Neuroprotective Effect of Nor-Prenylated Acylphloroglucinols from Hypericum perforatum L. (St John’s Wort) in the MPTP-Induced Zebrafish Model
Abstract
1. Introduction
2. Results
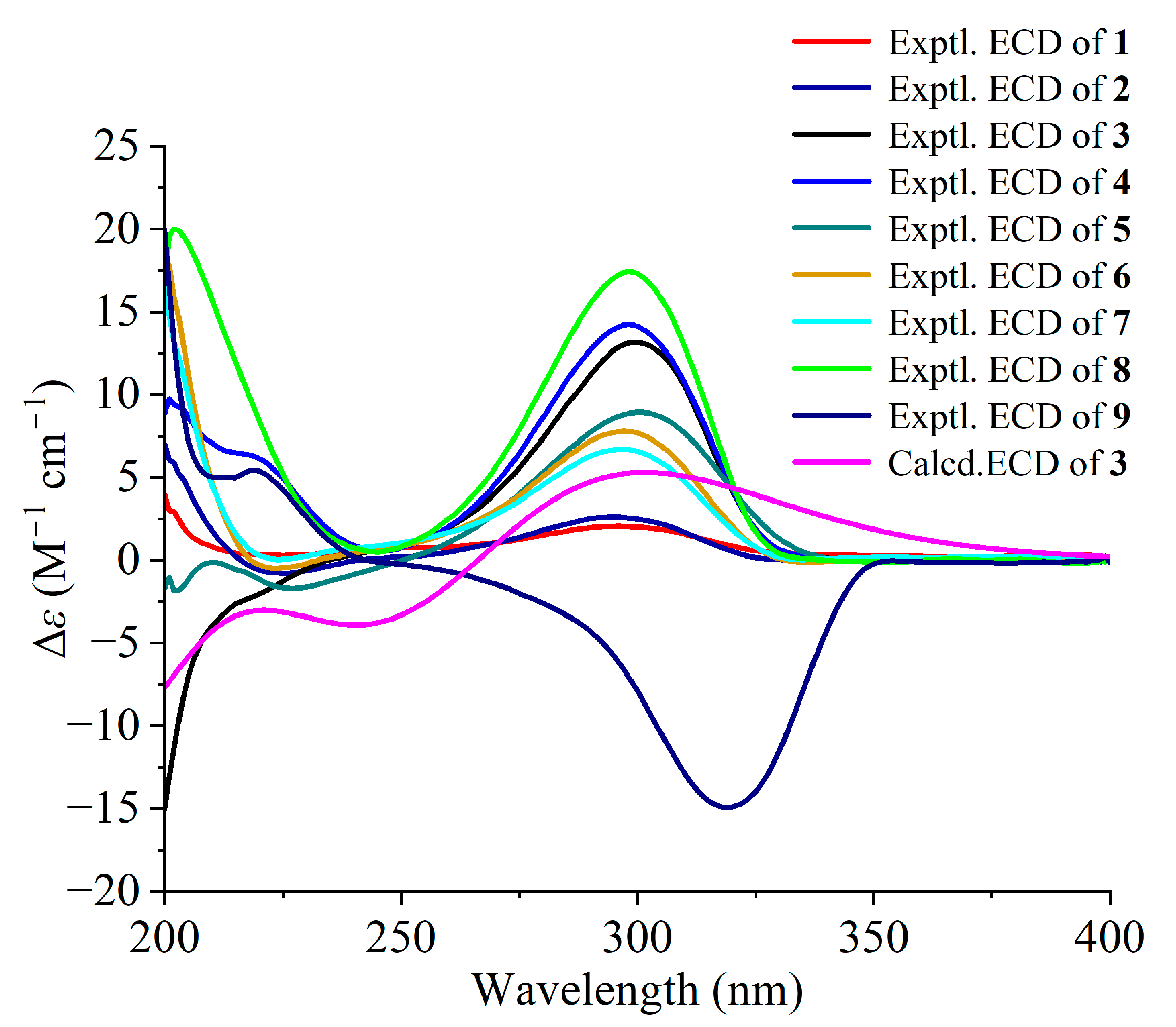
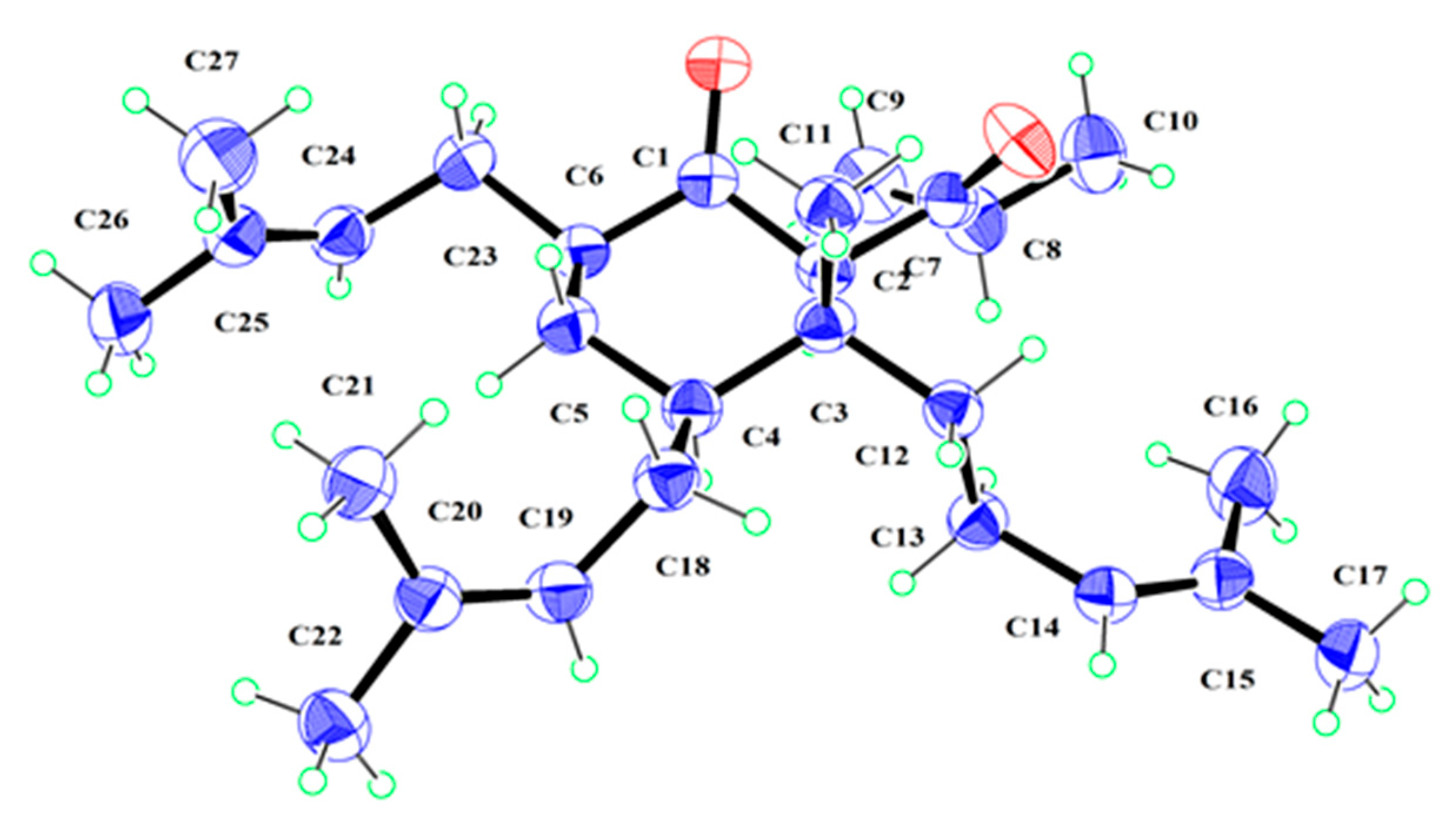
2.1. Structural Elucidation
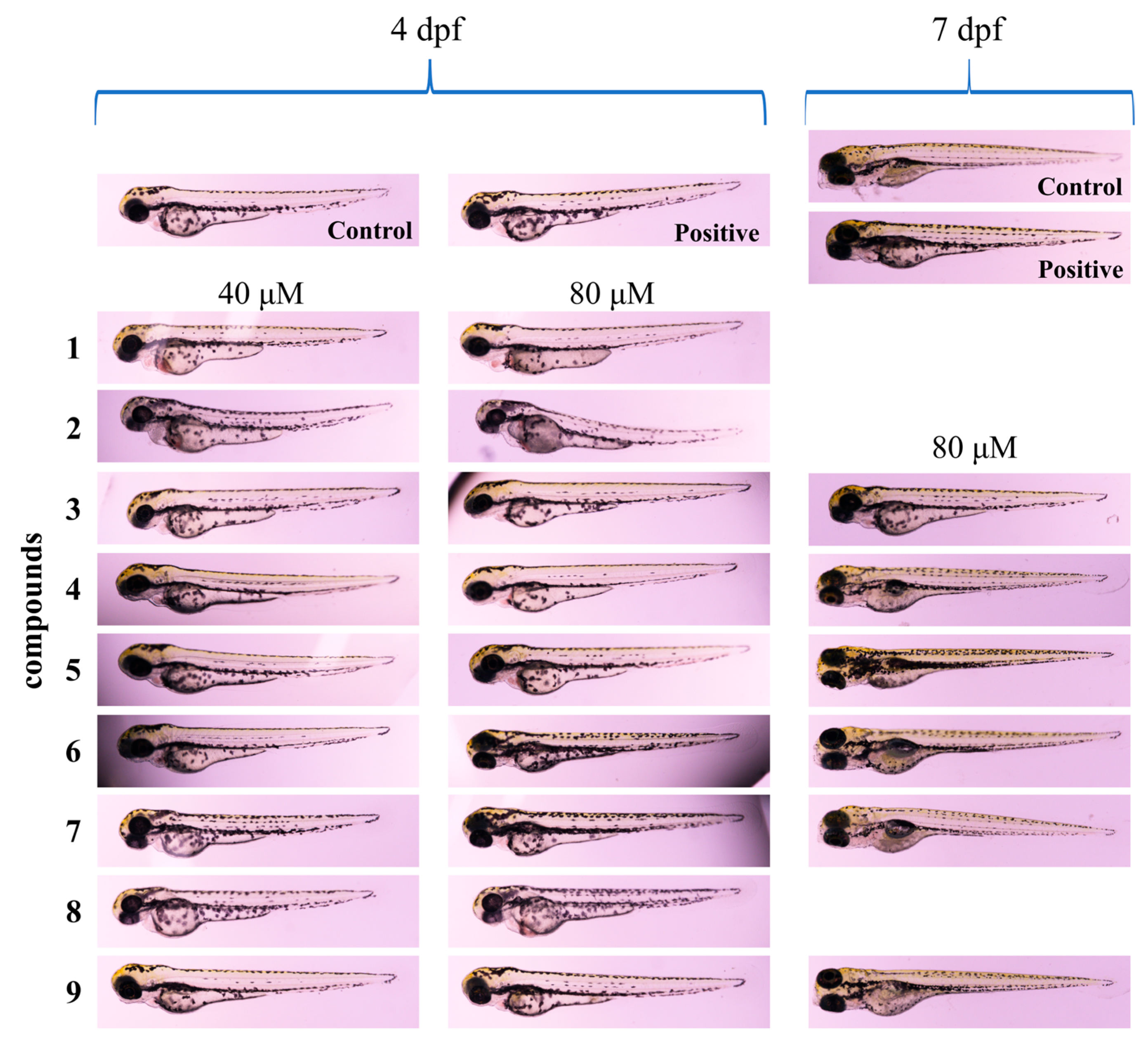
2.2. Acute Toxicity Assessment of Compounds
2.3. Effect of Compounds on MPTP-Induced PD-like Dyskinesia
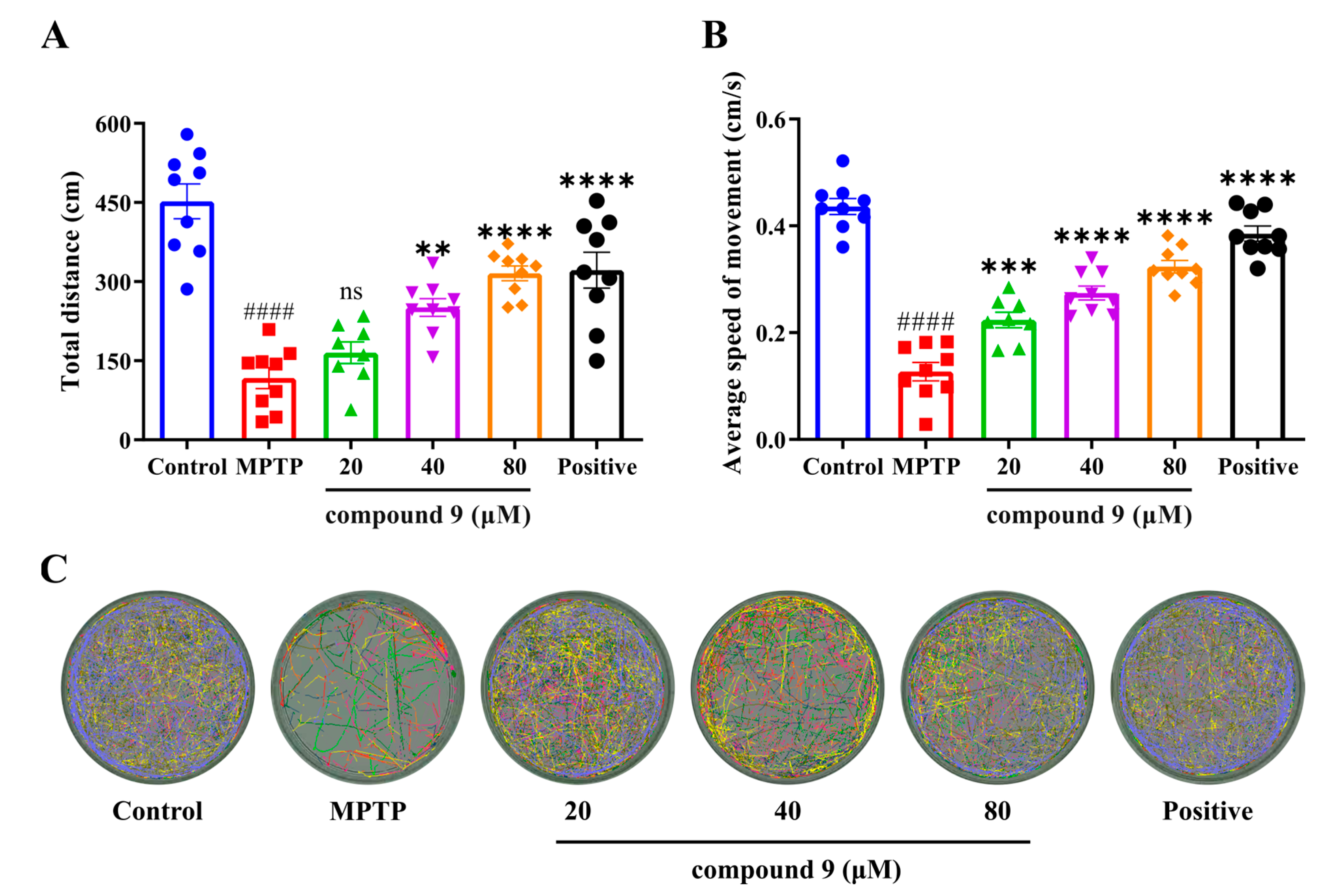
2.4. Effect of Compound 9 on MPTP-Induced PD-like Dyskinesia
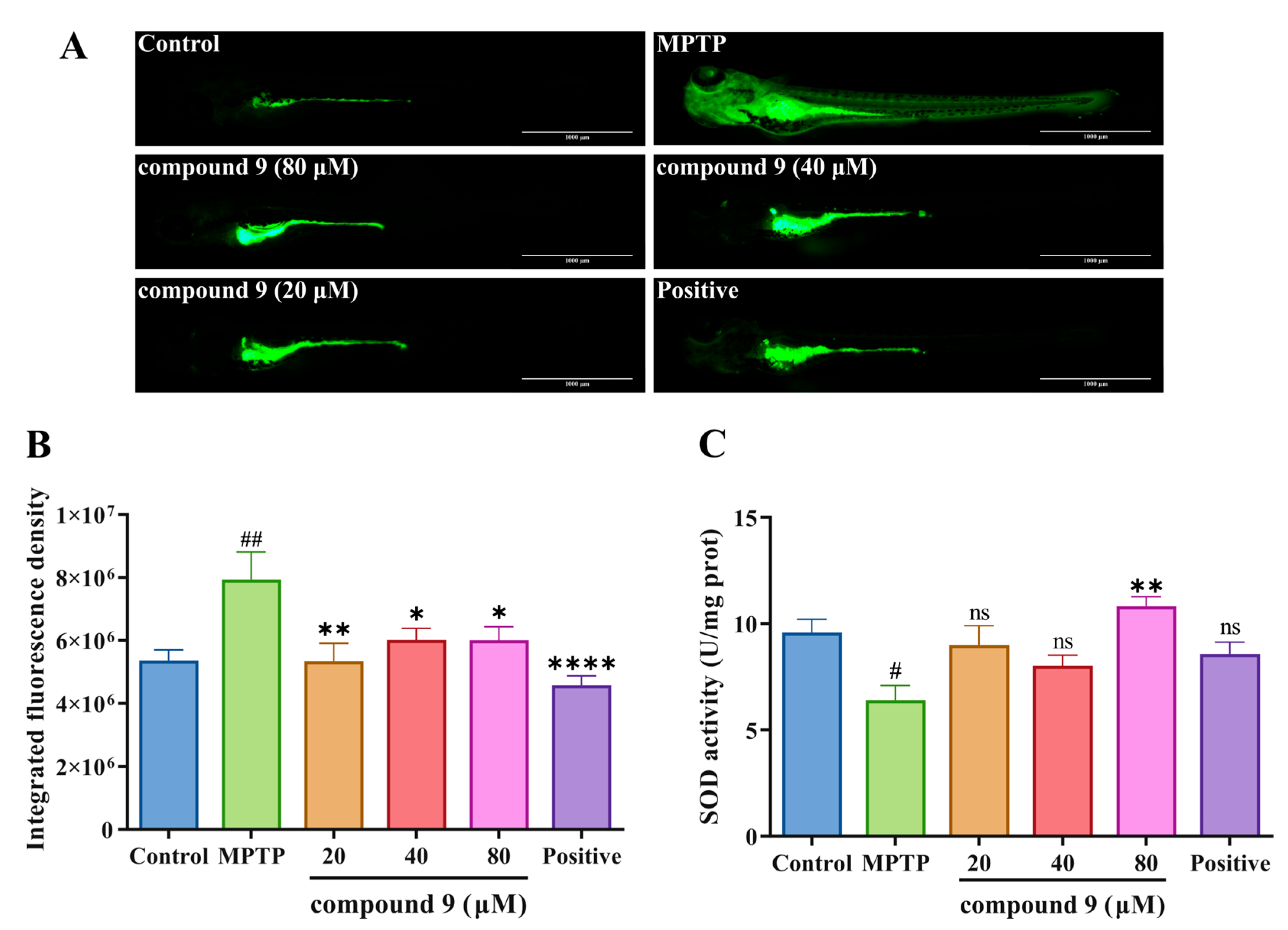
2.5. Effect of Compound 9 on Reactive Oxygen Species (ROS) Generation in PD Zebrafish Model
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. X-Ray Crystallographic Analysis
4.5. ECD Calculations
4.6. Acute Toxicity Assessment
4.7. Behavioral Observation of Zebrafish Larvae
4.8. Detection of ROS Generation in Zebrafish Larvae
4.9. Determination of SOD Activity of Zebrafish Larvae In Vivo
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, S.S.; Nöldner, M.; Koch, E.; Erdelmeier, C. Antidepressant Activity of Hypericum perforatum and Hyperforin: The Neglected Possibility. Pharmacopsychiatry 2007, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.I.; Dobrynin, V.N.; Kolosov, M.N.; Popravko, S.A.; Riabova, I.D. Antibiotic hyperforin from Hypericum perforatum L. Antibiotiki 1971, 16, 510–513. [Google Scholar] [PubMed]
- Bystrov, N.S.; Chernov, B.K.; Dobrynin, V.N.; Kolosov, M.N. The Structure of Hyperforin. Tetrahedron Lett. 1975, 16, 2791–2794. [Google Scholar] [CrossRef]
- Yang, X.W.; Grossman, R.B.; Xu, G. Research Progress of Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef]
- Xie, J.Y.; Li, P.F.; Yan, X.T.; Gao, J.M. Discovery from Hypericum elatoides and Synthesis of Hyperelanitriles as α-Aminopropionitrile-Containing Polycyclic Polyprenylated Acylphloroglucinols. Commun. Chem. 2024, 7, 1. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.Z.; Li, Y.N.; Yi, P.; Gu, W.; Yang, J.; Li, Y.M.; Hao, X.J.; Yuan, C.M. Hypersampones A–C, Three Nor-Polycyclic Polyprenylated Acylphloroglucinols with Lipid-Lowering Activity from Hypericum sampsonii. Org. Lett. 2022, 24, 5967–5971. [Google Scholar] [CrossRef]
- Sun, H.R.; Wang, J.J.; Zhen, B.; Wang, X.; Suo, X.Y.; Lin, M.B.; Jiang, J.D.; Ji, T.F. Polycyclic Polyprenylated Acylphloroglucinol Derivatives from Hypericum pseudohenryi. Phytochemistry 2021, 187, 112761. [Google Scholar] [CrossRef]
