Fermented Kamut Wheat Diet Prevents DSS-Induced Colitis via Modulating Gut Microbiota in Mice
Abstract
1. Introduction
2. Results
2.1. FKW Diet Helped Manage Colitis Symptoms in Mice Compared to Control Diet
2.2. FKW Diet Positively Impacted Colon Length, Spleen Weight, and Histological Score in Mice Compared to Control Diet
2.3. FKW Diet Reduced Inflammatory Markers in Mice Compared to Control Diet
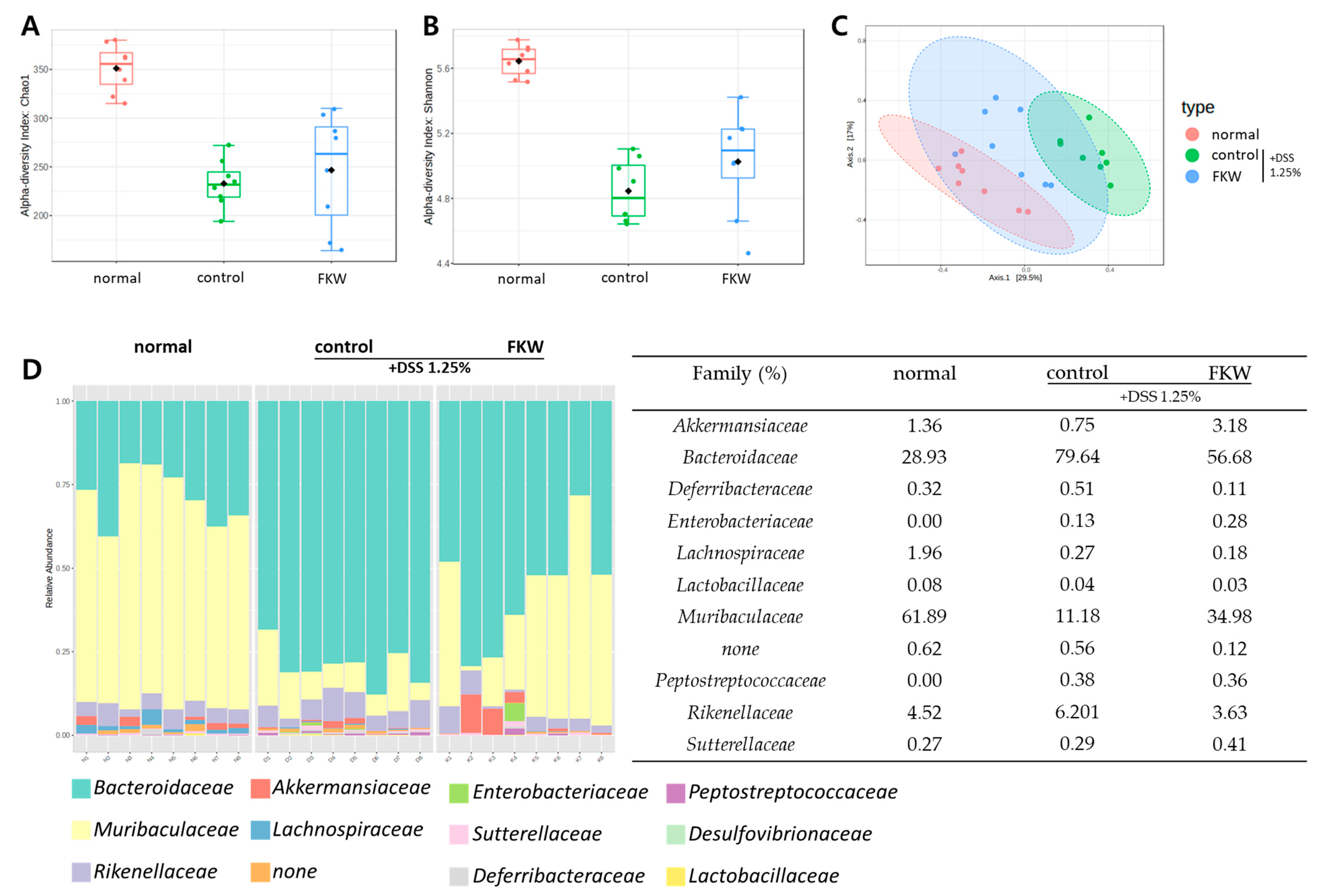
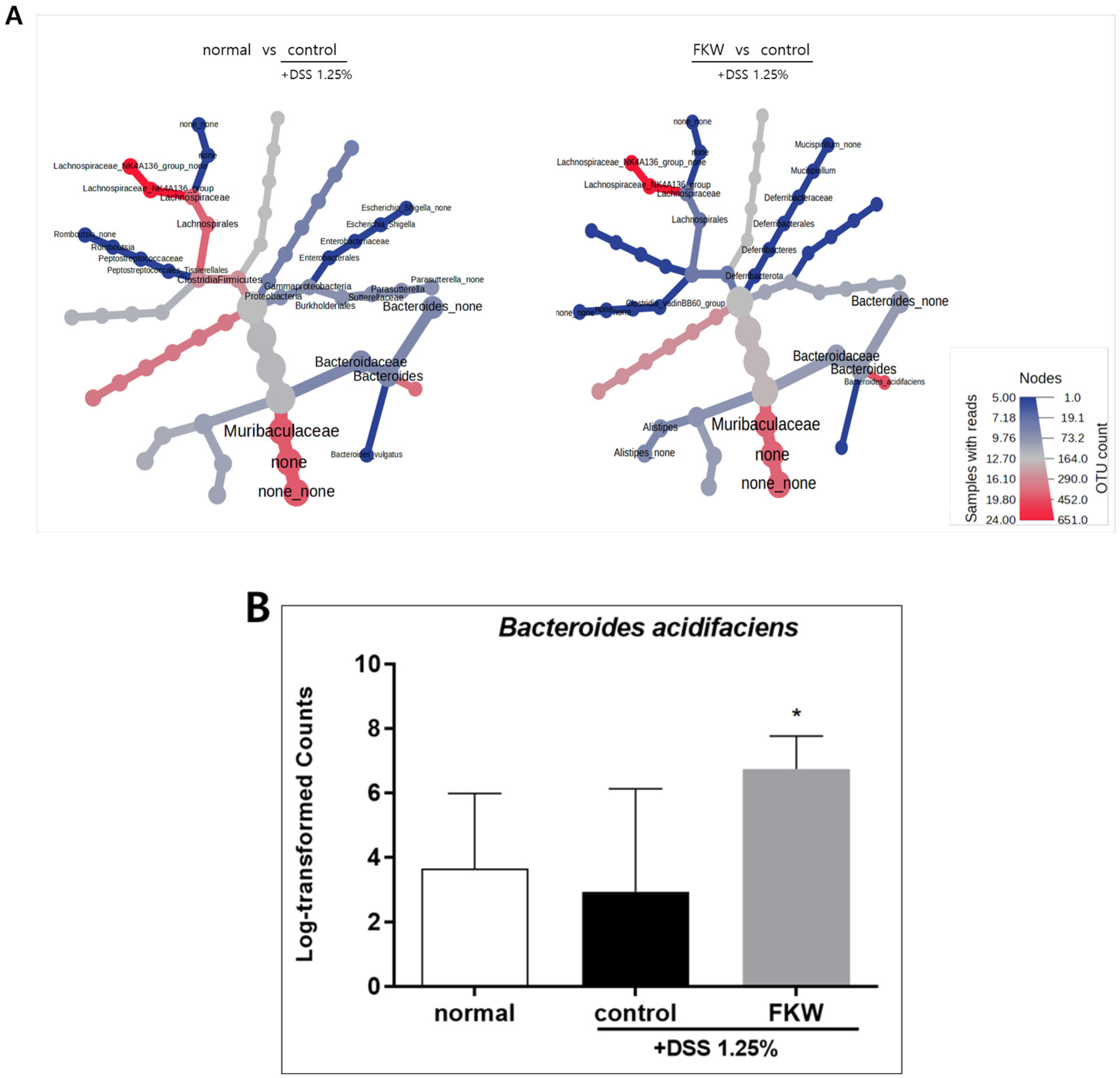
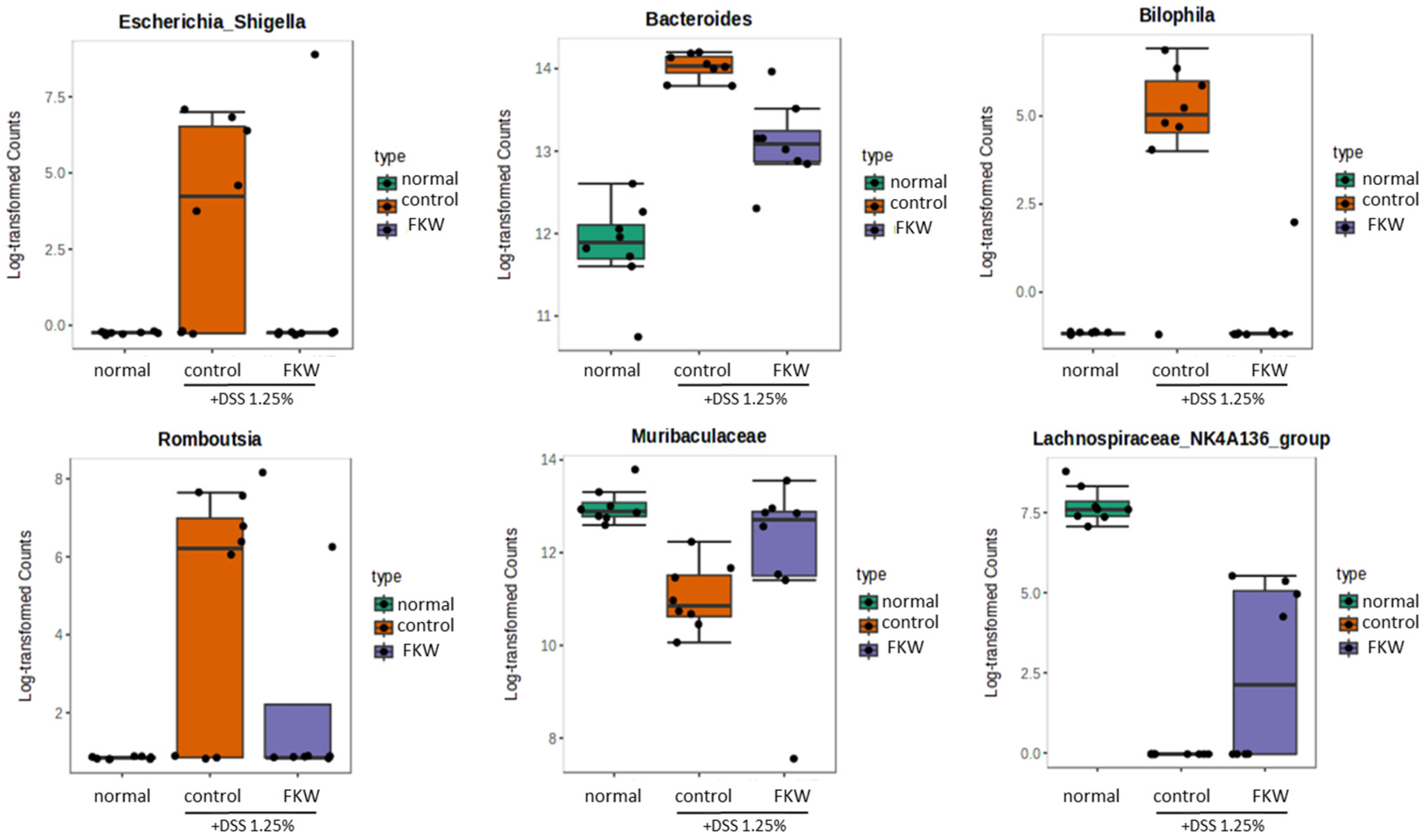
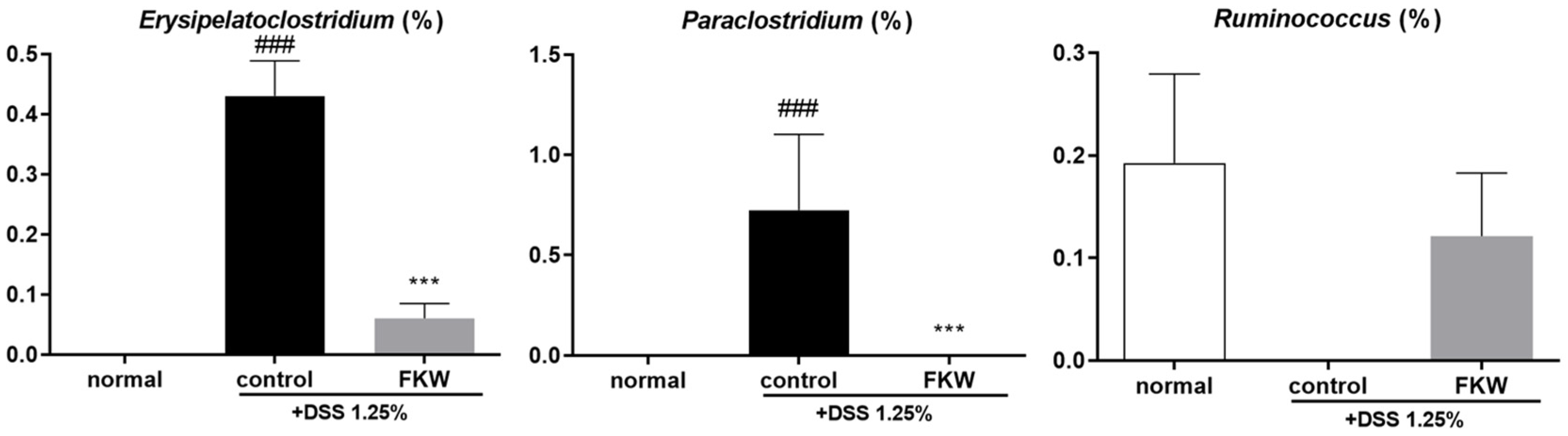
2.4. FKW Diet Modulated Gut Microbiota in DSS-Induced Colitis Mice
3. Discussion
3.1. Experimental Approach to Assess the Effects of FKW
3.2. FKW May Mitigate DSS-Induced Colonic Damage and Tissue Alterations
3.3. FKW May Restore Gut Microbiota Diversity and Protect Against DSS-Induced Dysbiosis
3.4. FKW May Regulate Immune Responses and Gut Microbial Balance to Suppress Intestinal Inflammation
3.5. FKW May Modulate Cytokine Production Through Gut Microbiome Regulation
3.6. FKW May Enhance Gut Health Through Microbial Balance and Enzymatic Activity
3.7. Limitations and Future Perspectives of FKW Research
4. Materials and Methods
4.1. Preparation of Fermented Kamut Wheat Enzyme (FKW)
4.2. Analysis of Nutritional Composition in FKW
4.3. Preparation of AIN-93G and FKW Diets
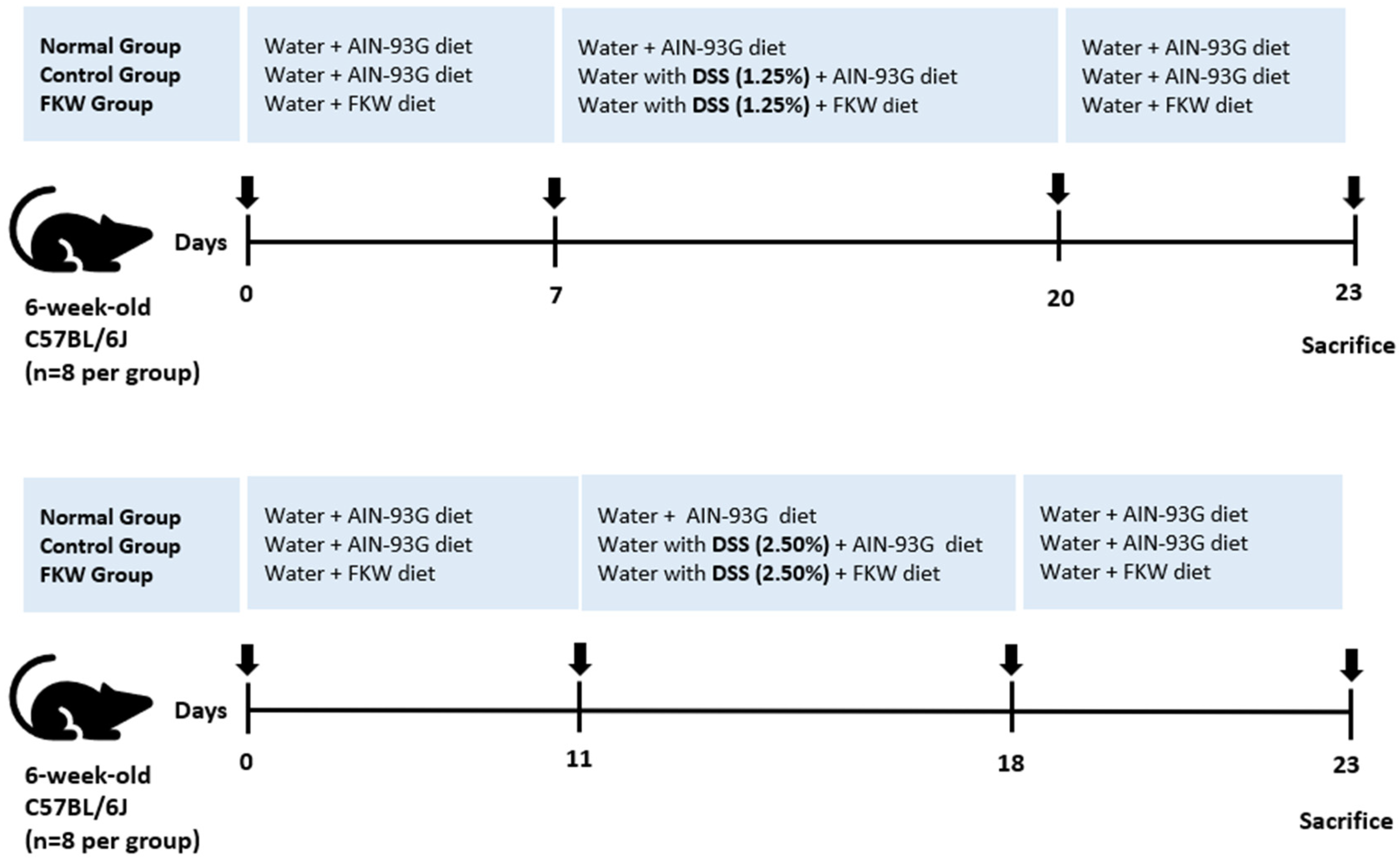
4.4. In Vivo Experimental Design
4.5. Assessment of Daily Disease Activity
4.6. Assessment of Colon Length and Spleen Weight
4.7. Histological Score in the Distal Colon
