GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes
Abstract
1. Introduction
2. Coronary Microvascular Dysfunction: Deciphering the Pathophysiology
2.1. Inflammation-Driven Dysregulation
2.2. Oxidative Stress-Induced Injury
2.3. Hyperglycemia and Insulin Resistance
3. Preclinical Models and Human Studies Investigating the Effect of GLP-1 Agonism on Coronary Microvascular Perfusion
3.1. Microvascular Dilation and Blood Pressure Lowering
3.2. Oxidative Stress and Vascular Inflammation Attenuation
3.3. Angiogenesis Stimulation
4. Clinical Evidence Supporting GLP-1 Receptor Agonists in Enhancing Myocardial Perfusion
Effect on Coronary Microcirculation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sinha, A.; Rahman, H.; Perera, D. Coronary Microvascular Disease: Current Concepts of Pathophysiology, Diagnosis and Management. Cardiovasc. Endocrinol. Metab. 2021, 10, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary Microvascular Dysfunction: An Update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Hammoud, R.; Drucker, D.J. Beyond the Pancreas: Contrasting Cardiometabolic Actions of GIP and GLP1. Nat. Rev. Endocrinol. 2023, 19, 201–216. [Google Scholar] [CrossRef]
- Ruff, C.T.; Baron, M.; Im, K.; O’Donoghue, M.L.; Fiedorek, F.T.; Sabatine, M.S. Subcutaneous Infusion of Exenatide and Cardiovascular Outcomes in Type 2 Diabetes: A Non-Inferiority Randomized Controlled Trial. Nat. Med. 2022, 28, 89–95. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Antoniadis, A.P.; Fragakis, N. Diabetes-Driven Atherosclerosis: Updated Mechanistic Insights and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2196. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Thirunavukarasu, S.; Joseph, T.; Jex, N.; Kotha, S.; Giannoudi, M.; Procter, H.; Cash, L.; Akkaya, S.; Broadbent, D.; et al. Liraglutide Improves Myocardial Perfusion and Energetics and Exercise Tolerance in Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2024, 84, 540–557. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Buckberg, G.D. The Myocardial Oxygen Supply: Demand Index Revisited. J. Am. Heart Assoc. 2014, 3, e000285. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Knaapen, P. Coronary Microvascular Function, Myocardial Metabolism, and Energetics in Hypertrophic Cardiomyopathy: Insights from Positron Emission Tomography. Eur. Hear. J. Cardiovasc. Imaging 2013, 14, 95–101. [Google Scholar] [CrossRef]
- Waters, S.L.; Alastruey, J.; Beard, D.A.; Bovendeerd, P.H.M.; Davies, P.F.; Jayaraman, G.; Jensen, O.E.; Lee, J.; Parker, K.H.; Popel, A.S.; et al. Theoretical Models for Coronary Vascular Biomechanics: Progress & Challenges. Prog. Biophys. Mol. Biol. 2011, 104, 49–76. [Google Scholar] [CrossRef]
- Varrichione, G.; Biccirè, F.G.; Di Pietro, R.; Prati, F.; Battisti, P. The Risk of Acute Coronary Events in Microvascular Disease. Eur. Hear. J. Suppl. J. Eur. Soc. Cardiol. 2022, 24, I127–I130. [Google Scholar] [CrossRef]
- Rehan, R.; Yong, A.; Ng, M.; Weaver, J.; Puranik, R. Coronary Microvascular Dysfunction: A Review of Recent Progress and Clinical Implications. Front. Cardiovasc. Med. 2023, 10, 1111721. [Google Scholar] [CrossRef]
- Devesa, A.; Fuster, V.; García-Lunar, I.; Oliva, B.; García-Alvarez, A.; Moreno-Arciniegas, A.; Vazirani, R.; Pérez-Herreras, C.; Marina, P.; Bueno, H.; et al. Coronary Microvascular Function in Asymptomatic Middle-Aged Individuals with Cardiometabolic Risk Factors. JACC. Cardiovasc. Imaging 2025, 18, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Lüscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and Vascular Disease: Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, J.M.; Li, Y.D.; Block, E.R. Proinflammatory Cytokines Downregulate Gene Expression and Activity of Constitutive Nitric Oxide Synthase in Porcine Pulmonary Artery Endothelial Cells. Res. Commun. Mol. Pathol. Pharmacol. 1997, 96, 71–87. [Google Scholar]
