DNA Methylation Patterns Provide Insights into the Epigenetic Regulation of Intersex Formation in the Chinese Mitten Crab (Eriocheir sinensis)
Abstract
1. Introduction
2. Results
2.1. Sequencing Data Quality Control and Reference Genome Alignment
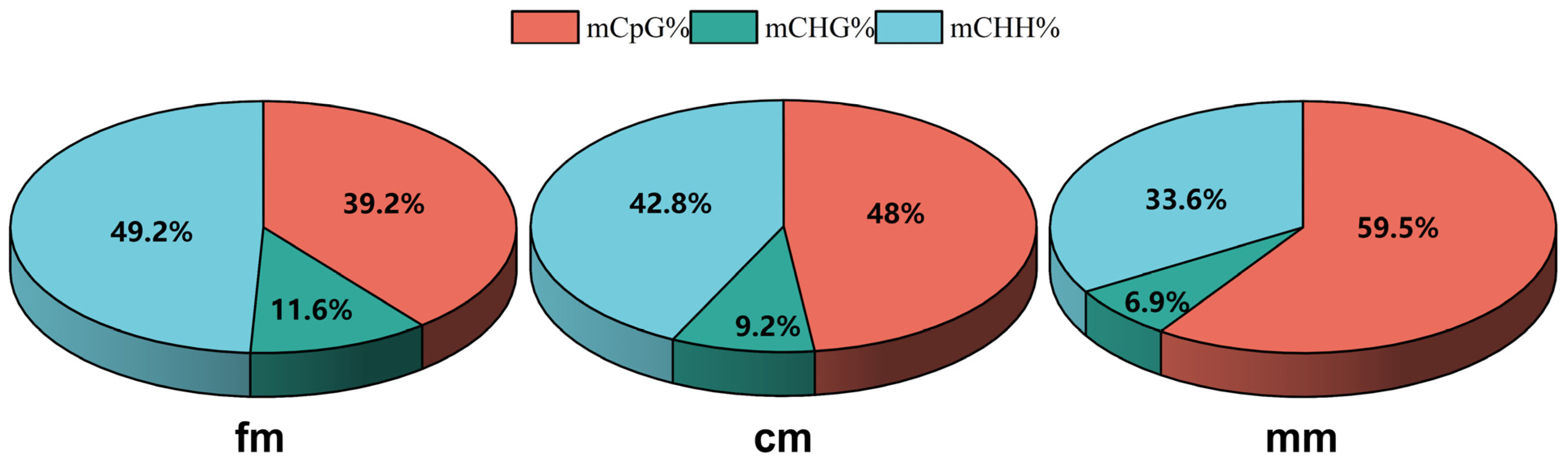
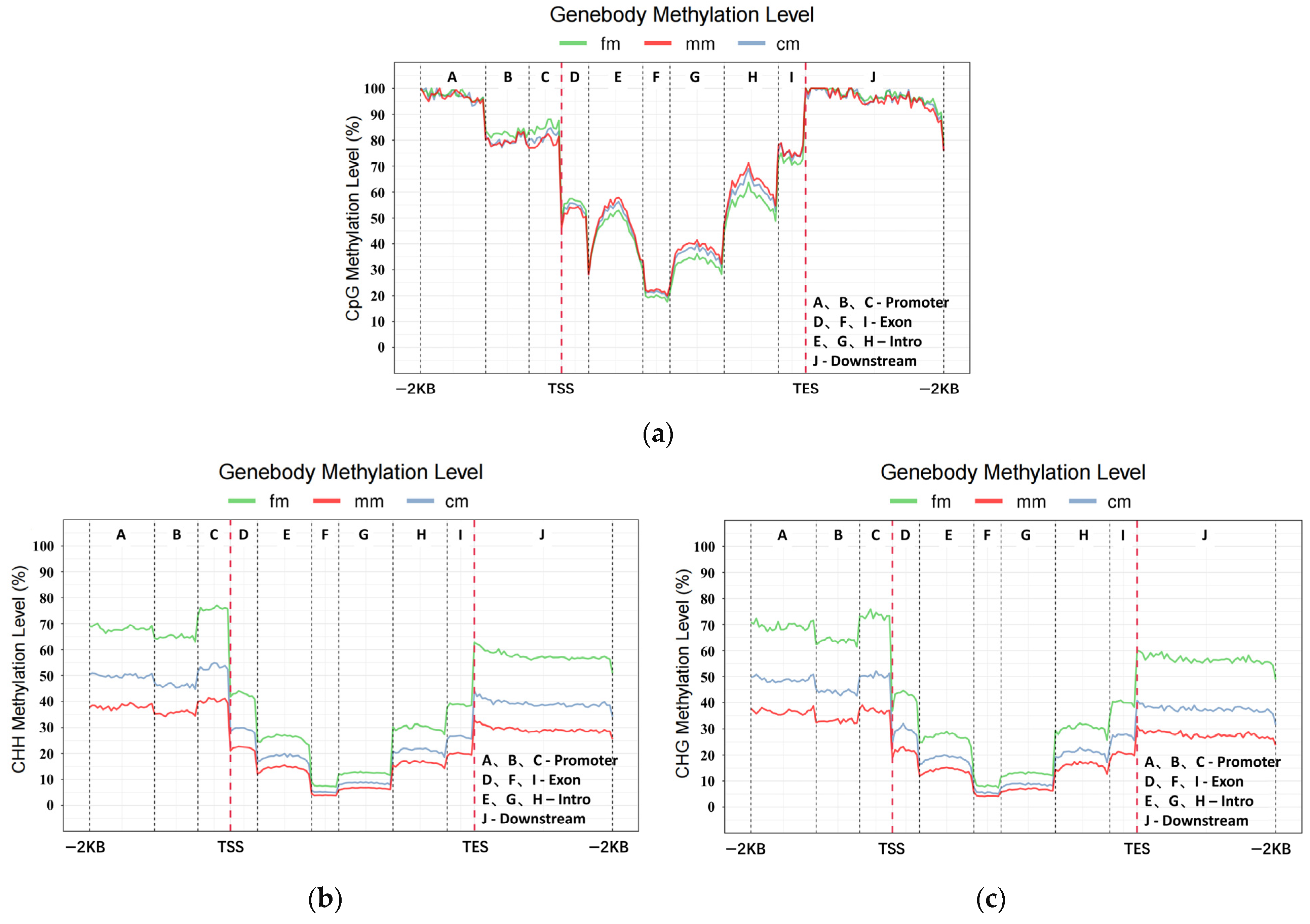
2.2. Genome-Wide DNA Methylation Profiles
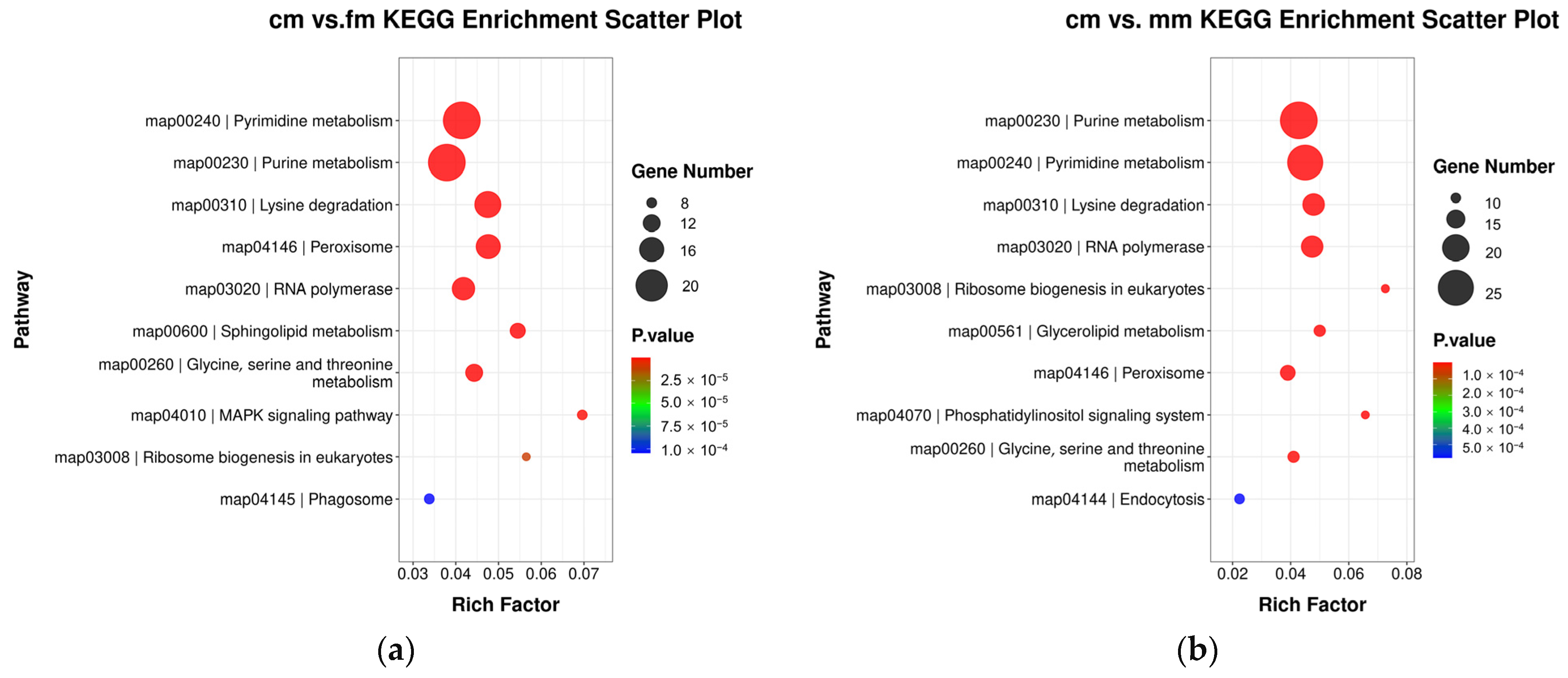
2.3. Functional Enrichment of Promoter-Region DMGs
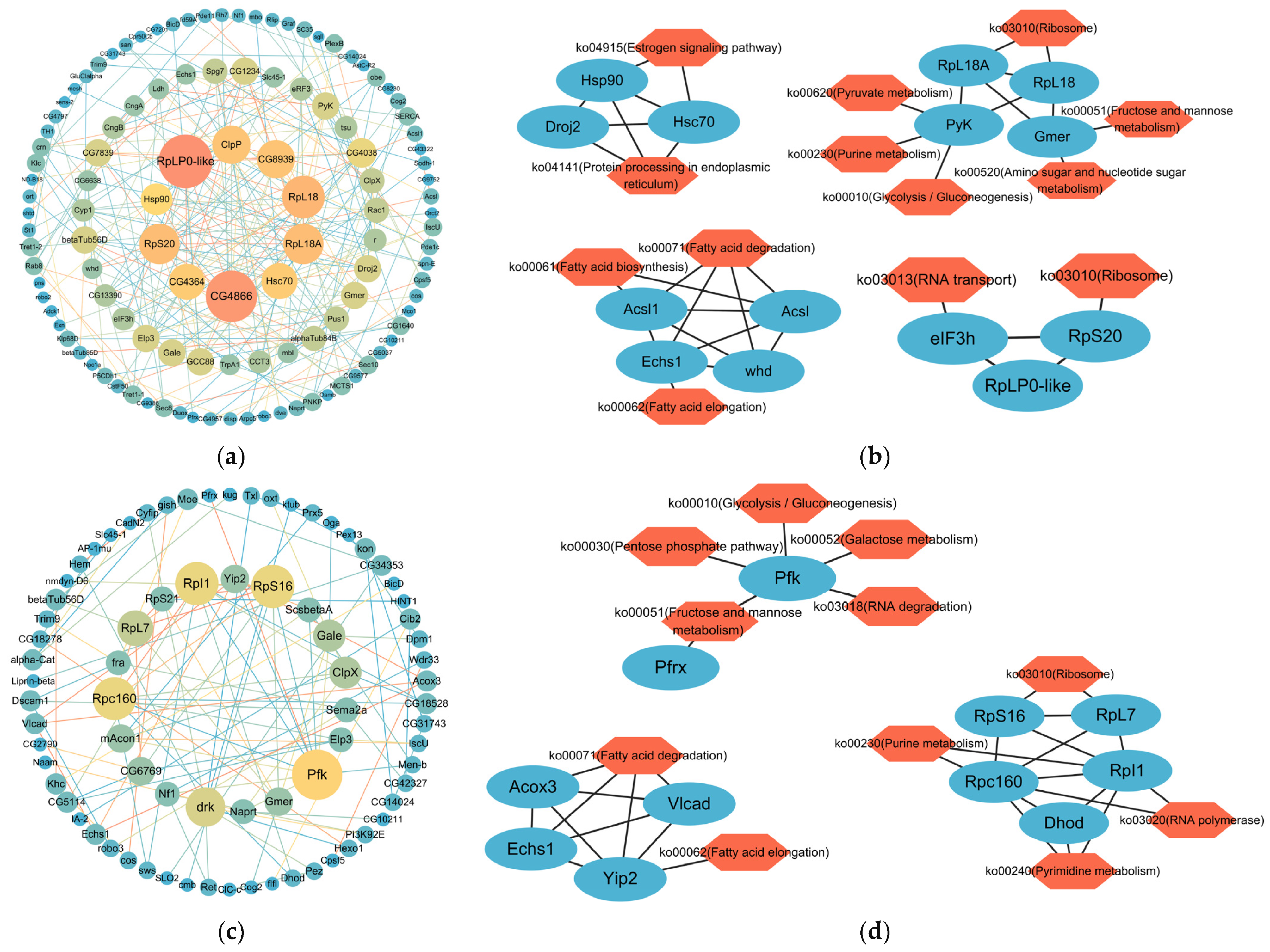
2.4. PPI Networks of DMGs in the Promoter Region of Intersex and Normal Crabs
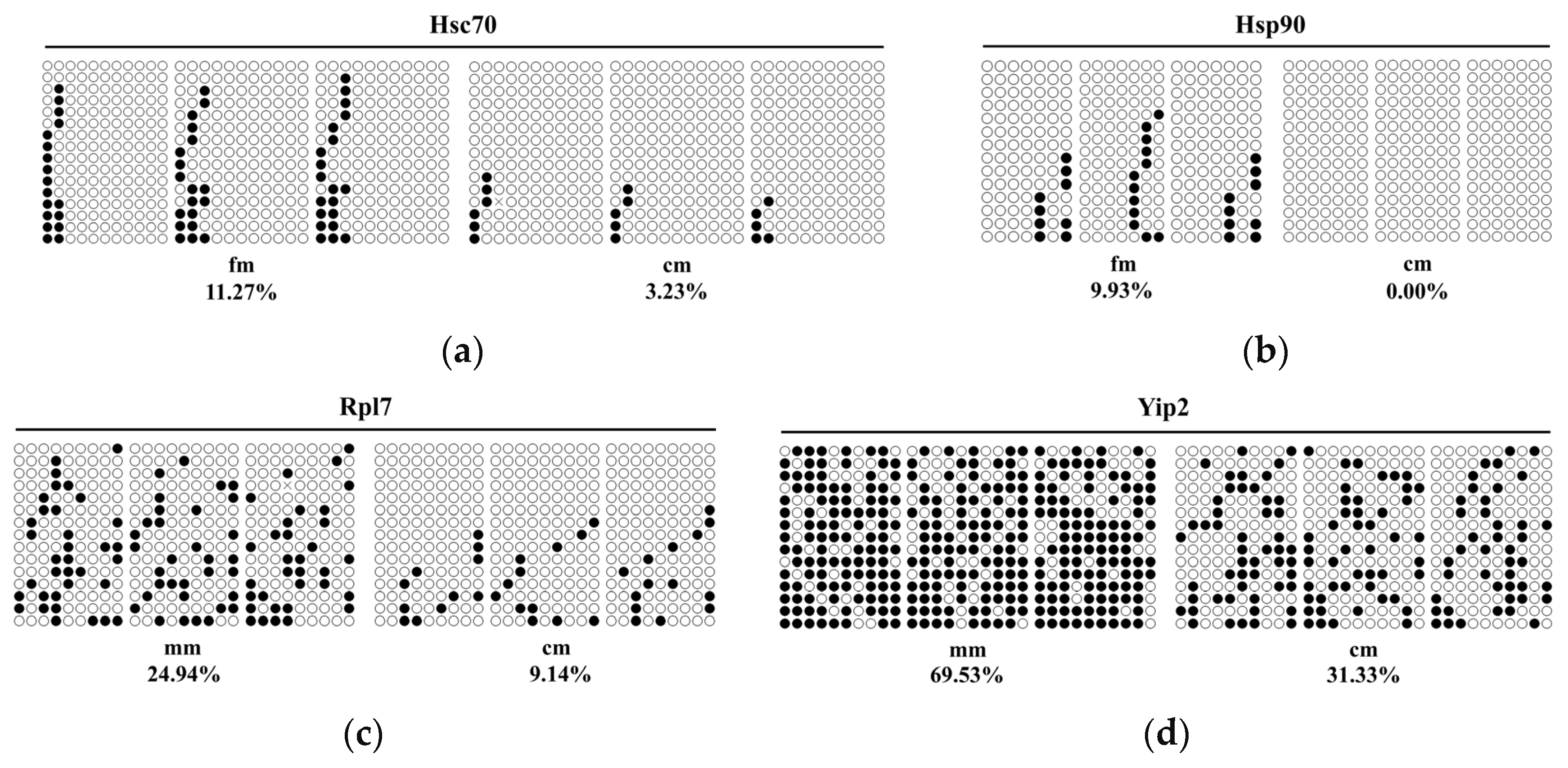
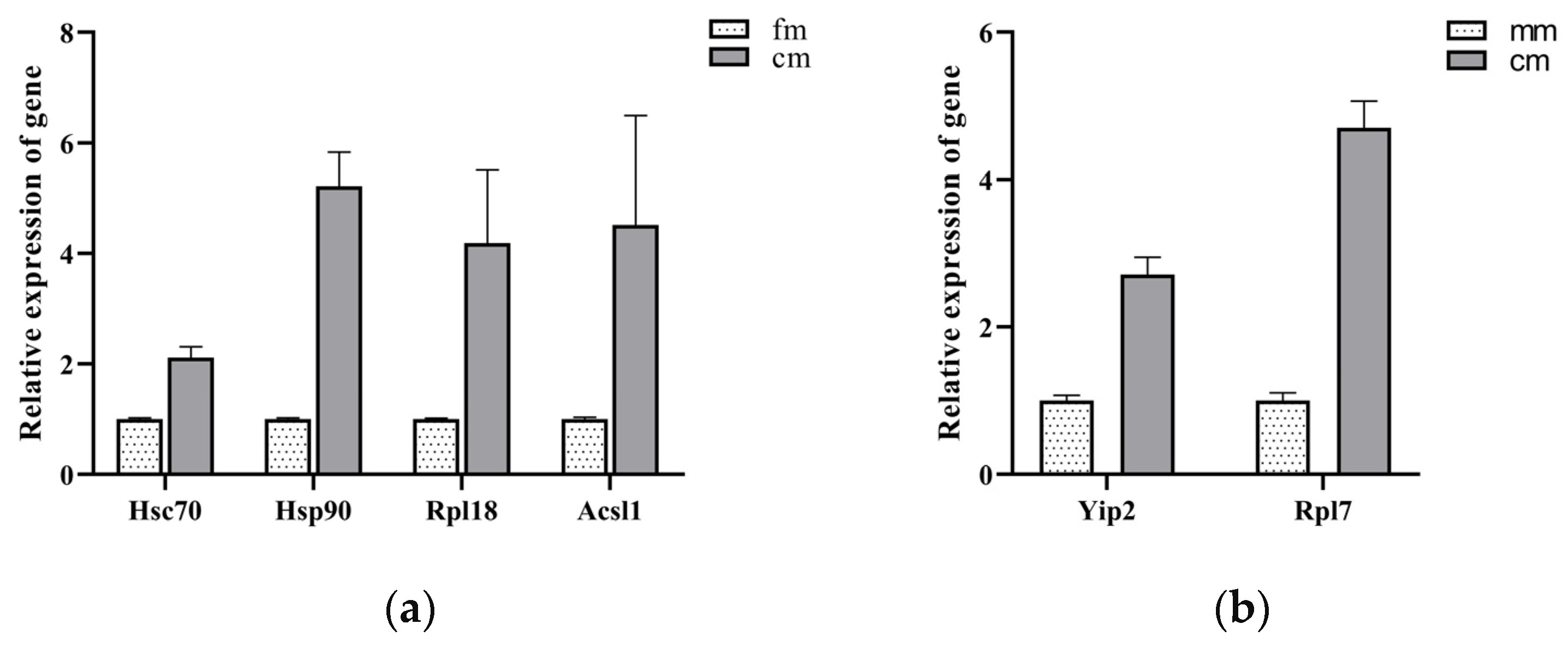
2.5. Regulation of Gene Expression by Differential Methylation of Promoter Region
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Sample Collection and Classification
4.3. Total Genomic DNA Extraction
4.4. Enzymatic Methyl Sequencing (EM-seq)
4.5. Identification of Differentially Methylated Regions (DMRs)
4.6. Functional Enrichment Analysis of Differentially Methylated Genes (DMGs) in Promoter Region
4.7. PPI Analysis of DMGs in Promoter Region
4.8. Bisulfite Sequencing PCR (BSP)
4.9. Quantitative Real-Time PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [PubMed]
- Li, G.-L.; Xu, Y.-J.; Huang, X.-M.; Xiao, J.; Nong, S.; Li, C.-G. MeDIP-Seq Reveals the Features of Mitochondrial Genomic Methylation in Immature Testis of Chinese Mitten Crab Eriocheir sinensis. Mitochondrial DNA Part A 2018, 29, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Pearson, M.; Blaxter, M.; Wilson, P.J.; Little, T.J. Genome-Wide Methylation Is Modified by Caloric Restriction in Daphnia magna. BMC Genom. 2019, 20, 197. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Wang, W.; Que, H.; Zhang, G.; Li, L. DNA Methylation Mediates Differentiation in Thermal Responses of Pacific Oyster (Crassostrea gigas) Derived from Different Tidal Levels. Heredity 2021, 126, 10–22. [Google Scholar] [CrossRef]
