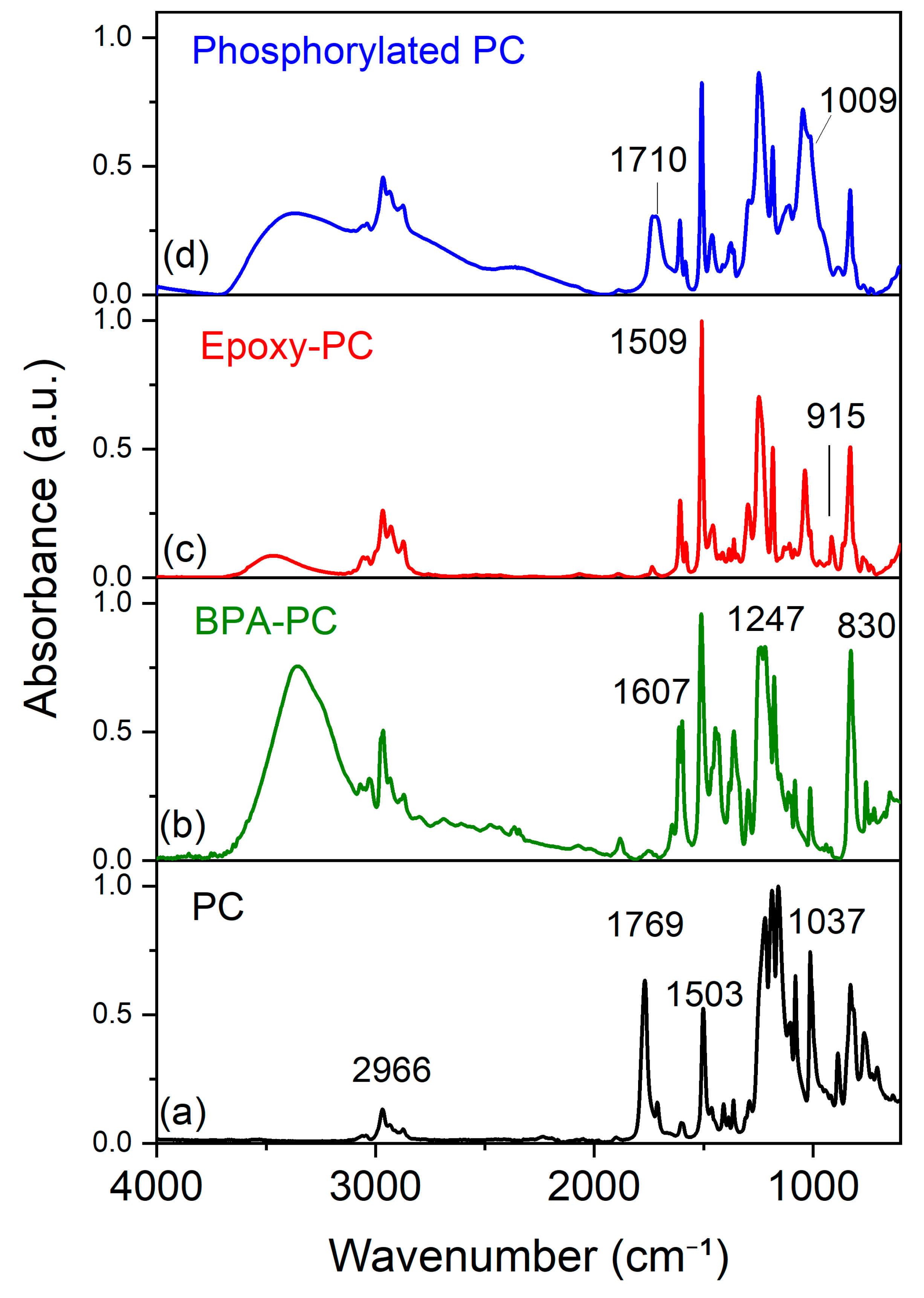
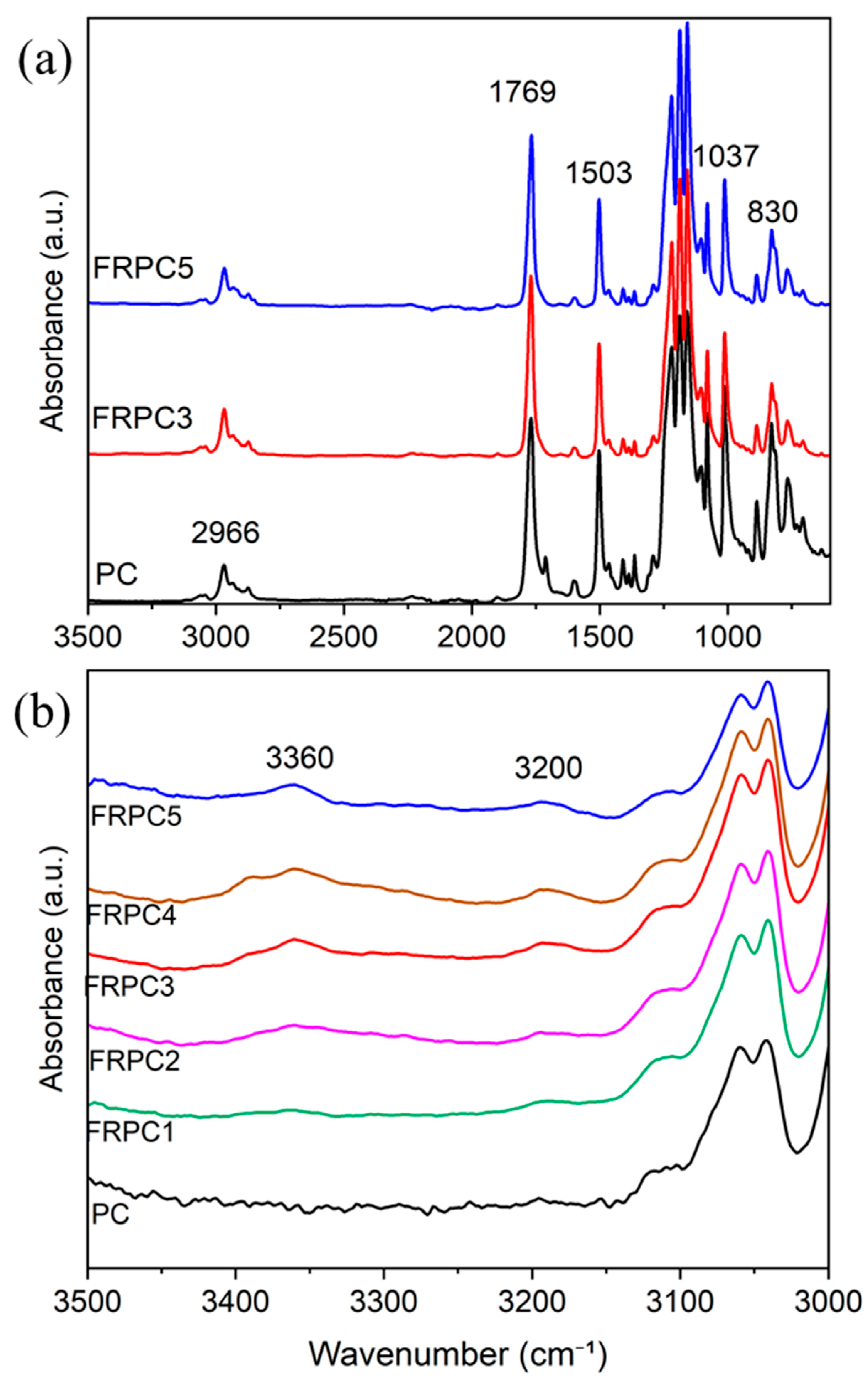
2.1. Structure and Morphology
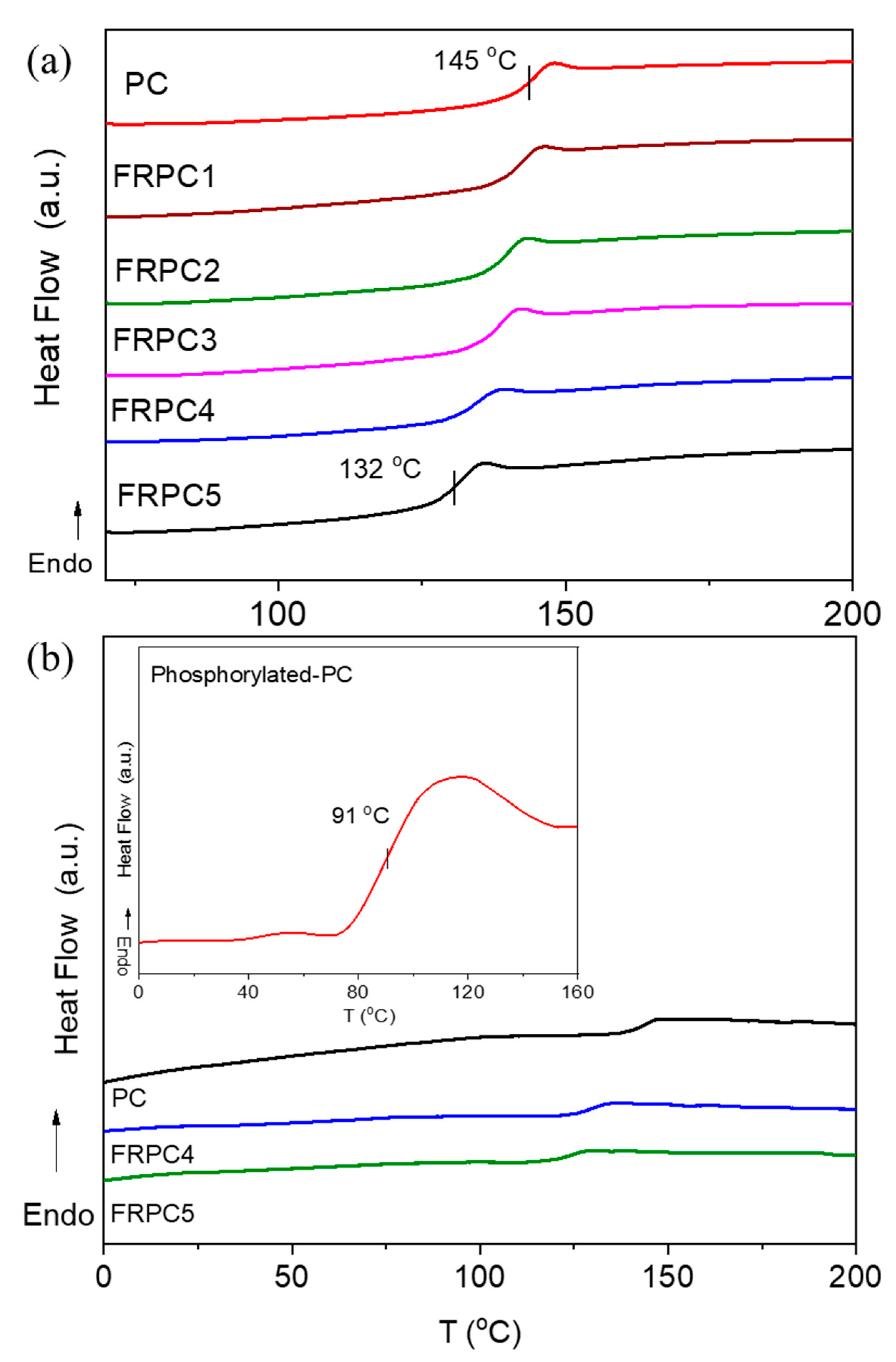
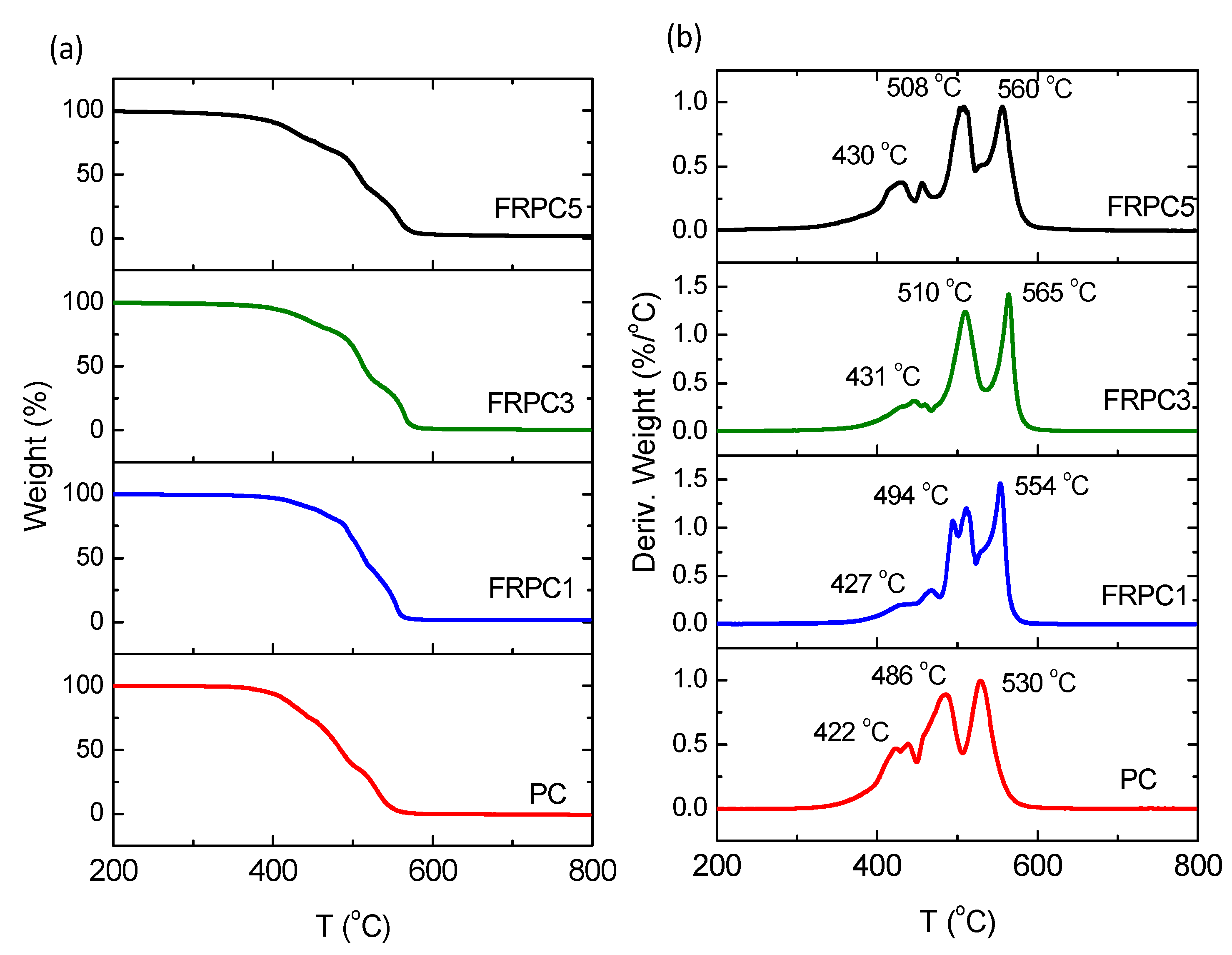
2.2. Glass Transition Temperatures and Thermo-Oxidative Properties
2.3. Flame Retardant Property
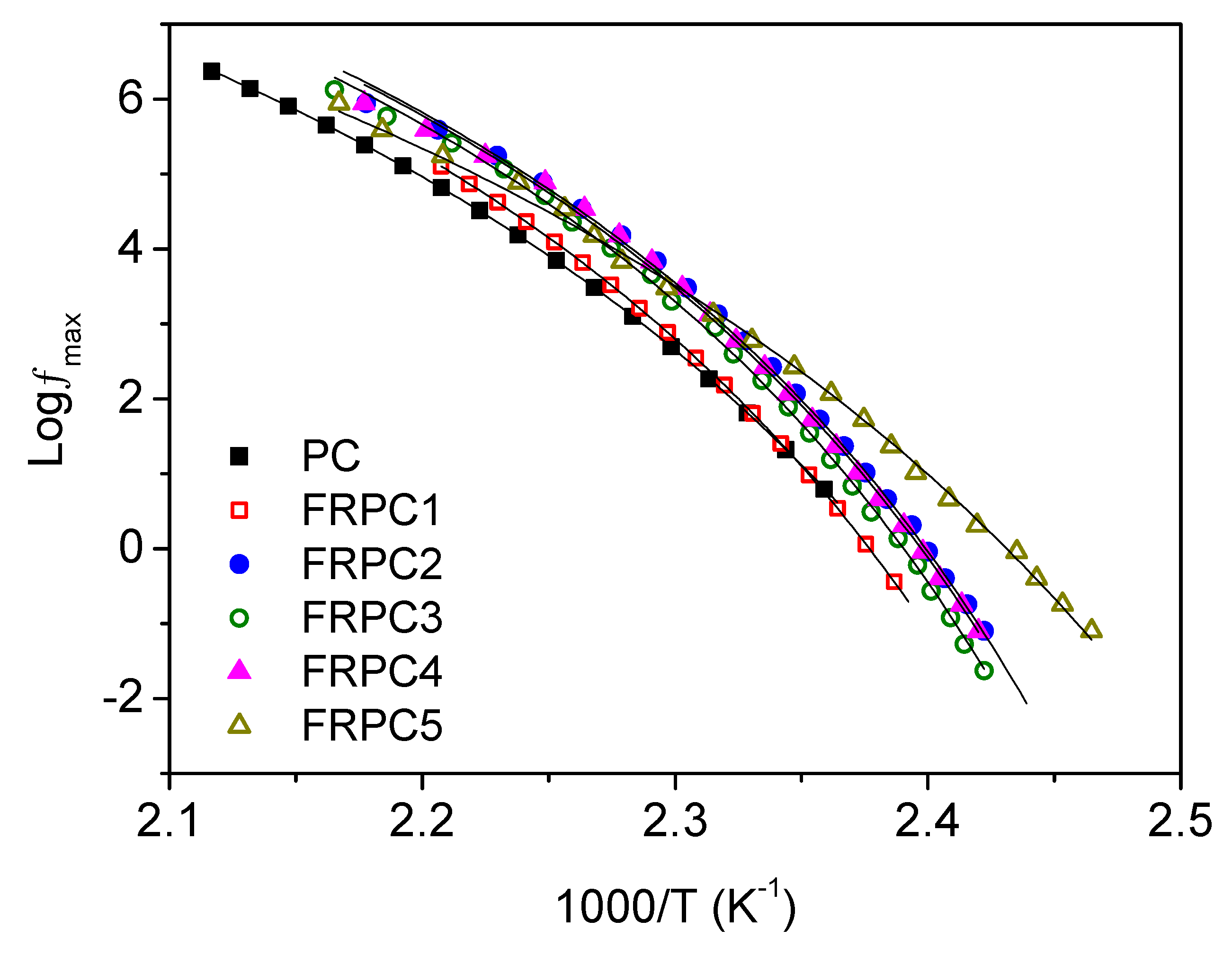
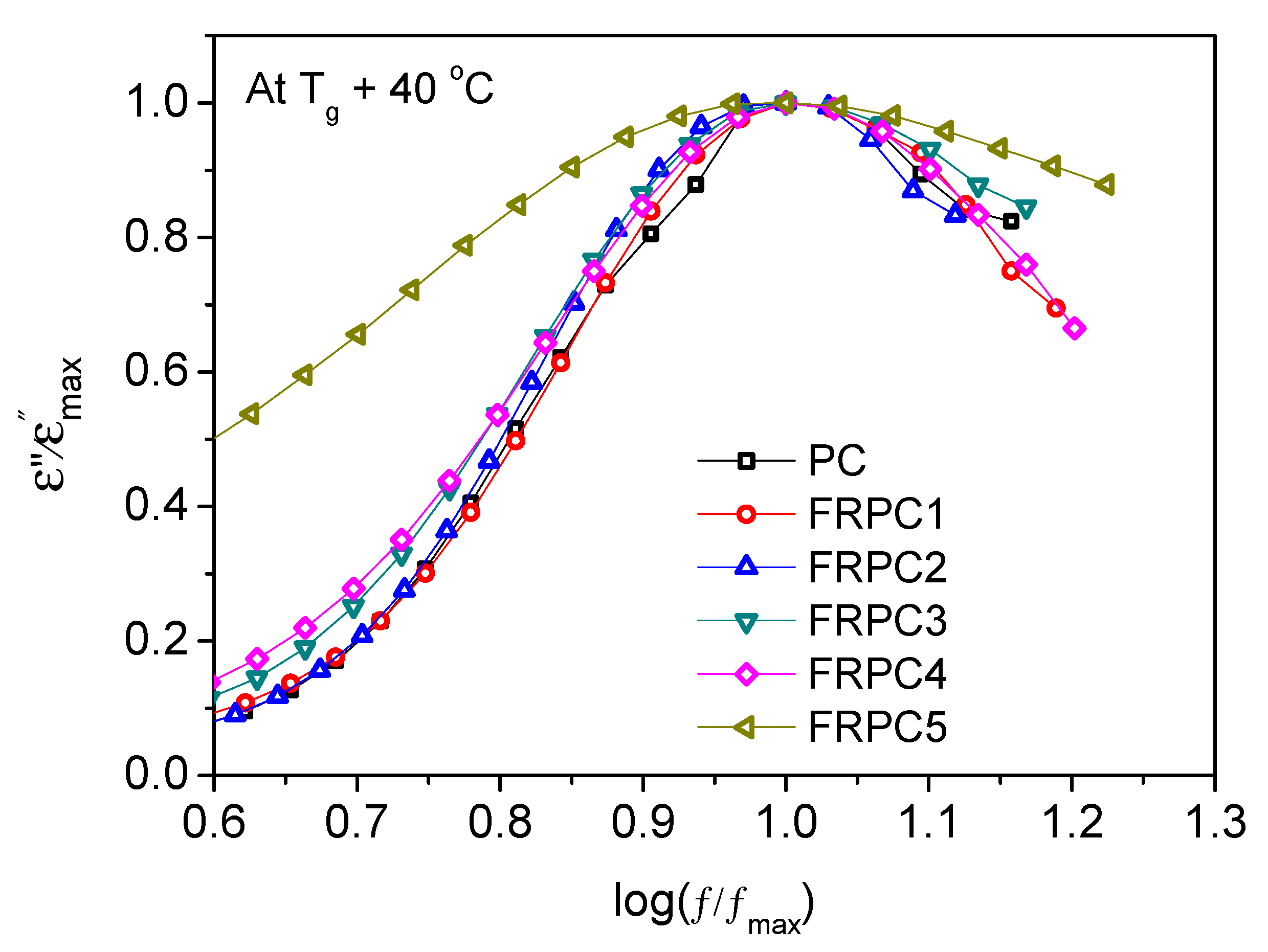
2.4. Dielectric Relaxations
3. Materials and Methods
3.1. Materials and Reagents
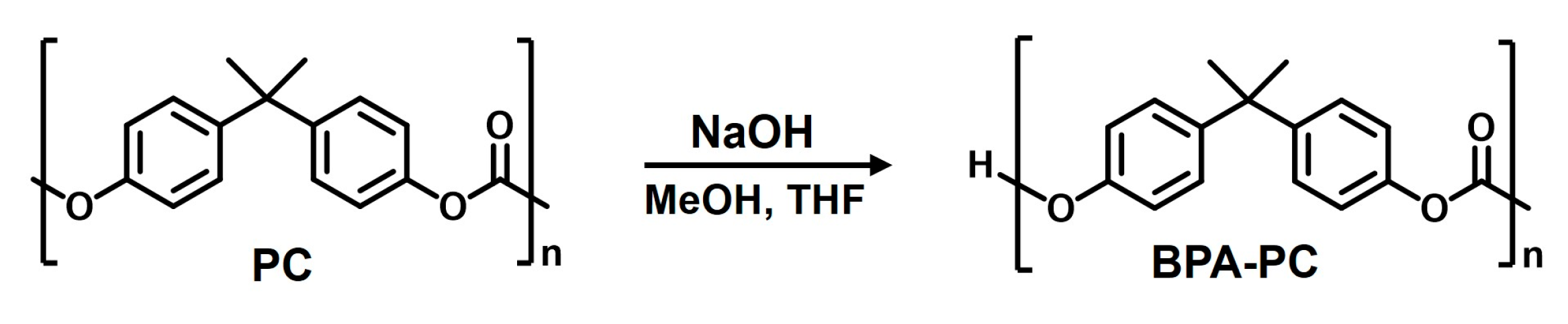
3.2. Preparation of Bisphenol-A from Polycarbonate (BPA-PC)
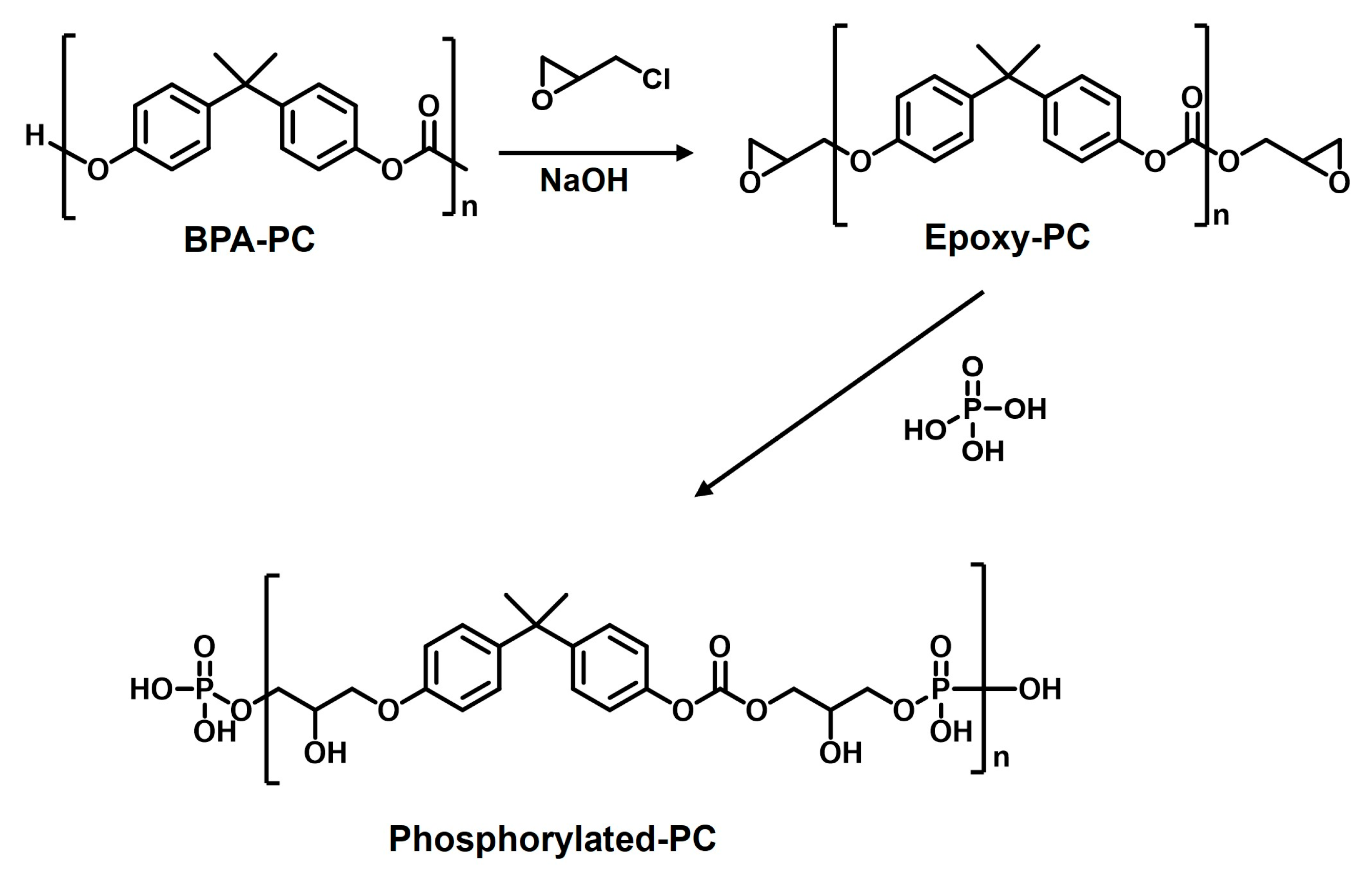
3.3. Preparation of Phosphorylated-PC from BPA-PC
3.4. Preparation of Flame-Retardant PC Blends (FRPC)
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horrocks, A.R.; Price, D. Fire retardant Materials; Woodhead Publishing Ltd.: Abington, Cambridge, UK, 2001; pp. 264–290. [Google Scholar]
- Veen, I.V.D.; Boer, J.D. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity, and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [PubMed]
