Genetic Variants of the Human Thiamine Transporter (SLC19A3, THTR2)—Potential Relevance in Metabolic Diseases
Abstract
1. Introduction
2. Results
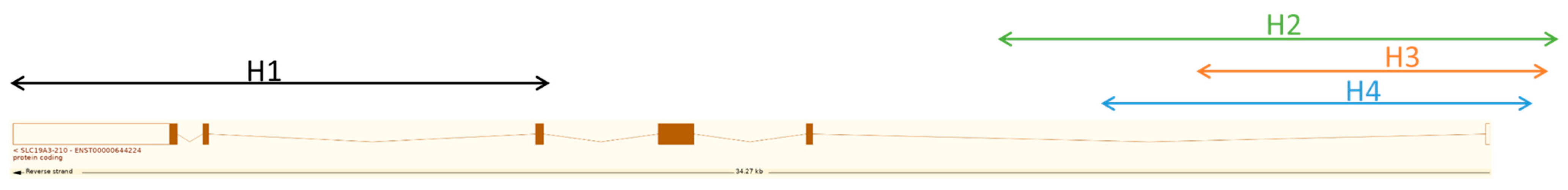
2.1. In Silico Haplotype and SNP Analysis in SLC19A3
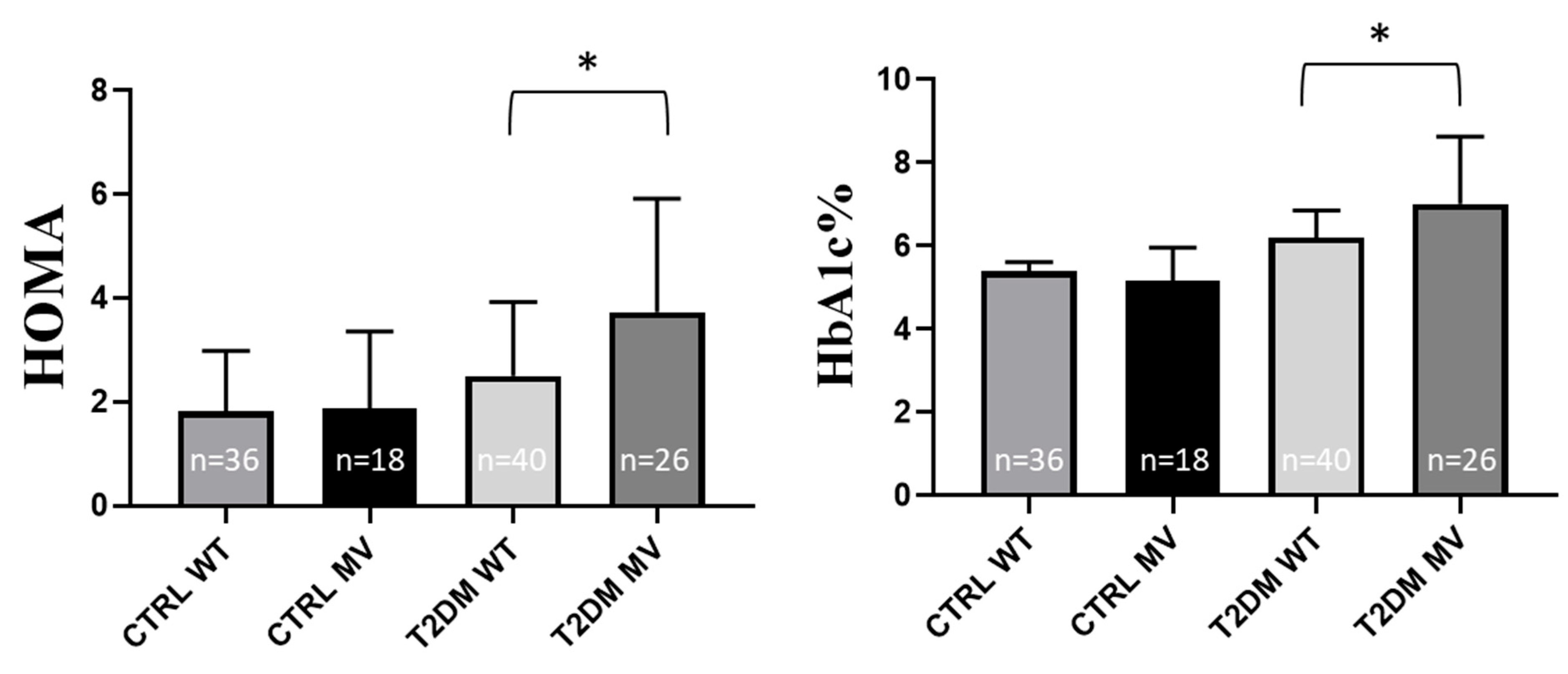
2.2. Experimental SNP Analysis in T2DM Patients—Correlations with Laboratory Data
