Increased Expression of Superoxide Dismutase 2 Is an Indicator of Worse Prognosis of Oropharyngeal Cancer
Abstract
1. Introduction
2. Results
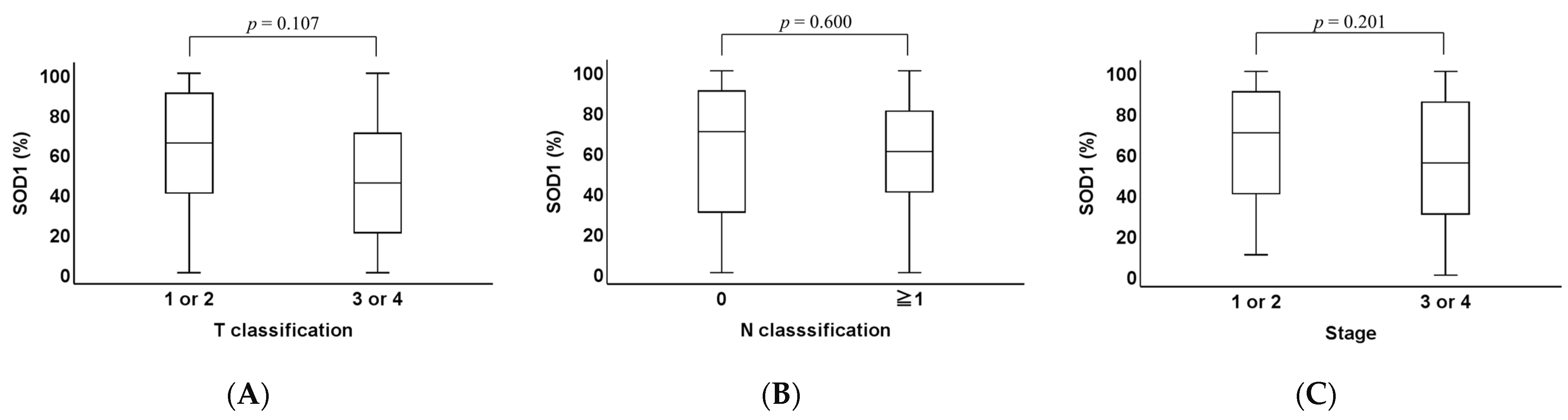
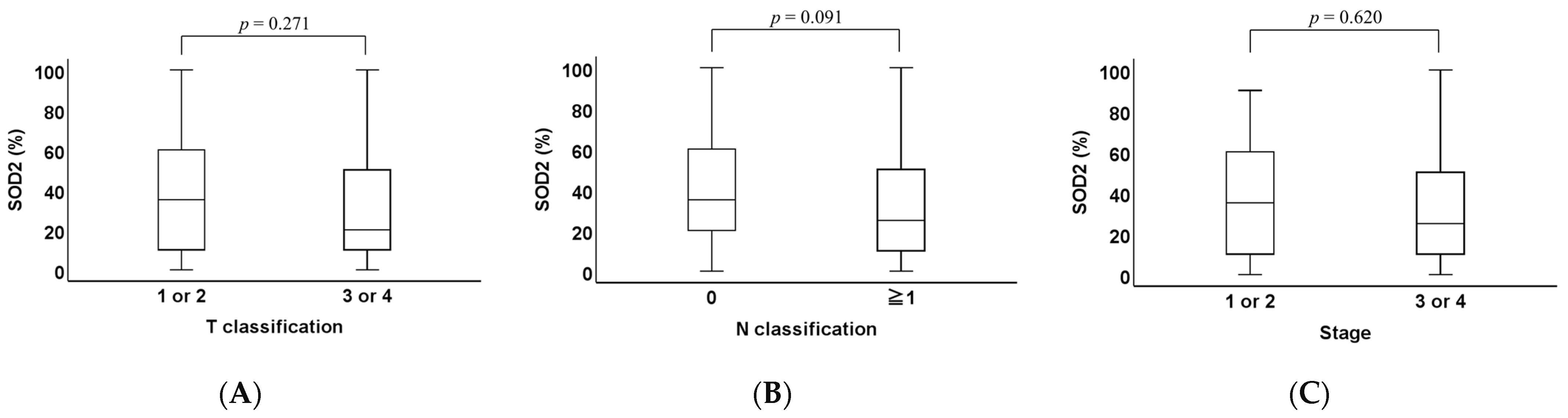
2.1. Clinicopathological Analysis of SOD1 and SOD2 Expression Levels in OPC Tissues
2.2. Immunohistochemical Analysis of SOD1 and SOD2 Expression Levels in OPC Tissues
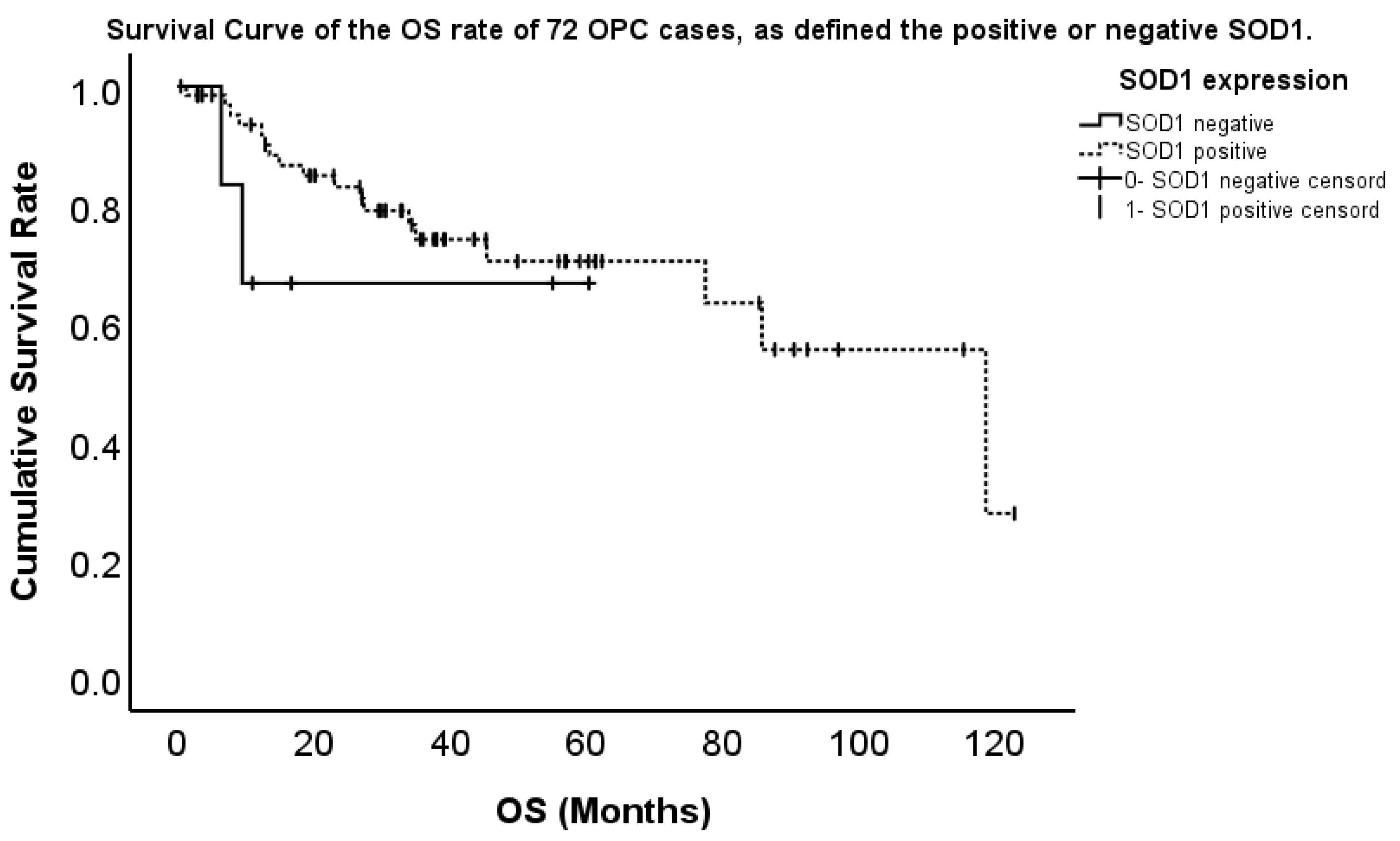
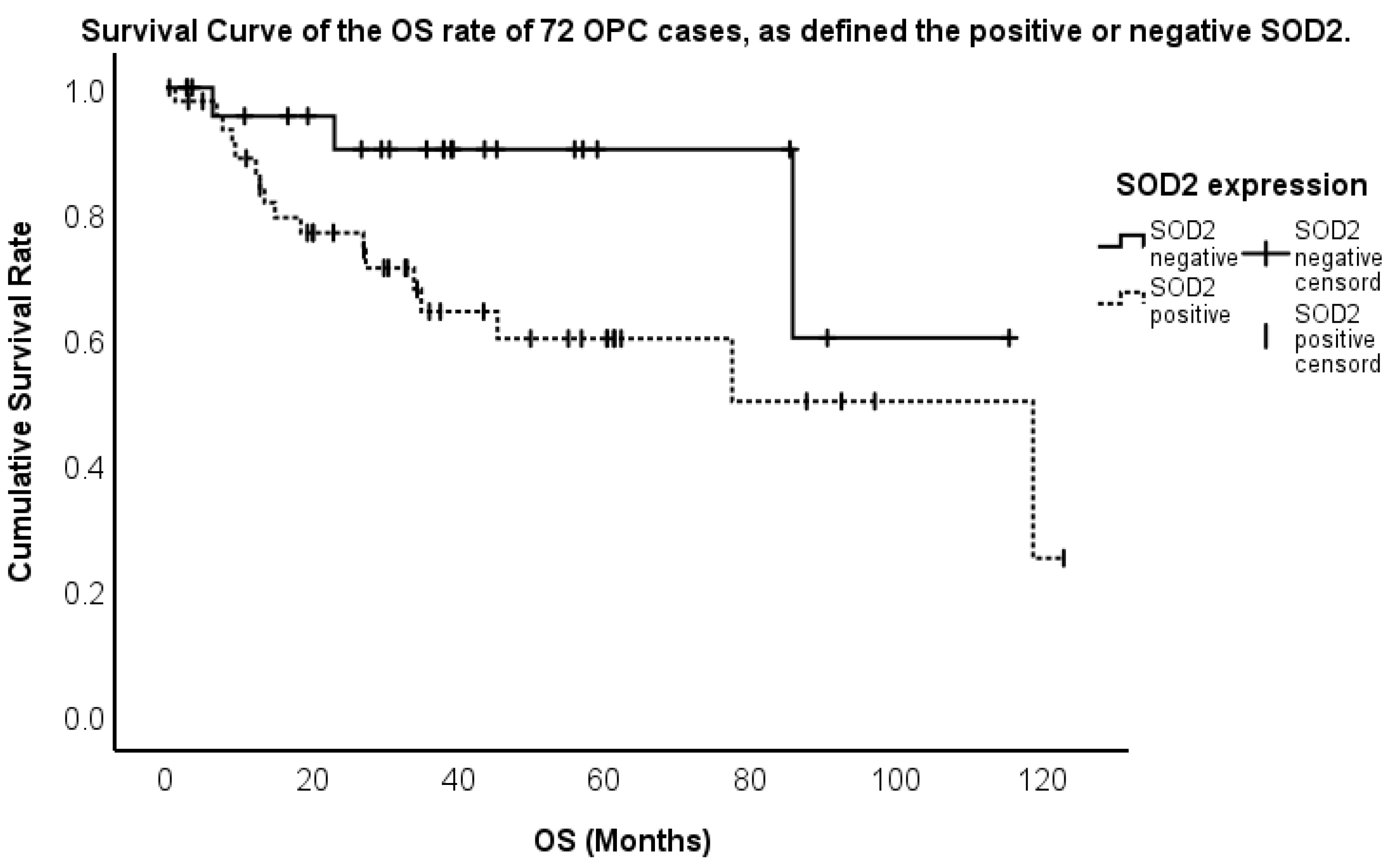
2.3. Association Between the Expression and Overall Survival (OS)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Evaluation of SOD1 and SOD2 Immunoreactivity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | human papillomavirus |
| OPC | oropharyngeal cancer |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| OS | overall survival |
| UICC | Union for International Cancer Control |
| TNM | tumor–node–metastasis |
| Cu/Zn-SOD | copper/zinc superoxide dismutase |
| Mn-SOD | manganese superoxide dismutase |
| HR | hazard ratio |
| CI | confidence interval |
| HK-2 | hexokinase 2 |
| HOXA13 | homeobox A13 |
| D-loop | displacement loop |
| PBS | phosphate-buffered saline |
References
- Murono, S.; Yoshida, H.; Kobayashi, T.; Kawase, T.; Kikuchi, D.; Suzuki, T.; Nakanishi, Y.; Endo, K.; Kondo, S.; Wakisaka, N.; et al. Multifocal human papillomavirus detection in palatine and pharyngeal tonsils. Acta Otolaryngol. 2018, 138, 483–486. [Google Scholar]
- Yoshida, H.; Murono, S.; Ueno, T.; Nakanishi, Y.; Tsuji, A.; Hatano, M.; Endo, K.; Kondo, S.; Sugimoto, H.; Wakisaka, N.; et al. Usefulness of human papillomavirus detection in oral rinse as a biomarker of oropharyngeal cancer. Acta Otolaryngol. 2017, 137, 773–777. [Google Scholar] [PubMed]
