Sarcoglycans Role in Actin Cytoskeleton Dynamics and Cell Adhesion of Human Articular Chondrocytes: New Insights from siRNA-Mediated Gene Silencing
Abstract
1. Introduction
2. Results
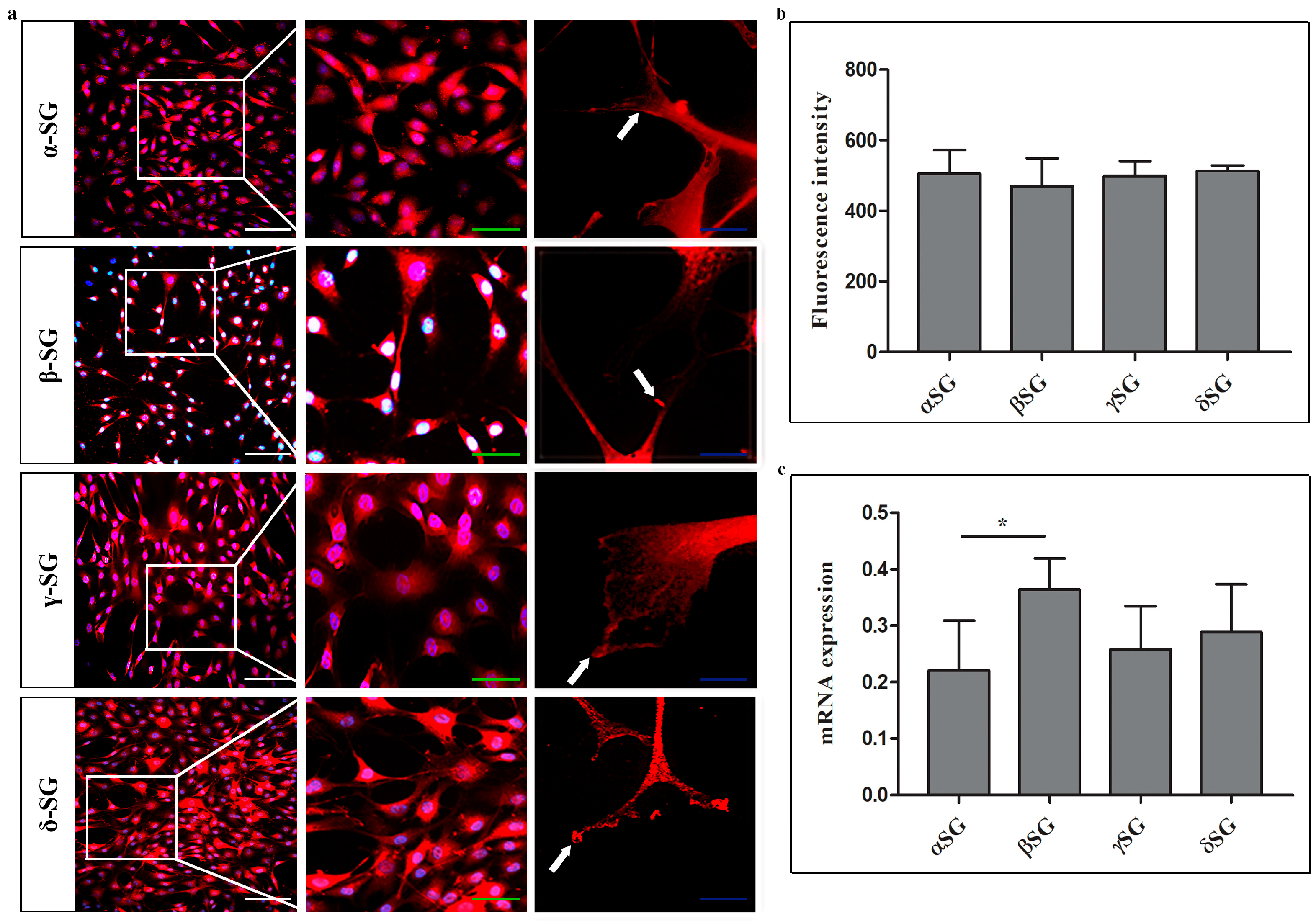
2.1. Immunofluorescence and RT-PCR on Control Chondrocytes
2.2. Immunofluorescence on Control and siRNA-Transfected Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chondrocyte Transfection
4.3. RNA Isolation, cDNA Synthesis, and qPCR
4.4. Immunofluorescence on Cells
4.5. Confocal Laser Microscope Observation
4.6. Image Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khajeh, S.; Bozorg-Ghalati, F.; Zare, M.; Panahi, G.; Razban, V. Cartilage Tissue and Therapeutic Strategies for Cartilage Repair. Curr. Mol. Med. 2021, 21, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Focsa, M.A.; Florescu, S.; Gogulescu, A. Emerging Strategies in Cartilage Repair and Joint Preservation. Medicina 2024, 61, 24. [Google Scholar] [CrossRef]
- Pueyo Moliner, A.; Ito, K.; Zaucke, F.; Kelly, D.J.; de Ruijter, M.; Malda, J. Restoring Articular Cartilage: Insights from Structure, Composition and Development. Nat. Rev. Rheumatol. 2025, 21, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-Cell Interaction of Cartilage Extracellular Matrix on Chondrocytes. Biomed. Res. Int. 2014, 2014, 648459. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef]
- Michelacci, Y.M.; Baccarin, R.Y.A.; Rodrigues, N.N.P. Chondrocyte Homeostasis and Differentiation: Transcriptional Control and Signaling in Healthy and Osteoarthritic Conditions. Life 2023, 13, 1460. [Google Scholar] [CrossRef]
- Mishra, Y.G.; Manavathi, B. Focal Adhesion Dynamics in Cellular Function and Disease. Cell. Signal. 2021, 85, 110046. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Calderwood, D.A. Organization, Dynamics and Mechanoregulation of Integrin-Mediated Cell-ECM Adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.A.; Deakin, N.O.; Turner, C.E. Chapter 156—The Focal Adhesion: A Network of Molecular Interactions. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 1259–1264. ISBN 978-0-12-374145-5. [Google Scholar]
- Loeser, R.F. Integrins and Cell Signaling in Chondrocytes. Biorheology 2002, 39, 119–124. [Google Scholar] [CrossRef]
- Loeser, R.F. Integrins and Chondrocyte-Matrix Interactions in Articular Cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Schoenwaelder, S.M.; Burridge, K. Bidirectional Signaling between the Cytoskeleton and Integrins. Curr. Opin. Cell Biol. 1999, 11, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Puklin-Faucher, E.; Sheetz, M.P. The Mechanical Integrin Cycle. J. Cell Sci. 2009, 122, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, W.H.; Gingras, A.R.; Critchley, D.R.; Emsley, J. Integrin Connections to the Cytoskeleton through Talin and Vinculin. Biochem. Soc. Trans. 2008, 36, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Integrin and Its Associated Proteins as a Mediator for Mechano-Signal Transduction. Biomolecules 2025, 15, 166. [Google Scholar] [CrossRef]