- Verma, S.; Wang, C.-H.; Li, S.-H.; Dumont, A.S.; Fedak, P.W.M.; Badiwala, M.V.; Dhillon, B.; Weisel, R.D.; Li, R.-K.; Mickle, D.A.G.; et al. A Self-Fulfilling Prophecy: C-Reactive Protein Attenuates Nitric Oxide Production and Inhibits Angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS Expression in Vascular Diseases. Front. Biosci. 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef]
- Faccini, A.; Kaski, J.C.; Camici, P.G. Coronary Microvascular Dysfunction in Chronic Inflammatory Rheumatoid Diseases. Eur. Heart J. 2016, 37, 1799–1806. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Stachteas, P.; Lefkou, E.; Dimitroulas, T.; Fragakis, N. Accelerated Atherosclerosis and Management of Cardiovascular Risk in Autoimmune Rheumatic Diseases: An Updated Review. Curr. Probl. Cardiol. 2023, 48, 101999. [Google Scholar] [CrossRef]
- Recio-Mayoral, A.; Rimoldi, O.E.; Camici, P.G.; Kaski, J.C. Inflammation and Microvascular Dysfunction in Cardiac Syndrome X Patients without Conventional Risk Factors for Coronary Artery Disease. JACC Cardiovasc. Imaging 2013, 6, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, A.; Pataky, Z.; Vincenti, G.; Makoundou, V.; Di Marzo, V.; Montecucco, F.; Carballo, S.; Thomas, A.; Staub, C.; Steffens, S.; et al. Elevated Endocannabinoid Plasma Levels Are Associated with Coronary Circulatory Dysfunction in Obesity. Eur. Heart J. 2011, 32, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Tona, F.; Serra, R.; Di Ascenzo, L.; Osto, E.; Scarda, A.; Fabris, R.; Montisci, R.; Famoso, G.; Tellatin, S.; Foletto, M.; et al. Systemic Inflammation Is Related to Coronary Microvascular Dysfunction in Obese Patients without Obstructive Coronary Disease. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; et al. Assessment and Pathophysiology of Microvascular Disease: Recent Progress and Clinical Implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High Glucose Level and Free Fatty Acid Stimulate Reactive Oxygen Species Production through Protein Kinase C--Dependent Activation of NAD(P)H Oxidase in Cultured Vascular Cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, H.; Ma, Y.; Tang, Z.; Zhao, N.; Wang, Y.; Pan, S. AGEs Exacerbates Coronary Microvascular Dysfunction in NoCAD by Activating Endoplasmic Reticulum Stress-Mediated PERK Signaling Pathway. Metabolism 2021, 117, 154710. [Google Scholar] [CrossRef]
- Fujimoto, W.Y. The Importance of Insulin Resistance in the Pathogenesis of Type 2 Diabetes Mellitus. Am. J. Med. 2000, 108 (Suppl. 6a), 9S–14S. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Wang, T.J.; Brown, N.J. The Vasculature in Prediabetes. Circ. Res. 2018, 122, 1135–1150. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Love, K.M.; Liu, J.; Regensteiner, J.G.; Reusch, J.E.B.; Liu, Z. GLP-1 and Insulin Regulation of Skeletal and Cardiac Muscle Microvascular Perfusion in Type 2 Diabetes. J. Diabetes 2020, 12, 488–498. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Negut, C.; De Kreutzenberg, S.V.; Tiengo, A.; Avogaro, A. Postprandial Myocardial Perfusion in Healthy Subjects and in Type 2 Diabetic Patients. Circulation 2005, 112, 179–184. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- St Onge, E.; Miller, S.; Clements, E.; Celauro, L.; Barnes, K. The Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Type 2 Diabetes. J. Transl. Intern. Med. 2017, 5, 79–89. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of Glucagon-like Peptide-1 Receptor Agonists and Co-Agonists on Body Composition: Systematic Review and Network Meta-Analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like Peptide 1 Receptor Agonists: Cardiovascular Benefits and Mechanisms of Action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Bernal-López, M.R.; Gómez-Huelgas, R. Glucagon-like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors Combination Therapy versus Monotherapy and Major Adverse Cardiovascular Events: Do the Benefits Add Up? Eur. J. Intern. Med. 2024, 130, 155–159. [Google Scholar]
- Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Yang, X.; Liu, K.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q.; et al. Signaling Pathways in Vascular Function and Hypertension: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 168. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (ENOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ma, S.; Wang, C.; Ke, J.; Yang, J.; Li, W.; Liu, Y.; Hou, W.; Feng, X.; Wang, G.; et al. Exenatide Exerts Direct Protective Effects on Endothelial Cells through the AMPK/Akt/ENOS Pathway in a GLP-1 Receptor-Dependent Manner. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E947–E957. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, V.; Tsuchimochi, H.; Sonobe, T.; Waddingham, M.T.; Shirai, M.; Pearson, J.T. Liraglutide Treatment Improves the Coronary Microcirculation in Insulin Resistant Zucker Obese Rats on a High Salt Diet. Cardiovasc. Diabetol. 2020, 19, 24. [Google Scholar] [CrossRef]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide in Mice with Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef]
- Chepurny, O.G.; Matsoukas, M.-T.; Liapakis, G.; Leech, C.A.; Milliken, B.T.; Doyle, R.P.; Holz, G.G. Nonconventional Glucagon and GLP-1 Receptor Agonist and Antagonist Interplay at the GLP-1 Receptor Revealed in High-Throughput FRET Assays for CAMP. J. Biol. Chem. 2019, 294, 3514–3531. [Google Scholar] [CrossRef]
- Lim, D.-M.; Park, K.-Y.; Hwang, W.-M.; Kim, J.-Y.; Kim, B.-J. Difference in Protective Effects of GIP and GLP-1 on Endothelial Cells According to Cyclic Adenosine Monophosphate Response. Exp. Ther. Med. 2017, 13, 2558–2564. [Google Scholar] [CrossRef]
- Yu, M.; Moreno, C.; Hoagland, K.M.; Dahly, A.; Ditter, K.; Mistry, M.; Roman, R.J. Antihypertensive Effect of Glucagon-like Peptide 1 in Dahl Salt-Sensitive Rats. J. Hypertens. 2003, 21, 1125–1135. [Google Scholar] [CrossRef]
- Basu, A.; Charkoudian, N.; Schrage, W.; Rizza, R.A.; Basu, R.; Joyner, M.J. Beneficial Effects of GLP-1 on Endothelial Function in Humans: Dampening by Glyburide but Not by Glimepiride. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1289-95. [Google Scholar] [CrossRef]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-Month Treatment with the Glucagon like Peptide-1 Analogue Liraglutide on Arterial Stiffness, Left Ventricular Myocardial Deformation and Oxidative Stress in Subjects with Newly Diagnosed Type 2 Diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 Receptor Activation and Epac2 Link Atrial Natriuretic Peptide Secretion to Control of Blood Pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Yu, Q.; Yu, P.; Yu, T.-L.; Zhang, Q.-M.; Lu, S.; Yu, D.-M. Changes in Liraglutide-Induced Body Composition Are Related to Modifications in Plasma Cardiac Natriuretic Peptides Levels in Obese Type 2 Diabetic Patients. Cardiovasc. Diabetol. 2014, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Rudovich, N.; Pivovarova, O.; Gögebakan, Ö.; Sparwasser, A.; Doehner, W.; Anker, S.D.; Arafat, A.M.; Bergmann, A.; Nauck, M.A.; Pfeiffer, A.F.H. Effect of Exogenous Intravenous Administrations of GLP-1 and/or GIP on Circulating pro-Atrial Natriuretic Peptide in Subjects with Different Stages of Glucose Tolerance. Diabetes Care 2015, 38, e7–e8. [Google Scholar] [PubMed]
