Role of Extracellular Vesicles in TSC Renal Cystogenesis
Abstract
1. Introduction
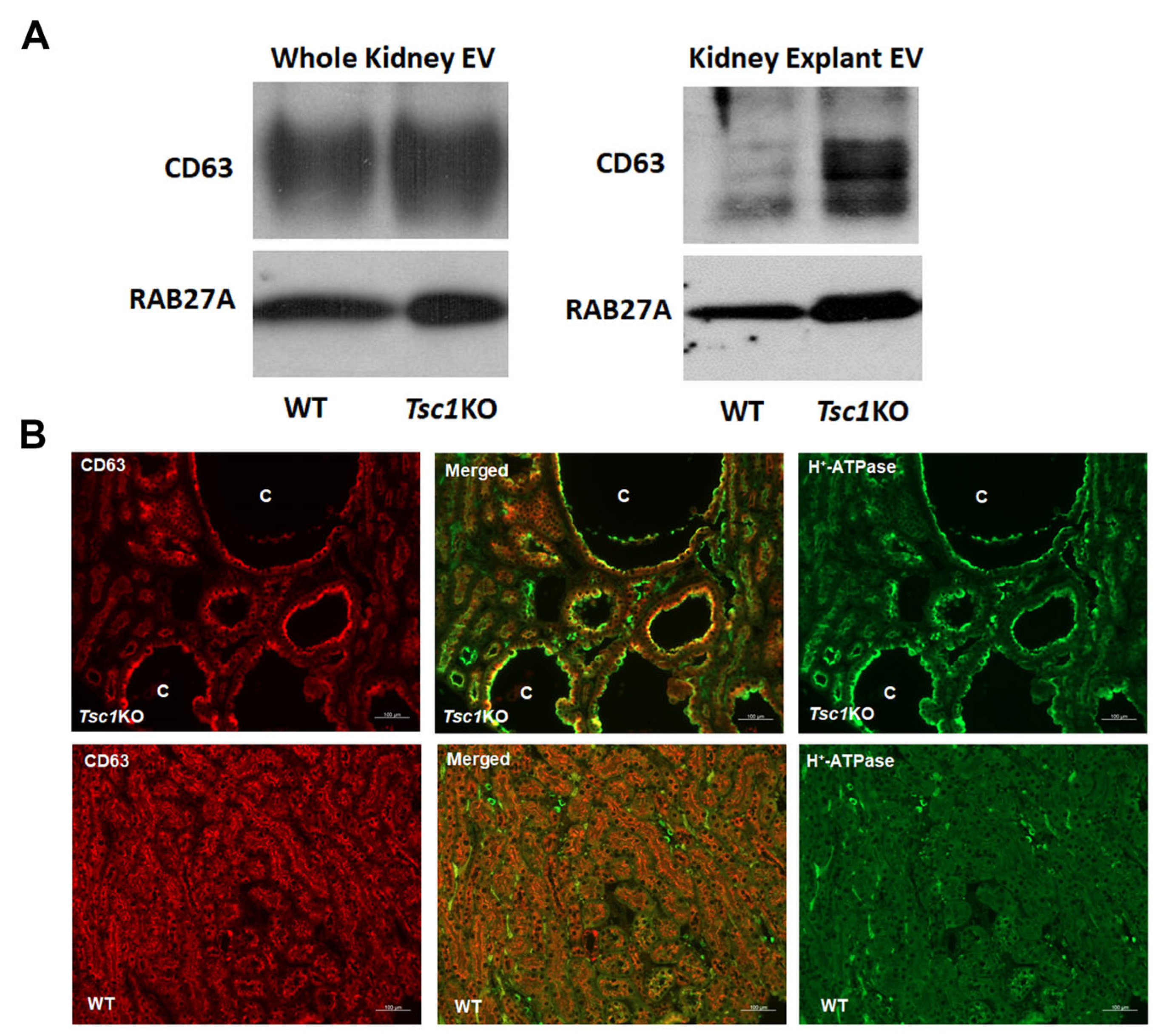
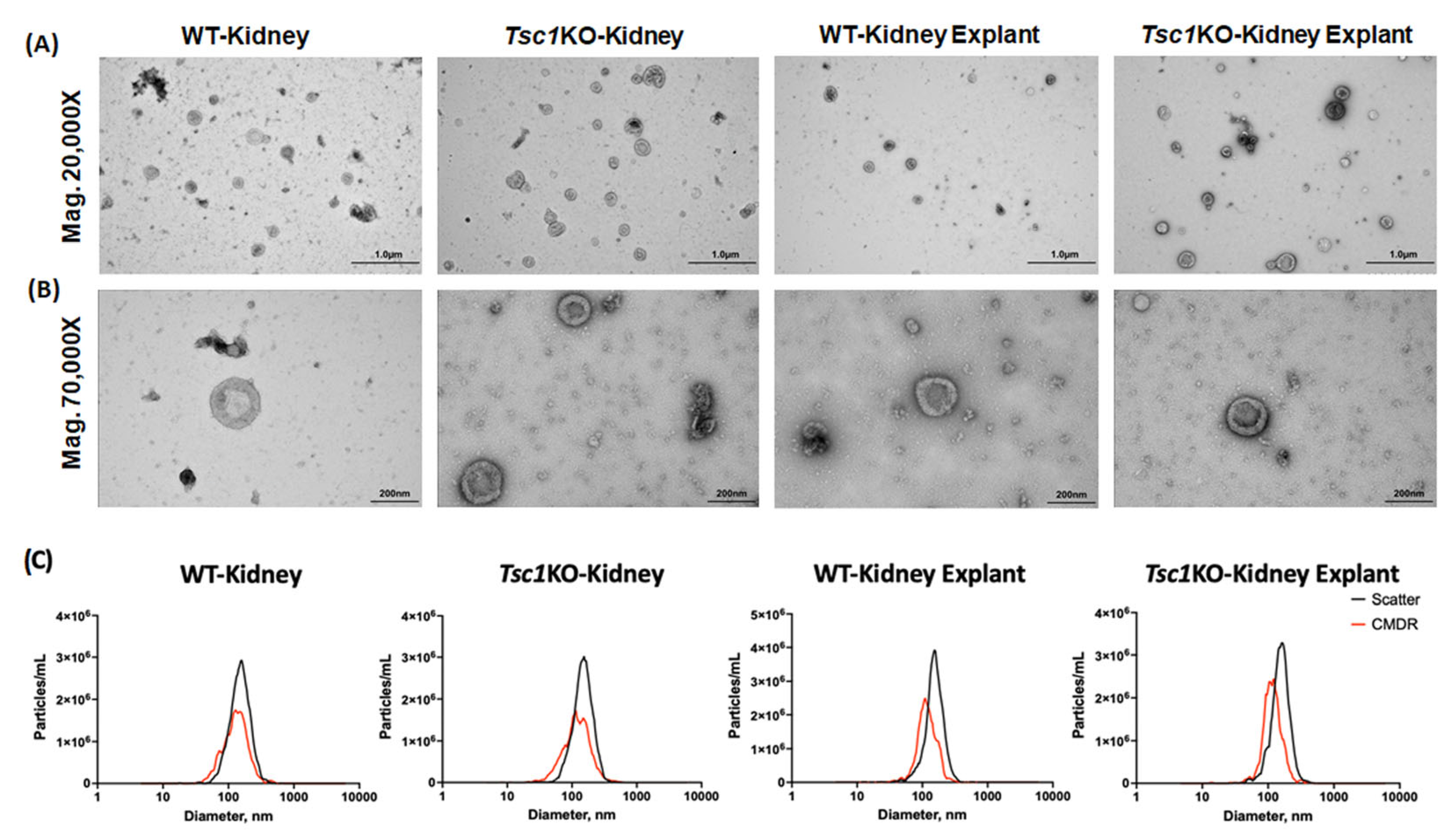
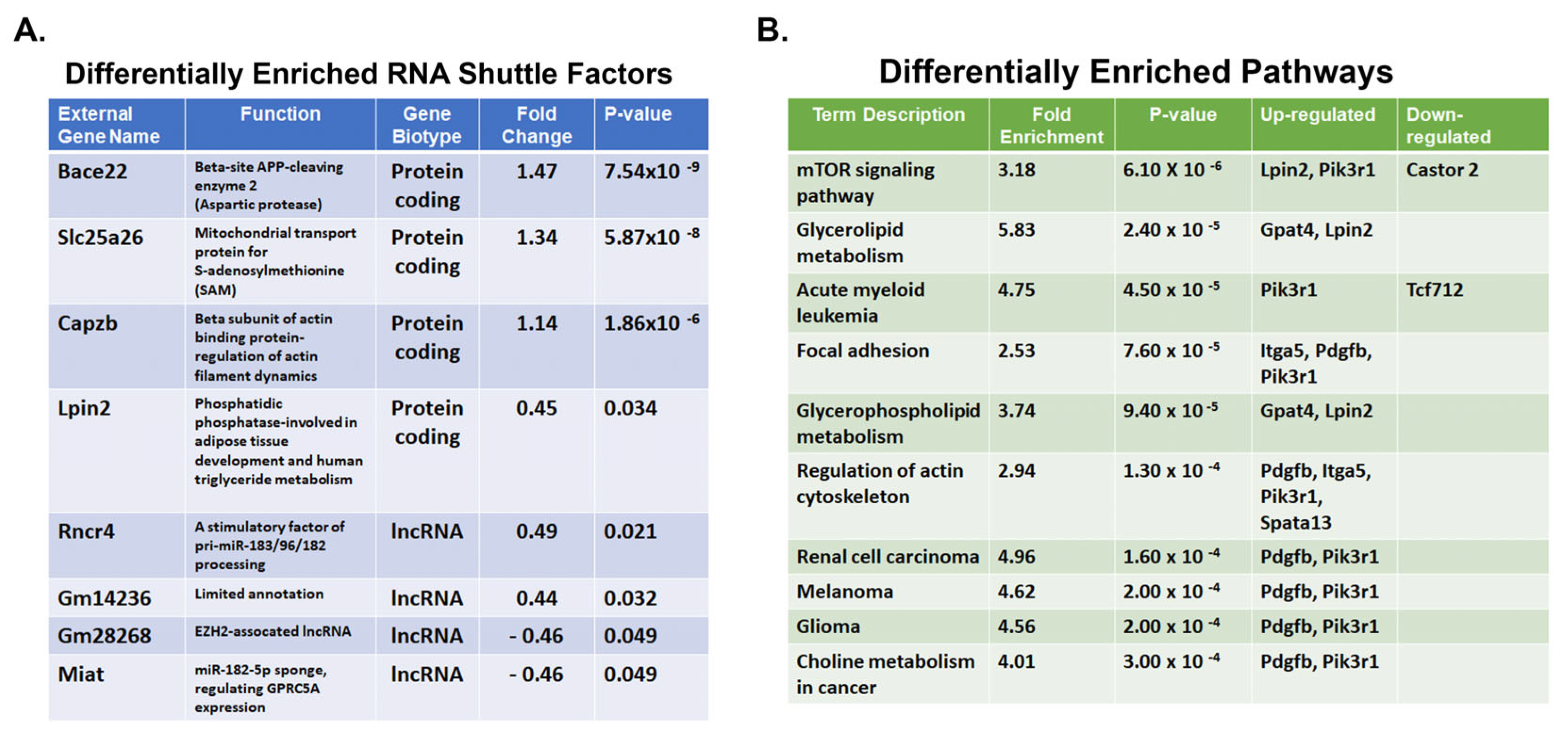
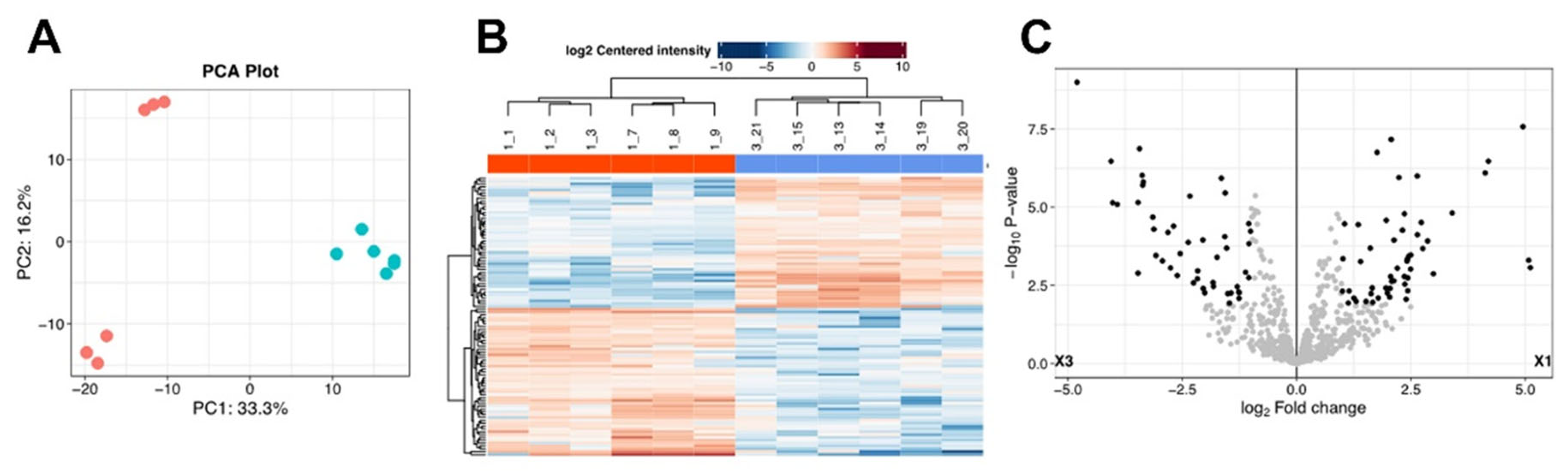
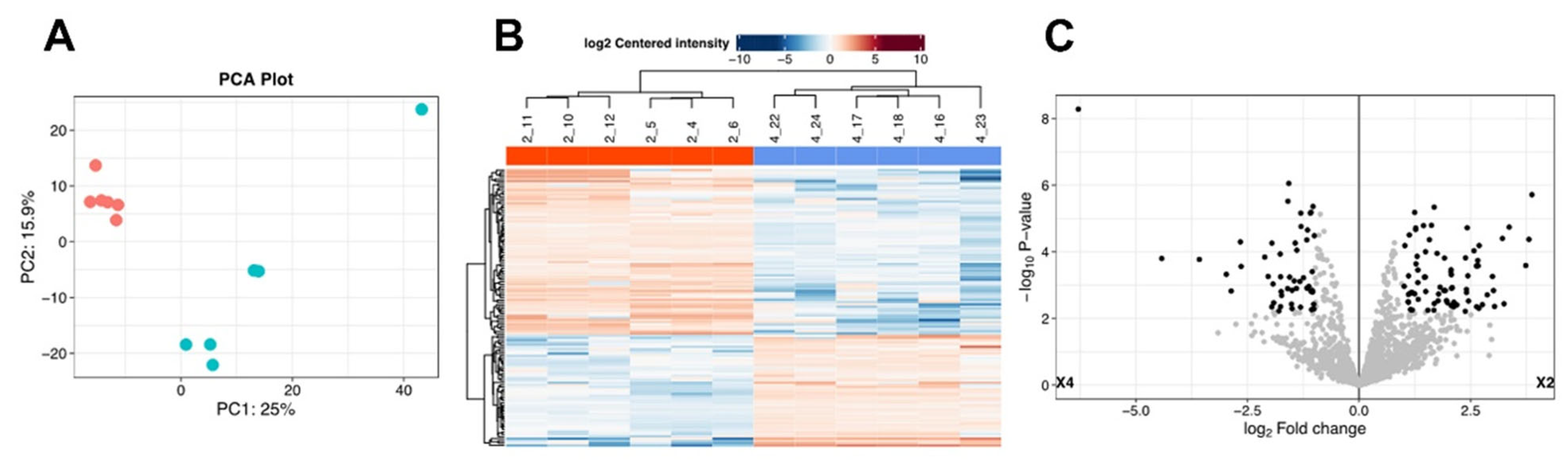
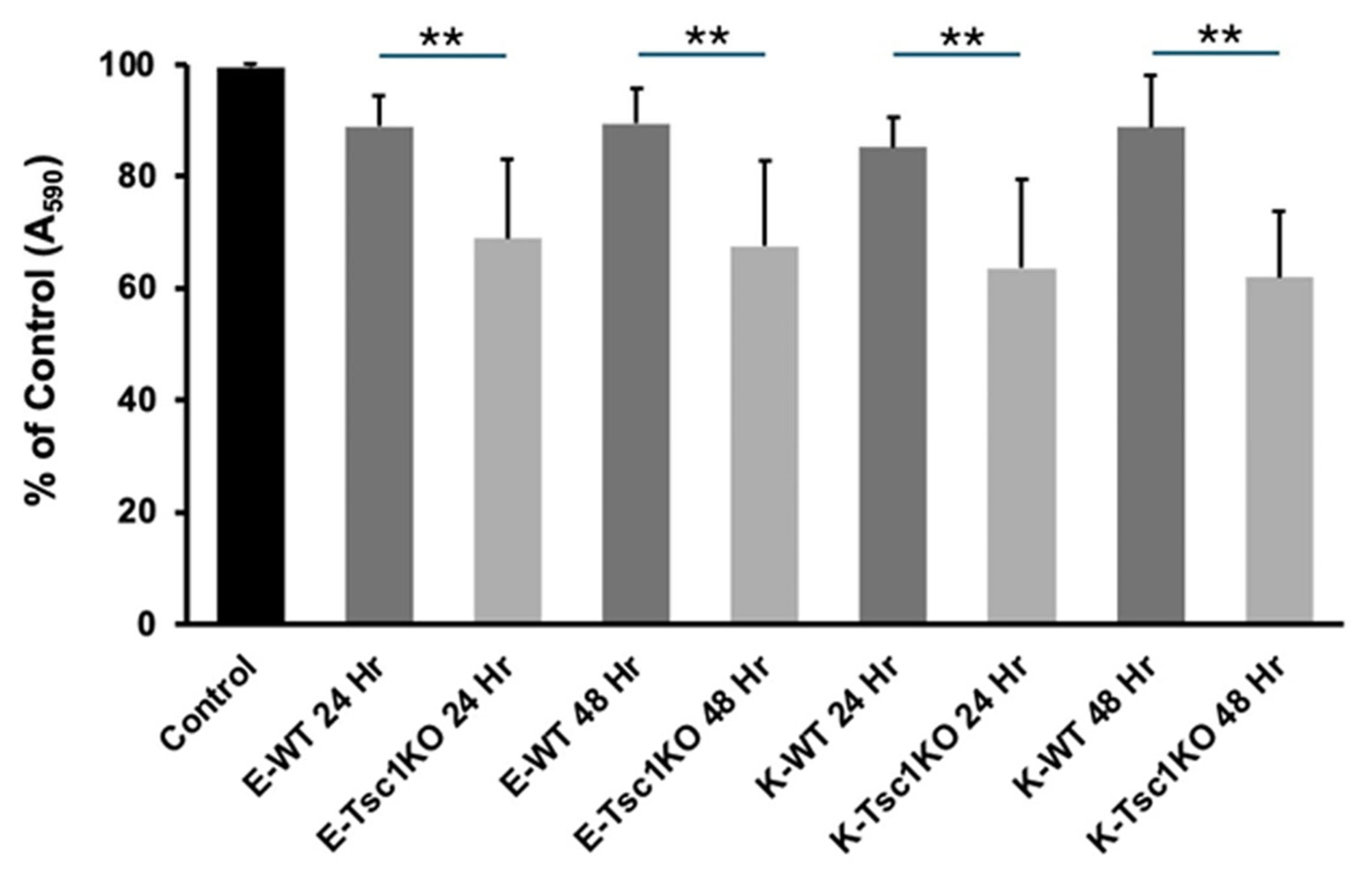
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of EVs
4.3. Western Blot Analyses
4.4. Immunofluorescence Microscopy
4.5. Transmission Electron Microscopy (TEM)
4.6. DLS and fNTA Analysis of EV Preparations
4.7. Examination of EV Shuttle Transcriptomes
4.8. Examination of EV Shuttle Proteomes
4.9. Cell Proliferation Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-IC | A-Intercalated Cells |
| AML | Angiomyolipoma |
| ADPKD | Autosomal Dominant Polycystic Kidney Disease |
| CCD | Cortical Collecting Duct |
| DLS | Dynamic Light Scattering |
| EV | Extracellular Vesicle |
| fTNA | Fluorescence Nanoparticle Tracking |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| PC | Principal Cell |
| PCA | Principal Component Analysis |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| TEM | Transmission Electron Microscopy |
| TSC | Tuberous Sclerosis Complex |
| TSC1 | Hamartin |
| TSC2 | Tuberin |
| WT | Wildtype Mice |
References
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Dabora, S.L.; Jozwiak, S.; Franz, D.N.; Roberts, P.S.; Nieto, A.; Chung, J.; Choy, Y.S.; Reeve, M.P.; Thiele, E.; Egelhoff, J.C.; et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001, 68, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Henske, E.P.; Jozwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016, 2, 16035. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.R.; Harris, P.C. The molecular genetics of tuberous sclerosis. Hum. Mol. Genet. 1994, 3, 1477–1480. [Google Scholar] [CrossRef]
- Rakowski, S.K.; Winterkorn, E.B.; Paul, E.; Steele, D.J.; Halpern, E.F.; Thiele, E.A. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int. 2006, 70, 1777–1782. [Google Scholar] [CrossRef]
- Dibble, C.C.; Manning, B.D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 2013, 15, 555–564. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef]
- Lam, H.C.; Siroky, B.J.; Henske, E.P. Renal disease in tuberous sclerosis complex: Pathogenesis and therapy. Nat. Rev. Nephrol. 2018, 14, 704–716. [Google Scholar] [CrossRef]
- Dixon, B.P.; Hulbert, J.C.; Bissler, J.J. Tuberous sclerosis complex renal disease. Nephron Exp. Nephrol. 2011, 118, e15–e20. [Google Scholar] [CrossRef]