- Hu, Y.L.; Hu, K.; Kong, L.M.; Xia, F.; Yang, X.W.; Xu, G. Norascyronones A and B, 2,3,4-nor-Polycyclic Polyprenylated Acylphloroglucinols from Hypericum ascyron. Org. Lett. 2019, 21, 1007–1010. [Google Scholar] [CrossRef]
- Sun, M.X.; Sun, H.R.; Zhu, T.; Zhang, S.; Jiang, B.; Wang, J.; Du, G.; Ji, T.F. Hypseudohenrin H, a Highly-Cyclized Polycyclic Polyprenylated Acylphloroglucinol Derivative from Hypericum pseudohenryi. Tetrahedron 2024, 152, 133820. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, F.; Sun, W.G.; Zhou, Y.; Chen, C.M.; Qi, C.X.; Yang, J.; Li, X.N.; Luo, Z.W.; Zhu, H.C.; et al. Unprecedented Polycyclic Polyprenylated Acylphloroglucinols with Anti-Alzheimer’s Activity from St. John’s Wort. Chem. Sci. 2021, 12, 11438–11446. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, W.Y.; Chen, S.; Gao, Y.S.; Tian, J.m.; Gao, J.M. Four New Polyprenylated Acylphloroglucinols from Hypericum perforatum L. Molecules 2024, 29, 1756. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, Y.F.; Qi, C.X.; Tong, Q.Y.; Chen, C.M.; Yang, J.; Zhu, H.C.; Zhang, Y.H. Polycyclic Polyprenylated Acylphloroglucinols with Immunosuppressive Activity from Hypericum perforatum and Absolute Configurations Assignment of Previously Reported Analogues. Bioorg. Chem. 2021, 114, 105144. [Google Scholar] [CrossRef]
- Deng, X.; Xia, J.; Qian, M.Y.; Wang, X.R.; Hu, B.; Liu, X.S.; Wu, L. Ascyrones A–E, Type B Bicyclic Ployprenylated Acylphloroglucinol Derivatives from Hypericum ascyron. Chin. J. Nat. Med. 2022, 20, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.T.; An, Z.; Huangfu, Y.C.; Zhang, Y.T.; Li, C.-H.; Chen, X.; Liu, P.L.; Gao, J.M. Polycyclic Polyprenylated Acylphloroglucinol and Phenolic Metabolites from the Aerial Parts of Hypericum elatoides and Their Neuroprotective and Anti-Neuroinflammatory Activities. Phytochemistry 2019, 159, 65–74. [Google Scholar] [CrossRef]
- Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17014. [CrossRef]
- Razali, K.; Othman, N.; Mohd Nasir, M.H.; Doolaanea, A.A.; Kumar, J.; Ibrahim, W.N.; Mohamed Ibrahim, N.; Mohamed, W.M.Y. The Promise of the Zebrafish Model for Parkinson’s Disease: Today’s Science and Tomorrow’s Treatment. Front. Genet. 2021, 12, 655550. [Google Scholar] [CrossRef]
- Liu, Y.H.; Deng, Y.C.; Wang, F.Y.; Liu, X.Y.; Wang, J.Q.; Xiao, J.; Zhang, C.L.; Zhang, Q. A New Mechanism for Ginsenoside Rb1 to Promote Glucose Uptake, Regulating Riboflavin Metabolism and Redox Homeostasis. Metabolites 2022, 12, 1011. [Google Scholar] [CrossRef]
