Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change
Abstract
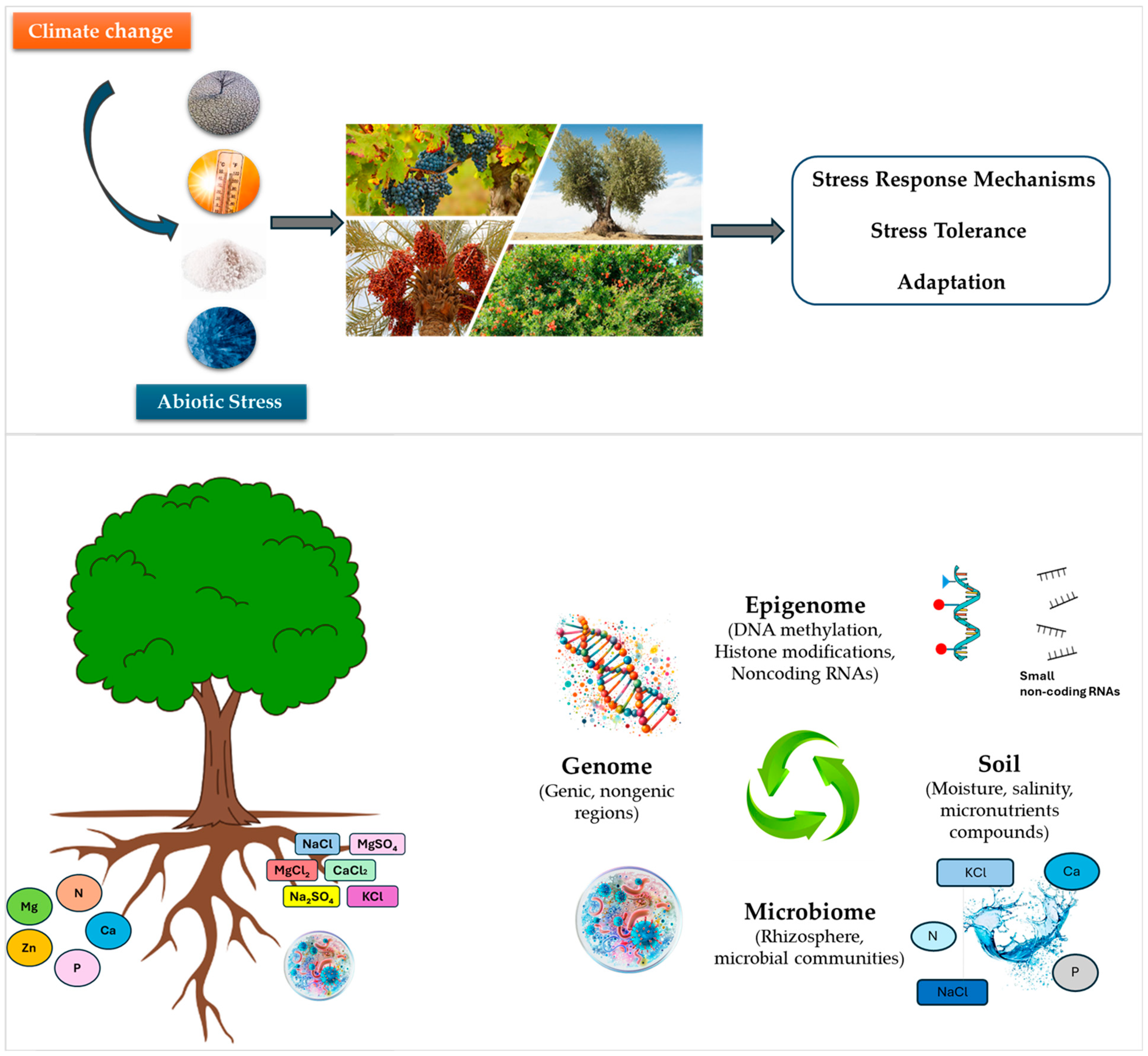
1. Introduction
2. Grapevine
2.1. Climate Challenges
2.2. Genetic and Epigenetic Attributes of Abiotic Stress
| Species | Stress Type | Molecular Tools | Molecular Response/Tolerance-Associated Genes | References |
|---|---|---|---|---|
| Grapevine | Drought | Genome-wide association studies (GWASs) | Candidate genes and SNPs associated with stomatal conductance and drought responsiveness, e.g., raffinose synthase. | [65,77] |
| Transcriptomics-RNA Seq/Quantitative PCR | Co-expression of gene networks related to signal transduction cascades, phenyl propanoid metabolism, sugar-metabolizing enzymes, heat-shock protein transcription factor regulation, and histone modification factors. | [79] | ||
| TF families VvAGL15, VvLBD41, and VvMYB86 | [78] | |||
| Up- and downregulation of responsive miRNAs VvmiR159 and VvmiR156. | [83] | |||
| Induction of miRNAs VvmiR159 and VvmiR156 and anticorrelated expression of TF genes MYB1 and TPR. | [85] | |||
| Drought-induced VvmiR169d and VvmiR156b upregulation and VvmiR398a downregulation. | [84] | |||
| Activation of the module miR156b-VvSBP8/13. | [87] | |||
| Heat | Transcriptomics-RNA seq/Quantitative PCR | Transcription factor families WRKYs, MYBs, and NACs; auxin and ABA signaling; starch and sucrose metabolism. | [80] | |
| Induction of heat-stress-responsive miRNA VvmiR167. | [71] | |||
| Aluminum (Al) toxicity | Whole-genome bisulfite sequencing (WGBS) | DNA methylation reduction/enhanced tolerance to Al. | [91] | |
| Cold | Chromatin immunoprecipitation (ChIP) Transcriptomics-RNA seq | H3K27 trimethylation alterations/gene target downregulation. | [92] | |
| Novel cold-stress-responsive microRNAs. | [90] | |||
| Olive tree | Drought | Transcriptomics/RNA-seq | Transmembrane transport and metal ion binding processes, and abscisic acid, gibberellin, brassinosteroid, and ethylene-activated signaling. | [94] |
| Salt | Transcriptomics/RNA-seq | TF families JERF and bZIP. | [95] | |
| Upregulation of OeNHX7, OeP5CS, OeRD19A, and OePetD. | [96] | |||
| Date Palm | Combined heat and drought | Proteomics | Increased abundance of heat shock proteins (HSPs), redox homeostasis proteins, and proteins involved in isoprene production. | [97] |
| Salt | Multi-omics | Converging gene expression and protein abundance associated with osmotic adjustment, reactive oxygen species scavenging in leaves, and remodeling of the ribosome-associated proteome in salt-exposed root cells. | [98] | |
| Induction of salt overly sensitive (SOS) genes PdSOS2;1, PdSOS2;2, PdSOS4, PdSOS5, and PdCIPK11. | [99] | |||
| Whole-genome bisulfite sequencing (WGBS) | Differential DNA methylation and gene expression alterations in the roots. | [100] | ||
| Pomegranate | Salt | Transcriptomics/RNA-seq | Spatiotemporal regulation of SWEET genes. | [101] |
| DEGs associated with ABA- and Ca2+-related and MAPK signal transduction pathways (ABA-receptors, Ca2+-sensors, MAPK cascades, TFs) and downstream functional genes coding for HSPs, LEAs, AQPs, and PODs. | [102] | |||
| Induction of proline, total soluble sugar, and SOD/POD activities and differential gene expression. | [103] | |||
| Cold | Transcriptomics/RNA-seq | Upregulation of CBF genes PgCBF3 and PgCBF7. | [104] | |
| Differentially expressed genes related to TFs, photosynthesis, the osmotic regulation system, hormone signal transduction, and sucrose metabolism. | [105] | |||
| Induction of beta-amylase, PgBAM4, and increase in soluble sugar content. | [106] |
2.3. Microbiota Attributes Related to Abiotic Stress
| Species | Stress Type | Microbe Type | Microbial Effect—Molecular Response | References |
|---|---|---|---|---|
| Grapevine | Drought | Rhizosphere-associated bacteria | Protection against reactive oxygen species (ROS)—accumulation of terpenes. | [108] |
| Drought | Root-associated microbiome | Water stress protection. | [107] | |
| Drought | Arbuscular mycorrhizal fungi (AMF) | Drought tolerance by increasing the accumulation of osmolytes, triggering antioxidant processes, and regulating the expression of key stress-responsive genes. | [111] | |
| Heat | Marine plant growth-promoting rhizobacteria consortia | Heat stress tolerance. | [113] | |
| Heat | AMF | Enhancement in physiological indices; modulation of miRNAs and stress-related transcription factors and proteins related to antioxidant pathways. | [121] | |
| Olive tree | Drought | Pseudomonas reactans Ph3R3 | Enhancement in plant performance by reducing water loss and improving N levels, net CO2 assimilation rate, and antioxidant capacity. | [123] |
| Drought | PGPB consortia sampled from the soil and rhizosphere of Tunisian olive orchards | Conferred tolerance to both drought-susceptible and drought-tolerant cultivars. | [124] | |
| Drought | AMF (Rhizophagus irregularis) | Reinforced tolerance to water deficit by enhancing olive plant growth and improving water status, accumulation of osmolytes and antioxidants, and phytohormone regulation. | [125] | |
| Drought | AMF (Rhizophagus irregularis) | Enhanced water deficit tolerance by increasing net carbon fixation, water use efficiency, and antioxidant defenses. | [126] | |
| Salt | PGPB Bacillus G7 | Improved physiological and metabolic parameters, and increased photosynthetic capacity, net carbon fixation, water use efficiency, and accumulation of osmolytes and antioxidants. | [121] | |
| Salt | AMF mixture of Glomus deserticola and Gigaspora margarita | Alleviation of the stress imposed by irrigation with salt-enriched wastewater. | [127] | |
| Date Palm | Drought | Selected date plam root bacterial endophytes | Increased the biomass of date palms exposed to recurrent drought stress cycles in a greenhouse experiment. | [128] |
| Salt | Piriformospora indica endophyte | Mitigated the detrimental effects of salt stress through ion homeostasis and nutrient uptake, antioxidant activity, and upregulation of stress-responsive genes. | [129,130] | |