- Henske, E.P.; Rasooly, R.; Siroky, B.; Bissler, J. Tuberous sclerosis complex, mTOR, and the kidney: Report of an NIDDK-sponsored workshop. Am. J. Physiol. Renal Physiol. 2014, 306, F279–F283. [Google Scholar] [CrossRef]
- Bissler, J.J.; Christopher Kingswood, J. Renal manifestation of tuberous sclerosis complex. Am. J. Med. Genet. C Semin. Med. Genet. 2018, 178, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; McCormack, F.X.; Young, L.R.; Elwing, J.M.; Chuck, G.; Leonard, J.M.; Schmithorst, V.J.; Laor, T.; Brody, A.S.; Bean, J.; et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008, 358, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Zahedi, K.; Brooks, M.; Henske, E.P.; Yang, Y.; Zhang, E.; Bissler, J.J.; Yu, J.J.; Soleimani, M. Kidney intercalated cells and the transcription factor FOXi1 drive cystogenesis in tuberous sclerosis complex. Proc. Natl. Acad. Sci. USA 2021, 118, e2020190118. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Zadjali, F.; Bridges, D.; Astrinidis, A.; Barone, S.; Yao, Y.; Redd, J.R.; Siroky, B.J.; Wang, Y.; Finley, J.T.; et al. Tuberous sclerosis complex exhibits a new renal cystogenic mechanism. Physiol. Rep. 2019, 7, e13983. [Google Scholar] [CrossRef]
- Bonsib, S.M.; Boils, C.; Gokden, N.; Grignon, D.; Gu, X.; Higgins, J.P.; Leroy, X.; McKenney, J.K.; Nasr, S.H.; Phillips, C.; et al. Tuberous sclerosis complex: Hamartin and tuberin expression in renal cysts and its discordant expression in renal neoplasms. Pathol. Res. Pract. 2016, 212, 972–979. [Google Scholar] [CrossRef]
- Onda, H.; Lueck, A.; Marks, P.W.; Warren, H.B.; Kwiatkowski, D.J. Tsc2+/− mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Investig. 1999, 104, 687–695. [Google Scholar] [CrossRef]
- Carbonara, C.; Longa, L.; Grosso, E.; Borrone, C.; Garre, M.G.; Brisigotti, M.; Migone, N. 9q34 loss of heterozygosity in a tuberous sclerosis astrocytoma suggests a growth suppressor-like activity also for the TSC1 gene. Hum. Mol. Genet. 1994, 3, 1829–1832. [Google Scholar] [CrossRef]
- Henske, E.P.; Neumann, H.P.; Scheithauer, B.W.; Herbst, E.W.; Short, M.P.; Kwiatkowski, D.J. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer 1995, 13, 295–298. [Google Scholar] [CrossRef]
- Green, A.J.; Smith, M.; Yates, J.R. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat. Genet. 1994, 6, 193–196. [Google Scholar] [CrossRef]