- Kim, S.; Linh, P.T.T.; Kang, J.; Kim, I. Phosphorus-containing thermoplastic poly(ether ester) elastomers showing intrinsic flame retardancy. J. Appl. Polym. Sci. 2017, 134, 45478. [Google Scholar]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Flame retardancy and thermal properties of solid bisphenol A bis(diphenyl phosphate) combined with montmorillonite in polycarbonate. Polym. Degrad. Stab. 2010, 95, 2041–2048. [Google Scholar]
- Perret, B.; Schartel, B. The effect of different impact modifiers in halogen-free flame retarded polycarbonate blends—I. Pyrolysis. Polym. Degrad. Stab. 2009, 94, 2194–2203. [Google Scholar]
- Mauerer, O. New reactive, halogen-free flame retardant system for epoxy resins. Polym. Degrad. Stab. 2005, 88, 70–73. [Google Scholar]
- Chen, L.; Luo, Y.; Hu, Z.; Lin, G.-P.; Zhao, B.; Wang, Y.-Z. An efficient halogen-free flame retardant for glass-fibre-reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2012, 97, 158–165. [Google Scholar]
- Zhang, W.; Li, X.; Guo, X.; Yang, R. Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites. Polym. Degrad. Stab. 2010, 95, 2541–2546. [Google Scholar]