- Caballero, A.E. Endothelial Dysfunction in Obesity and Insulin Resistance: A Road to Diabetes and Heart Disease. Obes. Res. 2003, 11, 1278–1289. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef]
- Bonacina, F.; Baragetti, A.; Catapano, A.L.; Norata, G.D. The Interconnection Between Immuno-Metabolism, Diabetes, and CKD. Curr. Diab. Rep. 2019, 19, 21. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Negut, C.; de Kreutzenberg, S.V.; Tiengo, A.; Avogaro, A. Effects of Different Insulin Regimes on Postprandial Myocardial Perfusion Defects in Type 2 Diabetic Patients. Diabetes Care 2006, 29, 95–100. [Google Scholar]
- Zhang, Y.; Zhou, H.; Wu, W.; Shi, C.; Hu, S.; Yin, T.; Ma, Q.; Han, T.; Zhang, Y.; Tian, F.; et al. Liraglutide Protects Cardiac Microvascular Endothelial Cells against Hypoxia/Reoxygenation Injury through the Suppression of the SR-Ca(2+)-XO-ROS Axis via Activation of the GLP-1R/PI3K/Akt/Survivin Pathways. Free Radic. Biol. Med. 2016, 95, 278–292. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The Protective Effect of the Mediterranean Diet on Endothelial Resistance to GLP-1 in Type 2 Diabetes: A Preliminary Report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef]
- Aravindhan, K.; Bao, W.; Harpel, M.R.; Willette, R.N.; Lepore, J.J.; Jucker, B.M. Cardioprotection Resulting from Glucagon-like Peptide-1 Administration Involves Shifting Metabolic Substrate Utilization to Increase Energy Efficiency in the Rat Heart. PLoS ONE 2015, 10, e0130894. [Google Scholar] [CrossRef]
- Wang, D.; Luo, P.; Wang, Y.; Li, W.; Wang, C.; Sun, D.; Zhang, R.; Su, T.; Ma, X.; Zeng, C.; et al. Glucagon-like Peptide-1 Protects against Cardiac Microvascular Injury in Diabetes via a CAMP/PKA/Rho-Dependent Mechanism. Diabetes 2013, 62, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Li, D.; Xu, H.; Chen, J.; Min, X.; He, M.; Wu, T.; Zhong, J.; Yang, H.; et al. Effect of GLP-1/GLP-1R on the Polarization of Macrophages in the Occurrence and Development of Atherosclerosis. Mediat. Inflamm. 2021, 2021, 5568159. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Simultaneous GLP-1 and Insulin Administration Acutely Enhances Their Vasodilatory, Antiinflammatory, and Antioxidant Action in Type 2 Diabetes. Diabetes Care 2014, 37, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Harris, D.D.; Broadwin, M.; Kanuparthy, M.; Nho, J.-W.; Yalamanchili, K.; Hamze, J.; Abid, M.R.; Sellke, F.W. Semaglutide Improves Myocardial Perfusion and Performance in a Large Animal Model of Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 285–297. [Google Scholar] [CrossRef]
- Aronis, K.N.; Chamberland, J.P.; Mantzoros, C.S. GLP-1 Promotes Angiogenesis in Human Endothelial Cells in a Dose-Dependent Manner, through the Akt, Src and PKC Pathways. Metabolism 2013, 62, 1279–1286. [Google Scholar] [CrossRef]
- Marfella, R.; Sasso, F.C.; Rizzo, M.R.; Paolisso, P.; Barbieri, M.; Padovano, V.; Carbonara, O.; Gualdiero, P.; Petronella, P.; Ferraraccio, F.; et al. Dipeptidyl Peptidase 4 Inhibition May Facilitate Healing of Chronic Foot Ulcers in Patients with Type 2 Diabetes. Exp. Diabetes Res. 2012, 2012, 892706. [Google Scholar] [CrossRef]
- Erdogdu, O.; Nathanson, D.; Sjöholm, A.; Nyström, T.; Zhang, Q. Exendin-4 Stimulates Proliferation of Human Coronary Artery Endothelial Cells through ENOS-, PKA- and PI3K/Akt-Dependent Pathways and Requires GLP-1 Receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Shih, C.-M.; Tsao, N.-W.; Lin, Y.-W.; Huang, P.-H.; Wu, S.-C.; Lee, A.-W.; Kao, Y.-T.; Chang, N.-C.; Nakagami, H.; et al. Dipeptidyl Peptidase-4 Inhibitor Improves Neovascularization by Increasing Circulating Endothelial Progenitor Cells. Br. J. Pharmacol. 2012, 167, 1506–1519. [Google Scholar] [CrossRef]