- Wang, H.; Cui, J.; Qiu, X.; Wang, X. Differences in DNA Methylation between Slow and Fast Muscle in Takifugu rubripes. Gene 2021, 801, 145853. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lian, Z.; Xia, X.; Tian, H.; Li, Z. Integrated Chromatin Accessibility and DNA Methylation Analysis to Reveal the Critical Epigenetic Modification and Regulatory Mechanism in Gonadal Differentiation of the Sequentially Hermaphroditic Fish, Monopterus albus. Biol. Sex Differ. 2022, 13, 73. [Google Scholar] [CrossRef]
- Ford, A.T. Intersexuality in Crustacea: An Environmental Issue? Aquat. Toxicol. 2012, 108, 125–129. [Google Scholar] [CrossRef]
- Takahashi, T.; Araki, A.; Nomura, Y.; Koga, M.; Arizono, K. The Occurrence of Dual-Gender Imposex in Japanese Freshwater Crab. J. Health Sci. 2000, 46, 376–379. [Google Scholar] [CrossRef][Green Version]
- Rodgers-Gray, T.P.; Smith, J.E.; Ashcroft, A.E.; Isaac, R.E.; Dunn, A.M. Mechanisms of Parasite-Induced Sex Reversal in Gammarus duebeni. Int. J. Parasitol. 2004, 34, 747–753. [Google Scholar] [CrossRef]
- Parnes, S.; Khalaila, I.; Hulata, G.; Sagi, A. Sex Determination in Crayfish: Are Intersex Cherax quadricarinatus (Decapoda, Parastacidae) Genetically Females? Genet. Res. 2003, 82, 107–116. [Google Scholar] [CrossRef]
- Abdel-moneim, A.; Coulter, D.P.; Mahapatra, C.T.; Sepúlveda, M.S. Intersex in Fishes and Amphibians: Population Implications, Prevalence, Mechanisms and Molecular Biomarkers. J. Appl. Toxicol. 2015, 35, 1228–1240. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a Fish Population after Exposure to a Synthetic Estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [PubMed]
- Inui, N.; Oguchi, K.; Shinji, J.; Okanishi, M.; Shimomura, M.; Miura, T. Parasitism-Induced Intersexuality in a Sexually Dimorphic Varunid Crab, Ptychognathus ishii (Decapoda: Varunidae). Zool. Sci. 2021, 38, 416–426. [Google Scholar]
- Souissi, A.; Souissi, S.; Devreker, D.; Hwang, J.-S. Occurence of Intersexuality in a Laboratory Culture of the Copepod Eurytemora affinis from the Seine Estuary (France). Mar. Biol. 2010, 157, 851–861. [Google Scholar]
- Short, S.; Yang, G.; Kille, P.; Ford, A.T. A Widespread and Distinctive Form of Amphipod Intersexuality Not Induced by Known Feminising Parasites. Sex. Dev. 2012, 6, 320–324. [Google Scholar] [PubMed]
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z. Epigenetic Modification and Inheritance in Sexual Reversal of Fish. Genome Res. 2014, 24, 604–615. [Google Scholar]