- Barnhill, L.M.; Murata, H.; Bronstein, J.M. Studying the Pathophysiology of Parkinson’s Disease Using Zebrafish. Biomedicines 2020, 8, 197. [Google Scholar] [CrossRef]
- Babu, N.S.; Murthy, C.L.N.; Kakara, S.; Sharma, R.; Swamy, C.V.B.; Idris, M.M. 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Induced Parkinson’s Disease in Zebrafish. Proteomics 2016, 16, 1407–1420. [Google Scholar] [CrossRef]
- Zawada, W.M.; Banninger, G.P.; Thornton, J.; Marriott, B.; Cantu, D.; Rachubinski, A.L.; Das, M.; Griffin, W.S.T.; Jones, S.M. Generation of Reactive Oxygen Species in 1-Methyl-4-Phenylpyridinium (MPP+) Treated Dopaminergic Neurons Occurs as an NADPH Oxidase-Dependent Two-Wave Cascade. J. Neuroinflamm. 2011, 8, 129. [Google Scholar] [CrossRef]
- Robea, M.A.; Balmus, I.M.; Ciobica, A.; Strungaru, S.; Plavan, G.; Gorgan, L.D.; Savuca, A.; Nicoara, M. Parkinson’s Disease-Induced Zebrafish Models: Focussing on Oxidative Stress Implications and Sleep Processes. Oxid. Med. Cell Longev. 2020, 2020, 1370837. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Y.; Wang, Z.X.; Liu, W.Y.; Liu, H.W.; Li, D.; Sang, Y.F.; Yang, Z.; Gao, J.M.; Yan, X.T. Hyperelatolides A–D, Antineuroinflammatory Constituents with Unusual Carbon Skeletons from Hypericum elatoides. J. Nat. Prod. 2023, 86, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Qiu, H.; Li, H.M.; Zhang, R.; Pan, Y.K.; Cao, C.Y.; Tian, J.M.; Gao, J.M. Prenylated Flavonoids from Hypericum perforatum L. and Their Anti-Neuroinflammatory and Neuroprotective Activities. Ind. Crops Prod. 2024, 216, 118792. [Google Scholar] [CrossRef]
- Yan, X.T.; Chen, J.X.; Wang, Z.X.; Zhang, R.Q.; Xie, J.Y.; Kou, R.W.; Zhou, H.F.; Zhang, A.L.; Wang, M.C.; Ding, Y.X.; et al. Hyperhubeins A–I, Bioactive Sesquiterpenes with Diverse Skeletons from Hypericum hubeiense. J. Nat. Prod. 2023, 86, 119–130. [Google Scholar] [CrossRef]
- Liu, W.Y.; Lei, X.X.; Wang, W.J.; Tian, J.M.; Gao, Y.Q.; Gao, J.M. Hyperforatone A, the 1,8-Seco Rearranged Polycyclic Polyprenylated Acylphloroglucinol with a Unique Bicyclo [5.4.0] Undecane Core from Hypericum perforatum. Chin. Chem. Lett. 2024, 36, 110478. [Google Scholar] [CrossRef]
- Liu, W.Y.; Gao, L.L.; Zhou, W.; Ma, Y.N.; Tian, J.M.; Gao, J.M. Hypertums A−J, Bioactive Polycyclic Polyprenylated Acylphloroglucinols with Diverse Skeletons from Hypericum perforatum L. (St. John’s Wort). Phytochemistry 2025, 235, 114450. [Google Scholar] [CrossRef]