- Henske, E.P.; Scheithauer, B.W.; Short, M.P.; Wollmann, R.; Nahmias, J.; Hornigold, N.; van Slegtenhorst, M.; Welsh, C.T.; Kwiatkowski, D.J. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am. J. Hum. Genet. 1996, 59, 400–406. [Google Scholar]
- Henske, E.P.; Wessner, L.L.; Golden, J.; Scheithauer, B.W.; Vortmeyer, A.O.; Zhuang, Z.; Klein-Szanto, A.J.; Kwiatkowski, D.J.; Yeung, R.S. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am. J. Pathol. 1997, 151, 1639–1647. [Google Scholar] [PubMed]
- Richardson, E.P., Jr. Pathology of tuberous sclerosis. Neuropathologic aspects. Ann. N. Y. Acad. Sci. 1991, 615, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Niida, Y.; Stemmer-Rachamimov, A.O.; Logrip, M.; Tapon, D.; Perez, R.; Kwiatkowski, D.J.; Sims, K.; MacCollin, M.; Louis, D.N.; Ramesh, V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am. J. Hum. Genet. 2001, 69, 493–503. [Google Scholar] [CrossRef]
- Qin, W.; Chan, J.A.; Vinters, H.V.; Mathern, G.W.; Franz, D.N.; Taillon, B.E.; Bouffard, P.; Kwiatkowski, D.J. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010, 20, 1096–1105. [Google Scholar] [CrossRef]
- Kumar, P.; Zadjali, F.; Yao, Y.; Bissler, J.J. Renal cystic disease in tuberous sclerosis complex. Exp. Biol. Med. 2021, 246, 2111–2117. [Google Scholar] [CrossRef]
- Patel, B.; Patel, J.; Cho, J.H.; Manne, S.; Bonala, S.; Henske, E.; Roegiers, F.; Markiewski, M.; Karbowniczek, M. Exosomes mediate the acquisition of the disease phenotypes by cells with normal genome in tuberous sclerosis complex. Oncogene 2016, 35, 3027–3036. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Hershfield, M.R.; Linsenbardt, H.R.; Smith, J.; Mack, J.; Natesan, S.; Averitt, D.L.; Stark, T.R.; Sosanya, N.M. Biological function of Extracellular Vesicles (EVs): A review of the field. Mol. Biol. Rep. 2023, 50, 8639–8651. [Google Scholar] [CrossRef]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589 Pt 24, 6119–6127. [Google Scholar] [CrossRef]
- Kumar, P.; Zadjali, F.; Yao, Y.; Johnson, D.; Siroky, B.; Astrinidis, A.; Vogel, P.; Gross, K.W.; Bissler, J.J. Tsc2 mutation induces renal tubular cell nonautonomous disease. Genes Dis. 2022, 9, 187–200. [Google Scholar] [CrossRef]
- Kumar, P.; Zadjali, F.; Yao, Y.; Siroky, B.; Astrinidis, A.; Gross, K.W.; Bissler, J.J. Tsc Gene Locus Disruption and Differences in Renal Epithelial Extracellular Vesicles. Front. Physiol. 2021, 12, 630933. [Google Scholar] [CrossRef] [PubMed]