- Bouaouina, M.; Harburger, D.S.; Calderwood, D.A. Talin and Signaling through Integrins. Methods Mol. Biol. 2012, 757, 325–347. [Google Scholar] [CrossRef]
- Das, M.; Ithychanda, S.; Qin, J.; Plow, E.F. Mechanisms of Talin-Dependent Integrin Signaling and Crosstalk. Biochim. Biophys. Acta 2014, 1838, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kluger, C.; Braun, L.; Sedlak, S.M.; Pippig, D.A.; Bauer, M.S.; Miller, K.; Milles, L.F.; Gaub, H.E.; Vogel, V. Different Vinculin Binding Sites Use the Same Mechanism to Regulate Directional Force Transduction. Biophys. J. 2020, 118, 1344–1356. [Google Scholar] [CrossRef]
- Wilson, D.G.S.; Tinker, A.; Iskratsch, T. The Role of the Dystrophin Glycoprotein Complex in Muscle Cell Mechanotransduction. Commun. Biol. 2022, 5, 1022. [Google Scholar] [CrossRef]
- Tarakci, H.; Berger, J. The Sarcoglycan Complex in Skeletal Muscle. Front. Biosci. 2016, 21, 744–756. [Google Scholar] [CrossRef]
- Sánchez Riera, C.; Lozanoska-Ochser, B.; Testa, S.; Fornetti, E.; Bouché, M.; Madaro, L. Muscle Diversity, Heterogeneity, and Gradients: Learning from Sarcoglycanopathies. Int. J. Mol. Sci. 2021, 22, 2502. [Google Scholar] [CrossRef]
- Arco, A.; Favaloro, A.; Gioffrè, M.; Santoro, G. Sarcoglycans in the Normal and Pathological Breast Tissue of Humans: An Immunohistochemical and Molecular Study. Cells Tissues Organs 2012, 195, 550–562. [Google Scholar] [CrossRef]
- Cutroneo, G.; Bramanti, P.; Favaloro, A.; Anastasi, G.; Trimarchi, F.; Di Mauro, D.; Rinaldi, C.; Speciale, F.; Inferrera, A.; Santoro, G.; et al. Sarcoglycan Complex in Human Normal and Pathological Prostatic Tissue: An Immunohistochemical and RT-PCR Study. Anat. Rec. 2014, 297, 327–336. [Google Scholar] [CrossRef]
- Al-Maslamani, N.A.; Oldershaw, R.; Tew, S.; Curran, J.; D’Hooghe, P.; Yamamoto, K.; Horn, H.F. Chondrocyte De-Differentiation: Biophysical Cues to Nuclear Alterations. Cells 2022, 11, 4011. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Nie, Y.; Zheng, S.; Yao, Y. Recent Research on Chondrocyte Dedifferentiation and Insights for Regenerative Medicine. Biotechnol. Bioeng. 2025, 122, 749–760. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef] [PubMed]