- Shan, M.D.; Hu, L.H.; Chen, Z.L. Three New Hyperforin Analogues from Hypericum perforatum. J. Nat. Prod. 2001, 64, 127–130. [Google Scholar] [CrossRef]
- Ma, J.; Ji, T.F.; Yang, J.B.; Wang, A.G.; Su, Y.L. Three New Phloroglucinol Derivatives from Hypericum Scabrum. J. Asian Nat. Prod. Res. 2012, 14, 508–514. [Google Scholar] [CrossRef]
- Ma, J.; Zang, Y.D.; Zhang, J.J.; Li, C.J.; Li, Y.; Su, Y.L.; Wang, A.G.; Zhang, D.M. Nine Prenylated Acylphloroglucinols with Potential Anti-Depressive and Hepatoprotective Activities from Hypericum Scabrum. Bioorg. Chem. 2021, 107, 104529. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Song, Q.Y.; Zheng, H.H.; Wang, R.; Zhang, Q. Toxicity and Metabolomic Dysfunction Invoked by Febrifugin, a Harmful Component of Edible Nut of Swietenia macrophylla. Int. J. Mol. Sci. 2024, 25, 9753. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.X.; Guo, D.X.; Huang, W.; Ren, H.P.; Zhang, Q. Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin. Int. J. Mol. Sci. 2024, 25, 8985. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.Y.; Jiang, X.; Zhang, S.S.; Gao, X.; Paudel, Y.N.; Zhang, P.Y.; Wang, R.C.; Liu, K.C.; Jin, M. Neuroprotective Effect of YIAEDAER Peptide against Parkinson’s Disease like Pathology in Zebrafish. Biomed. Pharmacother. 2022, 147, 112629. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Q.; Bian, M.; Gong, G.H.; Bai, C.M.; Liu, C.Y.; Wei, C.X.; Quan, Z.X.; Du, H.H. Synthesis and Evaluation of Bakuchiol Derivatives as Potent Anti-Inflammatory Agents In Vitro and In Vivo. J. Nat. Prod. 2022, 85, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Y.; Ma, F.W.; Yi, P.; Hu, Z.X.; Gu, W.; Huang, L.J.; He, W.W.; Yuan, C.M.; Hao, X.J. Bioassay and UPLC-Q-Orbitrap-MS/MS Guided Isolation of Polycyclic Polyprenylated Acylphloroglucinols from St. John’s Wort and Their Neuroprotective Activity. Arab. J. Chem. 2022, 15, 104057. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, Q.Y.; Zhang, N.; Duan, X.Y.; Cao, Y.; Zhu, H.C.; Xie, S.S.; Yang, J.; Zhang, J.W.; Liu, Y.F.; et al. Highly Functionalized Cyclohexanone-Monocyclic Polyprenylated Acylphloroglucinols from Hypericum perforatum Induce Leukemia Cell Apoptosis. Org. Chem. Front. 2019, 6, 817–824. [Google Scholar] [CrossRef]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Chen, Y.; Wisner, A.S.; Schiefer, I.T.; Williams, F.E.; Hall, F.S. Methamphetamine-Induced Lethal Toxicity in Zebrafish Larvae. Psychopharmacology 2022, 239, 3833–3846. [Google Scholar] [CrossRef]