- Jia, X.W.; Mu, W.L.; Shao, Z.B.; Xu, Y.J. Flame-Retardant Cycloaliphatic Epoxy Systems with High Dielectric Performance for Electronic Packaging Materials. Int. J. Mol. Sci. 2023, 24, 2301. [Google Scholar] [CrossRef]
- Jiang, X.L.; Tang, R.C. Phosphorylation of Kapok Fiber with Phytic Acid for Enhanced Flame Retardancy. Int. J. Mol. Sci. 2022, 23, 14950. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, X. Recent progress in phosphorus-based flame retardants for polymers: Mechanisms and applications. Prog. Polym. Sci. 2018, 89, 21–58. [Google Scholar]
- Song, L.; Wang, D. Phosphorus-based flame retardants and their influence on the properties of polycarbonate. J. Appl. Polym. Sci. 2012, 124, 1187–1194. [Google Scholar]
- Wu, L.; Zhang, X.; He, S. The flame retardancy and thermal degradation behavior of phosphorus-containing flame retardants for polymeric materials. J. Fire Sci. 2019, 37, 337–355. [Google Scholar]
- Zhou, L.; Ding, L. Fire behavior of phosphorus-containing flame retardants and their application in polymeric materials. Polym. Int. 2015, 64, 761–777. [Google Scholar]
- Mouret, J.; Allais, F. Phosphorus-based flame retardants in the context of environmental and safety regulations. J. Hazard. Mater. 2011, 185, 82–89. [Google Scholar]
- Shen, J.; Liang, J.; Lin, X.; Yu, J.; Wang, S. The flame-retardant mechanisms and preparation of polymer composites and their potential application in construction engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef]
- Allcock, H.R.; Lampe, F.W. Contemporary Polymer Chemistry, 2nd ed.; Prentice-Hall International: New York, NY, USA, 1992; pp. 29–30. [Google Scholar]
- Levchik, S.V.; Weil, E.D. Flame retardants in commercial use or in advanced development in polycarbonates and polycarbonate blends. J. Fire Sci. 2006, 24, 137–155. [Google Scholar] [CrossRef]
- Liu, F.-S.; Li, Z.; Yu, S.-T.; Cui, X.; Xie, C.-X.; Ge, X.-P. Methanolysis and hydrolysis of polycarbonate under moderate conditions. J. Polym. Environ. 2009, 17, 208–211. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. A TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2004, 86, 419–430. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, K.; Kumar, M.; Awasthi, K. Functionalized Pd-decorated and aligned MWCNTs in polycarbonate as a selective membrane for hydrogen separation. Int. J. Hydrogen Energy 2016, 41, 23057–23066. [Google Scholar] [CrossRef]
- Zuo, J.D.; Liu, S.M.; Sheng, Q. Synthesis and application in polypropylene of novel of phosphorus-containing intumescent flame retardant. Molecules 2010, 15, 7593–7602. [Google Scholar] [CrossRef]
- Murad, M.S.; Hamzat, A.K.; Asmatulu, E.; Bahçeci, E.; Bakir, M.; Asmatulu, R. Studying flame-retardancy, smoke and toxicity of fiber-reinforced composites manufactured via modified resins and metallic coatings. Hybrid Adv. 2025, 8, 100373. [Google Scholar]
- Atorngitjawat, P. Effects of processing conditions and crystallization on dynamic relaxations in semicrystalline poly(vinylidene fluoride) films. Macromol. Res. 2017, 25, 391–399. [Google Scholar] [CrossRef]
- Masser, K.A.; Runt, J. Dynamics of polymer blends of a strongly inter-associating homopolymer with poly(vinyl methyl ether) and poly(2-vinyl pyridine). Macromolecules 2010, 43, 6414–6421. [Google Scholar] [CrossRef]
- Atorngitjawat, P.; Klein, R.J.; Runt, J. Dynamics of sulfonated polystyrene copolymers using broadband dielectric spectroscopy. Macromolecules 2006, 39, 1815–1820. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 375–379. [Google Scholar]
- Angell, C.A. Formation of glasses from liquids and biopolymers. Science 2002, 267, 1924–1935. [Google Scholar] [CrossRef]
- Bohn, P.L.; Avramov, I. The relationship between fragility and cooperatively rearranging domains in polymers. J. Non-Cryst. Solids. 2004, 345, 73–81. [Google Scholar]
- Novikov, V.N.; Sokolov, A. Temperature dependence of structural relaxation in Glass-Forming Liquids and Polymers. Entropy 2022, 24, 1101. [Google Scholar] [CrossRef]
- Atorngitjawat, P.; Runt, J. Dynamics of sulfonated polystyrene ionomers using broadband dielectric spectroscopy. Macromolecules 2007, 40, 991–996. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]


