The Significance of Nectin Family Proteins in Various Cancerogenous Processes
Abstract
1. Introduction
1.1. Nectins Family
1.2. Structure
1.3. Mechanism of Action
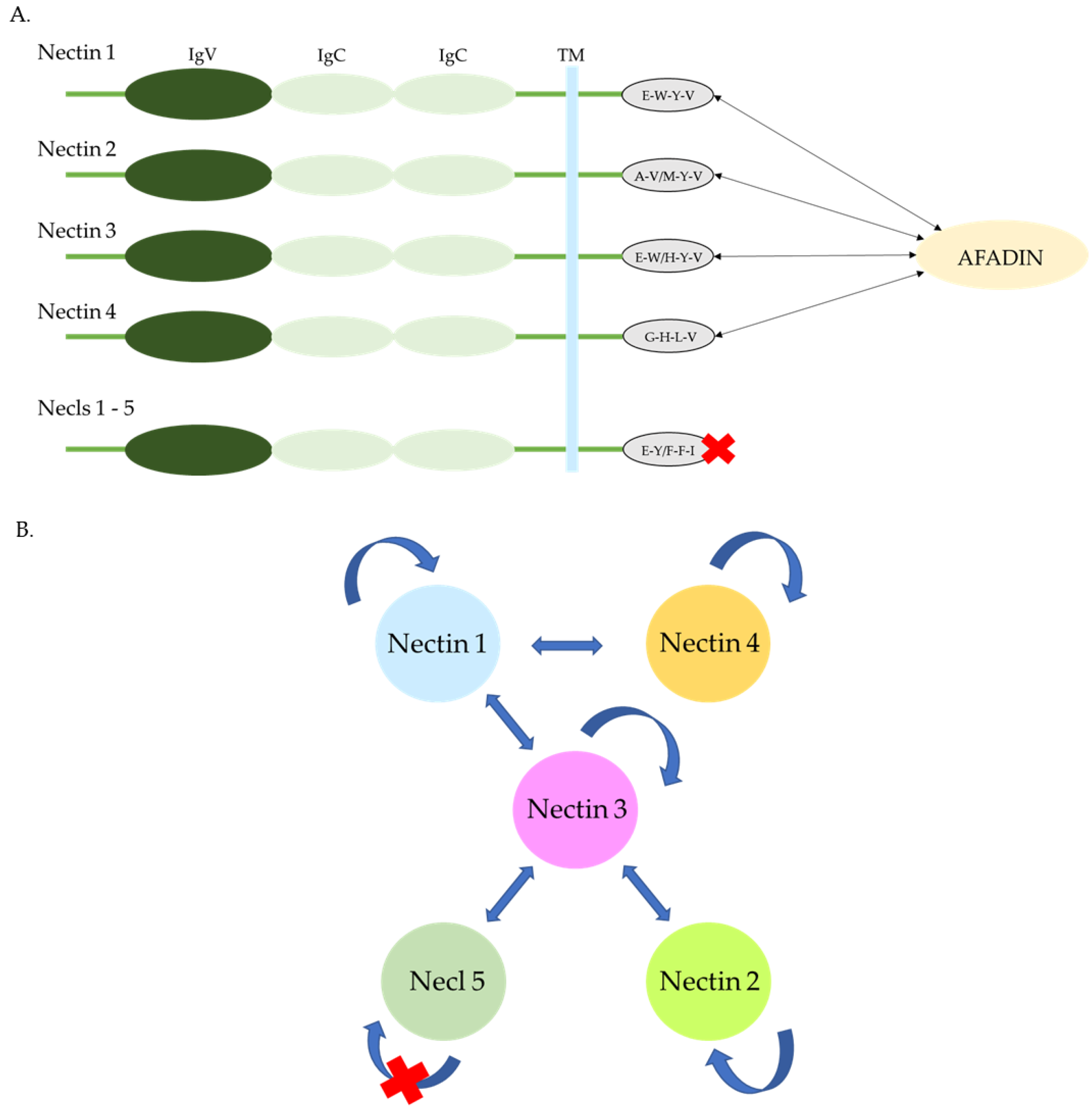
1.3.1. Interactions of Extracellular Regions
Nectins
Nectine-Like Proteins (Necls)
1.3.2. Interactions of Cytoplasmic Regions
1.4. Function
2. The Role of Nectins in Tumorigenesis
2.1. Nectin -1
2.2. Nectin-2
2.3. Nectin-3
2.4. Nectin-4
2.4.1. High Expression
2.4.2. Positive Aspects of High Expression
2.4.3. Drug Interactions
2.5. Necl-5
Clinical Use of Necl-5
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| necl-5 | nectin-like protein 5. |
| CAMs | Cell adhesion molecules. |
| IgSF | Immunoglobulin superfamily. |
| AJs | Adherence junctions. |
| TJs | Tight junctions. |
| CAFs | Cancer-associated fibroblasts. |
| DFS | Disease-free survival. |
| DRFS | Distant recurrence-free survival. |
| LRFS | Local recurrence-free survival. |
| UC | Urothelial carcinoma. |
| PUC | Plasmacytoid urothelial carcinoma. |
| UCSD | Urothelial carcinoma with squamous differentiation. |
| SCC | Squamous cell carcinoma. |
| NEs | Neuroendocrine bladder tumors. |
| PVR | Poliovirus receptor. |
| AML | Acute myeloid leukemia. |
| R-ISS | Revised International Staging System. |
References
- Ogita, H.; Rikitake, Y.; Miyoshi, J.; Takai, Y. Cell adhesion molecules nectins and associating proteins: Implications for physiology and pathology. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 621–629. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Nectin and nectin-like molecules: Biology and pathology. Am. J. Nephrol. 2007, 27, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; van Beers, L.; Broux, B. Nectin Family Ligands Trigger Immune Effector Functions in Health and Autoimmunity. Biology 2023, 12, 452. [Google Scholar] [CrossRef]
- Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Nectins and Nectin-like Molecules in Colorectal Cancer: Role in Diagnostics, Prognostic Values, and Emerging Treatment Options: A Literature Review. Diagnostics 2022, 12, 3076. [Google Scholar] [CrossRef] [PubMed]
- Duraivelan, K.; Samanta, D. Emerging roles of the nectin family of cell adhesion molecules in tumour-associated pathways. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188589. [Google Scholar] [CrossRef]
- Martin, T.A.; Lane, J.; Harrison, G.M.; Jiang, W.G. The expression of the Nectin complex in human breast cancer and the role of Nectin-3 in the control of tight junctions during metastasis. PLoS ONE 2013, 8, e82696. [Google Scholar] [CrossRef]