- Todd, E.V.; Ortega-Recalde, O.; Liu, H.; Lamm, M.S.; Rutherford, K.M.; Cross, H.; Black, M.A.; Kardailsky, O.; Marshall Graves, J.A.; Hore, T.A. Stress, Novel Sex Genes, and Epigenetic Reprogramming Orchestrate Socially Controlled Sex Change. Sci. Adv. 2019, 5, eaaw7006. [Google Scholar]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The Histone Demethylase KDM6B Regulates Temperature-Dependent Sex Determination in a Turtle Species. Science 2018, 360, 645–648. [Google Scholar]
- Lu, W.; Aamoum, O.; Feng, T.; Mo, N.; Han, R.; Shao, S.; Cui, Z. The Comparison of Morphological Characters, Ovarian Histology and Gene Expression Levels between Intersex and Normal Eriocheir sinensis (Decapoda, Varunidae). Crustaceana 2024, 97, 1303–1322. [Google Scholar]
- Zhang, P.; Yang, Y.; Xu, Y.; Cui, Z. Analyses of the Dmrt family in a decapod crab, Eriocheir sinensis uncover new facets on the evolution of DM domain genes. Front. Physiol. 2023, 14, 1201846. [Google Scholar]
- Zhu, D.; Feng, T.; Mo, N.; Han, R.; Lu, W.; Shao, S.; Cui, Z. New insights for the regulatory feedback loop between type 1 crustacean female sex hormone (CFSH-1) and insulin-like androgenic gland hormone (IAG) in the Chinese mitten crab (Eriocheir sinensis). Front. Physiol. 2022, 13, 1054773. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Xu, Y.; Ma, Y.; Zhang, D.; Li, X.; Zhao, S. The DNA Methylation Status of Wnt and Tgfβ Signals Is a Key Factor on Functional Regulation of Skeletal Muscle Satellite Cell Development. Front. Genet. 2019, 10, 220. [Google Scholar] [CrossRef]
- Cribiu, P.; Chaumot, A.; Geffard, O.; Ravanat, J.-L.; Bastide, T.; Delorme, N.; Quéau, H.; Caillat, S.; Devaux, A.; Bony, S. Natural Variability and Modulation by Environmental Stressors of Global Genomic Cytosine Methylation Levels in a Freshwater Crustacean, Gammarus fossarum. Aquat. Toxicol. 2018, 205, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, J. Elucidating the Role of DNA Methylation in Low-Salinity Adaptation of the Swimming Crab Portunus trituberculatus. Aquaculture 2025, 595, 741707. [Google Scholar]
