The Role of Phosphorus Fertilization in Antioxidant Responses of Drought-Stressed Common Beech and Sessile Oak Provenances
Abstract
1. Introduction
- To investigate the impact of drought, phosphorus fertilization, and provenance on the antioxidative capacity of common beech and sessile oak;
- To assess the impact of phosphorus fertilization under drought conditions on the antioxidative capacity in common beech and sessile oak;
- To investigate the differences in antioxidative capacity in common beech and sessile oak provenances.
2. Results
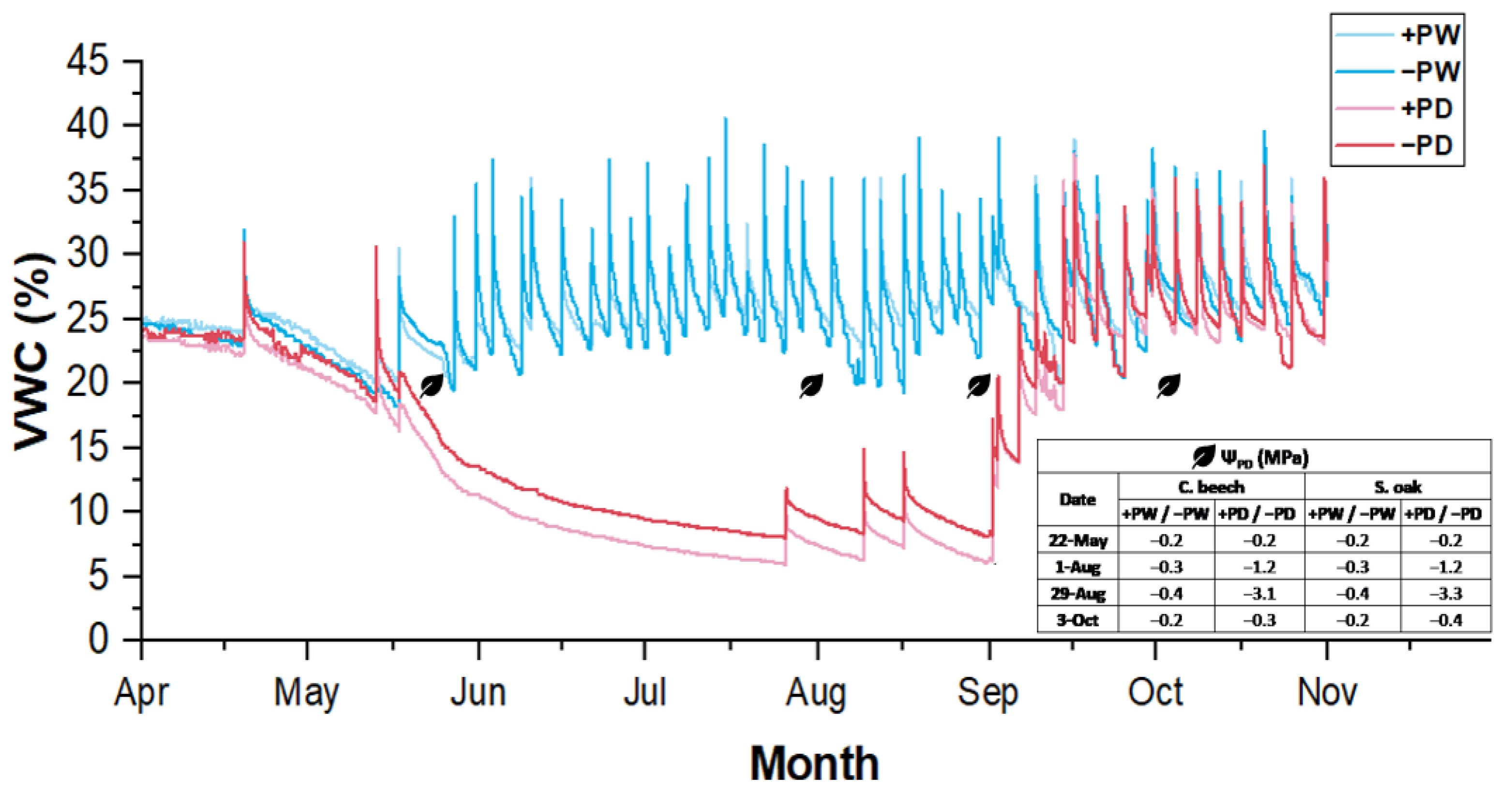
2.1. Water Availability in Soil and Leaf Water Potential
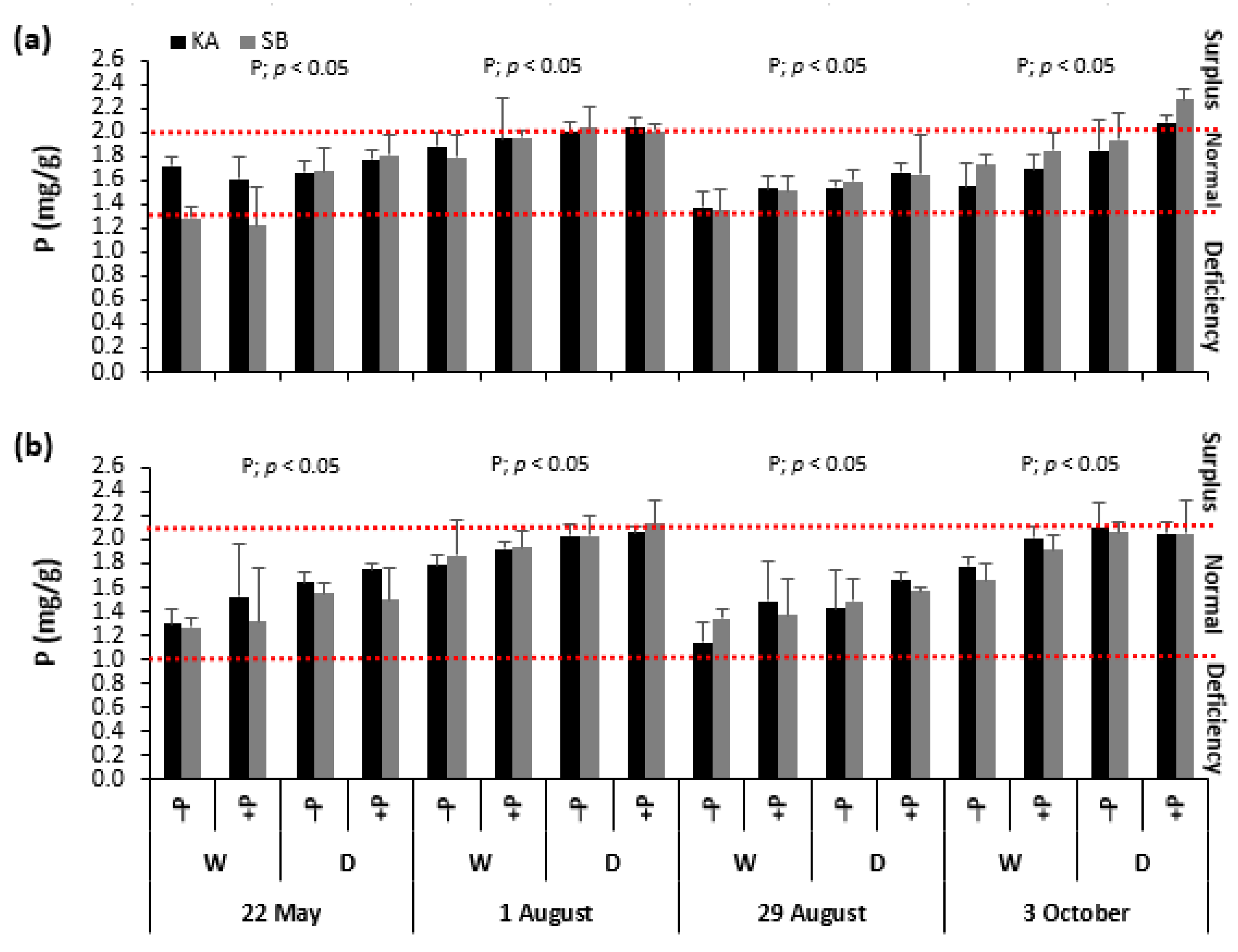
2.2. Leaf Phosphorus Concentrations
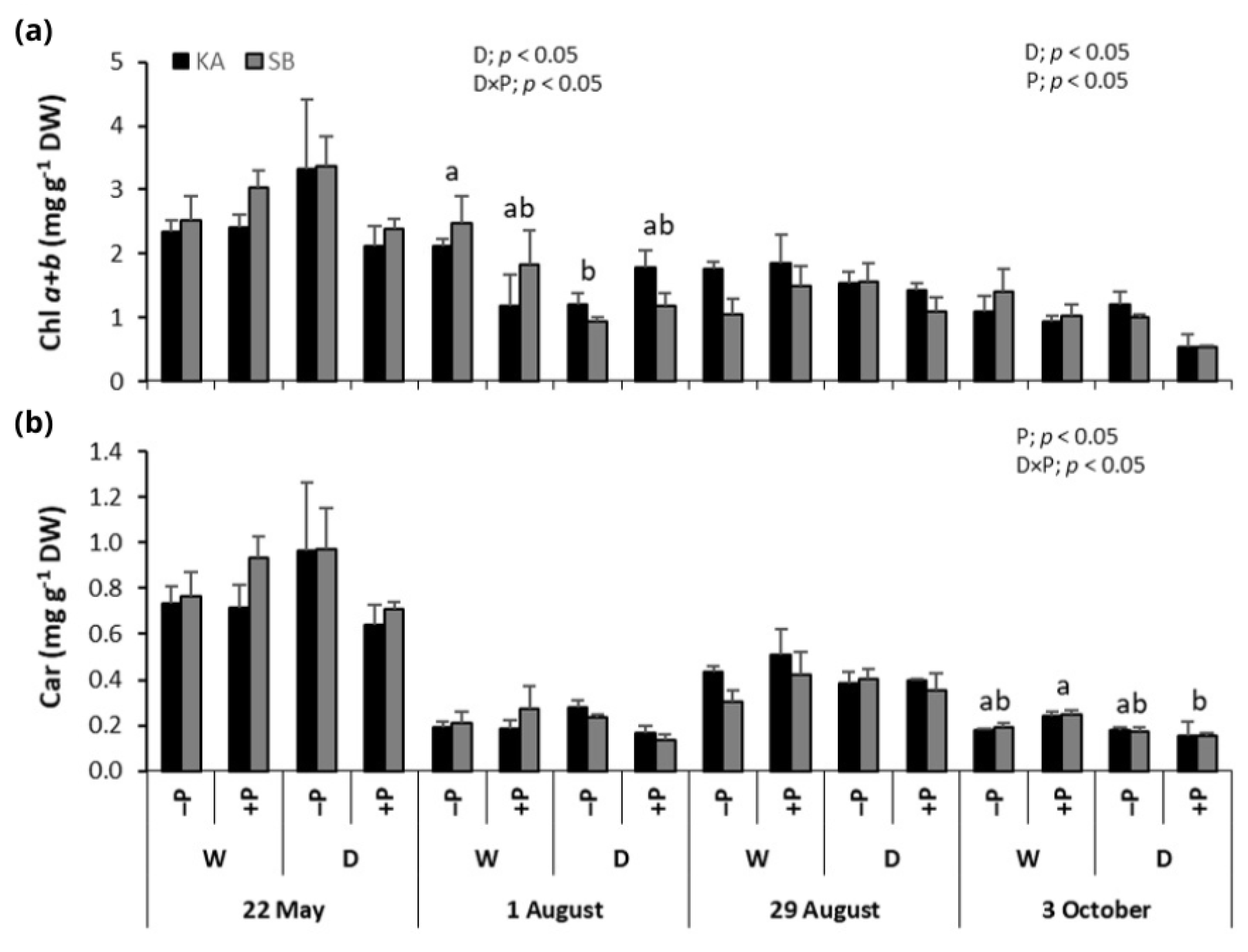
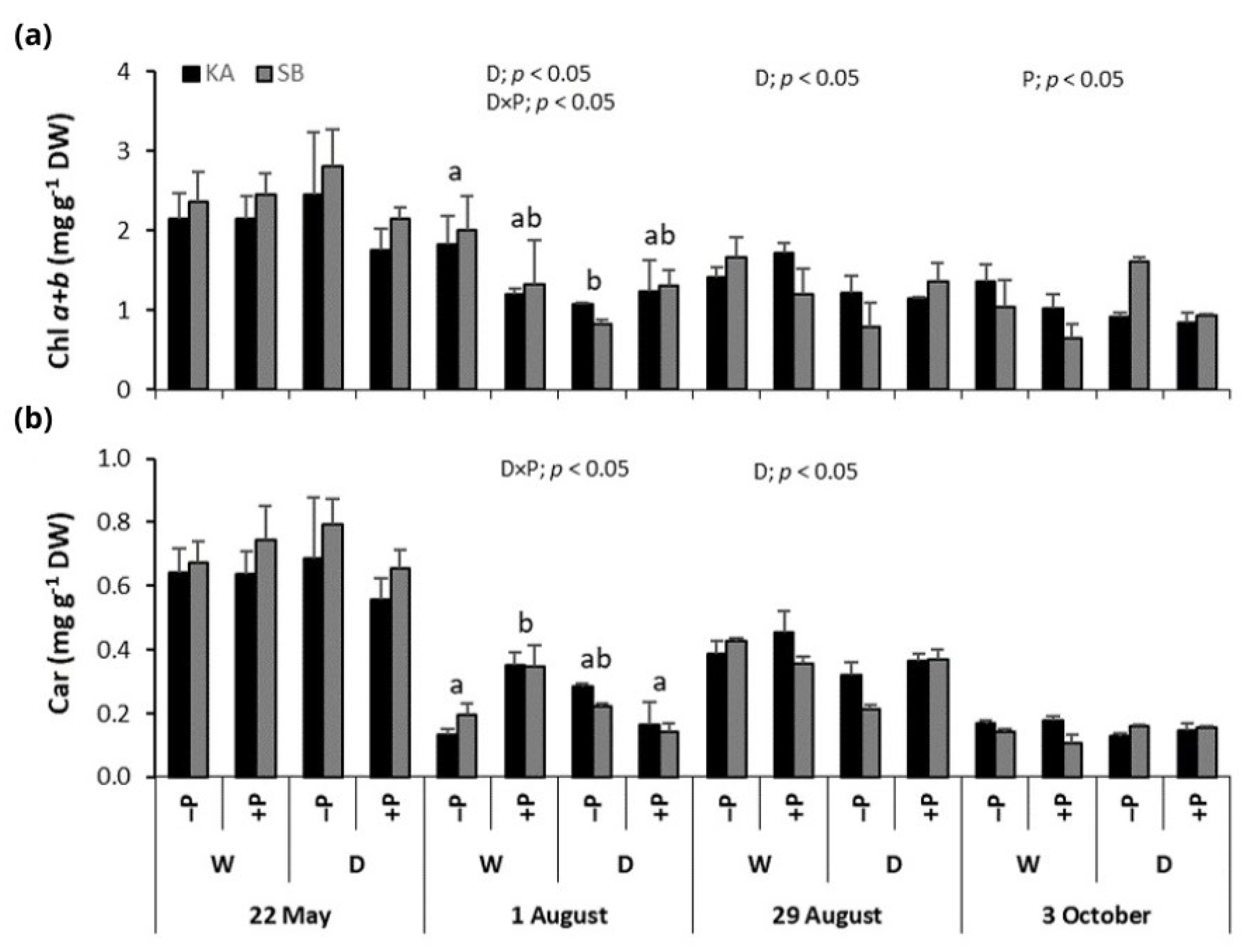
2.3. Photosynthetic Pigments
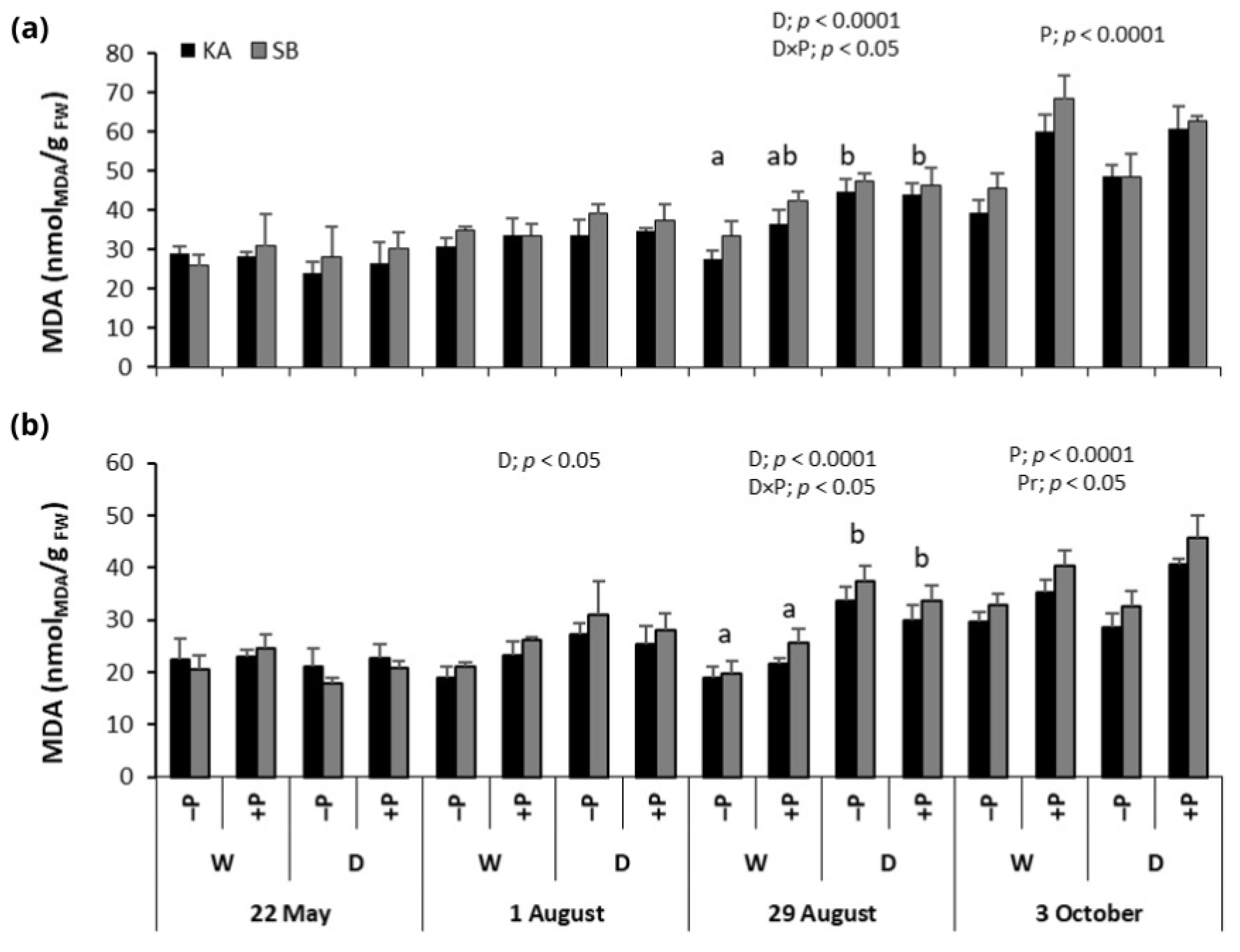
2.4. Malondialdehyde Concentrations
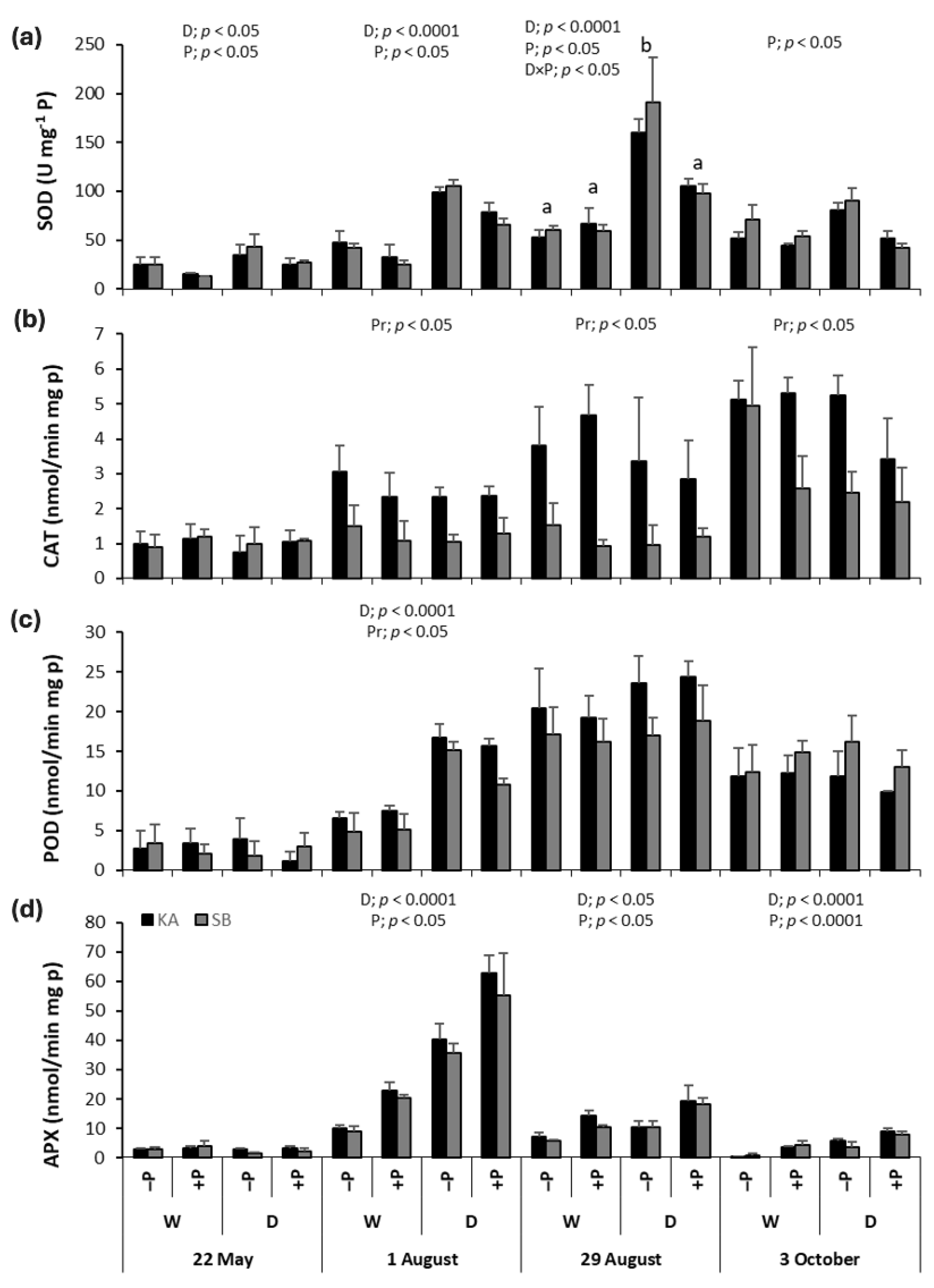
2.5. Antioxidant Enzyme Activities in Common Beech
2.6. Antioxidant Enzyme Activities in Sessile Oak
3. Discussion
3.1. Effect of Drought on Malondialdehyde Concentrations, Photosynthetic Pigments and Antioxidant Enzyme Activities
3.2. Effect of Phosphorus Fertilization and Drought × Phosphorus Fertilization Interaction on Malondialdehyde Concentrations, Photosynthetic Pigments and Antioxidant Enzyme Activities
3.3. Effect of Provenance on the Malondialdehyde Concentrations, Photosynthetic Pigments, and Antioxidant Enzyme Activities