| Salt | Enterobacter cloacae SQU-2 (SQU-2)’ | Improved the growth of Arabidopsis thaliana Columbia (Col-0) seedlings under both normal and salt stress conditions through the production of microbial volatile compounds (mVOCs). | [131] | |
| Pomegranate | Drought | AMF strains Rhizophagus intraradices (GA5 and GC2) | Early inoculation with AMF, especially for the GC2 strain, offers protection against drought; antioxidant defenses, specifically the ROS-scavenging enzymes superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), are enhanced in shoots. | [132] |
3. Olive Tree
3.1. Climate Challenges
3.2. The Genetic and Epigenetic Component
3.3. The Microbiota Component
4. Other Woody Fruit Crops
4.1. Date Palm
4.1.1. Genetic/Epigenetic Factors in Abiotic Stress
4.1.2. Microbiota Aspects and Abiotic Stress
4.2. Pomegranate
- Pomegranate (Punica granatum L.) is an ancient perennial species native to Central Asia and has been cultivated for over 3000 years. Today, it is commercially grown in more than 30 countries, including India, Iran, Spain, China, and the United States. It is also a major subtropical fruit crop in the Mediterranean region, grown especially in Turkey and Spain, and is valued for its fruits, leaves, and other plant parts due to its antioxidant properties. The tree thrives in arid and semi-arid regions, often facing challenges such as salinity and other environmental stressors, which can negatively impact transplantation survival rates, fruit yield, and quality [167,168].
- However, pomegranate trees thrive in warm climates and have a low tolerance for cold temperatures, leading to their predominant cultivation in tropical and subtropical regions. Cold stress restricts plant growth, development, and yield, with sudden cold snaps in winter and late spring posing a particular threat to pomegranate trees. These temperature drops can cause freezing damage, significantly reducing fruit yield and quality, thereby affecting market availability [169].
4.2.1. Genetic Aspects and Transcriptional Regulation
4.2.2. Microbiome and Abiotic Stress
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Working Group II Contribution to the IPCC Sixth Assessment Report. 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 20 March 2025).
- Jägermeyr, J.; Müller, C.; Ruane, A.C.; Elliott, J.; Balkovic, J.; Castillo, O.; Faye, B.; Foster, I.; Folberth, C.; Franke, J.A.; et al. Climate Impacts on Global Agriculture Emerge Earlier in New Generation of Climate and Crop Models. Nat. Food 2021, 2, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Ainsworth, E.A.; Bahuguna, R.N.; Broadley, M.R.; Busch, W.; Carpita, N.C.; Castrillo, G.; Chory, J.; DeHaan, L.R.; Duarte, C.M.; et al. Climate Change Challenges, Plant Science Solutions. Plant Cell 2023, 35, 24–66. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Bailey-Serres, J.; Brodersen, C.; Buckley, T.N.; Conti, L.; Christmann, A.; Dinneny, J.R.; Grill, E.; Hayes, S.; Heckman, R.W.; et al. Burning Questions for a Warming and Changing World: 15 Unknowns in Plant Abiotic Stress. Plant Cell 2023, 35, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Leisner, C.P. Review: Climate Change Impacts on Food Security-Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef]
- Salama, A.-M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate Fruit Trees under Climate Change: Challenges for Dormancy and Chilling Requirements in Warm Winter Regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Luedeling, E. Climate Change Impacts on Winter Chill for Temperate Fruit and Nut Production: A Review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Measham, P.F.; Darbyshire, R.; Turpin, S.R.; Murphy-White, S. Complexity in Chill Calculations: A Case Study in Cherries. Sci. Hortic. 2017, 216, 134–140. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic Selection in the Era of Next Generation Sequencing for Complex Traits in Plant Breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef]
- Lamichhane, S.; Thapa, S. Advances from Conventional to Modern Plant Breeding Methodologies. Plant Breed. Biotechnol. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern Plant Breeding Techniques in Crop Improvement and Genetic Diversity: From Molecular Markers and Gene Editing to Artificial Intelligence—A Critical Review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Varotto, S.; Tani, E.; Abraham, E.; Krugman, T.; Kapazoglou, A.; Melzer, R.; Radanović, A.; Miladinović, D. Epigenetics: Possible Applications in Climate-Smart Crop Breeding. J. Exp. Bot. 2020, 71, 5223–5236. [Google Scholar] [CrossRef] [PubMed]
- Diwan, D.; Rashid, M.M.; Vaishnav, A. Current Understanding of Plant-Microbe Interaction through the Lenses of Multi-Omics Approaches and Their Benefits in Sustainable Agriculture. Microbiol. Res. 2022, 265, 127180. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Patil, S.; Shaikh, A.; Jamla, M.; Kumar, V. Modern Omics Toolbox for Producing Combined and Multifactorial Abiotic Stress Tolerant Plants. Plant Stress. 2024, 11, 100301. [Google Scholar] [CrossRef]
- Ravikiran, K.T.; Thribhuvan, R.; Anilkumar, C.; Kallugudi, J.; Prakash, N.R.; Adavi, B.S.; Sunitha, N.C.; Abhijith, K.P. Harnessing the Power of Genomics to Develop Climate-Smart Crop Varieties: A Comprehensive Review. J. Environ. Manag. 2025, 373, 123461. [Google Scholar] [CrossRef]
- Ali, N.; Singh, S.; Garg, R. Unlocking Crops’ Genetic Potential: Advances in Genome and Epigenome Editing of Regulatory Regions. Curr. Opin. Plant Biol. 2025, 83, 102669. [Google Scholar] [CrossRef]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop Landraces and Indigenous Varieties: A Valuable Source of Genes for Plant Breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef]