| Sample | Mass of Volatile Product (%) | Char Residue (%) | ||
|---|---|---|---|---|
| 450 °C | 500 °C | 550 °C | 650 °C | |
| PC | 26.3 | 61.6 | 95.6 | 0.9 |
| FRPC1 | 11.3 | 35.0 | 80.7 | 1.7 |
| FRPC2 | 10.0 | 31.6 | 78.6 | 1.7 |
| FRPC3 | 15.8 | 34.3 | 72.5 | 1.7 |
| FRPC4 | 18.9 | 36.2 | 72.0 | 2.1 |
| FRPC5 | 23.8 | 43.3 | 77.5 | 2.3 |
| Sample | UL-94 Rating | Dripping | Weight Loss (%) |
|---|---|---|---|
| PC | V-2 | yes | 2.8 |
| FRPC1 | V-2 | yes | 1.2 |
| FRPC2 | V-2 | yes | 0.7 |
| FRPC3 | V-2 | yes | 0.6 |
| FRPC4 | V-1 | no | 0.3 |
| FRPC5 | V-1 | no | 0.2 |
| VFT Parameters | |||||
|---|---|---|---|---|---|
| Tg,DSC | Tg,DRS | B | T0 | Fragility | |
| Sample | (°C) | (°C) | (K) | (°C) | ° |
| PC | 145 | 144 | 2019 | 85 | 105 |
| FRPC1 | 143 | 144 | 1815 | 91 | 117 |
| FRPC2 | 140 | 141 | 1702 | 91 | 122 |
| FRPC3 | 138 | 142 | 1707 | 92 | 123 |
| FRPC4 | 135 | 141 | 1707 | 91 | 123 |