4. Materials and Methods
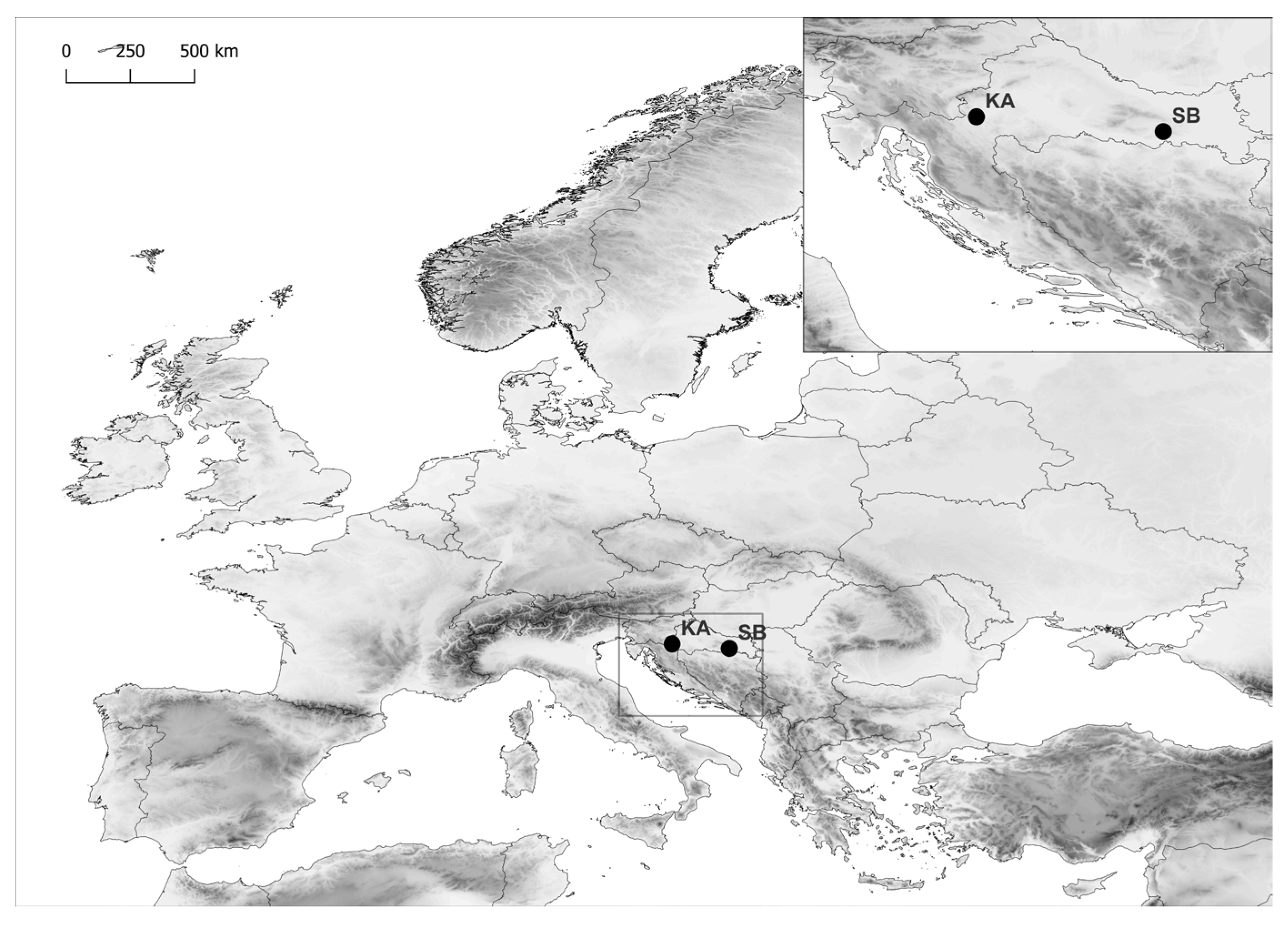
4.1. Plant Material and Provenance Habitat Conditions
4.2. Experimental Design and Growth Conditions
4.3. Soil Volumetric Water Content and Leaf Water Potential
4.4. Leaf Sampling for Chemical Analysis
4.5. Leaf Phosphorus Concentration
4.6. Chlorophyll and Carotenoid Concentrations
4.7. Determination of Malondialdehyde Concentration
4.8. Determination of Protein Concentration and Antioxidant Enzyme Activity
4.8.1. Determination of Total Soluble Protein Concentration by Bradford Method
4.8.2. Measurement of Ascorbate Peroxidase Activity
4.8.3. Measurement of Catalase Activity
4.8.4. Measurement of Peroxidase Activity
4.8.5. Measurement of Superoxide Dismutase Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arend, M.; Brem, A.; Kuster, T.M.; Günthardt-Goerg, M.S. Seasonal Photosynthetic Responses of European Oaks to Drought and Elevated Daytime Temperature. Plant Biol. 2013, 15, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Marino, S.; Maiuro, L.; Alvino, A.; Czajkowski, T.; Bolte, A.; Tognetti, R. Variation in Ecophysiological Traits and Drought Tolerance of Beech (Fagus sylvatica L.) Seedlings from Different Populations. Front. Plant Sci. 2016, 7, 886. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The Way Back: Recovery of Trees from Drought and Its Implication for Acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of Drought on Growth, Photosynthesis and Total Antioxidant Capacity of the Saharan Plant Oudeneya Africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Liñán, I.; Etzold, S.; Fonti, P.; et al. Climate Sensitivity and Drought Seasonality Determine Post-Drought Growth Recovery of Quercus Petraea and Quercus Robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; He, B.; Chen, Y.; Yuan, W.; Huang, L.; Guo, L.; Zhang, Y.; Xie, X. Higher Sensitivity of Planted Forests’ Productivity Than Natural Forests to Droughts in China. JGR Biogeosci. 2021, 126, e2021JG006306. [Google Scholar] [CrossRef]
- Iqbal, A.; Huiping, G.; Qiang, D.; Xiangru, W.; Hengheng, Z.; Xiling, Z.; Meizhen, S. Differential Responses of Contrasting Low Phosphorus Tolerant Cotton Genotypes under Low Phosphorus and Drought Stress. BMC Plant Biol 2023, 23, 168. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Ehsanullah Role of Mineral Nutrition in Alleviation of Drought Stress in Plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Choudhury, S.; Moulick, D.; Ghosh, D.; Soliman, M.; Alkhedaide, A.; Gaber, A.; Hossain, A. Drought-Induced Oxidative Stress in Pearl Millet (Cenchrus americanus L.) at Seedling Stage: Survival Mechanisms through Alteration of Morphophysiological and Antioxidants Activity. Life 2022, 12, 1171. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative Damage and Antioxidant Mechanism in Tomatoes Responding to Drought and Heat Stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. ISBN 978-0-12-799963-0. [Google Scholar]