- Ang, K.K.; Harris, J.; Weeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Gillison, M.L.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Xu, W.; et al. Development and validation of staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicenter cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar]
- Horne, Z.D.; Glaser, S.M.; Vargo, J.A.; Ferris, R.L.; Balasubramani, G.K.; Clump, D.A.; Heron, D.E.; Beriwal, S. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer 2016, 122, 2021–2030. [Google Scholar] [PubMed]
- Idelchik, M.D.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar]
- Maynard, S.; Schurman, S.H.; Harboe, C.; Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar]
- Xu, Q.; Xiaobo, N.; Wenhao, Y.; Konrad, K.; Holger, S.; Andreas, M.K.; Andreas, E.A. Reactive oxygen species in cancer stem cells of head and neck squamous cancer. Semin. Cancer Biol. 2018, 53, 248–257. [Google Scholar]
- Perry, J.P.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar]
- Liou, W.; Chang, L.Y.; Geuze, H.J.; Strous, G.J.; Crapo, J.D.; Slot, J.W. Distribution of CuZn superoxide dismutase in rat liver. Free Radic. Biol. Med. 1993, 14, 201–207. [Google Scholar]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar]
- Larissa, A.C.C.; Rodrigo, G.Q.; Alexandre, L.B.B.; Adamo, D.D.S.; Wilson, A.S.J.; Tiago, R.; Silvya, S.M.E. Redox-Related Proteins in Melanoma Progression. Antioxidants 2022, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Sapio, R.; Yang, J.; Wong, J.; Zhang, X.; Guo, J.Y.; Pine, S.; Remmen, H.V.; Zheng, X.F.S.; et al. SOD1 regulates ribosome biogenesis in KRAS mutant non-small cell lung cancer. Nat. Commun. 2021, 12, 2259. [Google Scholar] [PubMed]