| FRPC5 | 132 | 134 | 2285 | 67 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakulsaknimitr, W.; Wongsamut, C.; Atorngitjawat, P. Structural and Dynamic Properties of Flame-Retardant Phosphorylated-Polycarbonate/Polycarbonate Blends. Int. J. Mol. Sci. 2025, 26, 3241. https://doi.org/10.3390/ijms26073241
Sakulsaknimitr W, Wongsamut C, Atorngitjawat P. Structural and Dynamic Properties of Flame-Retardant Phosphorylated-Polycarbonate/Polycarbonate Blends. International Journal of Molecular Sciences. 2025; 26(7):3241. https://doi.org/10.3390/ijms26073241
Chicago/Turabian StyleSakulsaknimitr, Wissawat, Chompunut Wongsamut, and Pornpen Atorngitjawat. 2025. "Structural and Dynamic Properties of Flame-Retardant Phosphorylated-Polycarbonate/Polycarbonate Blends" International Journal of Molecular Sciences 26, no. 7: 3241. https://doi.org/10.3390/ijms26073241
APA StyleSakulsaknimitr, W., Wongsamut, C., & Atorngitjawat, P. (2025). Structural and Dynamic Properties of Flame-Retardant Phosphorylated-Polycarbonate/Polycarbonate Blends. International Journal of Molecular Sciences, 26(7), 3241. https://doi.org/10.3390/ijms26073241