- Feng, S.; Cokus, S.J.; Zhang, X.; Chen, P.-Y.; Bostick, M.; Goll, M.G.; Hetzel, J.; Jain, J.; Strauss, S.H.; Halpern, M.E.; et al. Conservation and Divergence of Methylation Patterning in Plants and Animals. Proc. Natl. Acad. Sci. USA 2010, 107, 8689–8694. [Google Scholar]
- Head, J.A. Patterns of DNA Methylation in Animals: An Ecotoxicological Perspective. Integr. Comp. Biol. 2014, 54, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, L.; Cheng, J.; Zhu, X.; Wu, P.; Bao, L.; Chu, W.; He, S.; Liang, X.; Zhang, J. Genome-Wide DNA Methylation Profiles Provide Insight into Epigenetic Regulation of Red and White Muscle Development in Chinese Perch Siniperca chuatsi. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110647. [Google Scholar]
- Liu, Q.; Liu, X.; Wang, G.; Wu, F.; Hou, Y.; Liu, H. Genome-Wide DNA Methylation Analysis of Astragalus and Danshen on the Intervention of Myofibroblast Activation in Idiopathic Pulmonary Fibrosis. BMC Pulm. Med. 2023, 23, 325. [Google Scholar] [CrossRef]
- Fan, Y.; Liang, Y.; Deng, K.; Zhang, Z.; Zhang, G.; Zhang, Y.; Wang, F. Analysis of DNA Methylation Profiles during Sheep Skeletal Muscle Development Using Whole-Genome Bisulfite Sequencing. BMC Genom. 2020, 21, 327. [Google Scholar]
- Begue, G.; Raue, U.; Jemiolo, B.; Trappe, S. DNA Methylation Assessment from Human Slow-and Fast-Twitch Skeletal Muscle Fibers. J. Appl. Physiol. 2017, 122, 952–967. [Google Scholar]
- Vogt, G. Studying Phenotypic Variation and DNA Methylation across Development, Ecology and Evolution in the Clonal Marbled Crayfish: A Paradigm for Investigating Epigenotype-Phenotype Relationships in Macro-Invertebrates. Sci. Nat. 2022, 109, 16. [Google Scholar]
- Boopathy, L.R.A.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms Tailoring the Expression of Heat Shock Proteins to Proteostasis Challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar]
- Jacob, P.; Hirt, H.; Bendahmane, A. The Heat-shock Protein/Chaperone Network and Multiple Stress Resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [PubMed]
- Kohno, S.; Katsu, Y.; Urushitani, H.; Ohta, Y.; Iguchi, T.; Guillette, L.J., Jr. Potential Contributions of Heat Shock Proteins to Temperature-Dependent Sex Determination in the American Alligator. Sex. Dev. 2010, 4, 73–87. [Google Scholar]