- Agius, D.R.; Kapazoglou, A.; Avramidou, E.; Baranek, M.; Carneros, E.; Caro, E.; Castiglione, S.; Cicatelli, A.; Radanovic, A.; Ebejer, J.-P.; et al. Exploring the Crop Epigenome: A Comparison of DNA Methylation Profiling Techniques. Front. Plant Sci. 2023, 14, 1181039. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Gerakari, M.; Lazaridi, E.; Kleftogianni, K.; Sarri, E.; Tani, E.; Bebeli, P.J. Crop Wild Relatives: A Valuable Source of Tolerance to Various Abiotic Stresses. Plants 2023, 12, 328. [Google Scholar] [CrossRef]
- Ramesh, S.; Rajesh, M.; Das, A.; Hebbar, K. CRISPR/Cas9 –Based Genome Editing to Expedite the Genetic Improvement of Palms: Challenges and Prospects. Front. Plant Sci. 2024, 15, 1385037. [Google Scholar] [CrossRef]
- Campa, M.; Miranda, S.; Licciardello, C.; Lashbrooke, J.G.; Dalla Costa, L.; Guan, Q.; Spök, A.; Malnoy, M. Application of New Breeding Techniques in Fruit Trees. Plant Physiol. 2024, 194, 1304–1322. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Resende, M.D.V. Genomic Selection in Forest Tree Breeding. Tree Genet. Genomes 2011, 7, 241–255. [Google Scholar] [CrossRef]
- Magon, G.; De Rosa, V.; Martina, M.; Falchi, R.; Acquadro, A.; Barcaccia, G.; Portis, E.; Vannozzi, A.; De Paoli, E. Boosting Grapevine Breeding for Climate-Smart Viticulture: From Genetic Resources to Predictive Genomics. Front. Plant Sci. 2023, 14, 1293186. [Google Scholar] [CrossRef] [PubMed]
- Jighly, A. Boosting Genome-Wide Association Power and Genomic Prediction Accuracy for Date Palm Fruit Traits with Advanced Statistics. Plant Sci. 2024, 344, 112110. [Google Scholar] [CrossRef] [PubMed]
- Seyum, E.G.; Bille, N.H.; Abtew, W.G.; Munyengwa, N.; Bell, J.M.; Cros, D. Genomic Selection in Tropical Perennial Crops and Plantation Trees: A Review. Mol. Breed. 2022, 42, 58. [Google Scholar] [CrossRef]
- Ventouris, Y.E.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Chorianopoulou, S.N.; Vlachostergios, D.N.; Papadopoulos, G.; Kapazoglou, A. Recurrent Water Deficit and Epigenetic Memory in Medicago Sativa L. Varieties. Appl. Sci. 2020, 10, 3110. [Google Scholar] [CrossRef]
- Drosou, V.; Kapazoglou, A.; Letsiou, S.; Tsaftaris, A.S.; Argiriou, A. Drought Induces Variation in the DNA Methylation Status of the Barley HvDME Promoter. J. Plant Res. 2021, 134, 1351–1362. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, Z.; Wu, H.; Huang, C.; Shuai, P.; Ye, C.-Y.; Tang, S.; Wang, Y.; Yang, L.; Wang, J.; et al. Single-Base-Resolution Methylomes of Populus Trichocarpa Reveal the Association between DNA Methylation and Drought Stress. BMC Genet. 2014, 15, S9. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Lu, L.; Yan, M.; Fang, N.; Xu, J.; Wang, L.; Yan, Y.; Zhao, T.; van Nocker, S.; et al. Contribution of Methylation Regulation of MpDREB2A Promoter to Drought Resistance of Mauls Prunifolia. Plant Soil. 2019, 441, 15–32. [Google Scholar] [CrossRef]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing Rhizosphere Microbiomes for Drought-Resilient Crop Production. Science (1979) 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–Microbiome Interactions under a Changing World: Responses, Consequences and Perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V.S. Interference of Climate Change on Plant-Microbe Interaction: Present and Future Prospects. Front. Agron. 2022, 3, 725804. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Miller, T.; Kisiel, A.; Cembrowska-Lech, D.; Mikiciuk, M.; Łobodzińska, A.; Bokszczanin, K. Harnessing Beneficial Microbes for Drought Tolerance: A Review of Ecological and Agricultural Innovations. Agriculture 2024, 14, 2228. [Google Scholar] [CrossRef]
- Sharma, A.; Choudhary, P.; Chakdar, H.; Shukla, P. Molecular Insights and Omics-Based Understanding of Plant–Microbe Interactions under Drought Stress. World J. Microbiol. Biotechnol. 2024, 40, 42. [Google Scholar] [CrossRef]
- Salvi, P.; Mahawar, H.; Agarrwal, R.; Kajal; Gautam, V.; Deshmukh, R. Advancement in the Molecular Perspective of Plant-Endophytic Interaction to Mitigate Drought Stress in Plants. Front. Microbiol. 2022, 13, 981355. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Li, J.-H.; Muhammad Aslam, M.; Gao, Y.-Y.; Dai, L.; Hao, G.-F.; Wei, Z.; Chen, M.-X.; Dini-Andreote, F. Microbiome-Mediated Signal Transduction within the Plant Holobiont. Trends Microbiol. 2023, 31, 616–628. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Kowalchuk, G.A.; Jousset, A. Targeted Plant Hologenome Editing for Plant Trait Enhancement. New Phytol. 2021, 229, 1067–1077. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Singh, R.; Gong, Q.; Wu, Q.; Shi, J. Omics Approaches in Understanding the Benefits of Plant-Microbe Interactions. Front. Microbiol. 2024, 15, 1391059. [Google Scholar] [CrossRef]
- Doddavarapu, B.; Lata, C.; Shah, J.M. Epigenetic Regulation Influenced by Soil Microbiota and Nutrients: Paving Road to Epigenome Editing in Plants. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2024, 1868, 130580. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.-L.; Caddell, D.; et al. Genome-Resolved Metagenomics Reveals Role of Iron Metabolism in Drought-Induced Rhizosphere Microbiome Dynamics. Nat. Commun. 2021, 12, 3209. [Google Scholar] [CrossRef]
- Zombardo, A.; Meneghetti, S.; Morreale, G.; Calò, A.; Costacurta, A.; Storchi, P. Study of Inter- and Intra-Varietal Genetic Variability in Grapevine Cultivars. Plants 2022, 11, 397. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual Domestications and Origin of Traits in Grapevine Evolution. Science (1979) 2023, 379, 892–901. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the World’s Future Wine-Growing Regions. Nat. Clim. Change 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Ollat, N.; Marguerit, E.; de Miguel, M.; Coupel-Ledru, A.; Cookson, S.J.; van Leeuwen, C.; Vivin, P.; Gallusci, P.; Segura, V.; Duchêne, E. Moving towards Grapevine Genotypes Better Adapted to Abiotic Constraints. Vitis-J. Grapevine Res. 2023, 62, 67–76. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). Available online: https://www.Oiv.Int/What-We-Do/Global-Report?Oiv (accessed on 20 March 2025).