- Rzepski, A.T.; Schofield, M.M.; Richardson-Solorzano, S.; Arranguez, M.L.; Su, A.W.; Parreno, J. Targeting the Reorganization of F-Actin for Cell-Based Implantation Cartilage Repair Therapies. Differentiation 2025, 143, 100847. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Matta, C.; Mobasheri, A. Physicochemical and Biomechanical Stimuli in Cell-Based Articular Cartilage Repair. Curr. Rheumatol. Rep. 2015, 17, 22. [Google Scholar] [CrossRef]
- Shin, H.; Lee, M.N.; Choung, J.S.; Kim, S.; Choi, B.H.; Noh, M.; Shin, J.H. Focal Adhesion Assembly Induces Phenotypic Changes and Dedifferentiation in Chondrocytes. J. Cell. Physiol. 2016, 231, 1822–1831. [Google Scholar] [CrossRef]
- Rizzo, G.; Di Mauro, D.; Cutroneo, G.; Schembri-Wismayer, P.; Brunetto, D.; Spoto, C.; Vermiglio, G.; Centofanti, A.; Favaloro, A. An Immunofluorescence Study About Staining Pattern Variability of Sarcoglycans in Rat’s Cerebral and Cerebellar Cortex. Eur. J. Exp. Biol. 2018, 8, 7. [Google Scholar] [CrossRef]
- Haudenschild, D.R.; Chen, J.; Steklov, N.; Lotz, M.K.; D’Lima, D.D. Characterization of the Chondrocyte Actin Cytoskeleton in Living Three-Dimensional Culture: Response to Anabolic and Catabolic Stimuli. Mol. Cell. Biomech. 2009, 6, 135–144. [Google Scholar]
- Delve, E.; Co, V.; Kandel, R.A. Superficial and Deep Zone Articular Chondrocytes Exhibit Differences in Actin Polymerization Status and Actin-Associated Molecules in Vitro. Osteoarthr. Cartil. Open 2020, 2, 100071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Zhao, H. The Effect of Matrix Stiffness on Biomechanical Properties of Chondrocytes. Acta Biochim. Biophys. Sin. 2016, 48, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Tatsumi, H.; Lim, C.T.; Sokabe, M. Force-Dependent Vinculin Binding to Talin in Live Cells: A Crucial Step in Anchoring the Actin Cytoskeleton to Focal Adhesions. Am. J. Physiol. Cell Physiol. 2014, 306, C607–C620. [Google Scholar] [CrossRef]
- Rothenberg, K.E.; Scott, D.W.; Christoforou, N.; Hoffman, B.D. Vinculin Force-Sensitive Dynamics at Focal Adhesions Enable Effective Directed Cell Migration. Biophys. J. 2018, 114, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Cutroneo, G.; Vermiglio, G.; Centofanti, A.; Rizzo, G.; Runci, M.; Favaloro, A.; Piancino, M.G.; Bracco, P.; Ramieri, G.; Bianchi, F.A.; et al. Morphofunctional Compensation of Masseter Muscles in Unilateral Posterior Crossbite Patients. Eur. J. Histochem. 2016, 60, 2605. [Google Scholar] [CrossRef]
- Bruschetta, D.; Anastasi, G.; Andronaco, V.; Cascio, F.; Rizzo, G.; Di Mauro, D.; Bonanno, L.; Izzo, V.; Buda, D.; Vermiglio, G.; et al. Human Calf Muscles Changes after Strength Training as Revealed by Diffusion Tensor Imaging. J. Sports Med. Phys. Fitness 2019, 59, 853–860. [Google Scholar] [CrossRef]