- Uchimura, T.; Hara, S.; Yazawa, T.; Kamei, Y.; Kitano, T. Involvement of Heat Shock Proteins on the Transcriptional Regulation of Corticotropin-Releasing Hormone in Medaka. Front. Endocrinol. 2019, 10, 529. [Google Scholar]
- Wang, C.; Chen, X.; Dai, Y.; Zhang, Y.; Sun, Y.; Cui, X. Comparative Transcriptome Analysis of Heat-Induced Domesticated Zebrafish during Gonadal Differentiation. BMC Genom. Data 2022, 23, 39. [Google Scholar]
- Liu, Q.; Wang, Y.; Tan, L.; Ma, W.; Zhao, X.; Shao, C.; Wang, Q. The Role of the Heat Shock Cognate Protein 70 Genes in Sex Determination and Differentiation of Chinese Tongue Sole (Cynoglossus semilaevis). Int. J. Mol. Sci. 2023, 24, 3761. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Feng, B.; Zhang, Z.; Wang, R.; Tang, L.; Li, W.; Li, Q.; Piferrer, F.; Shao, C. Transcriptome of Gonads From High Temperature Induced Sex Reversal During Sex Determination and Differentiation in Chinese Tongue Sole, Cynoglossus semilaevis. Front. Genet. 2019, 10, 1128. [Google Scholar] [CrossRef]
- Knee, D.A.; Froesch, B.A.; Nuber, U.; Takayama, S.; Reed, J.C. Structure-Function Analysis of Bag1 Proteins: Effects on Androgen Receptor Transcriptional Activity. J. Biol. Chem. 2001, 276, 12718–12724. [Google Scholar]
- Knorr, E.; Vilcinskas, A. Post-Embryonic Functions of HSP90 in Tribolium castaneum Include the Regulation of Compound Eye Development. Dev. Genes Evol. 2011, 221, 357–362. [Google Scholar]
- Khan, M.M.; Ashiqul Islam, S.M.; Mustafa, A. Identification of Stress Related Molecular Biomarkers in Zebrafish Employing an In-Silico Approach to Access Toxicity Based Risks in Aquaculture. Poult. Fish. Wildl. Sci. 2015, 3, 1000137. [Google Scholar]
- Quinn, N.L.; McGowan, C.R.; Cooper, G.A.; Koop, B.F.; Davidson, W.S. Ribosomal Genes and Heat Shock Proteins as Putative Markers for Chronic, Sublethal Heat Stress in Arctic Charr: Applications for Aquaculture and Wild Fish. Physiol. Genom. 2011, 43, 1056–1064. [Google Scholar]
- Vieira, J.C.S.; Braga, C.P.; de Oliveira, G.; Padilha, C. do C.F.; de Moraes, P.M.; Zara, L.F.; Leite, A. de L.; Buzalaf, M.A.R.; Padilha, P. de M. Mercury Exposure: Protein Biomarkers of Mercury Exposure in Jaraqui Fish from the Amazon Region. Biol. Trace Elem. Res. 2018, 183, 164–171. [Google Scholar]