- Luena, P.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 16–21. [Google Scholar]
- Wang, R.; Yin, C.; Li, X.X.; Yang, X.Z.; Yang, Y.; Zhang, M.Y.; Wang, H.Y.; Zheng, X.F.S. Reduced SOD2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging 2016, 8, 6. [Google Scholar]
- Bastos, D.R.; Longatto-Filho, A.; Conceição, M.P.F.; Termini, L. High levels of superoxide dismutase 2 are associated with worse prognosis in patients with breast cancer. Eur. J. Breast Health 2024, 20, 185–193. [Google Scholar]
- Rabelo-Santos, S.H.; Termini, L.; Boccardo, E.; Derchain, S.; Longatto-Filho, A.; Andreoli, M.A.; Costa, M.C.; Nunes, R.A.L.; Ângelo-Andrade, L.A.L.; Zeferino, L.C.; et al. Strong SOD2 expression and HPV-16/18 positivity are independent events in cervical cancer. Oncotarget 2018, 9, 21630–21640. [Google Scholar]
- Diego, C.B.; Julio, C.O.; Rancés, B.; Carolina, O.; Enrique, B.; Francisco, A. Interaction between cigarette smoke and human papillomavirus 16 E6/E7 oncoproteins to induce SOD2 expression and DNA damage in head and neck cancer. Int. J. Mol. Sci. 2023, 24, 6907. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Coronado-Martínez, I.; Pedraza-Chaverri, J.; Lizano, M. E6 Oncoproteins from high-risk human papillomavirus induce mitochondrial metabolism in a head and neck squamous cell carcinoma model. Biomolecules 2019, 9, 351. [Google Scholar] [CrossRef]
- Chang, B.; Yang, H.; Jiao, Y.; Wang, K.; Liu, Z.; Wu, P.; Li, S.; Wang, A. SOD2 deregulation enhances migration, invasion and has poor prognosis in salivary adenoid cystic carcinoma. Sci. Rep. 2016, 6, 25918. [Google Scholar]