- Li, S. Novel Insight into Functions of Ascorbate Peroxidase in Higher Plants: More than a Simple Antioxidant Enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Twala, P.; Mitema, A.; Baburam, C.; Aliye Feto, N. Breakthroughs in the Discovery and Use of Different Peroxidase Isoforms of Microbial Origin. AIMS Microbiol. 2020, 6, 330–349. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. ISBN 978-981-10-9043-1. [Google Scholar]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous Fertilization Alleviates Drought Effects on Alnus Cremastogyne by Regulating Its Antioxidant and Osmotic Potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Ullah, A.; Tariq, A.; Zeng, F.; Sardans, J.; Graciano, C.; Ullah, S.; Chai, X.; Zhang, Z.; Keyimu, M.; Asghar, M.A.; et al. Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum. Plants 2022, 11, 3054. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Li, N.; Song, D.; Sun, F.; Wu, X.; Dakhil, M.A.; et al. Impact of Phosphorus Application on Drought Resistant Responses of Eucalyptus grandis Seedlings. Physiol. Plant. 2019, 166, 894–908. [Google Scholar] [CrossRef]
- Yang, F.; Magh, R.-K.; Ivanković, M.; Lanšćak, M.; Haberstroh, S.; Du, B.; Dannenmann, M.; Rennenberg, H.; Herschbach, C. Foliar P Nutrition of European Beech (Fagus sylvatica L.) Depends on the Season but Remains Unaffected by Co-Cultivation with Silver Fir (Abies alba Mill.). Eur. J. For. Res. 2020, 139, 853–868. [Google Scholar] [CrossRef]