- Izumi, H.; Hirabayashi, K.; Nakamura, N.; Nakagohri, T. Nectin expression in pancreatic adenocarcinoma: Nectin-3 is associated with a poor prognosis. Surg. Today 2015, 45, 487–494. [Google Scholar] [CrossRef]
- Yamada, M.; Hirabayashi, K.; Kawanishi, A.; Hadano, A.; Takanashi, Y.; Izumi, H.; Kawaguchi, Y.; Mine, T.; Nakamura, N.; Nakagohri, T. Nectin-1 expression in cancer-associated fibroblasts is a predictor of poor prognosis for pancreatic ductal adenocarcinoma. Surg. Today 2018, 48, 510–516. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Z.; Chen, H.; Yang, H.; Wang, Q.; Xing, T. High expression of nectin-1 indicates a poor prognosis and promotes metastasis in hepatocellular carcinoma. Front. Oncol. 2022, 12, 953529. [Google Scholar] [CrossRef]
- Tampakis, A.; Tampaki, E.C.; Nonni, A.; Droeser, R.; Posabella, A.; Tsourouflis, G.; Kontzoglou, K.; Patsouris, E.; von Flüe, M.; Kouraklis, G. Nectin-1 Expression in Colorectal Cancer: Is There a Group of Patients with High Risk for Early Disease Recurrence? Oncology 2019, 96, 318–325. [Google Scholar] [CrossRef]
- Guzman, G.; Oh, S.; Shukla, D.; Valyi-Nagy, T. Nectin-1 Expression in the Normal and Neoplastic Human Uterine Cervix. Arch. Pathol. Lab. Med. 2006, 130, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Miyata, M.; Shiotani, H.; Kameyama, T.; Takai, Y. Nectin-2 in general and in the brain. Mol. Cell Biochem. 2022, 477, 167–180. [Google Scholar] [CrossRef]
- Bekes, I.; Löb, S.; Holzheu, I.; Janni, W.; Baumann, L.; Wöckel, A.; Wulff, C. Nectin-2 in ovarian cancer: How is it expressed and what might be its functional role? Cancer Sci. 2019, 110, 1872–1882. [Google Scholar] [CrossRef]
- Miao, X.; Yang, Z.L.; Xiong, L.; Zou, Q.; Yuan, Y.; Li, J.; Liang, L.; Chen, M.; Chen, S. Nectin-2 and DDX3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Int. J. Clin. Exp. Pathol. 2013, 6, 179–190. [Google Scholar] [PubMed]
- Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study. Int. J. Mol. Sci. 2023, 24, 15900. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Miyamoto, T.; Shimizu, T.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Oda, Y.; Morizawa, Y.; Hori, S.; Gotoh, D.; et al. Tumor Expression of Nectin-1-4 and Its Clinical Implication in Muscle Invasive Bladder Cancer: An Intra-Patient Variability of Nectin-4 Expression. Pathol. Res. Pract. 2022, 237, 154072. [Google Scholar] [CrossRef]
- Li, M.; Qiao, D.; Pu, J.; Wang, W.; Zhu, W.; Liu, H. Elevated Nectin-2 Expression Is Involved in Esophageal Squamous Cell Carcinoma by Promoting Cell Migration and Invasion. Oncol. Lett. 2018, 15, 4731–4736. [Google Scholar] [CrossRef]