- Church, S.L.; Grant, J.W.; Ridnour, L.A.; Oberley, L.W.; Swanson, P.E.; Meltzer, P.S.; Trent, J.M. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc. Natl. Acad. Sci. USA 1993, 90, 3113–3117. [Google Scholar] [PubMed]
- Liu, R.; Oberley, T.D.; Oberley, L.W. Transfection and expression of MnSOD cDNA decreases tumor malignancy of human oral squamous carcinoma SCC-25 cells. Hum. Gene Ther. 1997, 8, 585–595. [Google Scholar] [CrossRef]
- Ough, M.; Lewis, A.; Zhang, Y.; Hinkhouse, M.M.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic. Res. 2004, 38, 1223–1233. [Google Scholar] [CrossRef]
- Janssen, A.M.; Bosman, C.B.; Duijn, W.; Oostendorp-van de Ruit, M.M.; Kubben, F.J.; Griffioen, G.; Lamers, C.B.; Krieken, J.H.; Velde, C.J.; Verspaget, H.W. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin. Cancer Res. 2000, 6, 3183–3192. [Google Scholar]
- Kattan, Z.; Minig, V.; Leroy, P.; Dauça, M.; Becuwe, P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res. Treat. 2008, 108, 203–215. [Google Scholar] [PubMed]
- Ma, R.L.; Shen, L.Y.; Chen, K.N. Coexpression of ANXA2, SOD2 and HOXA13 predicts poor prognosis of esophageal squamous cell carcinoma. Oncol. Rep. 2014, 31, 2157–2164. [Google Scholar] [PubMed]
- Chung-man Ho, J.; Zheng, S.; Comhair, S.A.; Farver, C.; Erzurum, S.C. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001, 61, 8578–8585. [Google Scholar]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef]
- Svensk, A.M.; Soini, Y.; Pääkkö, P.; Hirvikoski, P.; Kinnula, V.L. Differential expression of superoxide dismutases in lung cancer. Am. J. Clin. Pathol. 2004, 122, 395–404. [Google Scholar]
- Termini, L.; Fregnani, J.H.; Boccardo, E.; Costa, W.H.; Longatto-Filho, A.; Andreoli, M.A.; Costa, M.C.; Lopes, A.; Cunha, I.W.; Guimarães, G.C.; et al. SOD2 immunoexpression predicts lymph node metastasis in penile cancer. BMC Clin. Pathol. 2015, 15, 3. [Google Scholar]