- Kuster, T.M.; Arend, M.; Günthardt-Goerg, M.S.; Schulin, R. Root Growth of Different Oak Provenances in Two Soils under Drought Stress and Air Warming Conditions. Plant Soil 2013, 369, 61–71. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.-P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of Mixed versus Pure Stands of Oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) and European Beech (Fagus sylvatica L.) along an Ecological Gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Wang, F.; Israel, D.; Ramírez-Valiente, J.-A.; Sánchez-Gómez, D.; Aranda, I.; Aphalo, P.J.; Robson, T.M. Seedlings from Marginal and Core Populations of European Beech (Fagus sylvatica L.) Respond Differently to Imposed Drought and Shade. Trees 2021, 35, 53–67. [Google Scholar] [CrossRef]
- Schlosser, F.; Böhm, S.; Horder, N.; Seegmüller, S. Antioxidative Capacity of Oak Trees—Tannins as a Substantial Prerequisite for a Provenance-Specific Response to Drought. Austrian J. For. Sci. 2022, 139, 73–94. [Google Scholar]
- Jafarnia, S.; Akbarinia, M.; Hosseinpour, B.; Modarres Sanavi, S.; Salami, S. Effect of Drought Stress on Some Growth, Morphological, Physiological, and Biochemical Parameters of Two Different Populations of Quercus brantii. iForest 2018, 11, 212–220. [Google Scholar] [CrossRef]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological Response to Drought Stress in Camptotheca acuminata Seedlings from Two Provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef]