- Kelly, A.S.; Bergenstal, R.M.; Gonzalez-Campoy, J.M.; Katz, H.; Bank, A.J. Effects of Exenatide vs. Metformin on Endothelial Function in Obese Patients with Pre-Diabetes: A Randomized Trial. Cardiovasc. Diabetol. 2012, 11, 64. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023, 82, 833–955. [Google Scholar] [CrossRef]
- Damluji, A.A.; Forman, D.E.; Wang, T.Y.; Chikwe, J.; Kunadian, V.; Rich, M.W.; Young, B.A.; Page, R.L., 2nd; DeVon, H.A.; Alexander, K.P. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement from the American Heart Association. Circulation 2023, 147, e32–e62. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Benedetto, U. Coronary Artery Bypass Grafting (CABG) vs. Percutaneous Coronary Intervention (PCI) in the Treatment of Multivessel Coronary Disease: Quo Vadis?—A Review of the Evidences on Coronary Artery Disease. Ann. Cardiothorac. Surg. 2018, 7, 506–515. [Google Scholar]
- Subaran, S.C.; Sauder, M.A.; Chai, W.; Jahn, L.A.; Fowler, D.E.; Aylor, K.W.; Basu, A.; Liu, Z. GLP-1 at Physiological Concentrations Recruits Skeletal and Cardiac Muscle Microvasculature in Healthy Humans. Clin. Sci. 2014, 127, 163–170. [Google Scholar] [CrossRef]
- Wang, N.; Tan, A.W.K.; Jahn, L.A.; Hartline, L.; Patrie, J.T.; Lin, S.; Barrett, E.J.; Aylor, K.W.; Liu, Z. Vasodilatory Actions of Glucagon-Like Peptide 1 Are Preserved in Skeletal and Cardiac Muscle Microvasculature but not in Conduit Artery in Obese Humans with Vascular Insulin Resistance. Diabetes Care 2020, 43, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Gejl, M.; Søndergaard, H.M.; Stecher, C.; Bibby, B.M.; Møller, N.; Bøtker, H.E.; Hansen, S.B.; Gjedde, A.; Rungby, J.; Brock, B. Exenatide Alters Myocardial Glucose Transport and Uptake Depending on Insulin Resistance and Increases Myocardial Blood Flow in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1165–E1169. [Google Scholar] [CrossRef]
- Clarke, S.J.; Giblett, J.P.; Yang, L.L.; Hubsch, A.; Zhao, T.; Aetesam-Ur-Rahman, M.; West, N.E.J.; O’Sullivan, M.; Figg, N.; Bennett, M.; et al. GLP-1 Is a Coronary Artery Vasodilator in Humans. J. Am. Heart Assoc. 2018, 7, e010321. [Google Scholar] [CrossRef]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of Liraglutide on Reperfusion Injury in Patients with ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2016, 9, e005146. [Google Scholar] [CrossRef]
- Rezinkina, P.; Sergienko, I.; Ansheles, A. Assessment of Myocardial Perfusion with SPECT in Obese Patients with High and Very High Cardiovascular Risk during GLP-1 Receptor Agonists Therapy, Preliminary Results. Atherosclerosis 2023, 379, S107. [Google Scholar] [CrossRef]
- Nielsen, R.; Jorsal, A.; Iversen, P.; Tolbod, L.P.; Bouchelouche, K.; Sørensen, J.; Harms, H.J.; Flyvbjerg, A.; Tarnow, L.; Kistorp, C.; et al. Effect of Liraglutide on Myocardial Glucose Uptake and Blood Flow in Stable Chronic Heart Failure Patients: A Double-Blind, Randomized, Placebo-Controlled LIVE Sub-Study. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2019, 26, 585–597. [Google Scholar] [CrossRef]
- Faber, R.; Zander, M.; Pena, A.; Michelsen, M.M.; Mygind, N.D.; Prescott, E. Effect of the Glucagon-like Peptide-1 Analogue Liraglutide on Coronary Microvascular Function in Patients with Type 2 Diabetes—A Randomized, Single-Blinded, Cross-over Pilot Study. Cardiovasc. Diabetol. 2015, 14, 41. [Google Scholar] [CrossRef]
- Nilsson, M.; Bové, K.B.; Suhrs, E.; Hermann, T.; Madsbad, S.; Holst, J.J.; Prescott, E.; Zander, M. The Effect of DPP-4-Protected GLP-1 (7-36) on Coronary Microvascular Function in Obese Adults. Int. J. Cardiol. Hear. Vasc. 2019, 22, 139–144. [Google Scholar] [CrossRef]