4.8. Measurement of Cytokine and Myeloperoxidase Level
4.9. Gut Microbiota Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, E.W.; Turpin, W.; Croitoru, K.; Colombel, J.-F.; Torres, J. Prediction and Prevention of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 396–405.e1. [Google Scholar] [CrossRef] [PubMed]
- Meade, S.; Liu Chen Kiow, J.; Massaro, C.; Kaur, G.; Squirell, E.; Bressler, B.; Lunken, G. Gut Microbiome-Associated Predictors as Biomarkers of Response to Advanced Therapies in Inflammatory Bowel Disease: A Systematic Review. Gut Microbes 2023, 15, 2287073. [Google Scholar] [CrossRef]
- He, P.; Yu, L.; Tian, F.; Zhang, H.; Chen, W.; Zhai, Q. Dietary Patterns and Gut Microbiota: The Crucial Actors in Inflammatory Bowel Disease. Adv. Nutr. 2022, 13, 1628–1651. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef]
- O’Reilly, C.; Mills, S.; Rea, M.C.; Lavelle, A.; Ghosh, S.; Hill, C.; Ross, R.P. Interplay between Inflammatory Bowel Disease Therapeutics and the Gut Microbiome Reveals Opportunities for Novel Treatment Approaches. Microbiome Res. Rep. 2023, 2, 35. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients with Inflammatory Bowel Disease. Off. J. Am. Coll. Gastroenterol. 2012, 107, 1051. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef]
- Basile, G.; De Maio, A.C.; Catalano, A.; Ceramella, J.; Iacopetta, D.; Bonofiglio, D.; Saturnino, C.; Sinicropi, M.S. Ancient Wheat as Promising Nutraceuticals for the Prevention of Chronic and Degenerative Diseases. Curr. Med. Chem. 2023, 30, 3384–3403. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Di Nunzio, M.; Taccari, A.; Valli, V. Ancient Wheat and Health: A Legend or the Reality? A Review on KAMUT Khorasan Wheat. Int. J. Food Sci. Nutr. 2017, 68, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Razem, M.; Morozova, K.; Ding, Y.; Ferrentino, G.; Scampicchio, M. Determination of Free and Bound Antioxidants in Kamut® Wheat by HPLC with Triple Detector (DAD-CAD-MS). Food Chem. X 2024, 21, 101216. [Google Scholar] [CrossRef]
- Cai, J.; Su, W.; Chen, X.; Zheng, H. Advances in the Study of Selenium and Human Intestinal Bacteria. Front. Nutr. 2022, 9, 1059358. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Cen, S.; Li, P.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Effects of Dietary Selenium Supplementation on Intestinal Barrier and Immune Responses Associated with Its Modulation of Gut Microbiota. Environ. Sci. Technol. Lett. 2018, 5, 724–730. [Google Scholar] [CrossRef]
- Sofi, F.; Whittaker, A.; Cesari, F.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Marotti, I.; Dinelli, G.; Casini, A.; Abbate, R.; et al. Characterization of Khorasan Wheat (Kamut) and Impact of a Replacement Diet on Cardiovascular Risk Factors: Cross-over Dietary Intervention Study. Eur. J. Clin. Nutr. 2013, 67, 190–195. [Google Scholar]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Taneyo Saa, D.; Turroni, S.; Serrazanetti, D.I.; Rampelli, S.; Maccaferri, S.; Candela, M.; Severgnini, M.; Simonetti, E.; Brigidi, P.; Gianotti, A. Impact of Kamut® Khorasan on Gut Microbiota and Metabolome in Healthy Volunteers. Food Res. Int. 2014, 63, 227–232. [Google Scholar] [CrossRef]