- Mellert, K.H.; Göttlein, A. Comparison of New Foliar Nutrient Thresholds Derived from van Den Burg’s Literature Compilation with Established Central European References. Eur. J. For. Res 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous Application Improves Drought Tolerance of Phoebe Zhennan. Front. Plant Sci. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ullah, A.; Tariq, A.; Zeng, F.; Asghar, M.A.; Sardans, J.; Graciano, C.; Ali, I.; Peñuelas, J. Drought Priming Improves Tolerance of Alhagi sparsifolia to Subsequent Drought: A Coordinated Interplay of Phytohormones, Osmolytes, and Antioxidant Potential. Plant Stress 2024, 12, 100469. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dey, T.; Galiba, G.; Kocsy, G.K.; Dey, N.; Kar, R.K. Effect of Combination of Light and Drought Stress on Physiology and Oxidative Metabolism of Rice Plants. Plant Sci. Today 2021, 8, 762–777. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, Y.; Li, P.; Zhang, F.; Liu, H.; Zheng, G. Drought Stress Induces Oxidative Stress and the Antioxidant Defense System in Ascorbate-Deficient Vtc1 Mutants of Arabidopsis Thaliana. Acta Physiol Plant 2013, 35, 1189–1200. [Google Scholar] [CrossRef]
- Özden, H.; Bayçu Kahyaoğlu, G. Physiological, Oxidative, and Antioxidative Responses of Quercus Vulcanica Boiss. and Quercus Aucheri Jaub. & Spach. under Drought Stress Conditions. İstanb. J. Pharm. 2024, 54, 69–79. [Google Scholar] [CrossRef]