- Stamm, H.; Klingler, F.; Grossjohann, E.M.; Muschhammer, J.; Vettorazzi, E.; Heuser, M.; Mock, U.; Thol, F.; Vohwinkel, G.; Latuske, E.; et al. Immune Checkpoints PVR and PVRL2 Are Prognostic Markers in AML and Their Blockade Represents a New Therapeutic Option. Oncogene 2018, 37, 5269–5280. [Google Scholar] [CrossRef]
- Graf, M.; Reif, S.; Hecht, K.; Kroell, T.; Nuessler, V.; Schmetzer, H. Expression of Poliovirus Receptor-Related Proteins PRR1 and PRR2 in Acute Myeloid Leukemia: First Report of Surface Marker Analysis, Contribution to Diagnosis, Prognosis and Implications for Future Therapeutical Strategies. Eur. J. Haematol. 2005, 75, 477–484. [Google Scholar] [CrossRef]
- Mandai, K.; Rikitake, Y.; Mori, M.; Takai, Y. Nectins and Nectin-Like Molecules in Development and Disease. Curr. Top. Dev. Biol. 2015, 112, 197–231. [Google Scholar] [CrossRef]
- Xu, F.; Si, X.; Wang, J.; Yang, A.; Qin, T.; Yang, Y. Nectin-3 Is a New Biomarker That Mediates the Upregulation of MMP2 and MMP9 in Ovarian Cancer Cells. Biomed. Pharmacother. 2019, 110, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, Y.; Nishio, W.; Okita, Y.; Yoshimura, M. Expression of Nectin 3: Novel Prognostic Marker of Lung Adenocarcinoma. Thorac. Cancer 2012, 3, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.; Lau, J.F.; Dietrich, D.; Strieth, S.; Brossart, P.; Kristiansen, G. Nectin-4 Is Widely Expressed in Head and Neck Squamous Cell Carcinoma. Oncotarget 2022, 13, 1166–1173. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adélaïde, J.; Geneix, J.; et al. Nectin-4 Is a New Histological and Serological Tumor Associated Marker for Breast Cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Rajc, J.; Gugić, D.; Fröhlich, I.; Marjanović, K.; Dumenčić, B. Prognostic Role of Nectin-4 Expression in Luminal B (HER2 Negative) Breast Cancer. Pathol. Res. Pract. 2017, 213, 110. [Google Scholar] [CrossRef]
- Lattanzio, R.; Ghasemi, R.; Brancati, F.; Sorda, R.L.; Tinari, N.; Perracchio, L.; Iacobelli, S.; Mottolese, M.; Natali, P.G.; Piantelli, M. Membranous Nectin-4 Expression Is a Risk Factor for Distant Relapse of T1-T2, N0 Luminal-A Early Breast Cancer. Oncogenesis 2014, 3, e118. [Google Scholar] [CrossRef]
- Bekos, C.; Muqaku, B.; Dekan, S.; Horvat, R.; Polterauer, S.; Gerner, C.; Aust, S.; Pils, D. NECTIN4 (PVRL4) as Putative Therapeutic Target for a Specific Subtype of High Grade Serous Ovarian Cancer—An Integrative Multi-Omics Approach. Cancers 2019, 11, 698. [Google Scholar] [CrossRef]