- Noh, J.K.; Woo, S.R.; Yun, M.; Lee, M.K.; Kong, M.; Min, S.; Kim, S.I.; Lee, Y.C.; Eun, Y.G.; Ko, S.G. SOD2- and NRF2-associated gene signature to predict radioresistance in head and neck cancer. Cancer Genom. Proteom. 2021, 18, 675–684. [Google Scholar] [CrossRef]
- Kuninaka, S.; Ichinose, Y.; Koja, K.; Toh, Y. Suppression of manganese superoxide dismutase augments sensitivity to radiation, hyperthermia and doxorubicin in colon cancer cell lines by inducing apoptosis. Br. J. Cancer 2000, 83, 928–934. [Google Scholar] [CrossRef]
- Juliachs, M.; Pujals, M.; Bellio, C.; Meo-Evoli, N.; Duran, J.M.; Zamora, E.Z.; Parés, M.; Suñol, A.; Méndez, O.; Villanueva, J.; et al. Circulating SOD2 is a candidate response biomarker for neoadjuvant therapy in breast cancer. Cancers 2022, 14, 3858. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Zhao, L.; Sun, J.; He, Q.; Yan, W.; Lu, Z.; Wang, A. Hexokinase 2 enhances the metastatic potential of tongue squamous cell carcinoma via the SOD2-H2O2 pathway. Oncotarget 2017, 8, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.M.; Kim, J.H.; Park, I.S.; Rho, Y.S. Heavy alcohol drinking downregulates ALDH2 gene expression but heavy smoking up-regulates SOD2 gene expression in head and neck squamous cell carcinoma. World J. Surg. Oncol. 2017, 15, 163. [Google Scholar] [CrossRef]
- Kleist, B.; Meurer, T.; Poetsch, M. Mitochondrial DNA alteration in primary and metastatic colorectal cancer: Different frequency and association with selected clinicopathological and molecular markers. Tumor Biol. 2017, 3, 1010428317692246. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, W.; Yin, Z.; Guan, P.; Zhou, B. MDM2 SNP309, gene-gene interaction, and tumor susceptibility: An updated meta-analysis. BMC Cancer 2011, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Omae, Y.; Hitomi, Y.; Park, B.; Shin, H.J.; Yoon, K.A.; Sawai, H.; Tsuji, M.; Hayashi, T.; Kim, J.Y.; et al. The association of integration patterns of human papilloma virus and single nucleotide polymorphisms on immune- or DNA repair-related genes in cervical cancer patients. Sci. Rep. 2019, 9, 13132. [Google Scholar] [CrossRef]
- Li, K.; Liu, T.; Chen, J.; Ni, H.; Li, W. Survivin in breast cancer-derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 2020, 295, 13737–13752. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front. Cell Dev. Biol. 2020, 23, 213. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Ma, H.; Yin, M.; Yang, X.; Han, R.; Ohara, S.; Kim, D.; Wang, G. Bioinformatics analysis reveals SOD1 is a prognostic factor in lung adenocarcinoma. Transl. Cancer Res. 2024, 13, 5522–5534. [Google Scholar] [PubMed]
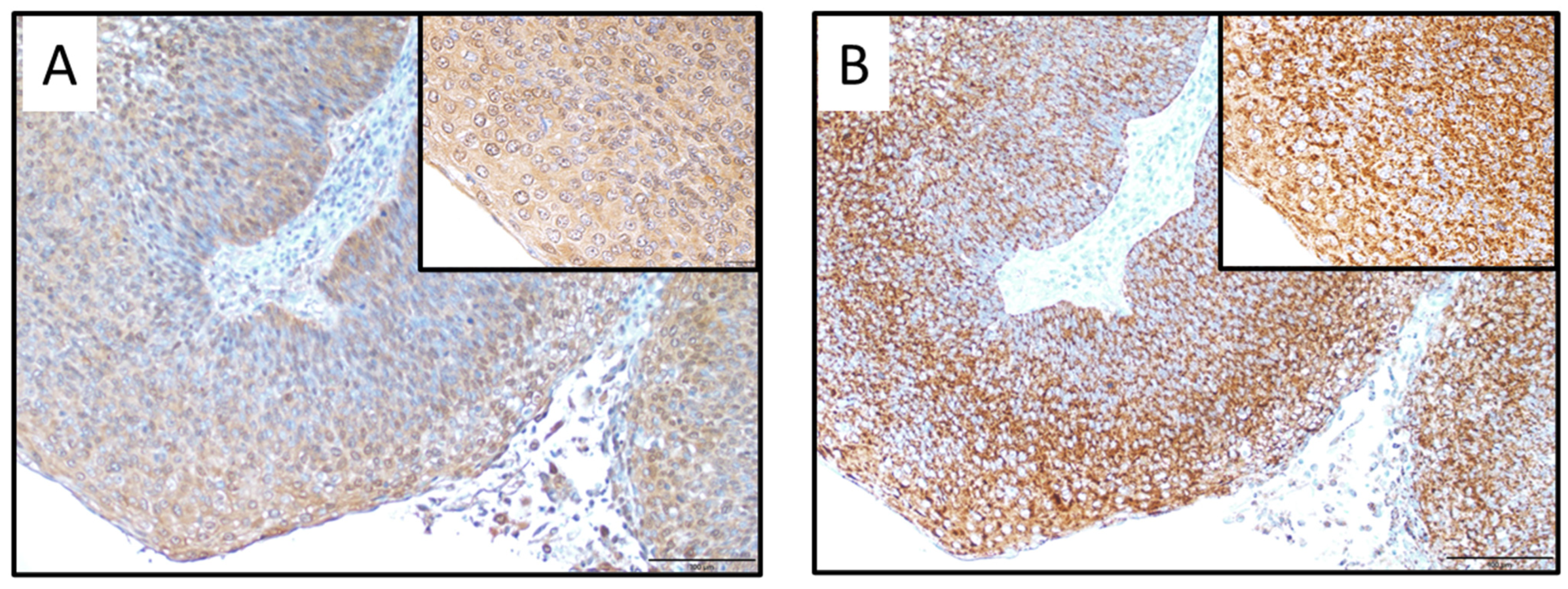
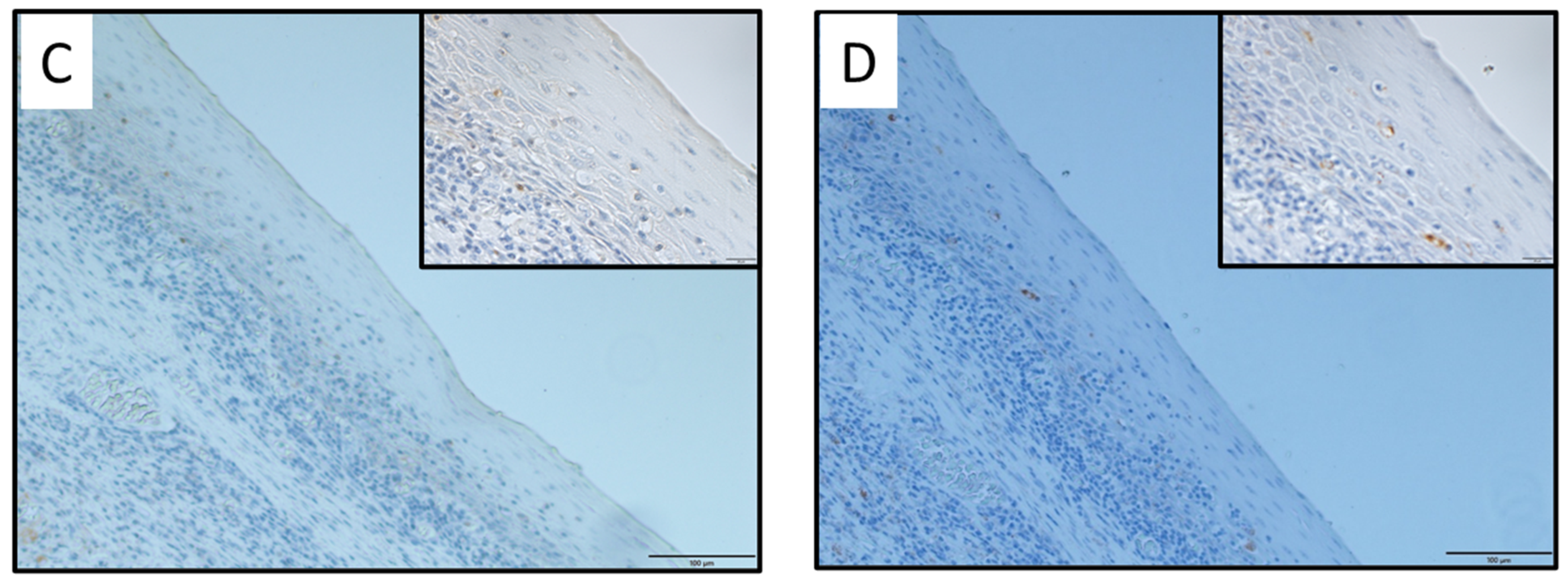
| Characteristics | Total | SOD1-Positive | SOD1-Negative | p Value |
|---|---|---|---|---|
| Number of cases | 72 | 66 | 6 | |
| Sex | 0.837 | |||
| Men | 62 | 57 | 5 | |
| Women | 10 | 9 | 1 | |
| Age | 0.431 | |||
| Mean | 63.5 | 64.9 | 52.8 | |
| Range | 33–89 | 43–89 | 33–74 | |
| Medium | 64 | 65 | 54 | |
| T categories | 1.000 | |||
| T1–2 | 48 | 44 | 4 | |
| T3–4 | 24 | 22 | 2 | |
| N categories | 0.459 | |||
| N0 | 26 | 23 | 3 | |
| N1–3 | 46 | 43 | 3 | |
| Stage | 0.560 | |||
| 1–2 | 44 | 41 | 3 | |
| 3–4 | 28 | 25 | 3 | |
| Therapy | 0.882 | |||
| Surgery | 46 | 42 | 4 | |
| Chemoradiotherapy | 26 | 24 | 2 | |