- Bhaoighill, M.N.; Falcon-Perez, J.M.; Royo, F.; Tee, A.R.; Webber, J.P.; Dunlop, E.A. Tuberous Sclerosis Complex cell-derived EVs have an altered protein cargo capable of regulating their microenvironment and have potential as disease biomarkers. J. Extracell. Vesicles 2023, 12, e12336. [Google Scholar] [CrossRef] [PubMed]
- Zadjali, F.; Kumar, P.; Yao, Y.; Johnson, D.; Astrinidis, A.; Vogel, P.; Gross, K.W.; Bissler, J.J. Tuberous Sclerosis Complex Axis Controls Renal Extracellular Vesicle Production and Protein Content. Int. J. Mol. Sci. 2020, 21, 1729. [Google Scholar] [CrossRef]
- Giannikou, K.; Malinowska, I.A.; Pugh, T.J.; Yan, R.; Tseng, Y.Y.; Oh, C.; Kim, J.; Tyburczy, M.E.; Chekaluk, Y.; Liu, Y.; et al. Whole Exome Sequencing Identifies TSC1/TSC2 Biallelic Loss as the Primary and Sufficient Driver Event for Renal Angiomyolipoma Development. PLoS Genet. 2016, 12, e1006242. [Google Scholar] [CrossRef]
- Badenas, C.; Torra, R.; Perez-Oller, L.; Mallolas, J.; Talbot-Wright, R.; Torregrosa, V.; Darnell, A. Loss of heterozygosity in renal and hepatic epithelial cystic cells from ADPKD1 patients. Eur. J. Hum. Genet. 2000, 8, 487–492. [Google Scholar] [CrossRef]
- Brasier, J.L.; Henske, E.P. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J. Clin. Investig. 1997, 99, 194–199. [Google Scholar] [CrossRef]
- Barone, S.; Brooks, M.; Zahedi, K.; Holliday, L.S.; Bissler, J.; Yu, J.J.; Soleimani, M. Identification of an Electrogenic 2Cl−/H+ Exchanger, ClC5, as a Chloride-Secreting Transporter Candidate in Kidney Cyst Epithelium in Tuberous Sclerosis. Am. J. Pathol. 2023, 193, 191–200. [Google Scholar] [CrossRef]
- Mohieldin, A.M.; Pala, R.; Sherpa, R.T.; Alanazi, M.; Alanazi, A.; Shamloo, K.; Ahsan, A.; AbouAlaiwi, W.A.; Moresco, J.J.; Yates, J.R., 3rd; et al. Proteomic Identification Reveals the Role of Ciliary Extracellular-Like Vesicle in Cardiovascular Function. Adv. Sci. 2020, 7, 1903140. [Google Scholar] [CrossRef]
- Latta, H.; Maunsbach, A.B.; Madden, S.C. Cilia in different segments of the rat nephron. J. Biophys. Biochem. Cytol. 1961, 11, 248–252. [Google Scholar] [CrossRef]
- Aronow, M.E.; Nakagawa, J.A.; Gupta, A.; Traboulsi, E.I.; Singh, A.D. Tuberous sclerosis complex: Genotype/phenotype correlation of retinal findings. Ophthalmology 2012, 119, 1917–1923. [Google Scholar] [CrossRef]
