Harnessing the Role of ESR1 in Breast Cancer: Correlation with microRNA, lncRNA, and Methylation
Abstract
1. Introduction
2. Results and Discussion
2.1. Expression of the ESR1 in the TCGA BRCA Datasets with Various BC Subtypes
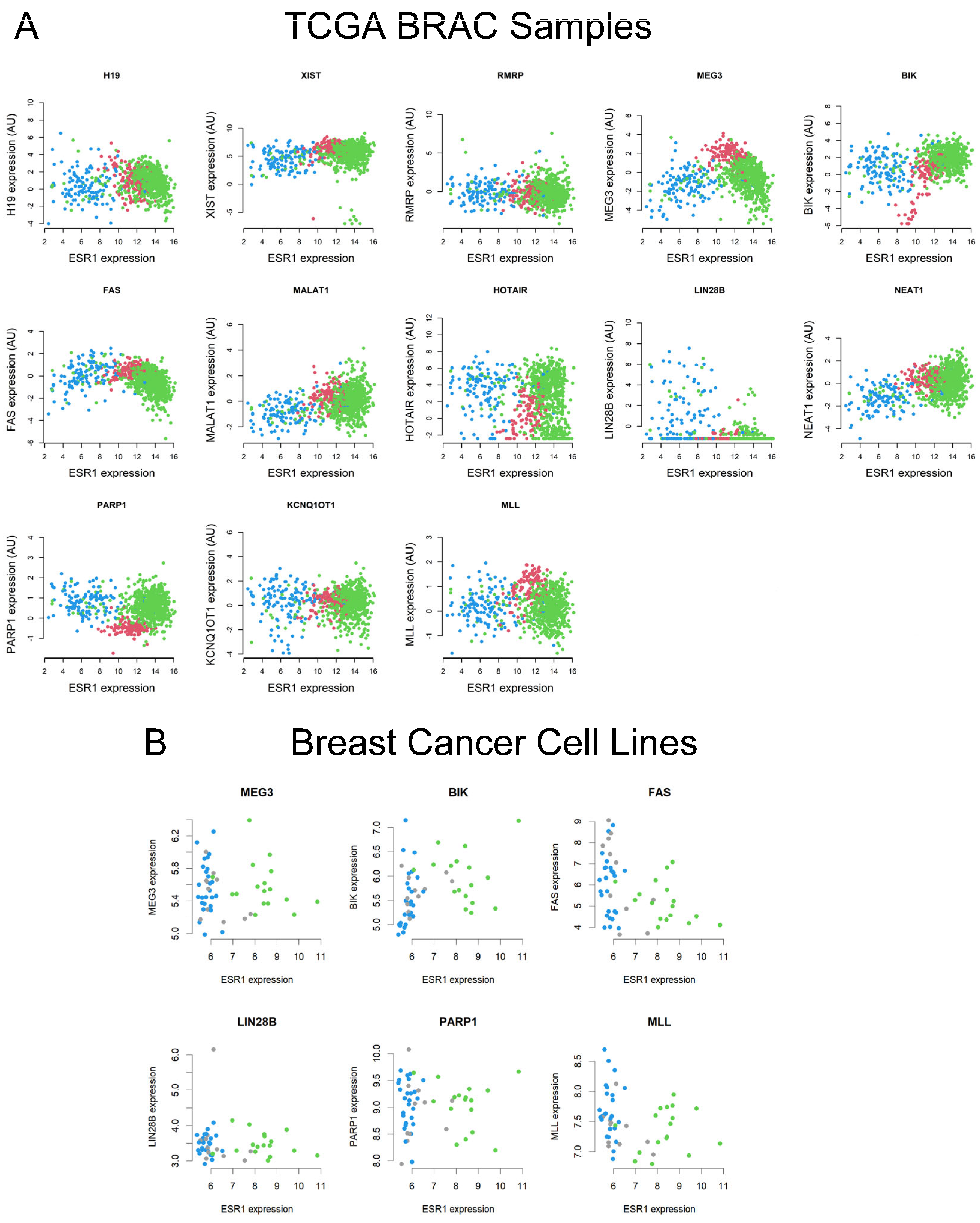
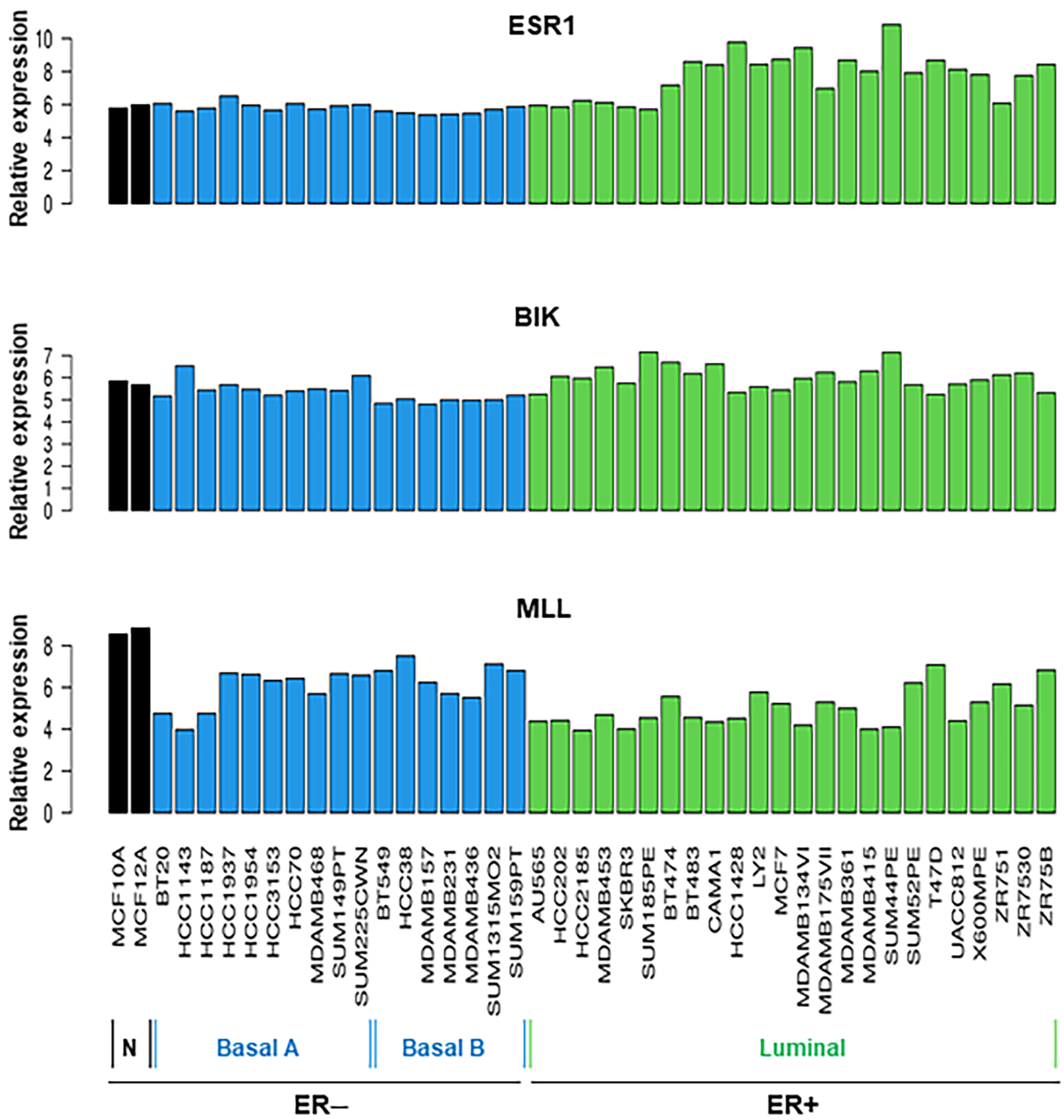
2.2. Analyses of TCGA BRCA and Breast Cell Line RNA-Seq Datasets for a Number of lncRNAs and Their Correlation to ESR1
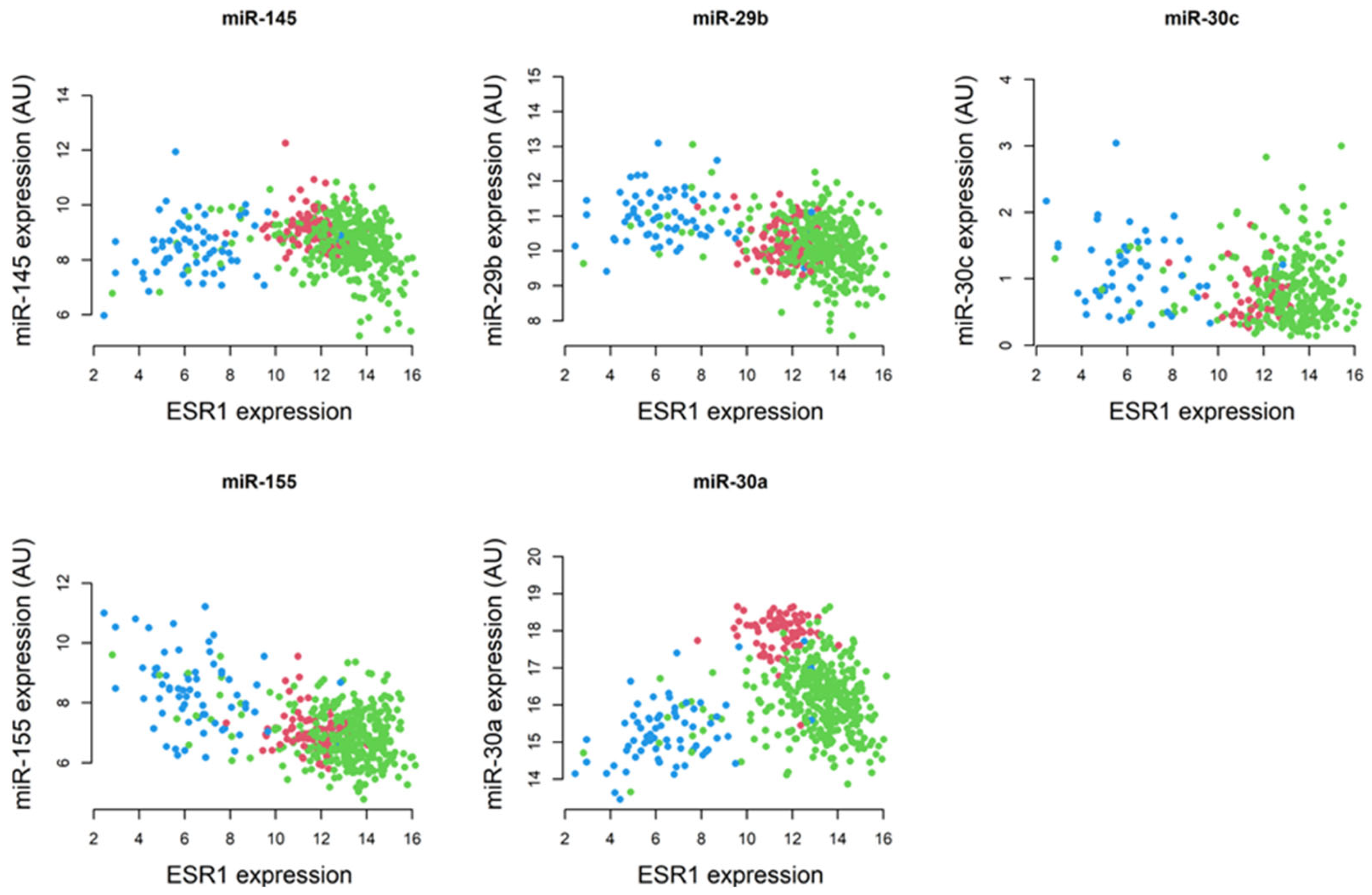
2.3. Correlation of Specific miRNAs with ESR1 and Their Relevance to Breast Pathogenesis
2.4. Analyses of TCGA BRCA Data for ESR1 DNA Methylation Patterns
2.5. Generation of a Heatmap Depicting a Correlation Matrix with lncRNAs, miRNAs, and ESR1
3. Materials and Methods
3.1. Analyses of the TCGA BRCA and Breast Cell Line RNA-Seq Datasets
3.2. Generation of Box and Scatter Plots Utilizing TCGA BRCA Data
3.3. Determination of the Spearman’s Rank Correlation Coefficients with TCGA BRCA Data
3.4. Development of a Heatmap for Correlation Matrix
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| ESR1 | Estrogen receptor 1 |
| lncRNA | long non-coding RNA |
| miRNA | Micro RNA |
| TNBC | Triple-negative breast cancer |
| BRCA | Breast invasive carcinoma |
| RNA-Seq | RNA sequencing |
| FAS | Fatty acid synthetase |
| TCGA | The cancer genomic atlas |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J. Update on the Use of Molecular Subtyping in Breast Cancer. Adv. Anat. Pathol. 2023, 30, 368–373. [Google Scholar]
- Hurson, A.N.; Ahearn, T.U.; Koka, H.; Jenkins, B.D.; Harris, A.R.; Roberts, S.; Fan, S.; Franklin, J.; Butera, G.; Keeman, R.; et al. Risk factors for breast cancer subtypes by race and ethnicity: A scoping review. J. Natl. Cancer Inst. 2024, 116, 1992–2002. [Google Scholar]
- Rios-Hoyo, A.; Shan, N.L.; Karn, P.L.; Pusztai, L. Clinical Implications of Breast Cancer Intrinsic Subtypes. Adv. Exp. Med. Biol. 2025, 1464, 435–448. [Google Scholar]
- Manna, P.R.; Ahmed, A.U.; Molehin, D.; Narasimhan, M.; Pruitt, K.; Reddy, P.H. Hormonal and Genetic Regulatory Events in Breast Cancer and Its Therapeutics: Importance of the Steroidogenic Acute Regulatory Protein. Biomedicines 2022, 10, 1313. [Google Scholar] [CrossRef]
- Dikoglu, E.; Pareja, F. Molecular Basis of Breast Tumor Heterogeneity. Adv. Exp. Med. Biol. 2025, 1464, 237–257. [Google Scholar] [PubMed]
- Manna, P.R.; Ramachandran, S.; Pradeepkiran, J.A.; Molehin, D.; Castro-Piedras, I.; Pruitt, K.; Ganapathy, V.; Reddy, P.H. Expression and Function of StAR in Cancerous and Non-Cancerous Human and Mouse Breast Tissues: New Insights into Diagnosis and Treatment of Hormone-Sensitive Breast Cancer. Int. J. Mol. Sci. 2023, 24, 758. [Google Scholar] [CrossRef]
- Manna, P.R.; Yang, S.; Reddy, P.H. Epigenetic Dysregulation and Its Correlation with the Steroidogenic Machinery Impacting Breast Pathogenesis: Data Mining and Molecular Insights into Therapeutics. Int. J. Mol. Sci. 2023, 24, 16488. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef]