- Qin, H.; Wu, H.; Shen, K.; Liu, Y.; Li, M.; Wang, H.; Qiao, Z.; Mu, Z. Fermented Minor Grain Foods: Classification, Functional Components, and Probiotic Potential. Foods 2022, 11, 3155. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yeo, S.; Kang, S.; Huh, C.S. Longitudinal Microbiome Analysis in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Microorganisms 2021, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-Induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kang, Y.-M.; Lee, A.; Kim, Y.-J.; Kim, K.-H.; Hwang, Y.-H. Hydroethanolic Extract of Lepidium Apetalum Willdenow Alleviates Dextran Sulfate Sodium-Induced Colitis by Enhancing Intestinal Barrier Integrity and Inhibiting Oxidative Stress and Inflammasome Activation. Antioxidants 2024, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-I.; Cho, S.; Choi, N.-J. Effect of Dietary Probiotics on Colon Length in an Inflammatory Bowel Disease-Induced Murine Model: A Meta-Analysis. J. Dairy Sci. 2020, 103, 1807–1819. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A Guide to Histomorphological Evaluation of Intestinal Inflammation in Mouse Models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Yang, X.; Fang, L.; Shen, J.; Tan, Z.; Zeng, W.; Peng, M.; Xiao, N. Lipid-Lowering and Antioxidant Effects of Polygonatum Fermented Liquor: A Study on Intestinal Microbiota and Brain–Gut Axis in Mice. Front. Nutr. 2024, 11, 1428228. [Google Scholar] [CrossRef]