- Sancak, O.; Nellist, M.; Goedbloed, M.; Elfferich, P.; Wouters, C.; Maat-Kievit, A.; Zonnenberg, B.; Verhoef, S.; Halley, D.; van den Ouweland, A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: Genotype—Phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur. J. Hum. Genet. 2005, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, D.J.; Zhang, H.; Bandura, J.L.; Heiberger, K.M.; Glogauer, M.; el-Hashemite, N.; Onda, H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 2002, 11, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Carrella, S.; Banfi, S.; Karali, M. Sophisticated Gene Regulation for a Complex Physiological System: The Role of Non-coding RNAs in Photoreceptor Cells. Front. Cell Dev. Biol. 2020, 8, 629158. [Google Scholar] [CrossRef]
- Chen, J.Y.; Liu, W.J.; Niu, S.R.; Zheng, Y.S.; Lin, S.; Hong, Y. Insight into the role of non-coding RNA in the diagnosis and treatment of retinitis pigmentosa. Noncoding RNA Res. 2024, 9, 44–54. [Google Scholar] [CrossRef]
- Guo, K.; Qian, K.; Shi, Y.; Sun, T.; Wang, Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. lncRNA MIAT promotes proliferation and invasion of HCC cells via sponging miR-214. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G559–G565. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Wu, X.; Huang, Q.; Chen, W.; Zhu, H.; Qu, X.; Xie, L.; Ma, X.; Huang, G. Long non-coding RNA MIAT is estrogen-responsive and promotes estrogen-induced proliferation in ER-positive breast cancer cells. Biochem. Biophys. Res. Commun. 2018, 503, 45–50. [Google Scholar] [CrossRef]
- Lu, F.; Song, Y.; Cui, S.; Zhao, H.; Chen, Y.; Du, H. LncRNA MIAT promotes the proliferation, migration, and invasion of melanoma cells through recruiting TCF12 and activating NFAT5. Am. J. Transl. Res. 2021, 13, 12588–12600. [Google Scholar]
- Sha, M.; Lin, M.; Wang, J.; Ye, J.; Xu, J.; Xu, N.; Huang, J. Long non-coding RNA MIAT promotes gastric cancer growth and metastasis through regulation of miR-141/DDX5 pathway. J. Exp. Clin. Cancer Res. 2018, 37, 58. [Google Scholar] [CrossRef]
- Song, F.; Yang, Y.; Liu, J. Long non-coding RNA MIAT promotes the proliferation and invasion of laryngeal squamous cell carcinoma cells by sponging microRNA-613. Exp. Ther. Med. 2021, 21, 232. [Google Scholar] [CrossRef]
- Wu, L.; Liu, C.; Zhang, Z. Knockdown of lncRNA MIAT inhibits proliferation and cisplatin resistance in non-small cell lung cancer cells by increasing miR-184 expression. Oncol. Lett. 2020, 19, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Hao, A.; Wang, Y.; Stovall, D.B.; Wang, Y.; Sui, G. Emerging Roles of LncRNAs in the EZH2-regulated Oncogenic Network. Int. J. Biol. Sci. 2021, 17, 3268–3280. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Hushmandi, K.; Hashemi, F.; Zabolian, A.; Canadas, I.; Zarrabi, A.; Nabavi, N.; Aref, A.R.; Crea, F.; et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J. Hematol. Oncol. 