- Manna, P.R.; Molehin, D.; Ahmed, A.U. Dysregulation of Aromatase in Breast, Endometrial, and Ovarian Cancers: An Overview of Therapeutic Strategies. Prog. Mol. Biol. Transl. Sci. 2016, 144, 487–537. [Google Scholar] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanovic, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar]
- Kerdivel, G.; Flouriot, G.; Pakdel, F. Modulation of estrogen receptor alpha activity and expression during breast cancer progression. Vitam. Horm. 2013, 93, 135–160. [Google Scholar] [PubMed]
- Farcas, A.M.; Nagarajan, S.; Cosulich, S.; Carroll, J.S. Genome-Wide Estrogen Receptor Activity in Breast Cancer. Endocrinology 2021, 162, bqaa224. [Google Scholar]
- Clusan, L.; Ferriere, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Shi, W.; Zhou, F.; Yan, Y.; Li, Y.; Xu, Y. The role of estrogen receptors in intracellular estrogen signaling pathways, an overview. J. Steroid Biochem. Mol. Biol. 2025, 245, 106632. [Google Scholar]
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 mutations in breast cancer. Cancer 2019, 125, 3714–3728. [Google Scholar]
- Herzog, S.K.; Fuqua, S.A.W. ESR1 mutations and therapeutic resistance in metastatic breast cancer: Progress and remaining challenges. Br. J. Cancer 2022, 126, 174–186. [Google Scholar] [PubMed]
- Hancock, G.R.; Gertz, J.; Jeselsohn, R.; Fanning, S.W. Estrogen Receptor Alpha Mutations, Truncations, Heterodimers, and Therapies. Endocrinology 2024, 165, bqae051. [Google Scholar]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [PubMed]
- Sun, T.; Lian, R.; Liang, X.; Sun, D. Association Between ESR1 XBAI and Breast Cancer Susceptibility: A Systematic Review and Meta-Analysis. Clin. Investig. Med. 2022, 45, E21–E34. [Google Scholar]
- Xie, J.; Gan, L.; Xue, B.; Wang, X.; Pei, X. Emerging roles of interactions between ncRNAs and other epigenetic modifications in breast cancer. Front. Oncol. 2023, 13, 1264090. [Google Scholar]
- Noyan, S.; Gur Dedeoglu, B. Upregulation of miR-99b-5p Modulates ESR1 Expression as an Adaptive Mechanism to Circumvent Drug Response via Facilitating ER/HER2 Crosstalk. Balkan Med. J. 2025, 42, 150–156. [Google Scholar]
- Fan, N.; Fu, H.; Feng, X.; Chen, Y.; Wang, J.; Wu, Y.; Bian, Y.; Li, Y. Long non-coding RNAs play an important regulatory role in tumorigenesis and tumor progression through aerobic glycolysis. Front. Mol. Biosci. 2022, 9, 941653. [Google Scholar]
- Huang, Y.; Mo, W.; Ding, X.; Ding, Y. Long non-coding RNAs in breast cancer stem cells. Med. Oncol. 2023, 40, 177. [Google Scholar]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar]
- Garcia-Padilla, C.; Duenas, A.; Garcia-Lopez, V.; Aranega, A.; Franco, D.; Garcia-Martinez, V.; Lopez-Sanchez, C. Molecular Mechanisms of lncRNAs in the Dependent Regulation of Cancer and Their Potential Therapeutic Use. Int. J. Mol. Sci. 2022, 23, 764. [Google Scholar] [CrossRef]