- Aetesam-Ur-Rahman, M.; Giblett, J.P.; Khialani, B.; Kyranis, S.; Clarke, S.J.; Zhao, T.X.; Braganza, D.M.; Clarke, S.C.; West, N.E.J.; Bennett, M.R.; et al. GLP-1 Vasodilatation in Humans with Coronary Artery Disease Is Not Adenosine Mediated. BMC Cardiovasc. Disord. 2021, 21, 223. [Google Scholar] [CrossRef]
- Alikhanova, N.; Musakhanova, C.; Nazarova, N.; Davronov, R.; Takhirova, F.; Akramova, G.; Abboskhujaeva, L.; Munavvara, S.; Trigulova, R. Effects of SGLT2 Inhibitors on Rest Myocardial Perfusion in Patients with Type 2 Diabetes and Diabetic Nephropathy. Am. Heart J. 2024, 267, 123–124. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Sabra, M.; Harris, D.D.; Malhotra, A.; Aboulgheit, A.; Stanley, M.; Abid, M.R.; Sellke, F.W. Canagliflozin Improves Myocardial Perfusion, Fibrosis, and Function in a Swine Model of Chronic Myocardial Ischemia. J. Am. Heart Assoc. 2023, 12, e028623. [Google Scholar] [CrossRef]
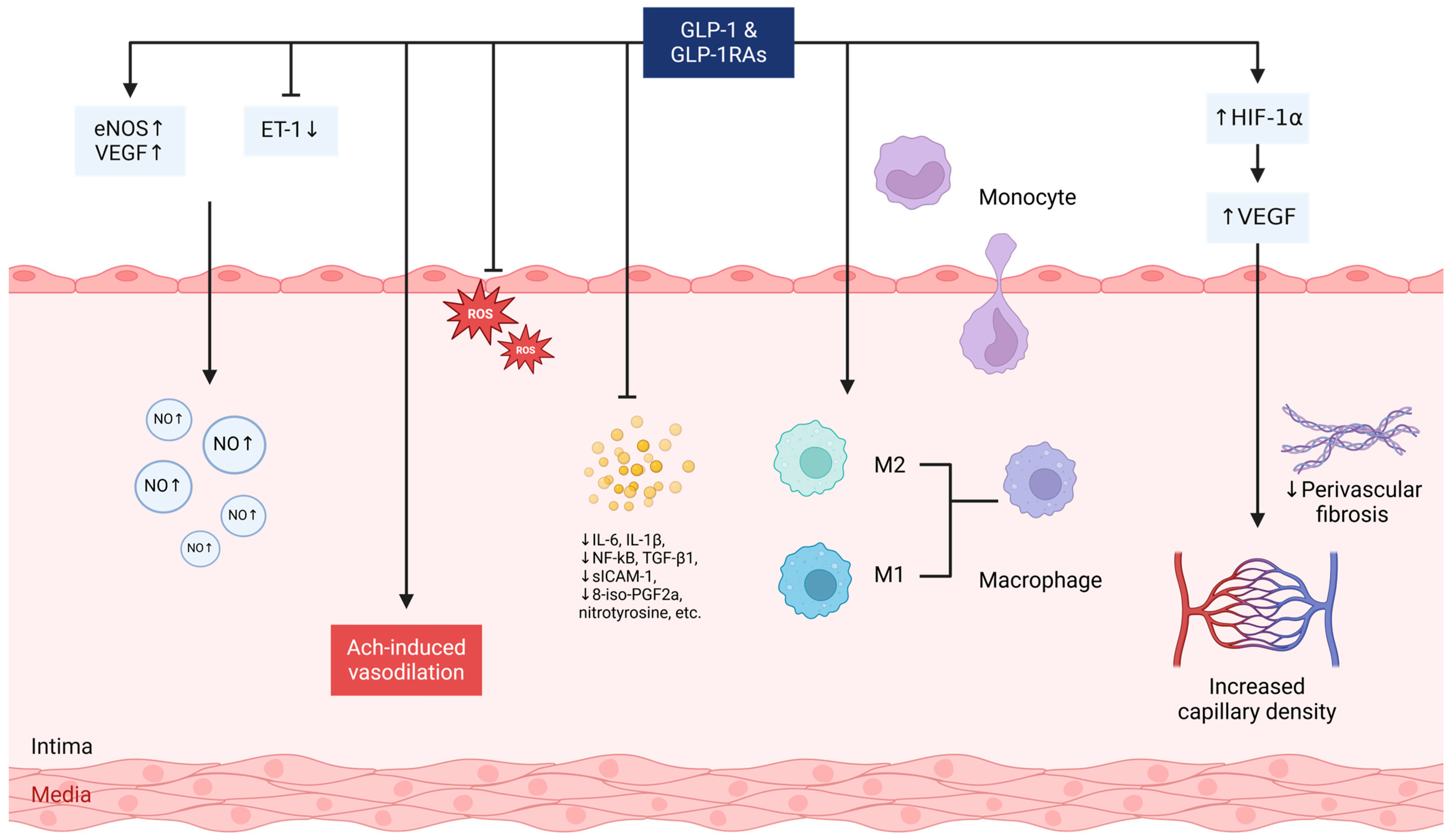
| Study (Year) | Model | Intervention | Dosing | Mechanisms |
|---|---|---|---|---|
| Helmstädter et al. (2020) [55] | Mouse model of angiotensin II-induced arterial hypertension | Liraglutide | N/R | Reduced vascular inflammation, oxidative stress, and endothelial dysfunction via endothelial GLP-1R activation. Prevented eNOS uncoupling, increased NO bioavailability, reduced leukocyte rolling and infiltration, and decreased expression of vascular adhesion molecules (VCAM-1, ICAM-1, P-selectin). |
| Kim & Platt et al. (2013) [61] | Mouse model of angiotensin II-induced hypertension | Liraglutide | 30 Βµg/kg intraperitoneally, twice daily for 3 weeks | GLP-1R activation in atrial cardiomyocytes increased atrial natriuretic peptide (ANP) secretion, which in turn reduced blood pressure via natriuresis and vasodilation. The mechanism involved Epac2-mediated ANP secretion, activation of natriuretic peptide receptor A, and increased cGMP signaling in vascular smooth muscle cells. |
| Kelly et al. (2012) [79] | Human (obese, pre-diabetic patients) | Exenatide | 5 mcg BID for 1 month, then 10 mcg BID for 2 months | No significant improvement in microvascular endothelial function (RHI), inflammation (CRP), oxidative stress (oxLDL), or vascular activation (VCAM-1) compared to metformin. However, exenatide significantly reduced triglycerides, which may have postprandial vascular benefits. |
| Ceriello et al. (2014) [73] | Human (patients with type 2 diabetes) | GLP-1 infusion with or without insulin | 0.4 pmol/kg/min infusion during 2 h glucose clamps | GLP-1 improved flow-mediated dilation (FMD), reduced markers of inflammation (IL-6, sICAM-1), and oxidative stress (8-iso-PGF2a, nitrotyrosine). Effects were enhanced when GLP-1 was combined with insulin, suggesting a synergistic vasodilatory, anti-inflammatory, and antioxidant action. |
| Stone et al. (2025) [74] | Large animal model (Yorkshire swine with coronary artery disease) | semaglutide | Oral 1.5 mg, increased to 3 mg over 2 weeks, continued for 5 weeks | Improved myocardial perfusion and systolic function through activation of the AMPK-eNOS pathway, leading to enhanced endothelial function and coronary vasodilation. Reduced perivascular fibrosis, interstitial fibrosis, and apoptosis, suggesting an additional role in myocardial remodeling and cellular survival. |
| Study (Year) | Population | Number of Participants | Imaging Modality | Results | Implications |
|---|---|---|---|---|---|
| Subaran et al. (2014) [83] | Healthy adults (18–35 years) | 26 | Contrast-enhanced ultrasound (CEU)/Myocardial contrast echocardiography (MCE) | GLP-1 infusion significantly increased myocardial microvascular blood volume (MBV) by ~53% at 30 min and ~57% at 150 min. Myocardial blood flow (MBF) increased by ~48% at 30 min and ~47% at 150 min. Microvascular flow velocity (MFV) slightly decreased. | GLP-1 receptor activation enhances myocardial microvascular recruitment, improving tissue oxygen and nutrient delivery. This suggests a potential role for GLP-1 receptor agonists in preserving coronary microvascular function. |