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Xie, L.; Zhang, J.; Liu, B.; Liu, X.; He, J.; Ma, D.; Dong, K. Determination of long non-coding RNAs associated with EZH2 in neuroblastoma by RIP-seq, RNA-seq and ChIP-seq. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Hong, S.H.; Hwang, H.J.; Son, D.H.; Kim, E.S.; Park, S.Y.; Yoon, Y.E. Inhibition of EZH2 exerts antitumorigenic effects in renal cell carcinoma via LATS1. FEBS Open Bio 2023, 13, 724–735. [Google Scholar] [CrossRef]
- Krol, J.; Krol, I.; Alvarez, C.P.; Fiscella, M.; Hierlemann, A.; Roska, B.; Filipowicz, W. A network comprising short and long noncoding RNAs and RNA helicase controls mouse retina architecture. Nat. Commun. 2015, 6, 7305. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, R.; Ahmed, S.A. MicroRNA-183/96/182 cluster in immunity and autoimmunity. Front. Immunol. 2023, 14, 1134634. [Google Scholar] [CrossRef]
- Lytovchenko, O.; Kunji, E.R.S. Expression and putative role of mitochondrial transport proteins in cancer. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 641–654. [Google Scholar] [CrossRef]
- Zhang, P.; Csaki, L.S.; Ronquillo, E.; Baufeld, L.J.; Lin, J.Y.; Gutierrez, A.; Dwyer, J.R.; Brindley, D.N.; Fong, L.G.; Tontonoz, P.; et al. Lipin 2/3 phosphatidic acid phosphatases maintain phospholipid homeostasis to regulate chylomicron synthesis. J. Clin. Investig. 2019, 129, 281–295. [Google Scholar] [CrossRef]
- Ben, J.; Jiang, B.; Wang, D.; Liu, Q.; Zhang, Y.; Qi, Y.; Tong, X.; Chen, L.; Liu, X.; Zhang, Y.; et al. Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-kappaB signaling mediated inflammation. Nat. Commun. 2019, 10, 1801. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, J.L.; Lee, K.; Richardson, L.E.; Johnson, B.H.; Lee, H.S.; Lee, J.S.; Kim, S.B.; Kwon, O.H.; Song, K.S.; et al. nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget 2014, 5, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Taube, M.; Lisiak, N.; Toton, E.; Rubis, B. Human Vault RNAs: Exploring Their Potential Role in Cellular Metabolism. Int. J. Mol. Sci. 2024, 25, 4072. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, C.; Qi, Y.; Xu, J.; Li, N.; Chen, L.; Jiang, B.; Zhu, X.; Zhang, H.; Li, X.; et al. Major vault protein suppresses lung cancer cell proliferation by inhibiting STAT3 signaling pathway. BMC Cancer 2019, 19, 454. [Google Scholar] [CrossRef]