- Hassani, B.; Taheri, M.; Asgari, Y.; Zekri, A.; Sattari, A.; Ghafouri-Fard, S.; Pouresmaeili, F. Expression Analysis of Long Non-Coding RNAs Related with FOXM1, GATA3, FOXA1 and ESR1 in Breast Tissues. Front. Oncol. 2021, 11, 671418. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.R.; Mohammed, E.A.; Toraih, E.A.; Kamel, M.M.; Abdelhafiz, A.S.; Badr, F.M. Circulating ESR1, long non-coding RNA HOTAIR and microRNA-130a gene expression as biomarkers for breast cancer stage and metastasis. Sci. Rep. 2023, 13, 22654. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Jeselsohn, R. ESR1 fusions and therapeutic resistance in metastatic breast cancer. Front. Oncol. 2022, 12, 1037531. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Treeck, O.; Haerteis, S.; Ortmann, O. Non-Coding RNAs Modulating Estrogen Signaling and Response to Endocrine Therapy in Breast Cancer. Cancers 2023, 15, 1632. [Google Scholar] [CrossRef]
- Drula, R.; Pardini, B.; Fu, X.; De Los Santos, M.C.; Jurj, A.; Pang, L.; El-Daly, S.M.; Fabris, L.; Knutsen, E.; Dragomir, M.P.; et al. 17beta-estradiol promotes extracellular vesicle release and selective miRNA loading in ERalpha-positive breast cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2122053120. [Google Scholar] [CrossRef]
- Martinez-Galan, J.; Torres-Torres, B.; Nunez, M.I.; Lopez-Penalver, J.; Del Moral, R.; Ruiz De Almodovar, J.M.; Menjon, S.; Concha, A.; Chamorro, C.; Rios, S.; et al. ESR1 gene promoter region methylation in free circulating DNA and its correlation with estrogen receptor protein expression in tumor tissue in breast cancer patients. BMC Cancer 2014, 14, 59. [Google Scholar] [CrossRef]
- Kirn, V.; Strake, L.; Thangarajah, F.; Richters, L.; Eischeid, H.; Koitzsch, U.; Odenthal, M.; Fries, J. ESR1-promoter-methylation status in primary breast cancer and its corresponding metastases. Clin. Exp. Metastasis 2018, 35, 707–712. [Google Scholar] [CrossRef]
- Quintas-Granados, L.I.; Cortes, H.; Carmen, M.G.; Leyva-Gomez, G.; Bustamante-Montes, L.P.; Rodriguez-Morales, M.; Villegas-Vazquez, E.Y.; Lopez-Reyes, I.; Alcaraz-Estrada, S.L.; Sandoval-Basilio, J.; et al. The high methylation level of a novel 151-bp CpG island in the ESR1 gene promoter is associated with a poor breast cancer prognosis. Cancer Cell Int. 2021, 21, 649. [Google Scholar]
- Pogribny, I.P.; Rusyn, I. Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 2013, 754, 215–232. [Google Scholar] [PubMed]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Casper, J.; Zweig, A.S.; Villarreal, C.; Tyner, C.; Speir, M.L.; Rosenbloom, K.R.; Raney, B.J.; Lee, C.M.; Lee, B.T.; Karolchik, D.; et al. The UCSC Genome Browser database: 2018 update. Nucleic Acids Res. 2018, 46, D762–D769. [Google Scholar]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e310. [Google Scholar] [PubMed]
- Manna, P.R.; Ahmed, A.U.; Yang, S.; Narasimhan, M.; Cohen-Tannoudji, J.; Slominski, A.T.; Pruitt, K. Genomic Profiling of the Steroidogenic Acute Regulatory Protein in Breast Cancer: In Silico Assessments and a Mechanistic Perspective. Cancers 2019, 11, 623. [Google Scholar] [CrossRef]
- Heiser, L.M.; Sadanandam, A.; Kuo, W.L.; Benz, S.C.; Goldstein, T.C.; Ng, S.; Gibb, W.J.; Wang, N.J.; Ziyad, S.; Tong, F.; et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2724–2729. [Google Scholar] [PubMed]