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine Abiotic Stress Assessment and Search for Sustainable Adaptation Strategies in Mediterranean-like Climates. A Review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Mavromatis, T.; Koufos, G.C.; Koundouras, S.; Jones, G.V. Adaptive Capacity of Winegrape Varieties Cultivated in Greece to Climate Change: Current Trends and Future Projections. OENO One 2020, 54, 1201–1219. [Google Scholar] [CrossRef]
- Venios, X.; Gkizi, D.; Nisiotou, A.; Korkas, E.; Tjamos, S.; Zamioudis, C.; Banilas, G. Emerging Roles of Epigenetics in Grapevine and Winegrowing. Plants 2024, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Carimi, F.; Zottini, M. The Role of the Endophytic Microbiome in the Grapevine Response to Environmental Triggers. Front. Plant Sci. 2019, 10, 01256. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Strack, T.; Dries, L.; Voss-Fels, K.P.; Geilfus, C. Roots of Resilience: Optimizing Microbe-rootstock Interactions to Enhance Vineyard Productivity. Plants People Planet 2024. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant Grafting: Molecular Mechanisms and Applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Cousins, P.; Jordan, L.M.; Myles, S.; Striegler, R.K.; Verdegaal, P.; Chitwood, D.H. Grapevine Rootstocks Affect Growth-related Scion Phenotypes. Plant Direct 2021, 5, e00324. [Google Scholar] [CrossRef]
- Chen, Y.; Fei, Y.; Howell, K.; Chen, D.; Clingeleffer, P.; Zhang, P. Rootstocks for Grapevines Now and into the Future: Selection of Rootstocks Based on Drought Tolerance, Soil Nutrient Availability, and Soil PH. Aust. J. Grape Wine Res. 2024, 2024, 6704238. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Gerakari, M.; Megariti, S.; Doupis, G.; Doulis, A.G. Epigenetic Changes and Transcriptional Reprogramming Upon Woody Plant Grafting for Crop Sustainability in a Changing Environment. Front. Plant Sci. 2021, 11, 613004. [Google Scholar] [CrossRef]
- Bettenfeld, P.; Cadena i Canals, J.; Jacquens, L.; Fernandez, O.; Fontaine, F.; van Schaik, E.; Courty, P.-E.; Trouvelot, S. The Microbiota of the Grapevine Holobiont: A Key Component of Plant Health. J. Adv. Res. 2022, 40, 1–15. [Google Scholar] [CrossRef]
- Darriaut, R.; Lailheugue, V.; Masneuf-Pomarède, I.; Marguerit, E.; Martins, G.; Compant, S.; Ballestra, P.; Upton, S.; Ollat, N.; Lauvergeat, V. Grapevine Rootstock and Soil Microbiome Interactions: Keys for a Resilient Viticulture. Hortic. Res. 2022, 9, uhac019. [Google Scholar] [CrossRef] [PubMed]
- Avramidou, E.; Masaoutis, I.; Pitsoli, T.; Kapazoglou, A.; Pikraki, M.; Trantas, E.; Nikolantonakis, M.; Doulis, A. Analysis of Wine-Producing Vitis vinifera L. Biotypes, Autochthonous to Crete (Greece), Employing Ampelographic and Microsatellite Markers. Life 2023, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Trenti, M.; Lorenzi, S.; Bianchedi, P.L.; Grossi, D.; Failla, O.; Grando, M.S.; Emanuelli, F. Candidate Genes and SNPs Associated with Stomatal Conductance under Drought Stress in Vitis. BMC Plant Biol. 2021, 21, 7. [Google Scholar] [CrossRef]
- Tsivelikas, A.L.; Avramidou, E.V.; Ralli, P.E.; Ganopoulos, I.V.; Moysiadis, T.; Kapazoglou, A.; Aravanopoulos, F.A.; Doulis, A.G. Genetic Diversity of Greek Grapevine (Vitis vinifera L.) Cultivars Using Ampelographic and Microsatellite Markers. Plant Genet. Resour. 2022, 20, 124–136. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Mercati, F.; Bergamini, C.; Cardone, M.F.; Lupini, A.; Mauceri, A.; Caputo, A.R.; Abbate, L.; Barbagallo, M.G.; Antonacci, D.; et al. SNP Genotyping Elucidates the Genetic Diversity of Magna Graecia Grapevine Germplasm and Its Historical Origin and Dissemination. BMC Plant Biol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Marinov, L.; Magris, G.; Di Gaspero, G.; Morgante, M.; Maletić, E.; Bubola, M.; Pejić, I.; Zdunić, G. Single Nucleotide Polymorphism (SNP) Analysis Reveals Ancestry and Genetic Diversity of Cultivated and Wild Grapevines in Croatia. BMC Plant Biol. 2024, 24, 975. [Google Scholar] [CrossRef]
- Villano, C.; Procino, S.; Blaiotta, G.; Carputo, D.; D’Agostino, N.; Di Serio, E.; Fanelli, V.; La Notte, P.; Miazzi, M.M.; Montemurro, C.; et al. Genetic Diversity and Signature of Divergence in the Genome of Grapevine Clones of Southern Italy Varieties. Front. Plant Sci. 2023, 14, 1201287. [Google Scholar] [CrossRef]
- Villano, C.; Aiese Cigliano, R.; Esposito, S.; D’Amelia, V.; Iovene, M.; Carputo, D.; Aversano, R. DNA-Based Technologies for Grapevine Biodiversity Exploitation: State of the Art and Future Perspectives. Agronomy 2022, 12, 491. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, D.; Li, H.; Chen, Q.; Zhang, Z.; Liu, M.; Liu, J.; Song, Y.; He, J.; Xu, W.; et al. Characterization and Identification of Grapevine Heat Stress-Responsive MicroRNAs Revealed the Positive Regulated Function of Vvi-MiR167 in Thermostability. Plant Sci. 2023, 329, 111623. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Y.; Li, J.; Liu, J.; Zhang, Z.; Xu, Y.; Fan, D.; Liu, M.; Ren, Y.; Xi, X.; et al. Development, Identification and Validation of a Novel SSR Molecular Marker for Heat Resistance of Grapes Based on MiRNA. Horticulturae 2023, 9, 931. [Google Scholar] [CrossRef]
- Shi, X.; Cao, S.; Wang, X.; Huang, S.; Wang, Y.; Liu, Z.; Liu, W.; Leng, X.; Peng, Y.; Wang, N.; et al. The Complete Reference Genome for Grapevine (Vitis vinifera L.) Genetics and Breeding. Hortic. Res. 2023, 10, uhad061. [Google Scholar] [CrossRef] [PubMed]
- Cochetel, N.; Minio, A.; Guarracino, A.; Garcia, J.F.; Figueroa-Balderas, R.; Massonnet, M.; Kasuga, T.; Londo, J.P.; Garrison, E.; Gaut, B.S.; et al. A Super-Pangenome of the North American Wild Grape Species. Genome Biol. 2023, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Su, Y.; Long, Q.; Peng, Y.; Shangguan, L.; Zhang, F.; Cao, S.; Wang, X.; Ge, M.; et al. Grapevine Pangenome Facilitates Trait Genetics and Genomic Breeding. Nat. Genet. 2024, 56, 2804–2814. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Q.; Li, W.; Yan, J.; Fernie, A.R. Raffinose Family Oligosaccharides: Crucial Regulators of Plant Development and Stress Responses. CRC Crit. Rev. Plant Sci. 2022, 41, 286–303. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, F.; Zheng, T.; Liu, Z.; Ji, X.; Zhang, Z.; Pervaiz, T.; Shangguan, L.; Fang, J. Whole-Genome Re-Sequencing, Diversity Analysis, and Stress-Resistance Analysis of 77 Grape Rootstock Genotypes. Front. Plant Sci. 2023, 14, 1102695. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Q.; Hao, Y.; Zhang, Y.; Gu, W.; Deng, Z.; Zhou, P.; Fang, Y.; Chen, K.; Zhang, K. Physiology and Transcriptome Profiling Reveal the Drought Tolerance of Five Grape Varieties under High Temperatures. J. Integr. Agric. 2024, in press. [Google Scholar] [CrossRef]