- Iwashita, K.; Ikeda, R.; Takeda, Y.; Sumizawa, T.; Furukawa, T.; Yamaguchi, T.; Akiyama, S.; Yamada, K. Major vault protein forms complexes with hypoxia-inducible factor (HIF)-1alpha and reduces HIF-1alpha level in ACHN human renal adenocarcinoma cells. Cancer Sci. 2010, 101, 920–926. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Brooks, M.; Murray Stewart, T.; Casero, R.A., Jr.; Soleimani, M. Renal Transcriptome and Metabolome in Mice with Principal Cell-Specific Ablation of the Tsc1 Gene: Derangements in Pathways Associated with Cell Metabolism, Growth and Acid Secretion. Int. J. Mol. Sci. 2022, 23, 10601. [Google Scholar] [CrossRef]
- Dowarha, D.; Chou, R.H.; Yu, C. S100A1 blocks the interaction between p53 and mdm2 and decreases cell proliferation activity. PLoS ONE 2020, 15, e0234152. [Google Scholar] [CrossRef]
- Nakagawa, M.; Higuchi, S.; Hashimura, M.; Oguri, Y.; Matsumoto, T.; Yokoi, A.; Ishibashi, Y.; Ito, T.; Saegusa, M. Functional interaction between S100A1 and MDM2 may modulate p53 signaling in normal and malignant endometrial cells. BMC Cancer 2022, 22, 184. [Google Scholar] [CrossRef]
- Conner, J.R.; Hirsch, M.S.; Jo, V.Y. HNF1beta and S100A1 are useful biomarkers for distinguishing renal oncocytoma and chromophobe renal cell carcinoma in FNA and core needle biopsies. Cancer Cytopathol. 2015, 123, 298–305. [Google Scholar] [CrossRef]
- Li, G.; Barthelemy, A.; Feng, G.; Gentil-Perret, A.; Peoc’h, M.; Genin, C.; Tostain, J. S100A1: A powerful marker to differentiate chromophobe renal cell carcinoma from renal oncocytoma. Histopathology 2007, 50, 642–647. [Google Scholar] [CrossRef]
- Tekletsadik, Y.K.; Sonn, R.; Osman, M.A. A conserved role of IQGAP1 in regulating TOR complex 1. J. Cell Sci. 2012, 125 Pt 8, 2041–2052. [Google Scholar] [CrossRef]
- Chen, J.Q.; Ou, Y.L.; Huang, Z.P.; Hong, Y.G.; Tao, Y.P.; Wang, Z.G.; Ni, J.S.; Hao, L.Q.; Lin, H. MicroRNA-212-3p inhibits the Proliferation and Invasion of Human Hepatocellular Carcinoma Cells by Suppressing CTGF expression. Sci. Rep. 2019, 9, 9820. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Lin, J.F.; Chou, K.Y.; Lin, Y.C.; Chen, H.E.; Hwang, T.I. miR-99a-5p acts as tumor suppressor via targeting to mTOR and enhances RAD001-induced apoptosis in human urinary bladder urothelial carcinoma cells. Onco Targets Ther. 2018, 11, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bongaarts, A.; Giannikou, K.; Reinten, R.J.; Anink, J.J.; Mills, J.D.; Jansen, F.E.; Spliet, G.M.W.; den Dunnen, W.F.A.; Coras, R.; Blumcke, I.; et al. Subependymal giant cell astrocytomas in Tuberous Sclerosis Complex have consistent TSC1/TSC2 biallelic inactivation, and no BRAF mutations. Oncotarget 2017, 8, 95516–95529. [Google Scholar] [CrossRef] [PubMed]