- Garcia-Chico, C.; Lopez-Ortiz, S.; Penin-Grandes, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Emanuele, E.; Ceci, C.; Graziani, G.; Fiuza-Luces, C.; Lista, S.; et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers 2023, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Murase, K.; Saito, M.; Imamura, M.; Oh, K. Mechanisms of estrogen receptor-alpha upregulation in breast cancers. Med. Mol. Morphol. 2010, 43, 193–196. [Google Scholar]
- Miziak, P.; Baran, M.; Blaszczak, E.; Przybyszewska-Podstawka, A.; Kalafut, J.; Smok-Kalwat, J.; Dmoszynska-Graniczka, M.; Kielbus, M.; Stepulak, A. Estrogen Receptor Signaling in Breast Cancer. Cancers 2023, 15, 4689. [Google Scholar] [CrossRef]
- Toumba, M.; Kythreotis, A.; Panayiotou, K.; Skordis, N. Estrogen receptor signaling and targets: Bones, breasts and brain (Review). Mol. Med. Rep. 2024, 30, 144. [Google Scholar]
- Dong, S.; Ma, M.; Li, M.; Guo, Y.; Zuo, X.; Gu, X.; Zhang, M.; Shi, Y. LncRNA MEG3 regulates breast cancer proliferation and apoptosis through miR-141-3p/RBMS3 axis. Genomics 2021, 113, 1689–1704. [Google Scholar] [PubMed]
- Wu, J.; Pang, R.; Li, M.; Chen, B.; Huang, J.; Zhu, Y. m6A-Induced LncRNA MEG3 Suppresses the Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cell Through miR-544b/BTG2 Signaling. OncoTargets Ther. 2021, 14, 3745–3755. [Google Scholar]
- Wei, Q.; Liu, G.; Huang, Z.; Huang, Y.; Huang, L.; Huang, Z.; Wu, X.; Wei, H.; Pu, J. LncRNA MEG3 Inhibits Tumor Progression by Modulating Macrophage Phenotypic Polarization via miR-145-5p/DAB2 Axis in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 1019–1035. [Google Scholar]
- Hussain, M.S.; Majami, A.A.; Ali, H.; Gupta, G.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Syed, R.U.; Khalifa, N.E.; Bin Break, M.K.; et al. The complex role of MEG3: An emerging long non-coding RNA in breast cancer. Pathol. Res. Pract. 2023, 251, 154850. [Google Scholar]
- Zhu, X.; Lv, L.; Wang, M.; Fan, C.; Lu, X.; Jin, M.; Li, S.; Wang, F. DNMT1 facilitates growth of breast cancer by inducing MEG3 hyper-methylation. Cancer Cell Int. 2022, 22, 56. [Google Scholar]
- Shi, Y. MEG3 regulates apoptosis of adipose-derived stem cells. Mol. Med. Rep. 2020, 21, 2435–2442. [Google Scholar]
- Li, Z.; Gao, J.; Sun, D.; Jiao, Q.; Ma, J.; Cui, W.; Lou, Y.; Xu, F.; Li, S.; Li, H. LncRNA MEG3: Potential stock for precision treatment of cardiovascular diseases. Front. Pharmacol. 2022, 13, 1045501. [Google Scholar] [CrossRef]
- Teinturier, R.; Abou Ziki, R.; Kassem, L.; Luo, Y.; Malbeteau, L.; Gherardi, S.; Corbo, L.; Bertolino, P.; Bachelot, T.; Treilleux, I.; et al. Reduced menin expression leads to decreased ERalpha expression and is correlated with the occurrence of human luminal B-like and ER-negative breast cancer subtypes. Breast Cancer Res. Treat. 2021, 190, 389–401. [Google Scholar]
- Jones, R.B.; Farhi, J.; Adams, M.; Parwani, K.K.; Cooper, G.W.; Zecevic, M.; Lee, R.S.; Hong, A.L.; Spangle JM: Targeting, M.L.L. Methyltransferases Enhances the Antitumor Effects of PI3K Inhibition in Hormone Receptor-positive Breast Cancer. Cancer Res. Commun. 2022, 2, 1569–1578. [Google Scholar]