- Yan, J.; Yang, Y.; Fan, X.; Tang, Y.; Tang, Z. Sp1-Mediated circRNA circHipk2 Regulates Myogenesis by Targeting Ribosomal Protein Rpl7. Genes 2021, 12, 696. [Google Scholar] [CrossRef]
- Martínez-Guitarte, J.L.; Planelló, R.; Morcillo, G. Characterization and Expression during Development and under Environmental Stress of the Genes Encoding Ribosomal Proteins L11 and L13 in Chironomus riparius. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 590–596. [Google Scholar] [CrossRef]
- de la Cruz, J.; Karbstein, K.; Woolford, J.L., Jr. Functions of Ribosomal Proteins in Assembly of Eukaryotic Ribosomes in Vivo. Annu. Rev. Biochem. 2015, 84, 93–129. [Google Scholar]
- Zhou, X.; Liao, W.-J.; Liao, J.-M.; Liao, P.; Lu, H. Ribosomal Proteins: Functions beyond the Ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [PubMed]
- Hu, W.; Zhu, Q.-L.; Zheng, J.-L.; Wen, Z.-Y. Cadmium Induced Oxidative Stress, Endoplasmic Reticulum (ER) Stress and Apoptosis with Compensative Responses towards the up-Regulation of Ribosome, Protein Processing in the ER, and Protein Export Pathways in the Liver of Zebrafish. Aquat. Toxicol. 2022, 242, 106023. [Google Scholar]
- Wang, J.; Liu, Q.; Zhang, X.; Gao, G.; Niu, M.; Wang, H.; Chen, L.; Wang, C.; Mu, C.; Wang, F. Metabolic Response in the Gill of Portunus Trituberculatus Under Short-Term Low Salinity Stress Based on GC-MS Technique. Front. Mar. Sci. 2022, 9, 881016. [Google Scholar]
- Jiang, Y.; Liu, X.; Shang, Y.; Li, J.; Gao, B.; Ren, Y.; Meng, X. Physiological and Transcriptomic Analyses Provide Insights into Nitrite Stress Responses of the Swimming Crab Portunus trituberculatus. Mar. Biotechnol. 2024, 26, 1040–1052. [Google Scholar]
- Zhang, X.; Yuan, J.; Zhang, X.; Yu, Y.; Li, F. Comparative Transcriptomic Analysis Unveils a Network of Energy Reallocation in Litopenaeus vannamei Responsive to Heat-Stress. Ecotoxicol. Environ. Saf. 2022, 238, 113600. [Google Scholar] [PubMed]