| Chowdhary et al. (2024) [14] | Patients with T2D without established cardiovascular disease | 41 | CMR and 31-phosphorus magnetic resonance spectroscopy (31P-MRS) | Liraglutide significantly improved stress myocardial blood flow (1.62 to 2.08 mL/g/min, p = 0.01) and myocardial perfusion reserve (2.40 to 2.90, p = 0.01). Rest and stress phosphocreatine-to-ATP ratios increased, indicating enhanced myocardial energetics. | GLP-1 receptor agonist liraglutide enhances myocardial perfusion and energetics, supporting its therapeutic potential in patients with T2D at risk of microvascular dysfunction. |
| Nilsson et al. (2019) [91] | Obese adults without diabetes | 12 | Trans-thoracic Doppler echocardiography | No significant difference in coronary flow velocity reserve (CFVR) between GLP-1 infusion (3.77 ± 1.25) and saline infusion (3.85 ± 1.32). No significant effect on peripheral endothelial function. | Acute GLP-1 infusion did not improve coronary microcirculation in obese, glucose-tolerant adults, suggesting its effects may depend on metabolic status or require long-term treatment. |
| Clarke et al. (2018) [86] | Patients with stable angina awaiting LAD stenting | 21 | Pressure-flow wire assessment of coronary blood flow | GLP-1 reduced resting coronary transit time (0.87 to 0.63 s, p = 0.02) and basal microcirculatory resistance (76.3 to 55.4 mmHg/s, p = 0.02), whereas controls exhibited an increase in both parameters. No significant effect on systemic hemodynamics or peripheral vascular tone. | GLP-1 promotes coronary microvascular dilation and enhances myocardial blood flow through ventricular-coronary crosstalk, suggesting a potential cardioprotective role independent of systemic vasodilation. |
| Aetesam-Ur-Rahman et al. (2021) [92] | Patients undergoing PCI for stable angina | 41 | Pressure wire assessment of coronary distal pressure and flow velocity (thermodilution transit time—Tmn) | GLP-1 caused a significant reduction in resting Tmn and basal microvascular resistance (BMR), indicating improved coronary microvascular function. The vasodilatory effect was not attenuated by theophylline, suggesting an adenosine-independent mechanism. | GLP-1 receptor activation improves coronary microvascular function via an adenosine-independent pathway, supporting its potential role in microvascular dysfunction management. Further research is needed to elucidate alternative mechanisms. |
| Chen et al. (2016) [87] | Patients with STEMI undergoing primary PCI | 92 | Transthoracic echocardiography | Liraglutide significantly improved left ventricular ejection fraction (LVEF) at 3 months compared to placebo (+4.1%, 95% CI: +1.1% to +6.9%, p < 0.001). Reduction in inflammatory markers and endothelial dysfunction indicators was observed. | Short-term liraglutide therapy post-STEMI may support myocardial recovery by enhancing left ventricular function and reducing endothelial inflammation, warranting larger-scale trials. |
| Faber et al. (2015) [90] | Patients with T2D and no coronary artery disease history | 24 | Trans-thoracic Doppler-flow echocardiography | Liraglutide led to a small, non-significant increase in coronary flow reserve (CFR) (change: 0.18, 95% CI: [−0.01, 0.36], p = 0.06). No significant difference in CFR between liraglutide and control (difference: 0.16, 95% CI: [−0.08, 0.40], p = 0.18). | Short-term liraglutide treatment did not significantly enhance coronary microvascular function. Future studies should explore long-term effects and higher dosing in patients with greater microvascular impairment. |