- Gadad, S.S.; Camacho, C.V.; Malladi, V.; Hutti, C.R.; Nagari, A.; Kraus, W.L. PARP-1 Regulates Estrogen-Dependent Gene Expression in Estrogen Receptor alpha-Positive Breast Cancer Cells. Mol. Cancer Res. 2021, 19, 1688–1698. [Google Scholar]
- Bohi, F.; Hottiger, M.O. Expanding the Perspective on PARP1 and Its Inhibitors in Cancer Therapy: From DNA Damage Repair to Immunomodulation. Biomedicines 2024, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar]
- Silvente-Poirot, S.; Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 2014, 343, 1445–1446. [Google Scholar] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase: A druggable driver of breast cancer brain metastasis. Expert. Opin. Ther. Targets 2022, 26, 427–444. [Google Scholar]
- Song, H.J.; Sneddon, A.A.; Heys, S.D.; Wahle, K.W. Regulation of fatty acid synthase (FAS) and apoptosis in estrogen-receptor positive and negative breast cancer cells by conjugated linoleic acids. Prostaglandins Leukot. Essent. Fatty Acids 2012, 87, 197–203. [Google Scholar]
- Menendez, J.A.; Lupu, R. Fatty acid synthase regulates estrogen receptor-alpha signaling in breast cancer cells. Oncogenesis 2017, 6, e299. [Google Scholar]
- Cairns, J.; Ingle, J.N.; Kalari, K.R.; Goetz, M.P.; Weinshilboum, R.M.; Gao, H.; Li, H.; Bari, M.G.; Wang, L. Anastrozole Regulates Fatty Acid Synthase in Breast Cancer. Mol. Cancer Ther. 2022, 21, 206–216. [Google Scholar]
- Kumar, S.; Morton, H.; Sawant, N.; Orlov, E.; Bunquin, L.E.; Pradeepkiran, J.A.; Alvir, R.; Reddy, P.H. MicroRNA-455-3p improves synaptic, cognitive functions and extends lifespan: Relevance to Alzheimer’s disease. Redox Biol. 2021, 48, 102182. [Google Scholar]
- Akshaya, R.L.; Saranya, I.; Selvamurugan, N. MicroRNAs mediated interaction of tumor microenvironment cells with breast cancer cells during bone metastasis. Breast Cancer 2023, 30, 910–925. [Google Scholar] [PubMed]
- Valle-Garcia, D.; Perez de la Cruz, V.; Flores, I.; Salazar, A.; Pineda, B.; Meza-Sosa, K.F. Use of microRNAs as Diagnostic, Prognostic, and Therapeutic Tools for Glioblastoma. Int. J. Mol. Sci. 2024, 25, 2464. [Google Scholar] [CrossRef]
- Mohan Lal, P.; Hamza Siddiqui, M.; Soulat, A.; Mohan, A.; Tanush, D.; Tirath, K.; Raja, S.; Khuzzaim Khan, M.; Raja, A.; Chaulagain, A.; et al. MicroRNAs as promising biomarkers and potential therapeutic agents in breast cancer management: A comprehensive review. Ann. Med. Surg. 2024, 86, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Wisham, A.A.; Wong, F.W.; Ahmad Najmi, H.R.; Ng, Z.F.; Diba, R.S. Mapping the function of MicroRNAs as a critical regulator of tumor-immune cell communication in breast cancer and potential treatment strategies. Front. Cell Dev. Biol. 2024, 12, 1390704. [Google Scholar] [CrossRef]
- Mirzaei, Z.; Barati, T.; Ebrahimi, A.; Derakhshan, S.M.; Khaniani, M.S. The role of mir-7-5p in cancer: Function, prognosis, diagnosis, and therapeutic implications. Mol. Biol. Rep. 2024, 52, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ding, B.; Lou, W. microRNA-Dependent Modulation of Genes Contributes to ESR1’s Effect on ERalpha Positive Breast Cancer. Front. Oncol. 2020, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, R.; Nicoloso, M.S.; Lupini, L.; Lu, Y.; Fogarty, J.; Rossi, S.; Zagatti, B.; Fabbri, M.; Veronese, A.; Liu, X.; et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010, 17, 246–254. [Google Scholar]