- Gregoretti, B.; Stebel, M.; Candussio, L.; Crivellato, E.; Bartoli, F.; Decorti, G. Toxicity of Hypericum perforatum (St. John’s wort) administered during pregnancy and lactation in rats. Toxicol. Appl. Pharm. 2004, 200, 201–205. [Google Scholar] [CrossRef]
- Hu, Y.L.; Yue, G.G.L.; Li, X.R.; Xu, G.; Lau, C.B.S. Structurally Diverse Spirocyclic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum ascyron Linn. and Their Anti-Tumor Activity. Phytochemistry 2023, 212, 113727. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s Wort ( Hypericum perforatum L.): A Review of Its Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2010, 53, 583–600. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s Wort (Hypericum perforatum): A Review of the Current Pharmacological, Toxicological, and Clinical Literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Patočka, J. The Chemistry, Pharmacology, and Toxicology of the Biologically Active Constituents of the Herb Hypericum perforatum L. J. Appl. Biomed. 2003, 1, 61–70. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. St. John’s Wort: Role of Active Compounds for Its Mechanism of Action and Efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Pellati, F.; Bellardi, M.G.; Benvenuti, S.; Paltrinieri, S.; Bertaccini, A.; Bianchi, A. Herbal Drug Quality and Phytochemical Composition of Hypericum perforatum L. Affected by Ash Yellows Phytoplasma Infection. J. Agric. Food Chem. 2005, 53, 964–968. [Google Scholar] [CrossRef]
- Hou, X.D.; Guan, X.Q.; Cao, Y.F.; Weng, Z.M.; Hu, Q.; Liu, H.B.; Jia, S.N.; Zang, S.Z.; Zhou, Q.; Yang, L.; et al. Inhibition of Pancreatic Lipase by the Constituents in St. John’s Wort: In Vitro and in Silico Investigations. Int. J. Biol. Macromol. 2020, 145, 620–633. [Google Scholar] [CrossRef]
- Sabesan, M.; Mohanasundari, M.; Sethupathy, S. The Effect of Hypericum perforatum Extract against the Neurochemical and Behavioural Changes Induced by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) in Mice. Indian J. Pharmacol. 2006, 38, 266. [Google Scholar] [CrossRef]
- Mohanasundari, M.; Srinivasan, M.S.; Sethupathy, S.; Sabesan, M. Enhanced Neuroprotective Effect by Combination of Bromocriptine and Hypericum perforatum Extract against MPTP-Induced Neurotoxicity in Mice. J. Neurol. Sci. 2006, 249, 140–144. [Google Scholar] [CrossRef]
- Ivetic, V.; Popovic, M.; Mimica-Dukic, N.; Barak, O.; Pilija, V. St. John’s Wort (Hypericum perforatum L.) and Kindling Epilepsy in Rabbit. Phytomedicine 2002, 9, 496–499. [Google Scholar] [CrossRef]