- Tan, J.W.; Shinde, H.; Tesfamicael, K.; Hu, Y.; Fruzangohar, M.; Tricker, P.; Baumann, U.; Edwards, E.J.; Rodríguez López, C.M. Global Transcriptome and Gene Co-Expression Network Analyses Reveal Regulatory and Non-Additive Effects of Drought and Heat Stress in Grapevine. Front. Plant Sci. 2023, 14, 1096225. [Google Scholar] [CrossRef]
- Dou, F.; Phillip, F.O.; Liu, G.; Zhu, J.; Zhang, L.; Wang, Y.; Liu, H. Transcriptomic and Physiological Analyses Reveal Different Grape Varieties Response to High Temperature Stress. Front. Plant Sci. 2024, 15, 1313832. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, Z.; Liu, Z.; Xia, R. Small RNAs, Emerging Regulators Critical for the Development of Horticultural Traits. Hortic. Res. 2018, 5, 63. [Google Scholar] [CrossRef]
- Ferrandino, A.; Pagliarani, C.; Pérez-Álvarez, E.P. Secondary Metabolites in Grapevine: Crosstalk of Transcriptional, Metabolic and Hormonal Signals Controlling Stress Defence Responses in Berries and Vegetative Organs. Front. Plant Sci. 2023, 14, 1124298. [Google Scholar] [CrossRef]
- Pagliarani, C.; Vitali, M.; Ferrero, M.; Vitulo, N.; Incarbone, M.; Lovisolo, C.; Valle, G.; Schubert, A. The Accumulation of MiRNAs Differentially Modulated by Drought Stress Is Affected by Grafting in Grapevine. Plant Physiol. 2017, 173, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, T.; Ju, Y.; Lei, Y.; Zhang, F.; Fang, Y.; Zhang, Z.; Jin, L.; Meng, J. MicroRNAs Behave Differently to Drought Stress in Drought-Tolerant and Drought-Sensitive Grape Genotypes. Environ. Exp. Bot. 2023, 207, 105223. [Google Scholar] [CrossRef]
- Maniatis, G.; Tani, E.; Katsileros, A.; Avramidou, E.V.; Pitsoli, T.; Sarri, E.; Gerakari, M.; Goufa, M.; Panagoulakou, M.; Xipolitaki, K.; et al. Genetic and Epigenetic Responses of Autochthonous Grapevine Cultivars from the ‘Epirus’ Region of Greece upon Consecutive Drought Stress. Plants 2023, 13, 27. [Google Scholar] [CrossRef]
- Zang, E.; Jiang, L.; Cui, H.; Li, X.; Yan, Y.; Liu, Q.; Chen, Z.; Li, M. Only Plant-Based Food Additives: An Overview on Application, Safety, and Key Challenges in the Food Industry. Food Rev. Int. 2023, 39, 5132–5163. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, M.; Feng, M.; Liu, G.; Torregrosa, L.; Tao, X.; Ren, R.; Fang, Y.; Zhang, Z.; Meng, J.; et al. MiR156b-Targeted VvSBP8/13 Functions Downstream of the Abscisic Acid Signal to Regulate Anthocyanins Biosynthesis in Grapevine Fruit under Drought. Hortic. Res. 2024, 11, uhad293. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, X.; Du, B.; Xiao, Y.; Wang, Y.; Sun, Y.; Zhou, X.; Wang, C.; Liu, Y.; Li, T.-H. MicroRNA156ab Regulates Apple Plant Growth and Drought Tolerance by Targeting Transcription Factor MsSPL13. Plant Physiol. 2023, 192, 1836–1857. [Google Scholar] [CrossRef]
- Wei, L.; Du, Y.; Xiang, J.; Zheng, T.; Cheng, J.; Wu, J. Integrated MRNA and MiRNA Transcriptome Analysis of Grape in Responses to Salt Stress. Front. Plant Sci. 2023, 14, 1173857. [Google Scholar] [CrossRef]
- Rooy, S.S.B.; Ghabooli, M.; Salekdeh, G.H.; Fard, E.M.; Karimi, R.; Fakhrfeshani, M.; Gholami, M. Identification of Novel Cold Stress Responsive MicroRNAs and Their Putative Targets in ‘Sultana’ Grapevine (Vitis vinifera) Using RNA Deep Sequencing. Acta Physiol. Plant 2023, 45, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, Z.; Li, X.; Ren, Z.; Xu, S.; Liu, Z.; Wang, K. 5-Azacytidine Enhances the Aluminum Tolerance of Grapes by Reducing the DNA Methylation Level. J. Soil. Sci. Plant Nutr. 2024, 24, 6922–6937. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Q.; Gichuki, D.K.; Hou, Y.; Liu, Y.; Zhou, H.; Xu, C.; Fang, L.; Gong, L.; Zheng, B.; et al. Genome-Wide Profiling of Histone H3 Lysine 27 Trimethylation and Its Modification in Response to Chilling Stress in Grapevine Leaves. Hortic. Plant J. 2023, 9, 496–508. [Google Scholar] [CrossRef]
- Ren, C.; Mohamed, M.S.M.; Aini, N.; Kuang, Y.; Liang, Z. CRISPR/Cas in Grapevine Genome Editing: The Best Is Yet to Come. Horticulturae 2024, 10, 965. [Google Scholar] [CrossRef]
- Benny, J.; Marchese, A.; Giovino, A.; Marra, F.P.; Perrone, A.; Caruso, T.; Martinelli, F. Gaining Insight into Exclusive and Common Transcriptomic Features Linked to Drought and Salinity Responses across Fruit Tree Crops. Plants 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Bazakos, C.; Manioudaki, M.E.; Therios, I.; Voyiatzis, D.; Kafetzopoulos, D.; Awada, T.; Kalaitzis, P. Comparative Transcriptome Analysis of Two Olive Cultivars in Response to NaCl-Stress. PLoS ONE 2012, 7, e42931. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, Epigenetic and Genetic Regulation in Some Olive Cultivars under Salt Stress. Sci. Rep. 2019, 9, 1093. [Google Scholar] [CrossRef]
- Ghirardo, A.; Nosenko, T.; Kreuzwieser, J.; Winkler, J.B.; Kruse, J.; Albert, A.; Merl-Pham, J.; Lux, T.; Ache, P.; Zimmer, I.; et al. Protein Expression Plasticity Contributes to Heat and Drought Tolerance of Date Palm. Oecologia 2021, 197, 903–919. [Google Scholar] [CrossRef]
- Mueller, H.M.; Franzisky, B.L.; Messerer, M.; Du, B.; Lux, T.; White, P.J.; Carpentier, S.C.; Winkler, J.B.; Schnitzler, J.; El-Serehy, H.A.; et al. Integrative Multi-omics Analyses of Date Palm (Phoenix dactylifera) Roots and Leaves Reveal How the Halophyte Land Plant Copes with Sea Water. Plant Genome 2024, 17, e20372. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, N.; Fu, H.; Wang, F.; Wen, M.; Chang, H.; Wu, J.; Abdelaala, W.B.; Luo, Q.; Li, Y.; et al. Salt Stress Modulates the Landscape of Transcriptome and Alternative Splicing in Date Palm (Phoenix dactylifera L.). Front. Plant Sci. 2022, 12, 807739. [Google Scholar] [CrossRef]
- Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W. Differential DNA Methylation and Transcription Profiles in Date Palm Roots Exposed to Salinity. PLoS ONE 2018, 13, e0191492. [Google Scholar] [CrossRef]
- Kumawat, S.; Sharma, Y.; Vats, S.; Sudhakaran, S.; Sharma, S.; Mandlik, R.; Raturi, G.; Kumar, V.; Rana, N.; Kumar, A.; et al. Understanding the Role of SWEET Genes in Fruit Development and Abiotic Stress in Pomegranate (Punica granatum L.). Mol. Biol. Rep. 2022, 49, 1329–1339. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Zhao, X.; Wang, J.; Gu, M.; Yuan, Z. Transcriptomic Profiling of Pomegranate Provides Insights into Salt Tolerance. Agronomy 2019, 10, 44. [Google Scholar] [CrossRef]