- Piasecka, D.; Braun, M.; Kordek, R.; Sadej, R.; Romanska, H. MicroRNAs in regulation of triple-negative breast cancer progression. J. Cancer Res. Clin. Oncol. 2018, 144, 1401–1411. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.Y.; Fu, S.; Wang, X.P.; Huang, M.Y.; Zhang, X.; Shao, Q.; Deng, L.; Zeng, M.S.; Zeng, Y.X.; et al. MicroRNA-30a promotes invasiveness and metastasis in vitro and in vivo through epithelial-mesenchymal transition and results in poor survival of nasopharyngeal carcinoma patients. Exp. Biol. Med. 2014, 239, 891–898. [Google Scholar]
- He, Q.; Chen, Z.; Deng, Y.; Mao, C. Plasma microRNA-30a expression in patients with chronic hepatitis B and its application value in the assessment of the severity of liver fibrosis. Eur. J. Gastroenterol. Hepatol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, N.P.; Klose, R. CpG island chromatin: A platform for gene regulation. Epigenetics 2011, 6, 147–152. [Google Scholar] [CrossRef]
- Sergeeva, A.; Davydova, K.; Perenkov, A.; Vedunova, M. Mechanisms of human DNA methylation, alteration of methylation patterns in physiological processes and oncology. Gene 2023, 875, 147487. [Google Scholar] [CrossRef]
- Bhootra, S.; Jill, N.; Shanmugam, G.; Rakshit, S.; Sarkar, K. DNA methylation and cancer: Transcriptional regulation, prognostic, and therapeutic perspective. Med. Oncol. 2023, 40, 71. [Google Scholar] [PubMed]
- Men, Y.; Fan, Y.; Shen, Y.; Lu, L.; Kallen, A.N. The Steroidogenic Acute Regulatory Protein (StAR) Is Regulated by the H19/let-7 Axis. Endocrinology 2017, 158, 402–409. [Google Scholar] [PubMed]
- Otsuka, K.; Matsubara, S.; Shiraishi, A.; Takei, N.; Satoh, Y.; Terao, M.; Takada, S.; Kotani, T.; Satake, H.; Kimura, A.P. A Testis-Specific Long Noncoding RNA, Start, Is a Regulator of Steroidogenesis in Mouse Leydig Cells. Front. Endocrinol. 2021, 12, 665874. [Google Scholar]
- Kumar, S.; Orlov, E.; Gowda, P.; Bose, C.; Swerdlow, R.H.; Lahiri, D.K.; Reddy, P.H. Synaptosome microRNAs regulate synapse functions in Alzheimer’s disease. NPJ Genom. Med. 2022, 7, 47. [Google Scholar] [PubMed]
- Islam, M.A.; Sultana, O.F.; Bandari, M.; Kshirsagar, S.; Manna, P.R.; Reddy, P.H. MicroRNA-455-3P as a peripheral biomarker and therapeutic target for mild cognitive impairment and Alzheimer’s disease. Ageing Res. Rev. 2024, 100, 102459. [Google Scholar]
- Guo, F.; Chen, H.; Chang, J.; Zhang, L. Mutation R273H confers p53 a stimulating effect on the IGF-1R-AKT pathway via miR-30a suppression in breast cancer. Biomed. Pharmacother. 2016, 78, 335–341. [Google Scholar] [CrossRef]
- Yeap, S.K.; Mohd Ali, N.; Akhtar, M.N.; Razak, N.A.; Chong, Z.X.; Ho, W.Y.; Boo, L.; Zareen, S.; Kurniawan, T.A.; Avtar, R.; et al. Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells. Molecules 2021, 26, 1277. [Google Scholar] [CrossRef]