- Li, X.; Gao, D.; Paudel, Y.N.; Li, X.; Zheng, M.; Liu, G.; Ma, Y.; Chu, L.; He, F.; Jin, M. Anti-Parkinson’s Disease Activity of Sanghuangprous vaninii Extracts in the MPTP-Induced Zebrafish Model. ACS Chem. Neurosci. 2022, 13, 330–339. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Mou, P.Y.; Huang, Y.Y.; Zeng, H.; Huang, Z.L.; Wei, X. Bioactive Polycyclic Polyprenylated Acylphloroglucinols from Hypericum scabrum. Fitoterapia 2022, 161, 105249. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Shi, W.P.; Zeng, H.; Huang, Y.Y.; Wei, X.; Pan, K.; Teng, L.P. Two New Polycyclic Polyprenylated Acylphloroglucinols from Hypericum curvisepalum N. Robson. Phytochem. Lett. 2022, 48, 43–46. [Google Scholar] [CrossRef]
- Ma, J.; Xia, G.Y.; Zang, Y.D.; Li, C.J.; Yang, J.B.; Huang, J.W.; Zhang, J.J.; Su, Y.L.; Wang, A.G.; Zhang, D.M. Three New Decarbonyl Prenylphloroglucinols Bearing Unusual Spirost Subunits from Hypericum scabrum and Their Neuronal Activities. Chin. Chem. Lett. 2021, 32, 1173–1176. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Gahtani, R.M.; Shoaib, S.; Hani, U.; Jayachithra, R.; Alomary, M.N.; Chauhan, W.; Jahan, R.; Tufail, S.; Ansari, M.A. Combating Parkinson’s Disease with Plant-Derived Polyphenols: Targeting Oxidative Stress and Neuroinflammation. Neurochem. Int. 2024, 178, 105798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Y.J.; Wang, Z.H.; Chen, T.R.; Qian, H.W.; He, J.; Li, J.; Han, B.; Wang, T. Rosmarinic Acid Protects against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Dopaminergic Neurotoxicity in Zebrafish Embryos. Toxicol. Vitr. 2020, 65, 104823. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Guo, C.; Qi, R.Y.; Ren, J.Y.; Xu, D.D.; Zhang, Q.; Gao, J.M.; Tang, J.J. Inubritantrimers A–D: Trimerized Sesquiterpenoid [4 + 2] Adducts Featuring a Distinctive Spiro-Polycyclic Scaffold from Inula britannica. J. Org. Chem. 2024, 89, 5029–5037. [Google Scholar] [CrossRef]
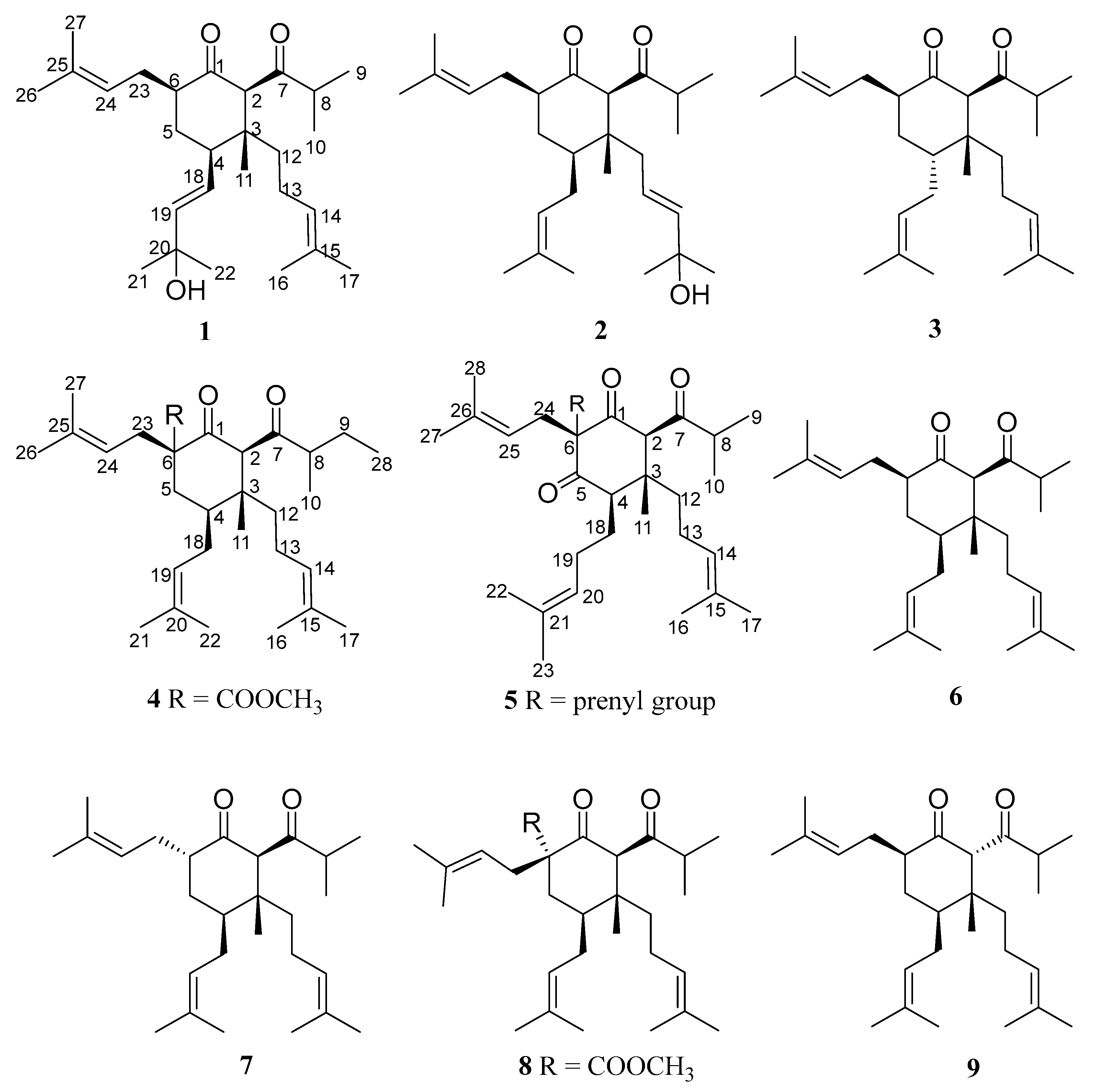
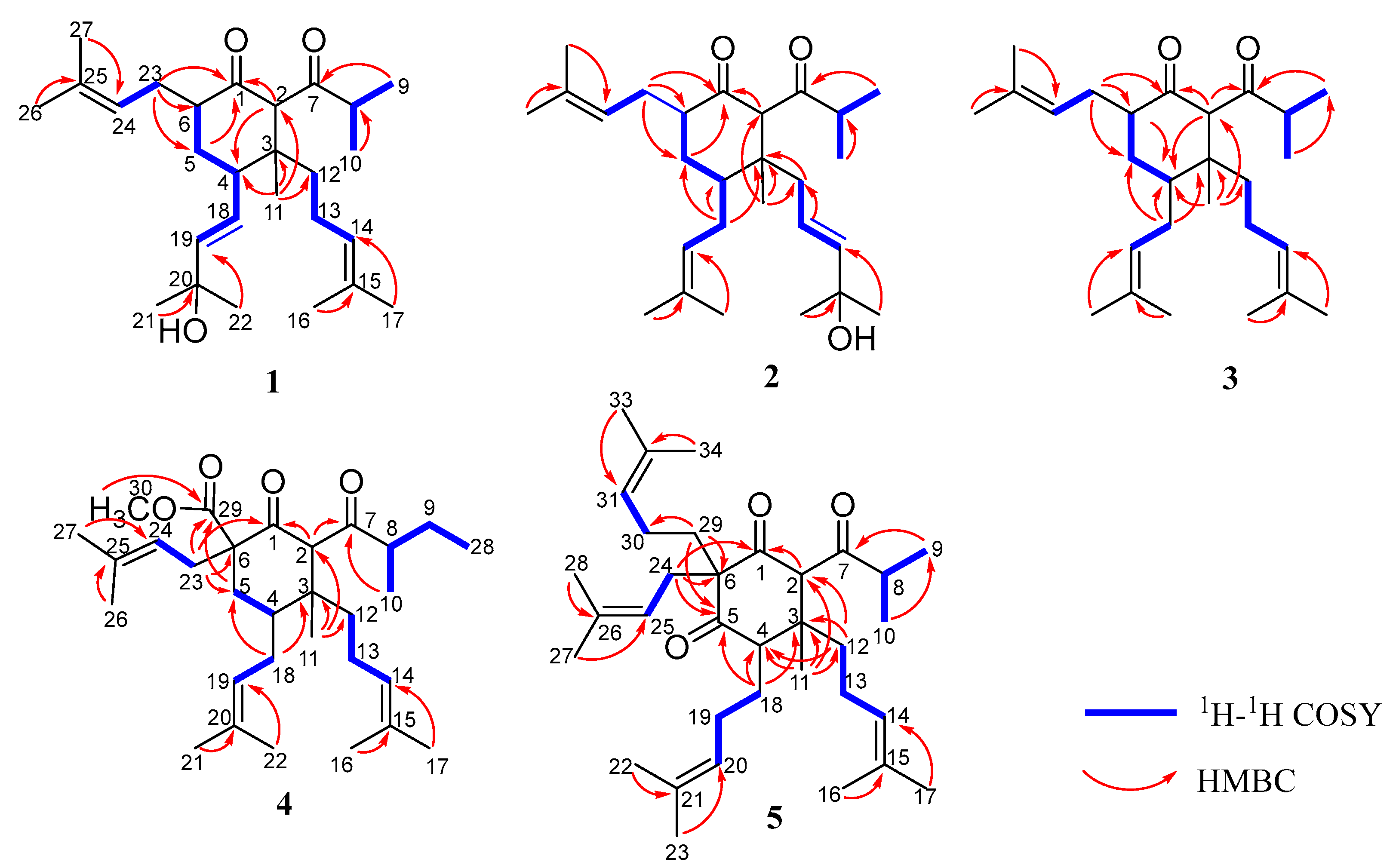
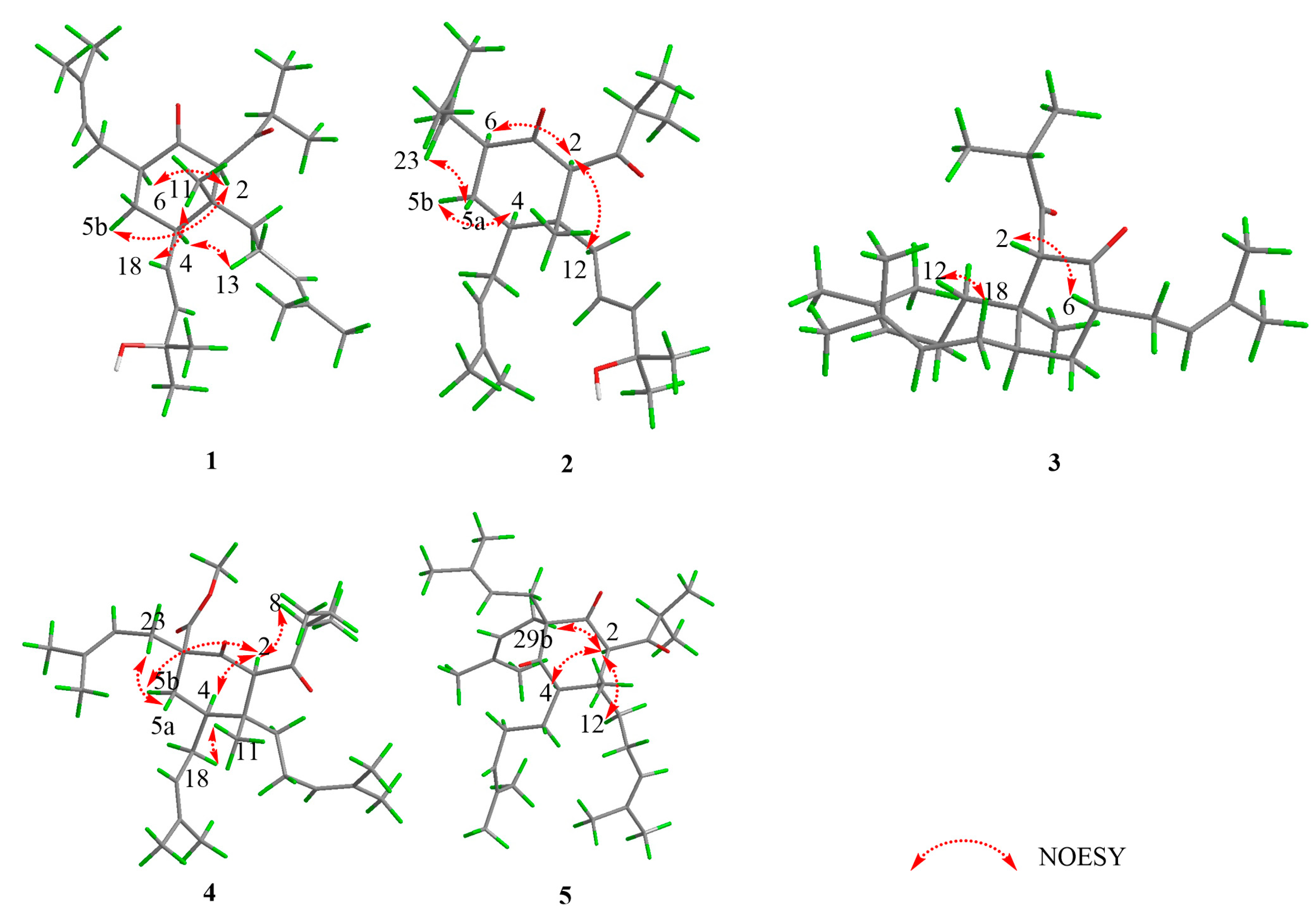

| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 3.83, s | 3.72, s | 3.88, s | 3.90, s | 3.82, s |
| 4 | 2.58, ddd (12.5, 8.6, 3.8) | 1.74, m | 1.79, m | 1.80, m | 1.69, m |
| 5a | 1.96, m | 2.12, m | 1.74, m | 2.49, m | |
| 5b | 1.53, d (5.4) | 1.16, m | 1.55, m | 1.29, m | |
| 6 | 2.45, m | 2.35, m | 2.39, m | ||