- Tang, H.; Wang, C.; Mei, J.; Feng, L.; Wu, Q.; Yin, Y. Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress. Agronomy 2024, 14, 2261. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Tong, R.; Wang, S.; Yao, J.; Jiao, J.; Wan, R.; Wang, M.; Shi, J.; Zheng, X. Overexpression of PgCBF3 and PgCBF7 Transcription Factors from Pomegranate Enhances Freezing Tolerance in Arabidopsis under the Promoter Activity Positively Regulated by PgICE1. Int. J. Mol. Sci. 2022, 23, 9439. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Chai, Y.; Hao, Q.; Ma, Y.; Wan, W.; Liu, H.; Diao, M. Transcriptomic and Physiological Analysis Reveals Crucial Biological Pathways Associated with Low-Temperature Stress in Tunisian Soft-Seed Pomegranate (Punica granatum L.). J. Plant Interact. 2023, 18, 2152887. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Zhang, L.; Zheng, J. A Genome-Wide Analysis of the BAM Gene Family and Identification of the Cold-Responsive Genes in Pomegranate (Punica granatum L.). Plants 2024, 13, 1321. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved Plant Resistance to Drought Is Promoted by the Root-associated Microbiome as a Water Stress-dependent Trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Salomon, M.V.; Purpora, R.; Bottini, R.; Piccoli, P. Rhizosphere Associated Bacteria Trigger Accumulation of Terpenes in Leaves of Vitis vinifera L. Cv. Malbec That Protect Cells against Reactive Oxygen Species. Plant Physiol. Biochem. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Saderi, S.; Corretto, E.; Mapelli, F.; Cherif, A.; Borin, S.; Valenti, L.; Sorlini, C.; Daffonchio, D. Root-Associated Bacteria Promote Grapevine Growth: From the Laboratory to the Field. Plant Soil. 2017, 410, 369–382. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Zamarreño, A.M.; Carmen Antolín, M. Mycorrhizal Symbiosis Affects ABA Metabolism during Berry Ripening in Vitis vinifera L. Cv. Tempranillo Grown under Climate Change Scenarios. Plant Sci. 2018, 274, 383–393. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular Mycorrhizal Fungi Enhance Drought Stress Tolerance by Regulating Osmotic Balance, the Antioxidant System, and the Expression of Drought-Responsive Genes in Vitis vinifera L. Aust. J. Grape Wine Res. 2023, 2023, 7208341. [Google Scholar] [CrossRef]
- Cardinale, M.; Minervini, F.; De Angelis, M.; Papadia, P.; Migoni, D.; Dimaglie, M.; Dinu, D.G.; Quarta, C.; Selleri, F.; Caccioppola, A.; et al. Vineyard Establishment under Exacerbated Summer Stress: Effects of Mycorrhization on Rootstock Agronomical Parameters, Leaf Element Composition and Root-Associated Bacterial Microbiota. Plant Soil. 2022, 478, 613–634. [Google Scholar] [CrossRef]
- Carreiras, J.; Cruz-Silva, A.; Fonseca, B.; Carvalho, R.C.; Cunha, J.P.; Proença Pereira, J.; Paiva-Silva, C.; Santos, S.A.; Janeiro Sequeira, R.; Mateos-Naranjo, E.; et al. Improving Grapevine Heat Stress Resilience with Marine Plant Growth-Promoting Rhizobacteria Consortia. Microorganisms 2023, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Zhong, H.; Patel, M.K.; Zhang, S.; Zhou, X.; Zhang, C.; Zhang, J.; Su, J.; Zhang, F.; Wu, X. Integrated Omics-Based Exploration for Temperature Stress Resilience: An Approach to Smart Grape Breeding Strategies. Plant Stress. 2024, 11, 100356. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A Global Microbiome Survey of Vineyard Soils Highlights the Microbial Dimension of Viticultural Terroirs. Commun. Biol. 2022, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Anand, L.; Gentimis, T.; Downie, A.B.; Lopez, C.M.R. Grapevine Rootstock and Scion Genotypes’ Symbiosis with Soil Microbiome: A Machine Learning Revelation for Climate-Resilient Viticulture 2024. bioRxiv 2024. [CrossRef]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 673810. [Google Scholar] [CrossRef]
- Colautti, A.; Mian, G.; Tomasi, D.; Bell, L.; Marcuzzo, P. Exploring the Influence of Soil Salinity on Microbiota Dynamics in Vitis vinifera Cv. “Glera”: Insights into the Rhizosphere, Carposphere, and Yield Outcomes. Diversity 2024, 16, 247. [Google Scholar] [CrossRef]
- Jatan, R.; Chauhan, P.S.; Lata, C. Pseudomonas Putida Modulates the Expression of MiRNAs and Their Target Genes in Response to Drought and Salt Stresses in Chickpea (Cicer arietinum L.). Genomics 2019, 111, 509–519. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-Term Effect of Epigenetic Modification in Plant–Microbe Interactions: Modification of DNA Methylation Induced by Plant Growth-Promoting Bacteria Mediates Promotion Process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef]
- Campos, C.; Coito, J.L.; Cardoso, H.; Marques da Silva, J.; Pereira, H.S.; Viegas, W.; Nogales, A. Dynamic Regulation of Grapevine’s MicroRNAs in Response to Mycorrhizal Symbiosis and High Temperature. Plants 2023, 12, 982. [Google Scholar] [CrossRef]
- Sportes, A.; Hériché, M.; Mounier, A.; Durney, C.; van Tuinen, D.; Trouvelot, S.; Wipf, D.; Courty, P.E. Comparative RNA Sequencing-Based Transcriptome Profiling of Ten Grapevine Rootstocks: Shared and Specific Sets of Genes Respond to Mycorrhizal Symbiosis. Mycorrhiza 2023, 33, 369–385. [Google Scholar] [CrossRef]
- Dias, M.C.; Silva, S.; Galhano, C.; Lorenzo, P. Olive Tree Belowground Microbiota: Plant Growth-Promoting Bacteria and Fungi. Plants 2024, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Azri, R.; Lamine, M.; Bensalem-Fnayou, A.; Hamdi, Z.; Mliki, A.; Ruiz-Lozano, J.M.; Aroca, R. Genotype-Dependent Response of Root Microbiota and Leaf Metabolism in Olive Seedlings Subjected to Drought Stress. Plants 2024, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of Arbuscular Mycorrhizal Fungi Inoculation on Growth and Physiology Performance of Olive Trees under Regulated Deficit Irrigation and Partial Rootzone Drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Tekaya, M.; Dabbaghi, O.; Guesmi, A.; Attia, F.; Chehab, H.; Khezami, L.; Algathami, F.K.; Ben Hamadi, N.; Hammami, M.; Prinsen, E.; et al. Arbuscular Mycorrhizas Modulate Carbohydrate, Phenolic Compounds and Hormonal Metabolism to Enhance Water Deficit Tolerance of Olive Trees (Olea europaea). Agric. Water Manag. 2022, 274, 107947. [Google Scholar] [CrossRef]
- Ben Hassena, A.; Zouari, M.; Trabelsi, L.; Decou, R.; Ben Amar, F.; Chaari, A.; Soua, N.; Labrousse, P.; Khabou, W.; Zouari, N. Potential Effects of Arbuscular Mycorrhizal Fungi in Mitigating the Salinity of Treated Wastewater in Young Olive Plants (Olea europaea L. Cv. Chetoui). Agric. Water Manag. 2021, 245, 106635. [Google Scholar] [CrossRef]
- Cherif, H.; Marasco, R.; Rolli, E.; Ferjani, R.; Fusi, M.; Soussi, A.; Mapelli, F.; Blilou, I.; Borin, S.; Boudabous, A.; et al. Oasis Desert Farming Selects Environment-specific Date Palm Root Endophytic Communities and Cultivable Bacteria That Promote Resistance to Drought. Environ. Microbiol. Rep. 2015, 7, 668–678. [Google Scholar] [CrossRef]
- Sabeem, M.; Abdul Aziz, M.; Mullath, S.K.; Brini, F.; Rouached, H.; Masmoudi, K. Enhancing Growth and Salinity Stress Tolerance of Date Palm Using Piriformospora Indica. Front. Plant Sci. 2022, 13, 1037273. [Google Scholar] [CrossRef]
- Ahmad, M.; Abdul Aziz, M.; Sabeem, M.; Kutty, M.S.; Sivasankaran, S.K.; Brini, F.; Xiao, T.T.; Blilou, I.; Masmoudi, K. Date Palm Transcriptome Analysis Provides New Insights on Changes in Response to High Salt Stress of Colonized Roots with the Endophytic Fungus Piriformospora Indica. Front. Plant Sci. 2024, 15, 1400215. [Google Scholar] [CrossRef]
- Jana, G.A.; Yaish, M.W. Isolation and Functional Characterization of a MVOC Producing Plant-Growth-Promoting Bacterium Isolated from the Date Palm Rhizosphere. Rhizosphere 2020, 16, 100267. [Google Scholar] [CrossRef]