| HPV | 0.104 | |||
| p16-positive | 46 | 44 | 2 | |
| p16-negative | 26 | 22 | 4 | |
| Smoking | 0.793 | |||
| Yes | 57 | 52 | 5 | |
| Never | 15 | 14 | 1 | |
| Alcohol | 1.000 | |||
| Yes | 60 | 55 | 5 | |
| Never | 12 | 11 | 1 |
| Characteristics | Total | SOD2-Positive | SOD2-Negative | p Value |
|---|---|---|---|---|
| Number of cases | 72 | 47 | 25 | |
| Sex | 0.274 | |||
| Men | 62 | 42 | 20 | |
| Women | 10 | 5 | 5 | |
| Age | 0.976 | |||
| Mean | 63.5 | 62.7 | 65.7 | |
| Range | 33–89 | 33–89 | 53–81 | |
| Medium | 64 | 63 | 66 | |
| T categories | 0.161 | |||
| T1–2 | 48 | 34 | 14 | |
| T3–4 | 24 | 13 | 11 | |
| N categories | 0.119 | |||
| N0 | 26 | 20 | 6 | |
| N1–3 | 46 | 27 | 19 | |
| Stage | 0.516 | |||
| 1–2 | 44 | 30 | 14 | |
| 3–4 | 28 | 17 | 11 | |
| Therapy | 0.002 | |||
| Surgery | 46 | 36 | 10 | |
| Chemoradiotherapy | 26 | 11 | 15 | |
| HPV | 0.395 | |||
| p16-positive | 46 | 36 | 10 | |
| p16-negative | 26 | 18 | 8 | |
| Smoking | 0.899 | |||
| Yes | 57 | 37 | 20 | |
| Never | 15 | 10 | 5 | |
| Alcohol | 0.580 | |||
| Yes | 60 | 40 | 20 | |
| Never | 12 | 7 | 5 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variates | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Sex | 0.847 (0.091–7.894) | 0.884 | NI | |
| Age (>60) | 1.576 (0.332–7.480) | 0.567 | NI | |
| Subsite (tonsil vs. other subsites) | 0.382 (0.103–1.412) | 0.149 | NI | |
| T stage (T1, 2 vs. T3, 4) | 1.036 (0.663–1.617) | 0.877 | NI | |
| N stage (N0 vs. N1,2,3) | 0.819 (0.221–3.039) | 0.765 | NI | |
| Stage (I/II vs. III/IV) | 1.669 (1.032–2.699) | 0.037 | 1.826 (1.298–2.569) | <0.001 |
| Surgery or Chemoradiotherapy | 2.081 (0.476–9.094) | 0.330 | NI | |
| p16-positive/negative | 0.943 (0.278–3.201) | 0.925 | NI | |