| 8 | 2.50, m | 2.45, m | 2.36, m | 2.37, m | 2.57, m |
| 9a | 1.05, d (6.8) | 1.03, d (6.8) | 0.96, overlapped | 1.62, m | 1.02, d (6.6) |
| 9b | 1.25, m | ||||
| 10 | 1.06, d (6.8) | 1.05, d (6.8) | 0.96, overlapped | 1.06, d (6.7) | 1.08, d (6.6) |
| 11 | 1.02, s | 1.02, s | 0.96, s | 0.96, s | 0.96, s |
| 12a | 1.49, m | 2.26, m | 1.39, m | 1.51, m | 1.41, m |
| 12b | 1.26, m | 1.41, m | |||
| 13a | 2.07, m | 5.61, overlapped | 1.99, m | 2.01, m | 1.93, m |
| 13b | 1.71, m | 1.74, m | 1.67, m | 1.68, m | |
| 14 | 4.93, t (6.5) | 5.61, overlapped | 4.92, overlapped | 4.93, t (7.1) | 4.91, t (6.7) |
| 16a | 1.63, s | 1.30, s | 1.61, s | 1.63, s | 1.65, s |
| 16b | |||||
| 17 | 1.56, s | 1.30, s | 1.56, s | 1.55, s | 1.52, s |
| 18a | 5.53, dd (15.6, 8.8) | 2.17, m | 2.04, m | 2.11, m | 2.39, m |
| 18b | 1.69, m | 1.70, m | 1.36, m | ||
| 19a | 5.74, d (15.6) | 5.09, overlapped | 5.00, overlapped | 5.13, overlapped | 2.08, m |
| 19b | 1.67, m | ||||
| 20 | 5.12, t (7.8) | ||||
| 21 | 1.32, s | 1.71, s | 1.67, s | 1.74, s | |
| 22 | 1.32, s | 1.59, s | 1.61, s | 1.59, s | 1.73, s |
| 23a | 2.39, m | 2.37, m | 2.32, m | 2.51, m | 1.61, s |
| 23b | 1.95, m | 1.92, m | 2.21, m | 2.26, dd (14.5, 7.6) | |
| 24a | 5.07, t (6.5) | 5.06, overlapped | 4.96, overlapped | 5.05, overlapped | 2.45, m |
| 24b | 2.28, m | ||||
| 25 | 4.95, t (7.0) | ||||
| 26 | 1.68, s | 1.68, s | 1.65, s | 1.68, s | |
| 27 | 1.60, s | 1.59, s | 1.54, s | 1.55, s | 1.73, s |
| 28 | 0.88, t (7.4) | 1.56, s | |||
| 29a | 2.52, m | ||||
| 29b | 2.33, m | ||||
| 30a | 3.76, OCH3 | 2.28, m | |||
| 30b | 2.20, m | ||||
| 31 | 5.02, t (7.3) | ||||
| 33 | 1.61, s | ||||
| 34 | 1.52, s |
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 209.7, C | 210.4, C | 212.4, C | 205.4, C | 207.9, C |
| 2 | 66.7, CH | 67.2, CH | 64.2, CH | 66.1, CH | 66.5, CH |
| 3 | 45.3, C | 46.4, C | 45.3, C | 45.8, C | 45.2, C |
| 4 | 45.8, CH | 42.8, CH | 38.0, CH | 40.0, CH | 40.2, CH |
| 5 | 35.7, CH2 | 34.8, CH2 | 31.3, CH2 | 36.4, CH2 | 211.2, C |
| 6 | 51.0, CH | 51.4, CH | 49.8, CH | 61.6, C | 67.8, C |
| 7 | 210.8, C | 211.3, C | 211.3, C | 209.9, C | 210.2, C |
| 8 | 42.7, CH | 42.7, CH | 42.7, CH | 49.2, CH | 42.5, CH |
| 9 | 18.9, CH3 | 19.0, CH3 | 18.4, CH3 | 25.8, CH2 | 18.8, CH3 |
| 10 | 17.8, CH3 | 17.9, CH3 | 17.7, CH3 | 14.5, CH3 | 17.7, CH3 |
| 11 | 17.4, CH3 | 16.8, CH3 | 17.6, CH3 | 17.2, CH3 | 17.5, CH3 |
| 12 | 37.6, CH2 | 39.1, CH2 | 37.3, CH2 | 36.6, CH2 | 36.7, CH2 |