- Zhao, T.; Ma, A.; Huang, Z.; Liu, Z.; Sun, Z.; Zhu, C.; Yang, J.; Li, Y.; Wang, Q.; Qiao, X.; et al. Transcriptome Analysis Reveals That High Temperatures Alter Modes of Lipid Metabolism in Juvenile Turbot (Scophthalmus maximus) Liver. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100887. [Google Scholar]
- Li, Q.-Q.; Zhang, J.; Wang, H.-Y.; Niu, S.-F.; Wu, R.-X.; Tang, B.-G.; Wang, Q.-H.; Liang, Z.-B.; Liang, Y.-S. Transcriptomic Response of the Liver Tissue in Trachinotus ovatus to Acute Heat Stress. Animals 2023, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shen, J.; Perrimon, N.; Kong, X.; Wang, D. The Endoribonuclease Arlr Is Required to Maintain Lipid Homeostasis by Downregulating Lipolytic Genes during Aging. Nat. Commun. 2023, 14, 6254. [Google Scholar]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar]
- Krueger, F.; Andrews, S.R. Bismark: A Flexible Aligner and Methylation Caller for Bisulfite-Seq Applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup the Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Song, Q.; Decato, B.; Hong, E.E.; Zhou, M.; Fang, F.; Qu, J.; Garvin, T.; Kessler, M.; Zhou, J.; Smith, A.D. A Reference Methylome Database and Analysis Pipeline to Facilitate Integrative and Comparative Epigenomics. PLoS ONE 2013, 8, e81148. [Google Scholar]
- Akalin, A.; Kormaksson, M.; Li, S.; Garrett-Bakelman, F.E.; Figueroa, M.E.; Melnick, A.; Mason, C.E. methylKit: A Comprehensive R Package for the Analysis of Genome-Wide DNA Methylation Profiles. Genome Biol. 2012, 13, R87. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Sample | Raw Data | Valid Data | Valid% | Q20% | Q30% | GC% | ||
|---|---|---|---|---|---|---|---|---|
| Read | Base | Read | Base | |||||
| fm | 385,311,926 | 57.80 G | 374,807,550 | 46.28 G | 97.27 | 97.19 | 93.44 | 22.31 |
| mm | 365,349,640 | 54.80 G | 351,897,242 | 44.02 G | 96.32 | 97.09 | 93.21 | 21.93 |
| cm | 330,057,373 | 49.51 G | 321,911,061 | 38.65 G | 97.54 | 97.24 | 93.60 | 22.13 |
| Sample | Total Reads | Mapped Reads | Mapping Rate (%) | EM Conversion Rate (%) | Total mC (%) |
|---|---|---|---|---|---|
| fm | 374,807,550 | 171,443,461 | 45.74 | 97.13 | 4.5 |
| mm | 351,897,242 | 161,322,433 | 45.84 | 99.06 | 2.6 |
| cm | 321,911,061 | 139,530,777 | 43.33 | 98.35 | 3.5 |
| Gene | Nucleotide Sequence (From 5′ to 3′) |
|---|---|