| SOD1-positive/negative | 0.908 (0.142–5.802) | 0.918 | NI | |
| SOD2-positive/negative | 8.422 (1.236–57.832) | 0.030 | 3.915 (1.210–15.699) | 0.024 |
| Smoking | 1.644 (0.327–8.269) | 0.546 | NI | |
| Alcohol | 0.253 (0.043–1.490) | 0.129 | NI | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, Y.; Kondo, S.; Kobayashi, E.; Moriyama-Kita, M.; Dochi, H.; Komura, S.; Nakanishi, Y.; Endo, K.; Wakisaka, N.; Yoshizaki, T. Increased Expression of Superoxide Dismutase 2 Is an Indicator of Worse Prognosis of Oropharyngeal Cancer. Int. J. Mol. Sci. 2025, 26, 3223. https://doi.org/10.3390/ijms26073223
Aoki Y, Kondo S, Kobayashi E, Moriyama-Kita M, Dochi H, Komura S, Nakanishi Y, Endo K, Wakisaka N, Yoshizaki T. Increased Expression of Superoxide Dismutase 2 Is an Indicator of Worse Prognosis of Oropharyngeal Cancer. International Journal of Molecular Sciences. 2025; 26(7):3223. https://doi.org/10.3390/ijms26073223
Chicago/Turabian StyleAoki, Yoshitaka, Satoru Kondo, Eiji Kobayashi, Makiko Moriyama-Kita, Hirotomo Dochi, Shigetaka Komura, Yosuke Nakanishi, Kazuhira Endo, Naohiro Wakisaka, and Tomokazu Yoshizaki. 2025. "Increased Expression of Superoxide Dismutase 2 Is an Indicator of Worse Prognosis of Oropharyngeal Cancer" International Journal of Molecular Sciences 26, no. 7: 3223. https://doi.org/10.3390/ijms26073223
APA StyleAoki, Y., Kondo, S., Kobayashi, E., Moriyama-Kita, M., Dochi, H., Komura, S., Nakanishi, Y., Endo, K., Wakisaka, N., & Yoshizaki, T. (2025). Increased Expression of Superoxide Dismutase 2 Is an Indicator of Worse Prognosis of Oropharyngeal Cancer. International Journal of Molecular Sciences, 26(7), 3223. https://doi.org/10.3390/ijms26073223