- Qian, X.-B.; Chen, T.; Xu, Y.-P.; Chen, L.; Sun, F.-X.; Lu, M.-P.; Liu, Y.-X. A Guide to Human Microbiome Research: Study Design, Sample Collection, and Bioinformatics Analysis. Chin. Med. J. 2020, 133, 1844. [Google Scholar] [CrossRef]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the Diversity of the Human Microbiota with Targeted Next-Generation Sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Hong, D.; Kim, H.K.; Yang, W.; Yoon, C.; Kim, M.; Yang, C.-S.; Yoon, S. Integrative Analysis of Single-Cell RNA-Seq and Gut Microbiome Metabarcoding Data Elucidates Macrophage Dysfunction in Mice with DSS-Induced Ulcerative Colitis. Commun. Biol. 2024, 7, 731. [Google Scholar] [CrossRef]
- Al-Amrah, H.; Saadah, O.I.; Mosli, M.; Annese, V.; Al-Hindi, R.; Edris, S.; Alshehri, D.; Alatawi, H.; Alatawy, M.; Bahieldin, A. Composition of the Gut Microbiota in Patients with Inflammatory Bowel Disease in Saudi Arabia: A Pilot Study. Saudi J. Gastroenterol. 2023, 29, 102–110. [Google Scholar] [CrossRef]
- Su, X. Elucidating the Beta-Diversity of the Microbiome: From Global Alignment to Local Alignment. mSystems 2021, 6, e0036321. [Google Scholar] [CrossRef] [PubMed]
- Casalegno Garduño, R.; Däbritz, J. New Insights on CD8+ T Cells in Inflammatory Bowel Disease and Therapeutic Approaches. Front. Immunol. 2021, 12, 738762. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wan, J.; Anderson, W.; Sun, H.; Zhang, H.; Peng, X.; Yu, Z.; Wang, T.; Yan, X.; Smith, W. Downregulation of IL-10 Secretion by Treg Cells in Osteoarthritis Is Associated with a Reduction in Tim-3 Expression. Biomed. Pharmacother. 