- Celikkol Akcay, U.; Ercan, O.; Kavas, M.; Yildiz, L.; Yilmaz, C.; Oktem, H.A.; Yucel, M. Drought-Induced Oxidative Damage and Antioxidant Responses in Peanut (Arachis hypogaea L.) Seedlings. Plant Growth Regul. 2010, 61, 21–28. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus Toxicity Disrupts Rubisco Activation and Reactive Oxygen Species Defence Systems by Phytic Acid Accumulation in Leaves. Plant Cell Environ. 2020, 43, 2033–2053. [Google Scholar] [CrossRef] [PubMed]
- Vukmirović, A.; Škvorc, Ž.; Bogdan, S.; Krstonošić, D.; Bogdan, I.K.; Karažija, T.; Bačurin, M.; Brener, M.; Sever, K. Photosynthetic Response to Phosphorus Fertilization in Drought-Stressed Common Beech and Sessile Oak from Different Provenances. Plants 2024, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Sever, K.; Vukmirović, A.; Hodak, L.; Bogdan, S.; Katičić Bogdan, I.; Krstonošić, D.; Karažija, T.; Franjić, J.; Škvorc, Ž. Funkcionalna Prilagodba Prirodnog Pomlatka Hrasta Kitnjaka i Obične Bukve na Različite Stanišne Prilike. Šumar. List Online 2022, 146, 293–307. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ambriović Ristov, A.; Brozović, A.; Bruvo Mađarić, B.; Ćetković, H.; Herak Bosnar, M.; Hranilović, D.; Katušić Hećimović, S.; Mihaljević, S.; Slade, N.; Vujaklija, D. Metode u Molekularnoj Biologiji; Institut Ruđer Bošković: Zagreb, Croatia, 2007; ISBN 978-953-6690-72-5. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukmirović, A.; Škvorc, Ž.; Bogdan, S.; Krstonošić, D.; Bogdan, I.K.; Karažija, T.; Bačurin, M.; Brener, M.; Sever, K. The Role of Phosphorus Fertilization in Antioxidant Responses of Drought-Stressed Common Beech and Sessile Oak Provenances. Int. J. Mol. Sci. 2025, 26, 3053. https://doi.org/10.3390/ijms26073053
Vukmirović A, Škvorc Ž, Bogdan S, Krstonošić D, Bogdan IK, Karažija T, Bačurin M, Brener M, Sever K. The Role of Phosphorus Fertilization in Antioxidant Responses of Drought-Stressed Common Beech and Sessile Oak Provenances. International Journal of Molecular Sciences. 2025; 26(7):3053. https://doi.org/10.3390/ijms26073053
Chicago/Turabian StyleVukmirović, Antonia, Željko Škvorc, Saša Bogdan, Daniel Krstonošić, Ida Katičić Bogdan, Tomislav Karažija, Marko Bačurin, Magdalena Brener, and Krunoslav Sever. 2025. "The Role of Phosphorus Fertilization in Antioxidant Responses of Drought-Stressed Common Beech and Sessile Oak Provenances" International Journal of Molecular Sciences 26, no. 7: 3053. https://doi.org/10.3390/ijms26073053
APA StyleVukmirović, A., Škvorc, Ž., Bogdan, S., Krstonošić, D., Bogdan, I. K., Karažija, T., Bačurin, M., Brener, M., & Sever, K. (2025). The Role of Phosphorus Fertilization in Antioxidant Responses of Drought-Stressed Common Beech and Sessile Oak Provenances. International Journal of Molecular Sciences, 26(7), 3053. https://doi.org/10.3390/ijms26073053