| Hsc70 | inner-F: GTTATTTAGAGAGATAAAGATYGAGAG |
| inner-R: CATTCAAAAAAACAACAATTCT | |
| outer-F: TTTTGAGGTTGTTTTGTGTAGG | |
| outer-R: CCAAACACACACATTTAACAAAC | |
| Hsp90 | inner-F: TGTTTTTTAGTTTGTGGTTTGT |
| inner-R: AAAAATTTCCTCAAAATCAAAT | |
| outer-F: AGAYGTTTTTATTTTTATTTTAGAGGGG | |
| outer-R: CCCTAAAAAAACAATAAACATCCAAAAA | |
| Rpl7 | F: ATTGAAGGTTTGTAAATTGGAGT |
| R: TTATAAAAAAAAACACATCCCAA | |
| Yip2 | F: TAGTGTATATTTGAAGGTGTTGAA |
| R: AATAATAATAACCCAAACAAACAC |
| Gene | Nucleotide Sequence (From 5′ to 3′) |
|---|---|
| Hsc70 | F: CGAGACCAAGTCGTTCTACCC |
| R: GCAGCACATTGAGACCAGAGA | |
| Hsp90 | F: TCGCAGTTCATTGGCTATCC |
| R: CCTCAATCTTGGGCTTCTCAT | |
| Rpl18 | F: GAACCCAAGTCGCAGGATG |
| R: CACCAGCCCAGAGAGTGAGAT | |
| Acsl1 | F: TCCGTAACCTTGTGGATGACTA |
| R: TTCTCCTTATCACACCCCTTTC | |
| Rpl7 | F: GCTCCGTTATTGTCCCTGC |
| R: GTAGCCCCAGGCAATGAAG | |
| Yip2 | F: CTGTCAGGAACATTCGCTTTG |
| R: CTTCATTCTGGGCGGTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.-J.; Shao, S.-C.; Ni, M.-Q.; Yang, Y.-N.; Cui, Z.-X. DNA Methylation Patterns Provide Insights into the Epigenetic Regulation of Intersex Formation in the Chinese Mitten Crab (Eriocheir sinensis). Int. J. Mol. Sci. 2025, 26, 3224. https://doi.org/10.3390/ijms26073224
Fang S-J, Shao S-C, Ni M-Q, Yang Y-N, Cui Z-X. DNA Methylation Patterns Provide Insights into the Epigenetic Regulation of Intersex Formation in the Chinese Mitten Crab (Eriocheir sinensis). International Journal of Molecular Sciences. 2025; 26(7):3224. https://doi.org/10.3390/ijms26073224
Chicago/Turabian StyleFang, Shu-Jian, Shu-Cheng Shao, Meng-Qi Ni, Ya-Nan Yang, and Zhao-Xia Cui. 2025. "DNA Methylation Patterns Provide Insights into the Epigenetic Regulation of Intersex Formation in the Chinese Mitten Crab (Eriocheir sinensis)" International Journal of Molecular Sciences 26, no. 7: 3224. https://doi.org/10.3390/ijms26073224
APA StyleFang, S.-J., Shao, S.-C., Ni, M.-Q., Yang, Y.-N., & Cui, Z.-X. (2025). DNA Methylation Patterns Provide Insights into the Epigenetic Regulation of Intersex Formation in the Chinese Mitten Crab (Eriocheir sinensis). International Journal of Molecular Sciences, 26(7), 3224. https://doi.org/10.3390/ijms26073224