- Volovat, S.R.; Volovat, C.; Hordila, I.; Hordila, D.A.; Mirestean, C.C.; Miron, O.T.; Lungulescu, C.; Scripcariu, D.V.; Stolniceanu, C.R.; Konsoulova-Kirova, A.A.; et al. MiRNA and LncRNA as Potential Biomarkers in Triple-Negative Breast Cancer: A Review. Front. Oncol. 2020, 10, 526850. [Google Scholar]
- Schwarzenbach, H.; Gahan, P.B. Interplay between LncRNAs and microRNAs in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8095. [Google Scholar] [CrossRef]
- Song, J.; Cui, Q.; Gao, J. Roles of lncRNAs related to the p53 network in breast cancer progression. Front. Oncol. 2024, 14, 1453807. [Google Scholar]
- Albrecht, J.; Muller, M.; Hafstaeth, V.; Kaminska, K.; Vallon-Christersson, J.; Honeth, G.; Persson, H. Dynamic methylation and expression of alternative promoters for oestrogen receptor alpha in cell line models of fulvestrant resistance. Mol. Oncol. 2025, 19, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Shukla, S.; Khan, S.; Tollefsbol, T.O.; Meeran, S.M. Epigenetic reactivation of p21CIP1/WAF1 and KLOTHO by a combination of bioactive dietary supplements is partially ERalpha-dependent in ERalpha-negative human breast cancer cells. Mol. Cell Endocrinol. 2015, 406, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Castro-Piedras, I.; Sharma, M.; den Bakker, M.; Molehin, D.; Martinez, E.G.; Vartak, D.; Pruitt, W.M.; Deitrick, J.; Almodovar, S.; Pruitt, K. DVL1 and DVL3 differentially localize to CYP19A1 promoters and regulate aromatase mRNA in breast cancer cells. Oncotarget 2018, 9, 35639–35654. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Ahmadi, F.A.M.; Husaini, N.A.; Al-Nuaimi, N.; Ahmad, S.M.S.; Pintus, G.; Zayed, H. microRNA 21 and long non-coding RNAs interplays underlie cancer pathophysiology: A narrative review. Noncoding RNA Res. 2024, 9, 831–852. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Zhao, H.; Ren, W.; Gao, D.; Wang, Z.; Lv, W.; Dong, Q. Niraparib restrains prostate cancer cell proliferation and metastasis and tumor growth in mice by regulating the lncRNA MEG3/miR-181-5p/GATA6 pathway. PeerJ 2023, 11, e16314. [Google Scholar] [CrossRef]
- Goncalves, R.; Warner, W.A.; Luo, J.; Ellis, M.J. New concepts in breast cancer genomics and genetics. Breast Cancer Res. 2014, 16, 460. [Google Scholar] [CrossRef]


| CpG | Correlation | Raw p Value | Adjusted p Value |
|---|---|---|---|
| cg23009221 | −0.43721 | 6.73 × 10−23 | 3.63 × 10−21 |
| cg00601836 | −0.65592 | 6.44 × 10−58 | 3.48 × 10−56 |
| cg19411146 | −0.44922 | 3.15 × 10−24 | 1.70 × 10−22 |
| cg09646983 | −0.49705 | 4.46 × 10−30 | 2.41 × 10−28 |
| cg03037684 | 0.086458 | 0.063919 | 1.000 |
| cg07584093 | −0.52222 | 4.67 × 10−33 | 2.52 × 10−31 |
| cg17706972 | −0.45091 | 2.03 × 10−24 | 1.10 × 10−22 |
| cg17264271 | −0.39943 | 4.77 × 10−19 | 2.58 × 10−17 |
| cg07671949 | −0.48692 | 9.27 × 10−29 | 5.00 × 10−27 |
| cg20627916 | −0.44493 | 9.55 × 10−24 | 5.16 × 10−22 |
| cg21157690 | −0.3816 | 2.15 × 10−17 | 1.16 × 10−15 |
| cg23164938 | −0.33446 | 1.74 × 10−13 | 9.42 × 10−12 |
| cg15543523 | −0.67757 | 4.20 × 10−63 | 2.27 × 10−61 |
| cg15980539 | −0.3692 | 2.66 × 10−16 | 1.44 × 10−14 |
| cg07455133 | −0.2602 | 1.49 × 10−8 | 8.02 × 10−7 |