- Ghali, F.; Vakar-Lopez, F.; Roudier, M.P.; Garcia, J.; Arora, S.; Cheng, H.H.; Schweizer, M.T.; Haffner, M.C.; Lee, J.K.; Yu, E.Y.; et al. Metastatic Bladder Cancer Expression and Subcellular Localization of Nectin-4 and Trop-2 in Variant Histology: A Rapid Autopsy Study. Clin. Genitourin. Cancer 2023, 21, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 Expression Contributes to Tumor Proliferation, Angiogenesis, and Patient Prognosis in Human Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef]
- Mayer, M.; Nachtsheim, L.; Prinz, J.; Shabli, S.; Suchan, M.; Klußmann, J.P.; Quaas, A.; Arolt, C.; Wolber, P. Nectin-4 Is Frequently Expressed in Primary Salivary Gland Cancer and Corresponding Lymph Node Metastases and Represents an Important Treatment-Related Biomarker. Clin. Exp. Metastasis 2023, 40, 395–405. [Google Scholar] [CrossRef]
- Deng, H.; Shi, H.; Chen, L.; Zhou, Y.; Jiang, J. Over-Expression of Nectin-4 Promotes Progression of Esophageal Cancer and Correlates with Poor Prognosis of the Patients. Cancer Cell Int. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients with Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2022, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High Expression of Nectin-4 Is Associated with Unfavorable Prognosis in Gastric Cancer. Oncol. Lett. 2018, 15, 8789–8795. [Google Scholar] [CrossRef]
- Lin, X.; Hu, H.; Pan, Y.; Gao, S. The Prognostic Role of Expression of Nectin-4 in Esophageal Cancer. Med. Sci. Monit. 2019, 25, 10089–10094. [Google Scholar] [CrossRef]
- Mikuteit, M.; Zschäbitz, S.; Autenrieth, M.; Weichert, W.; Hartmann, A.; Steffens, S.; Erlmeier, F. Expression of Nectin-4 in Chromophobe Renal Cell Carcinoma in a Multicenter Cohort: Early Prognostic and Therapeutic Considerations. Oncology 2024, 102, 503–509. [Google Scholar] [CrossRef]
- Das, D.; Satapathy, S.R.; Siddharth, S.; Nayak, A.; Kundu, C.N. NECTIN-4 Increased the 5-FU Resistance in Colon Cancer Cells by Inducing the PI3K-AKT Cascade. Cancer Chemother. Pharmacol. 2015, 76, 471–479. [Google Scholar] [CrossRef]
- Mendelsohn, C.L.; Wimmer, E.; Racaniello, V.R. Cellular Receptor for Poliovirus: Molecular Cloning, Nucleotide Sequence, and Expression of a New Member of the Immunoglobulin Superfamily. Cell 1989, 56, 855–865. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD155, an Onco-Immunologic Molecule in Human Tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Clinical Significance of CD155 Expression in Human Pancreatic Cancer. Anticancer Res. 2015, 35, 2287–2297. [Google Scholar] [PubMed]