- Urzì Brancati, V.; Aliquò, F.; Freni, J.; Pantano, A.; Galipò, E.; Puzzolo, D.; Minutoli, L.; Marini, H.R.; Campo, G.M.; D’Ascola, A. The Effects of Seleno-Methionine in Cadmium-Challenged Human Primary Chondrocytes. Pharmaceuticals 2024, 17, 936. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. [Google Scholar] [CrossRef]
- Freni, J.; Pallio, G.; Marini, H.R.; Micali, A.; Irrera, N.; Romeo, C.; Puzzolo, D.; Mannino, F.; Minutoli, L.; Pirrotta, I.; et al. Positive Effects of the Nutraceutical Association of Lycopene and Selenium in Experimental Varicocele. Int. J. Mol. Sci. 2023, 24, 13526. [Google Scholar] [CrossRef] [PubMed]




| Gene | Species | Sequence (5′→3′) |
|---|---|---|
| ACTB | Human | F: 5′-GCTGTCTCTCTATGCCTCTGGA-3′ R: 5′-CCAGATCCAGACGCATGAT-3′ |
| SGCA | Human | F: 5′-ACTTCCGCGTTGACTGGT-3′ R: 5′-AGTGGGTGGGCAGAAGAA-3′ |
| SGCB | Human | F: 5′-CTGACATGGGAGTGATCCAC-3 R: 5′-TGAGCTTTGTTGTCCCTTGC-3′ |
| SGCG | Human | F: 5′-CTGTAAATGCGCGCAACTC-3′ R: 5′-AAATAGTGGCTTGCCGTCGT-3′ |
| SGCD | Human | F: 5′-ATCAATGCAGAAGCTGGCA-3′ R:5′-GATCCATGAGGCAGTCTAGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centofanti, A.; Runci Anastasi, M.; Nicita, F.; Labellarte, D.; Scuruchi, M.; Pantano, A.; Freni, J.; Favaloro, A.; Vermiglio, G. Sarcoglycans Role in Actin Cytoskeleton Dynamics and Cell Adhesion of Human Articular Chondrocytes: New Insights from siRNA-Mediated Gene Silencing. Int. J. Mol. Sci. 2025, 26, 5732. https://doi.org/10.3390/ijms26125732
Centofanti A, Runci Anastasi M, Nicita F, Labellarte D, Scuruchi M, Pantano A, Freni J, Favaloro A, Vermiglio G. Sarcoglycans Role in Actin Cytoskeleton Dynamics and Cell Adhesion of Human Articular Chondrocytes: New Insights from siRNA-Mediated Gene Silencing. International Journal of Molecular Sciences. 2025; 26(12):5732. https://doi.org/10.3390/ijms26125732
Chicago/Turabian StyleCentofanti, Antonio, Michele Runci Anastasi, Fabiana Nicita, Davide Labellarte, Michele Scuruchi, Alice Pantano, Josè Freni, Angelo Favaloro, and Giovanna Vermiglio. 2025. "Sarcoglycans Role in Actin Cytoskeleton Dynamics and Cell Adhesion of Human Articular Chondrocytes: New Insights from siRNA-Mediated Gene Silencing" International Journal of Molecular Sciences 26, no. 12: 5732. https://doi.org/10.3390/ijms26125732
APA StyleCentofanti, A., Runci Anastasi, M., Nicita, F., Labellarte, D., Scuruchi, M., Pantano, A., Freni, J., Favaloro, A., & Vermiglio, G. (2025). Sarcoglycans Role in Actin Cytoskeleton Dynamics and Cell Adhesion of Human Articular Chondrocytes: New Insights from siRNA-Mediated Gene Silencing. International Journal of Molecular Sciences, 26(12), 5732. https://doi.org/10.3390/ijms26125732