| 13 | 21.9, CH2 | 121.4, CH | 22.0, CH2 | 22.1, CH2 | 22.0, CH2 |
| 14 | 123.7, CH | 142.2, CH | 123.7, CH | 123.7, CH | 123.6, CH |
| 15 | 131.7, C | 70.9, C | 131.7, C | 131.9, C | 131.8, C |
| 16 | 25.8, CH3 | 30.2, CH3 | 25.7, CH3 | 25.9, CH3 | 25.8, CH3 |
| 17 | 17.9, CH3 | 30.2, CH3 | 17.7, CH3 | 17.8, CH3 | 17.8, CH3 |
| 18 | 125.6, CH | 27.0, CH2 | 26.9, CH2 | 27.0, CH2 | 35.3, CH2 |
| 19 | 140.6, CH | 123.0, CH | 123.2, CH | 122.7, CH | 27.0, CH2 |
| 20 | 70.9, C | 133.1, C | 132.8, C | 133.1, C | 122.6, CH |
| 21 | 30.2, CH3 | 26.0, CH3 | 25.8, CH3 | 26.1, CH3 | 133.4, C |
| 22 | 29.9, CH3 | 18.1, CH3 | 18.0, CH3 | 18.2, CH3 | 26.0, CH3 |
| 23 | 27.6, CH2 | 27.7, CH2 | 30.6, CH2 | 33.4, CH2 | 18.1, CH3 |
| 24 | 121.4, CH | 121.6, CH | 121.0, CH | 118.2, CH | 33.0, CH2 |
| 25 | 133.7, C | 133.4, C | 133.9, C | 135.5, C | 118.4, CH |
| 26 | 26.0, CH3 | 26.0, CH3 | 25.9, CH3 | 26.0, CH3 | 135.1, C |
| 27 | 18.0, CH3 | 18.0, CH3 | 18.0, CH3 | 17.9, CH3 | 26.0, CH3 |
| 28 | 11.8, CH3 | 18.0, CH3 | |||
| 29 | 172.3, C | 37.9, CH2 | |||
| 30 | 52.5, OCH3 | 22.2, CH2 | |||
| 31 | 122.6, CH | ||||
| 32 | 133.3, C | ||||
| 33 | 25.8, CH3 | ||||
| 34 | 17.8, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhao, P.; Liu, Y.; Meng, X.; Xie, J.; Tian, J.; Gao, J. Neuroprotective Effect of Nor-Prenylated Acylphloroglucinols from Hypericum perforatum L. (St John’s Wort) in the MPTP-Induced Zebrafish Model. Int. J. Mol. Sci. 2025, 26, 3096. https://doi.org/10.3390/ijms26073096
Liu W, Zhao P, Liu Y, Meng X, Xie J, Tian J, Gao J. Neuroprotective Effect of Nor-Prenylated Acylphloroglucinols from Hypericum perforatum L. (St John’s Wort) in the MPTP-Induced Zebrafish Model. International Journal of Molecular Sciences. 2025; 26(7):3096. https://doi.org/10.3390/ijms26073096
Chicago/Turabian StyleLiu, Wuyang, Peng Zhao, Yihan Liu, Xiangyan Meng, Jinyan Xie, Junmian Tian, and Jinming Gao. 2025. "Neuroprotective Effect of Nor-Prenylated Acylphloroglucinols from Hypericum perforatum L. (St John’s Wort) in the MPTP-Induced Zebrafish Model" International Journal of Molecular Sciences 26, no. 7: 3096. https://doi.org/10.3390/ijms26073096
APA StyleLiu, W., Zhao, P., Liu, Y., Meng, X., Xie, J., Tian, J., & Gao, J. (2025). Neuroprotective Effect of Nor-Prenylated Acylphloroglucinols from Hypericum perforatum L. (St John’s Wort) in the MPTP-Induced Zebrafish Model. International Journal of Molecular Sciences, 26(7), 3096. https://doi.org/10.3390/ijms26073096