| cg25490334 | 0.210539 | 5.25 × 10−6 | 0.000284 |
| cg01715172 | −0.58812 | 3.84 × 10−44 | 2.07 × 10−42 |
| cg03732055 | −0.28882 | 2.74 × 10−10 | 1.48 × 10−8 |
| cg12209876 | −0.00373 | 0.936474 | 1.000 |
| cg20893956 | −0.33864 | 8.34 × 10−14 | 4.50 × 10−12 |
| cg00920970 | −0.36435 | 6.90 × 10−16 | 3.72 × 10−14 |
| cg04211581 | −0.40391 | 1.77 × 10−19 | 9.55 × 10−18 |
| cg24900983 | −0.48253 | 3.35 × 10−28 | 1.81 × 10−26 |
| cg07059469 | 0.004822 | 0.917859 | 1.000 |
| cg08884395 | −0.61707 | 1.30 × 10−49 | 7.00 × 10−48 |
| cg21608605 | −0.62193 | 1.37 × 10−50 | 7.40 × 10−49 |
| cg07619683 | −0.44714 | 1.49 × 10−23 | 8.05 × 10−22 |
| cg21614759 | −0.42071 | 3.72 × 10−21 | 2.01 × 10−19 |
| cg08907436 | −0.44323 | 1.47 × 10−23 | 7.96 × 10−22 |
| cg04063345 | −0.47197 | 6.79 × 10−27 | 3.67 × 10−25 |
| cg18007957 | −0.4478 | 4.56 × 10−24 | 2.46 × 10−22 |
| cg23467008 | −0.21278 | 4.14 × 10−6 | 0.000224 |
| cg21265702 | −0.6346 | 3.25 × 10−53 | 1.75 × 10−51 |
| cg01321962 | −0.53427 | 2.60 × 10−35 | 1.40 × 10−33 |
| cg11813455 | −0.42858 | 5.63 × 10−22 | 3.04 × 10−20 |
| cg26089753 | −0.66545 | 3.80 × 10−60 | 2.05 × 10−58 |
| cg23165623 | −0.48253 | 3.35 × 10−28 | 1.81 × 10−26 |
| cg09414638 | −0.15151 | 0.001116 | 0.060274 |
| cg22839866 | −0.20121 | 1.37 × 10−5 | 0.000741 |
| cg07746998 | −0.33584 | 1.37 × 10−13 | 7.39 × 10−12 |
| cg02285263 | −0.38209 | 1.94 × 10−17 | 1.05 × 10−15 |
| cg02720618 | −0.39546 | 1.14 × 10−18 | 6.15 × 10−17 |
| cg10441070 | −0.53198 | 5.70 × 10−35 | 3.08 × 10−33 |
| cg27316393 | −0.42427 | 1.59 × 10−21 | 8.60 × 10−20 |
| cg24764793 | −0.42745 | 7.40 × 10−22 | 4.00 × 10−20 |
| cg20253551 | −0.38795 | 5.68 × 10−18 | 3.07 × 10−16 |
| cg11251858 | −0.38646 | 7.80 × 10−18 | 4.21 × 10−16 |
| cg15626350 | −0.49488 | 8.64 × 10−30 | 4.67 × 10−28 |
| cg07189962 | −0.50757 | 1.72 × 10−31 | 9.30 × 10−30 |
| cg00655307 | −0.42447 | 1.52 × 10−21 | 8.19 × 10−20 |
| cg10939667 | −0.62799 | 7.89 × 10−52 | 4.26 × 10−50 |
| cg05171584 | −0.43805 | 5.45 × 10−23 | 2.94 × 10−21 |
| cg21950534 | −0.43511 | 1.14 × 10−22 | 6.14 × 10−21 |
| cg01777019 | −0.22291 | 1.37 × 10−6 | 7.42 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Manna, C.; Manna, P.R. Harnessing the Role of ESR1 in Breast Cancer: Correlation with microRNA, lncRNA, and Methylation. Int. J. Mol. Sci. 2025, 26, 3101. https://doi.org/10.3390/ijms26073101
Yang S, Manna C, Manna PR. Harnessing the Role of ESR1 in Breast Cancer: Correlation with microRNA, lncRNA, and Methylation. International Journal of Molecular Sciences. 2025; 26(7):3101. https://doi.org/10.3390/ijms26073101
Chicago/Turabian StyleYang, Shengping, Chayan Manna, and Pulak R. Manna. 2025. "Harnessing the Role of ESR1 in Breast Cancer: Correlation with microRNA, lncRNA, and Methylation" International Journal of Molecular Sciences 26, no. 7: 3101. https://doi.org/10.3390/ijms26073101
APA StyleYang, S., Manna, C., & Manna, P. R. (2025). Harnessing the Role of ESR1 in Breast Cancer: Correlation with microRNA, lncRNA, and Methylation. International Journal of Molecular Sciences, 26(7), 3101. https://doi.org/10.3390/ijms26073101