- Bompadre, M.J.; Silvani, V.A.; Bidondo, L.F.; Ríos de Molina, M.D.C.; Colombo, R.P.; Pardo, A.G.; Godeas, A.M. Arbuscular Mycorrhizal Fungi Alleviate Oxidative Stress in Pomegranate Plants Growing under Different Irrigation Conditions. Botany 2014, 92, 187–193. [Google Scholar] [CrossRef]
- Diez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive Domestication and Diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Langgut, D.; Garfinkel, Y. 7000-Year-Old Evidence of Fruit Tree Cultivation in the Jordan Valley, Israel. Sci. Rep. 2022, 12, 7463. [Google Scholar] [CrossRef] [PubMed]
- Cardoni, M.; Mercado-Blanco, J. Confronting Stresses Affecting Olive Cultivation from the Holobiont Perspective. Front. Plant Sci. 2023, 14, 1261754. [Google Scholar] [CrossRef]
- El Yamani, M.; Cordovilla, M.d.P. Tolerance Mechanisms of Olive Tree (Olea europaea) under Saline Conditions. Plants 2024, 13, 2094. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Hrameche, O.; Tul, S.; Manolikaki, I.; Digalaki, N.; Kaltsa, I.; Psarras, G.; Koubouris, G. Optimizing Agroecological Measures for Climate-Resilient Olive Farming in the Mediterranean. Plants 2024, 13, 900. [Google Scholar] [CrossRef]
- Nteve, G.-M.; Kostas, S.; Polidoros, A.N.; Madesis, P.; Nianiou-Obeidat, I. Adaptation Mechanisms of Olive Tree under Drought Stress: The Potential of Modern Omics Approaches. Agriculture 2024, 14, 579. [Google Scholar] [CrossRef]
- De Ollas, C.; Morillón, R.; Fotopoulos, V.; Puértolas, J.; Ollitrault, P.; Gómez-Cadenas, A.; Arbona, V. Facing Climate Change: Biotechnology of Iconic Mediterranean Woody Crops. Front. Plant Sci. 2019, 10, 427. [Google Scholar] [CrossRef]
- Bazakos, C.; Alexiou, K.G.; Ramos-Onsins, S.; Koubouris, G.; Tourvas, N.; Xanthopoulou, A.; Mellidou, I.; Moysiadis, T.; Vourlaki, I.; Metzidakis, I.; et al. Whole Genome Scanning of a Mediterranean Basin Hotspot Collection Provides New Insights into Olive Tree Biodiversity and Biology. Plant J. 2023, 116, 303–319. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Valeri, M.C.; Regni, L.; Lilli, E.; Albertini, E.; Proietti, P.; Businelli, D.; Baldoni, L. Characterization of Differentially Expressed Genes under Salt Stress in Olive. Int. J. Mol. Sci. 2021, 23, 154. [Google Scholar] [CrossRef]
- Bullones, A.; Castro, A.J.; Lima-Cabello, E.; Alché, J.d.D.; Luque, F.; Claros, M.G.; Fernandez-Pozo, N. OliveAtlas: A Gene Expression Atlas Tool for Olea europaea. Plants 2023, 12, 1274. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Araújo, M.; Ma, Y. A Bio-Based Strategy for Sustainable Olive Performance under Water Deficit Conditions. Plant Stress. 2024, 11, 100342. [Google Scholar] [CrossRef]
- Galicia-Campos, E.; García-Villaraco, A.; Montero-Palmero, M.B.; Gutiérrez-Mañero, F.J.; Ramos-Solano, B. Bacillus G7 Improves Adaptation to Salt Stress in Olea europaea L. Plantlets, Enhancing Water Use Efficiency and Preventing Oxidative Stress. Sci. Rep. 2023, 13, 22507. [Google Scholar] [CrossRef]
- Sallami, A.; Rachidi, F.; Lahsini, A.I.; El Khedri, H.; Douira, A.; El Modafar, C.; Medraoui, L.; Filali-Maltouf, A. Plant Growth Promoting (PGP) Performances and Diversity of Bacterial Species Isolated from Olive (Olea europaea L.) Rhizosphere in Arid and Semi-Arid Regions of Morocco. J. Pure Appl. Microbiol. 2023, 17, 2165–2178. [Google Scholar] [CrossRef]
- Malacrinò, A.; Mosca, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Schena, L. Plant Genotype Shapes the Bacterial Microbiome of Fruits, Leaves, and Soil in Olive Plants. Plants 2022, 11, 613. [Google Scholar] [CrossRef]
- Vita, F.; Sabbatini, L.; Sillo, F.; Ghignone, S.; Vergine, M.; Guidi Nissim, W.; Fortunato, S.; Salzano, A.M.; Scaloni, A.; Luvisi, A.; et al. Salt Stress in Olive Tree Shapes Resident Endophytic Microbiota. Front. Plant Sci. 2022, 13, 992395. [Google Scholar] [CrossRef]
- Kakagianni, M.; Tsiknia, M.; Feka, M.; Vasileiadis, S.; Leontidou, K.; Kavroulakis, N.; Karamanoli, K.; Karpouzas, D.G.; Ehaliotis, C.; Papadopoulou, K.K. Above- and below-Ground Microbiome in the Annual Developmental Cycle of Two Olive Tree Varieties. FEMS Microbes 2023, 4, xtad001. [Google Scholar] [CrossRef]
- Vergine, M.; Vita, F.; Casati, P.; Passera, A.; Ricciardi, L.; Pavan, S.; Aprile, A.; Sabella, E.; De Bellis, L.; Luvisi, A. Characterization of the Olive Endophytic Community in Genotypes Displaying a Contrasting Response to Xylella Fastidiosa. BMC Plant Biol. 2024, 24, 337. [Google Scholar] [CrossRef]
- Chao, C.T.; Krueger, R.R. The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarray, S.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Climate and Development Modulate the Metabolome and Antioxidative System of Date Palm Leaves. J. Exp. Bot. 2019, 70, 5959–5969. [Google Scholar] [CrossRef]
- Arab, L.; Kreuzwieser, J.; Kruse, J.; Zimmer, I.; Ache, P.; Alfarraj, S.; Al-Rasheid, K.A.S.; Schnitzler, J.-P.; Hedrich, R.; Rennenberg, H. Acclimation to Heat and Drought—Lessons to Learn from the Date Palm (Phoenix dactylifera). Environ. Exp. Bot. 2016, 125, 20–30. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Flowers, J.M.; Nelson, D.; Lemansour, A.; Masmoudi, K.; Amiri, K.M.A. Prospects for the Study and Improvement of Abiotic Stress Tolerance in Date Palms in the Post-Genomics Era. Front. Plant Sci. 2020, 11, 293. [Google Scholar] [CrossRef]
- Rahman, H.; Vikram, P.; Hammami, Z.; Singh, R.K. Recent Advances in Date Palm Genomics: A Comprehensive Review. Front. Genet. 2022, 13, 959266. [Google Scholar] [CrossRef]
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.M.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-Wide Expression Profiling in Leaves and Roots of Date Palm (Phoenix dactylifera L.) Exposed to Salinity. BMC Genom. 2017, 18, 246. [Google Scholar] [CrossRef]
- Safronov, O.; Kreuzwieser, J.; Haberer, G.; Alyousif, M.S.; Schulze, W.; Al-Harbi, N.; Arab, L.; Ache, P.; Stempfl, T.; Kruse, J.; et al. Detecting Early Signs of Heat and Drought Stress in Phoenix dactylifera (Date Palm). PLoS ONE 2017, 12, e0177883. [Google Scholar] [CrossRef]
- Rekik, I.; Chaâbene, Z.; Kriaa, W.; Rorat, A.; Franck, V.; Hafedh, M.; Elleuch, A. Transcriptome Assembly and Abiotic Related Gene Expression Analysis of Date Palm Reveal Candidate Genes Involved in Response to Cadmium Stress. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2019, 225, 108569. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Metabolic Responses of Date Palm (Phoenix dactylifera L.) Leaves to Drought Differ in Summer and Winter Climate. Tree Physiol. 2021, 41, 1685–1700. [Google Scholar] [CrossRef]
- Yaish, M.W.; Sunkar, R.; Zheng, Y.; Ji, B.; Al-Yahyai, R.; Farooq, S.A. A Genome-Wide Identification of the MiRNAome in Response to Salinity Stress in Date Palm (Phoenix dactylifera L.). Front. Plant Sci. 2015, 6, 946. [Google Scholar] [CrossRef]