| Gejl et al. (2012) [85] | Insulin-naive male patients with T2D without coronary artery disease | 8 | Positron emission tomography (PET) with 18F-fluorodeoxyglucose and 13N-ammonia | Exenatide increased myocardial blood flow (MBF) by 24% (0.69 ± 0.097 to 0.86 ± 0.09 mL/g/min, p = 0.0089), but had no effect on myocardial glucose uptake (MGU). | GLP-1 receptor activation with exenatide enhances myocardial perfusion without altering glucose uptake, suggesting a potential vasodilatory effect on coronary microcirculation in patients with T2D. Further research is needed to explore its long-term benefits. |
| Nielsen et al. (2019) [89] | Patients with stable chronic heart failure and reduced ejection fraction (≤45%) | 36 | Positron emission tomography (PET) with 18F-FDG and 15O-H2O | Liraglutide treatment for 24 weeks had no significant effect on myocardial glucose uptake (MGU), myocardial blood flow (MBF), or myocardial flow reserve (MFR) compared to placebo (p = 0.98, p = 0.76, and p = 0.89, respectively). | Liraglutide does not enhance myocardial perfusion or glucose metabolism in non-diabetic patients with heart failure. The absence of effects on myocardial perfusion may explain the lack of observed cardiovascular benefit in heart failure trials involving GLP-1 receptor agonists. |
| Wei et al. (2016) [53] | Patients with newly diagnosed T2D | 36 | Transthoracic Doppler echocardiography | Exenatide significantly improved coronary flow velocity reserve (CFVR) (baseline: 2.89 ± 0.60, post-treatment: 3.36 ± 0.58, p < 0.05). Significant reduction in inflammatory markers sICAM-1 and sVCAM-1 post-treatment. | Exenatide enhances coronary endothelial function and reduces vascular inflammation in newly diagnosed T2D patients, suggesting a potential role in mitigating cardiovascular risk. |
| Rezinkina et al. (2023) [88] | Obese patients with high and very high cardiovascular risk | 30 (15 with T2D/IGT, 15 without carbohydrate metabolism disorders) | 99mTc-MIBI SPECT (rest/stress) | After 6 months of GLP-1 receptor agonist therapy, myocardial perfusion inhomogeneity significantly improved, with reductions in stress σsev (26.8 ± 5.7 to 22.6 ± 4.7, p = 0.03) and stress σhet (10.6 ± 3.1 to 9.1 ± 2.5, p = 0.061). Improvements were more pronounced in the T2D/IGT group. | GLP-1 receptor agonists improve myocardial perfusion at the microcirculatory level in high-risk obese patients, particularly those with T2D/IGT. Further research is needed to refine patient selection and optimize cardiovascular outcome assessments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Patoulias, D.; Theofilis, P.; Pamporis, K.; Sagris, M.; Vlachakis, P.K.; Koufakis, T.; Antoniadis, A.P.; Fragakis, N. GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 3050. https://doi.org/10.3390/ijms26073050
Karakasis P, Patoulias D, Theofilis P, Pamporis K, Sagris M, Vlachakis PK, Koufakis T, Antoniadis AP, Fragakis N. GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. International Journal of Molecular Sciences. 2025; 26(7):3050. https://doi.org/10.3390/ijms26073050
Chicago/Turabian StyleKarakasis, Paschalis, Dimitrios Patoulias, Panagiotis Theofilis, Konstantinos Pamporis, Marios Sagris, Panayotis K. Vlachakis, Theocharis Koufakis, Antonios P. Antoniadis, and Nikolaos Fragakis. 2025. "GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes" International Journal of Molecular Sciences 26, no. 7: 3050. https://doi.org/10.3390/ijms26073050
APA StyleKarakasis, P., Patoulias, D., Theofilis, P., Pamporis, K., Sagris, M., Vlachakis, P. K., Koufakis, T., Antoniadis, A. P., & Fragakis, N. (2025). GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. International Journal of Molecular Sciences, 26(7), 3050. https://doi.org/10.3390/ijms26073050