2016, 79, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Yao, Y.; Orban, P.C.; Steiner, T.S.; Levings, M.K. Regulatory T-Cell Therapy for Inflammatory Bowel Disease: More Questions than Answers. Immunology 2012, 136, 115–122. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Ćesić, D.; Lugović Mihić, L.; Ozretić, P.; Lojkić, I.; Buljan, M.; Šitum, M.; Zovak, M.; Vidović, D.; Mijić, A.; Galić, N.; et al. Association of Gut Lachnospiraceae and Chronic Spontaneous Urticaria. Life 2023, 13, 1280. [Google Scholar] [CrossRef]
- Liu, C.; Qi, X.; Guan, K.; Chen, H.; Li, Q.; Mao, K.; Shen, G.; Ma, Y. Paraprobiotics 5-PA and 6-PA Restore Intestinal Homeostasis by Inhibiting NF-κB/NLRP3 Signaling Pathway and Alleviating Dysbiosis in Mice with Chronic Ulcerative Colitis. J. Funct. Foods 2024, 113, 106048. [Google Scholar] [CrossRef]
- Gao, X.; Chang, S.; Liu, S.; Peng, L.; Xie, J.; Dong, W.; Tian, Y.; Sheng, J. Correlations between α-Linolenic Acid-Improved Multitissue Homeostasis and Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00391-20. [Google Scholar] [CrossRef]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A Human Stool-Derived Bilophila Wadsworthia Strain Caused Systemic Inflammation in Specific-Pathogen-Free Mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901-19. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Sasaki, T.; Itoh, K.; Kitahara, T.; Takema, Y.; Hiramatsu, K.; Ishikawa, D.; Shibuya, T.; Kobayashi, O.; Osada, T.; et al. A Soluble Fiber Diet Increases Bacteroides Fragilis Group Abundance and Immunoglobulin A Production in the Gut. Appl. Environ. Microbiol. 2020, 86, e00405-20. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-S.; You, Y.-L.; Byun, H.-J.; Han, K.-H.; Kim, J.; Jung, J.-B.; Choi, H.-S.; Han, S.-H. Enzyme Activity and Lipogenesis Inhibition by Fermented Grain Using Natural Enzymes. Molecules 2023, 28, 7285. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Z.; Wu, G.; Xu, F.; Zhang, J.; Luo, X.; Ma, Y.; Pang, H.; Duan, Y.; Chen, J.; et al. Effects of Probiotic-Fermented Feed on the Growth Profile, Immune Functions, and Intestinal Microbiota of Bamei Piglets. Animals 2024, 14, 647. [Google Scholar] [CrossRef]
- Tovoli, F. Clinical and Diagnostic Aspects of Gluten Related Disorders. World J. Clin. Cases 2015, 3, 275. [Google Scholar] [CrossRef]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum Subsp Turanicum Wheat on Irritable Bowel Syndrome: A Double-Blinded Randomised Dietary Intervention Trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef]
- McCleary, B.V. Measurement of Dietary Fiber: Which AOAC Official Method of Analysis SM to Use. J. AOAC Int. 2023, 106, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Ferreira-Paes, T.; Seixas-Costa, P.; Almeida-Amaral, E.E. Validation of a Feed Protocol in a Mouse Model That Mimics Marasmic Malnutrition. Front. Vet. Sci. 2021, 8, 757136. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef] [PubMed]





| Compound | FKW |
|---|---|
| Carbohydrates (g/100 g) | 66.41 |
| Sugars (g/100 g) | 14.26 |
| Proteins (g/100 g) | 19.16 |
| Selenium (μg/100 g) | 112.43 |
| Ingredient (g) | AIN-93G | FKW |
|---|---|---|
| Casein | 200 | 134.7 |
| Sucrose | 100 | 100 |
| Dextrose | 132 | 132 |