- Parry, L.; Maynard, J.H.; Patel, A.; Hodges, A.K.; von Deimling, A.; Sampson, J.R.; Cheadle, J.P. Molecular analysis of the TSC1 and TSC2 tumour suppressor genes in sporadic glial and glioneuronal tumours. Hum. Genet. 2000, 107, 350–356. [Google Scholar] [CrossRef]
- Roux, T.; An-Gourfinkel, I.; Bertrand, A.; Bielle, F. Astrocytic tumor with large cells and worrisome features in two patients with tuberous sclerosis: Drastically different diagnoses and prognoses. Clin. Neuropathol. 2017, 36, 102–107. [Google Scholar] [CrossRef]
- Bojmar, L.; Kim, H.S.; Tobias, G.C.; Pelissier Vatter, F.A.; Lucotti, S.; Gyan, K.E.; Kenific, C.M.; Wan, Z.; Kim, K.A.; Kim, D.; et al. Extracellular vesicle and particle isolation from human and murine cell lines, tissues, and bodily fluids. STAR Protoc. 2021, 2, 100225. [Google Scholar] [CrossRef]
- MacKenzie, M.; Tigert, S.; Lovato, D.; Mir, H.; Zahedi, K.; Barone, S.L.; Brooks, M.; Soleimani, M.; Argyropoulos, C. To make a short story long: Simultaneous short and long RNA profiling on Nanopore devices. bioRxiv 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahedi, K.; Morgan, M.; Prieto, B.; Brooks, M.; Howard, T.A.; Barone, S.; Bissler, J.J.; Argyropoulos, C.; Soleimani, M. Role of Extracellular Vesicles in TSC Renal Cystogenesis. Int. J. Mol. Sci. 2025, 26, 3154. https://doi.org/10.3390/ijms26073154
Zahedi K, Morgan M, Prieto B, Brooks M, Howard TA, Barone S, Bissler JJ, Argyropoulos C, Soleimani M. Role of Extracellular Vesicles in TSC Renal Cystogenesis. International Journal of Molecular Sciences. 2025; 26(7):3154. https://doi.org/10.3390/ijms26073154
Chicago/Turabian StyleZahedi, Kamyar, Mackenzie Morgan, Brenda Prieto, Marybeth Brooks, Tamara A. Howard, Sharon Barone, John J. Bissler, Christos Argyropoulos, and Manoocher Soleimani. 2025. "Role of Extracellular Vesicles in TSC Renal Cystogenesis" International Journal of Molecular Sciences 26, no. 7: 3154. https://doi.org/10.3390/ijms26073154
APA StyleZahedi, K., Morgan, M., Prieto, B., Brooks, M., Howard, T. A., Barone, S., Bissler, J. J., Argyropoulos, C., & Soleimani, M. (2025). Role of Extracellular Vesicles in TSC Renal Cystogenesis. International Journal of Molecular Sciences, 26(7), 3154. https://doi.org/10.3390/ijms26073154