- Nakai, R.; Maniwa, Y.; Tanaka, Y.; Nishio, W.; Yoshimura, M.; Okita, Y.; Ohbayashi, C.; Satoh, N.; Ogita, H.; Takai, Y.; et al. Overexpression of Necl-5 Correlates with Unfavorable Prognosis in Patients with Lung Adenocarcinoma. Cancer Sci. 2010, 101, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, J.H.; Kang, K.W.; Lee, S.R.; Park, Y.; Sung, H.J.; Kim, B.S. PVR (CD155) Expression as a Potential Prognostic Marker in Multiple Myeloma. Biomedicines 2022, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; McLendon, R.; Sankey, E.; Kornahrens, R.; Lyne, A.M.; Cavalli, F.M.G.; McKay, Z.; Herndon, J.E., II; Remke, M.; Picard, D.; et al. CD155 Is a Putative Therapeutic Target in Medulloblastoma. Clin. Transl. Oncol. 2023, 25, 696–705. [Google Scholar] [CrossRef]
| Cancer Type | Most Important Correlation of Protein Overexpression with Clinicopathological Parameters | References | |
|---|---|---|---|
| Nectin-1 | |||
| Colorectal cancer |
| [10] | |
| Pancreatic cancer |
| [8] | |
| Breast cancer |
| [6] | |
| Nectin-2 | |||
| Pancreatic cancer |
| [8] | |
| Gallbladder adenocarcinoma |
| [14] | |
| Gallbladder squamous cell carcinoma and adenosquamous carcinoma |
| [14] | |
| Brest cancer |
| [6] | |
| Acute myeloid leukemia |
| [19] | |
| Nectin-3 | |||
| Ovarian cancer |
| [21] | |
| Colorectal cancer |
| [15] | |
| Glandular lung cancer |
| [22] | |
| Nectin-4 | |||
| Breast cancer (luminal-A) |
| [27] | |
| Breast cancer |
| [6] [25] | |
| Bladder cancers |
| [29] | |
| Gastric cancers |
| [31] | |
| Pancreatic cancers |
| [30] | |
| Squamous cell carcinoma of head and neck |
| [23] | |
| Salivary gland cancer |
| [32] | |
| Necl-5 | |||
| Pancreatic cancer |
| [41] | |
| Lung cancer |
| [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romańczyk, W.; Pryczynicz, A. The Significance of Nectin Family Proteins in Various Cancerogenous Processes. Int. J. Mol. Sci. 2025, 26, 3200. https://doi.org/10.3390/ijms26073200
Romańczyk W, Pryczynicz A. The Significance of Nectin Family Proteins in Various Cancerogenous Processes. International Journal of Molecular Sciences. 2025; 26(7):3200. https://doi.org/10.3390/ijms26073200
Chicago/Turabian StyleRomańczyk, Wiktoria, and Anna Pryczynicz. 2025. "The Significance of Nectin Family Proteins in Various Cancerogenous Processes" International Journal of Molecular Sciences 26, no. 7: 3200. https://doi.org/10.3390/ijms26073200
APA StyleRomańczyk, W., & Pryczynicz, A. (2025). The Significance of Nectin Family Proteins in Various Cancerogenous Processes. International Journal of Molecular Sciences, 26(7), 3200. https://doi.org/10.3390/ijms26073200