| Corn Starch | 397.5 | 124.4 |
| Cellulose | 50 | 0 |
| Soybean Oil | 70 | 60.4 |
| Mineral mix | 35 | 35 |
| Vitamin mix | 10 | 10 |
| L-Cystine | 3 | 3 |
| Choline Bitartrate | 2.5 | 2.5 |
| Kamut | - | 398 |
| Total | 1000.0 | 1000.0 |
| Score | Body Weight Decrease (%) | Stool Consistency | Bleeding |
|---|---|---|---|
| 0 | <1 | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stools | |
| 3 | 10–20 | ||
| 4 | >20 | Diarrhea | Gross Bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kil, B.J.; Choi, Y.; Chai, H.; Lee, D.; Jo, H.-G.; Lee, D. Fermented Kamut Wheat Diet Prevents DSS-Induced Colitis via Modulating Gut Microbiota in Mice. Int. J. Mol. Sci. 2025, 26, 3017. https://doi.org/10.3390/ijms26073017
Lee J, Kil BJ, Choi Y, Chai H, Lee D, Jo H-G, Lee D. Fermented Kamut Wheat Diet Prevents DSS-Induced Colitis via Modulating Gut Microbiota in Mice. International Journal of Molecular Sciences. 2025; 26(7):3017. https://doi.org/10.3390/ijms26073017
Chicago/Turabian StyleLee, Juni, Bum Ju Kil, Yeojin Choi, Hyungyung Chai, Donghoon Lee, Hee-Geun Jo, and Donghun Lee. 2025. "Fermented Kamut Wheat Diet Prevents DSS-Induced Colitis via Modulating Gut Microbiota in Mice" International Journal of Molecular Sciences 26, no. 7: 3017. https://doi.org/10.3390/ijms26073017
APA StyleLee, J., Kil, B. J., Choi, Y., Chai, H., Lee, D., Jo, H.-G., & Lee, D. (2025). Fermented Kamut Wheat Diet Prevents DSS-Induced Colitis via Modulating Gut Microbiota in Mice. International Journal of Molecular Sciences, 26(7), 3017. https://doi.org/10.3390/ijms26073017