- Kordrostami, M.; Mafakheri, M.; Al-Khayri, J.M. Date Palm (Phoenix dactylifera L.) Genetic Improvement via Biotechnological Approaches. Tree Genet. Genomes 2022, 18, 26. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Gros-Balthazard, M.; Flowers, J.M.; Copetti, D.; Lemansour, A.; Lebrun, M.; Masmoudi, K.; Ferrand, S.; Dhar, M.I.; Fresquez, Z.A.; et al. Genome-Wide Association Mapping of Date Palm Fruit Traits. Nat. Commun. 2019, 10, 4680. [Google Scholar] [CrossRef]
- Younuskunju, S.; Mohamoud, Y.A.; Mathew, L.S.; Mayer, K.F.X.; Suhre, K.; Malek, J.A. Genomic Analysis of Date Palm Fruit Size Traits and Identification of Candidate Genes through GWAS 2025. bioRxiv 2025. [CrossRef]
- Kumar, K.; Amaresan, N.; Bhagat, S.; Madhuri, K.; Srivastava, R.C. Isolation and Characterization of Rhizobacteria Associated with Coastal Agricultural Ecosystem of Rhizosphere Soils of Cultivated Vegetable Crops. World J. Microbiol. Biotechnol. 2011, 27, 1625–1632. [Google Scholar] [CrossRef]
- Yaish, M.W.; Al-Harrasi, I.; Alansari, A.S.; Al-Yahyai, R.; Glick, B.R. The Use of High Throughput DNA Sequence Analysis to Assess the Endophytic Microbiome of Date Palm Roots Grown under Different Levels of Salt Stress. Int. Microbiol. 2016, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Franzisky, B.L.; Muhammad, W.; Alfarraj, S.; Geilfus, C.; Rennenberg, H. How to Cope With Stress in the Desert—The Date Palm Approach. Plant Cell Environ. 2025, 48, 768–780. [Google Scholar] [CrossRef]
- Khayyat, M.; Vazifeshenas, M.; Akbari, M. Pomegranate and Salt Stress Responses—Assimilation Activities and Chlorophyll Fluorescence Performances: A Review. Appl. Fruit. Sci. 2024, 66, 2455–2468. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, X.; Yan, Y.; Wang, N.; Ge, W.; Zhou, Q.; Yang, Y. Unravelling the Molecular Mechanisms of Abscisic Acid-Mediated Drought-Stress Alleviation in Pomegranate (Punica granatum L.). Plant Physiol. Biochem. 2020, 157, 211–218. [Google Scholar] [CrossRef]
- Adiba, A.; Haddioui, A.; Hamdani, A.; El Kettabi, Z.; Outghouliast, H.; Charafi, J. Impact of Contrasting Climate Conditions on Pomegranate Development and Productivity: Implications for Breeding and Cultivar Selection in Colder Environments. Vegetos 2024. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars. Plant Signal Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Eom, J.-S.; Chen, L.-Q.; Sosso, D.; Julius, B.T.; Lin, I.; Qu, X.-Q.; Braun, D.M.; Frommer, W.B. SWEETs, Transporters for Intracellular and Intercellular Sugar Translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, J.; Chen, W.; Li, Y.; Lü, Y.; Guo, Y.; Wang, J.; Yuan, L.; Liu, Z.; Zhang, Y. A Genome-Wide Analysis of SWEET Gene Family in Cotton and Their Expressions under Different Stresses. J. Cotton Res. 2018, 1, 7. [Google Scholar] [CrossRef]
- Feng, C.-Y.; Han, J.-X.; Han, X.-X.; Jiang, J. Genome-Wide Identification, Phylogeny, and Expression Analysis of the SWEET Gene Family in Tomato. Gene 2015, 573, 261–272. [Google Scholar] [CrossRef]
- Qin, G.; Xu, C.; Ming, R.; Tang, H.; Guyot, R.; Kramer, E.M.; Hu, Y.; Yi, X.; Qi, Y.; Xu, X.; et al. The Pomegranate (Punica granatum L.) Genome and the Genomics of Punicalagin Biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef]
- Roopa Sowjanya, P.; Shilpa, P.; Patil, G.P.; Babu, D.K.; Sharma, J.; Sangnure, V.R.; Mundewadikar, D.M.; Natarajan, P.; Marathe, A.R.; Reddy, U.K.; et al. Reference Quality Genome Sequence of Indian Pomegranate Cv. ‘Bhagawa’ (Punica granatum L.). Front. Plant Sci. 2022, 13, 947164. [Google Scholar] [CrossRef]
- Yuan, Z.; Fang, Y.; Zhang, T.; Fei, Z.; Han, F.; Liu, C.; Liu, M.; Xiao, W.; Zhang, W.; Wu, S.; et al. The Pomegranate (Punica granatum L.) Genome Provides Insights into Fruit Quality and Ovule Developmental Biology. Plant Biotechnol. J. 2018, 16, 1363–1374. [Google Scholar] [CrossRef]
- Patankar, H.V.; Rivera, L.F.; Alzahrani, F.O.; Wing, R.A.; Blilou, I. A Chromosome Level Assembly of Pomegranate (Punica granatum L.) Variety Grown in Arid Environment. Sci. Data 2025, 12, 73. [Google Scholar] [CrossRef]
- Luo, X.; Shua, Z.; Zhao, D.; Liu, B.; Luo, H.; Chen, Y.; Meng, D.; Song, Z.; Yang, Q.; Wang, Z.; et al. Genome Assembly of Pomegranate Highlights Structural Variations Driving Population Differentiation and Key Loci Underpinning Cold Adaption. Hortic. Res. 2025, uhaf022. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.; Hong, X.; Agarwal, M.; Zhu, J.-K. ICE1: A Regulator of Cold-Induced Transcriptome and Freezing Tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lu, R.; Feng, L.; Zheng, M.; Zhang, H.; Yin, Y.; Zheng, L. Functional Characterization of Pomegranate CAMTA3 in Cold Stress Responses. Plants 2025, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, C.; Qi, X.; Chen, J.; Zhong, Y.; Muhammad, A.; Lin, M.; Fang, J. The AaCBF4-AaBAM3.1 Module Enhances Freezing Tolerance of Kiwifruit (Actinidia arguta). Hortic. Res. 2021, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Ravinath, R.; Das, A.J.; Usha, T.; Ramesh, N.; Middha, S.K. Targeted Metagenome Sequencing Reveals the Abundance of Planctomycetes and Bacteroidetes in the Rhizosphere of Pomegranate. Arch. Microbiol. 2022, 204, 481. [Google Scholar] [CrossRef]
- Hwarari, D.; Radani, Y.; Ke, Y.; Chen, J.; Yang, L. CRISPR/Cas Genome Editing in Plants: Mechanisms, Applications, and Overcoming Bottlenecks. Funct. Integr. Genom. 2024, 24, 50. [Google Scholar] [CrossRef]
- Mishra, S.; Nayak, S.; Tuteja, N.; Poosapati, S.; Swain, D.M.; Sahoo, R.K. CRISPR/Cas-Mediated Genome Engineering in Plants: Application and Prospectives. Plants 2024, 13, 1884. [Google Scholar] [CrossRef]
- Corbin, K.R.; Bolt, B.; Rodríguez López, C.M. Breeding for Beneficial Microbial Communities Using Epigenomics. Front. Microbiol. 2020, 11, 937. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapazoglou, A.; Tani, E.; Papasotiropoulos, V.; Letsiou, S.; Gerakari, M.; Abraham, E.; Bebeli, P.J. Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change. Int. J. Mol. Sci. 2025, 26, 3160. https://doi.org/10.3390/ijms26073160
Kapazoglou A, Tani E, Papasotiropoulos V, Letsiou S, Gerakari M, Abraham E, Bebeli PJ. Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change. International Journal of Molecular Sciences. 2025; 26(7):3160. https://doi.org/10.3390/ijms26073160
Chicago/Turabian StyleKapazoglou, Aliki, Eleni Tani, Vasileios Papasotiropoulos, Sophia Letsiou, Maria Gerakari, Eleni Abraham, and Penelope J. Bebeli. 2025. "Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change" International Journal of Molecular Sciences 26, no. 7: 3160. https://doi.org/10.3390/ijms26073160
APA StyleKapazoglou, A., Tani, E., Papasotiropoulos, V., Letsiou, S., Gerakari, M., Abraham, E., & Bebeli, P. J. (2025). Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change. International Journal of Molecular Sciences, 26(7), 3160. https://doi.org/10.3390/ijms26073160