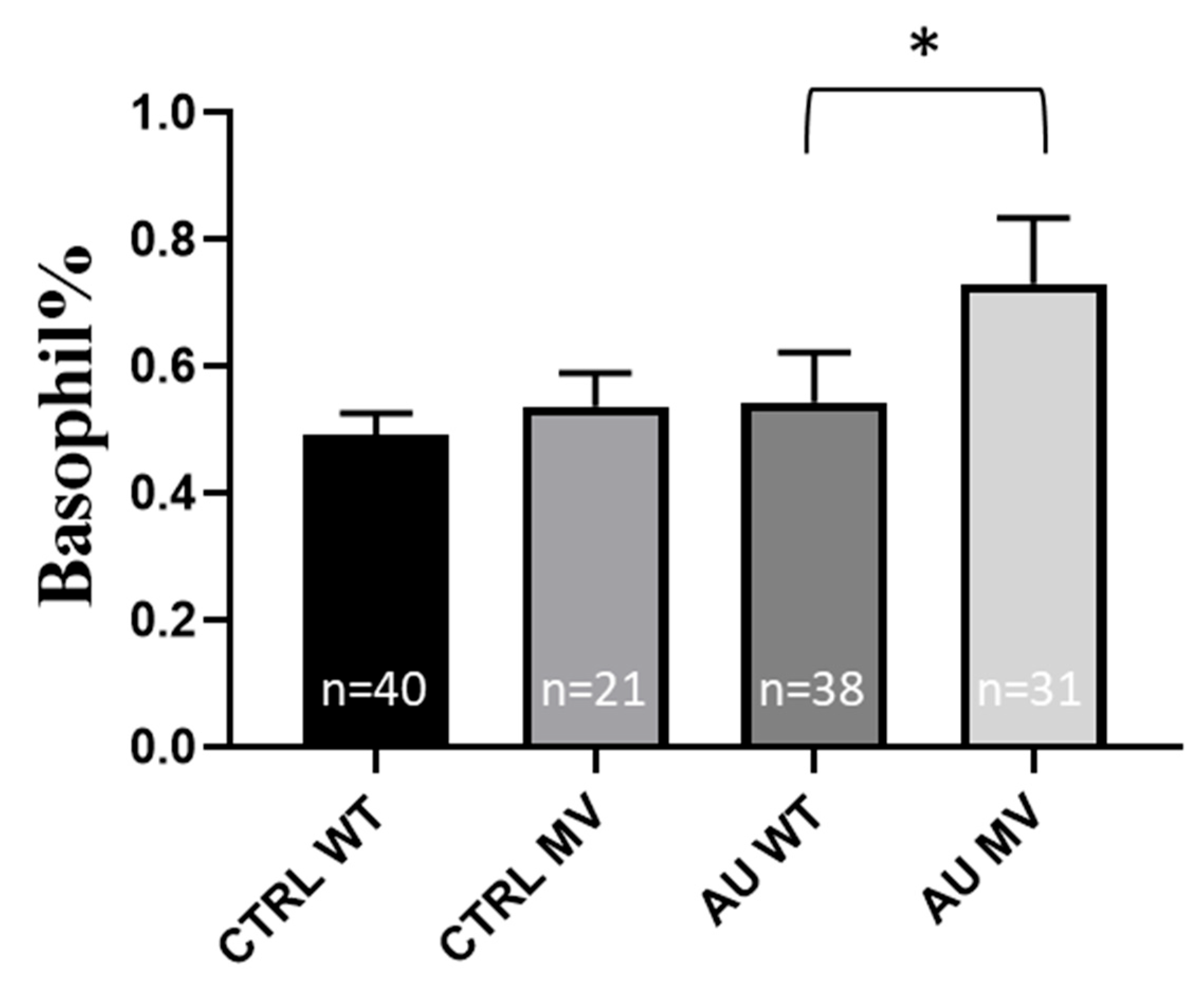
2.3. Experimental SNP Analysis in Gout Patients—Correlation with Laboratory Data
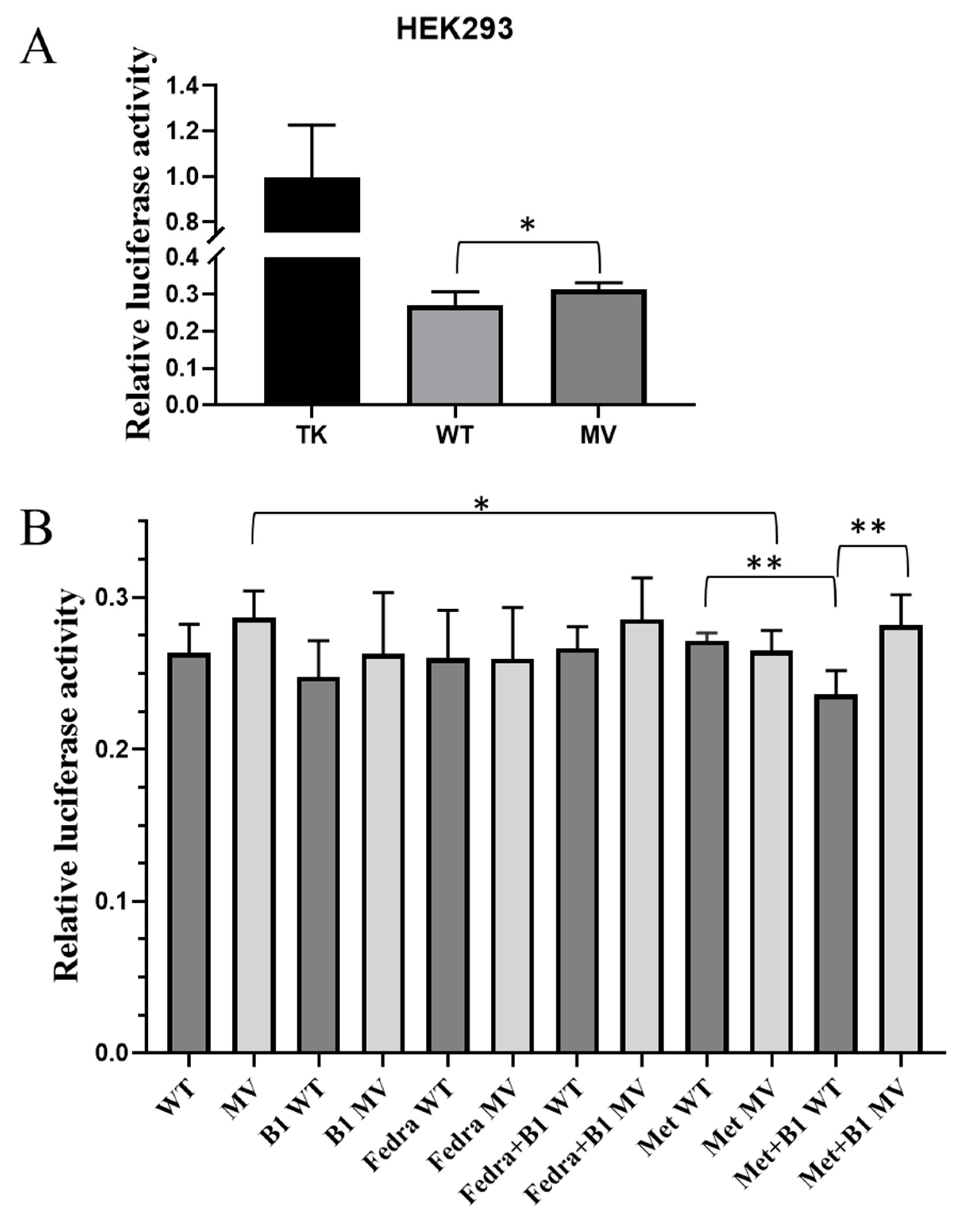
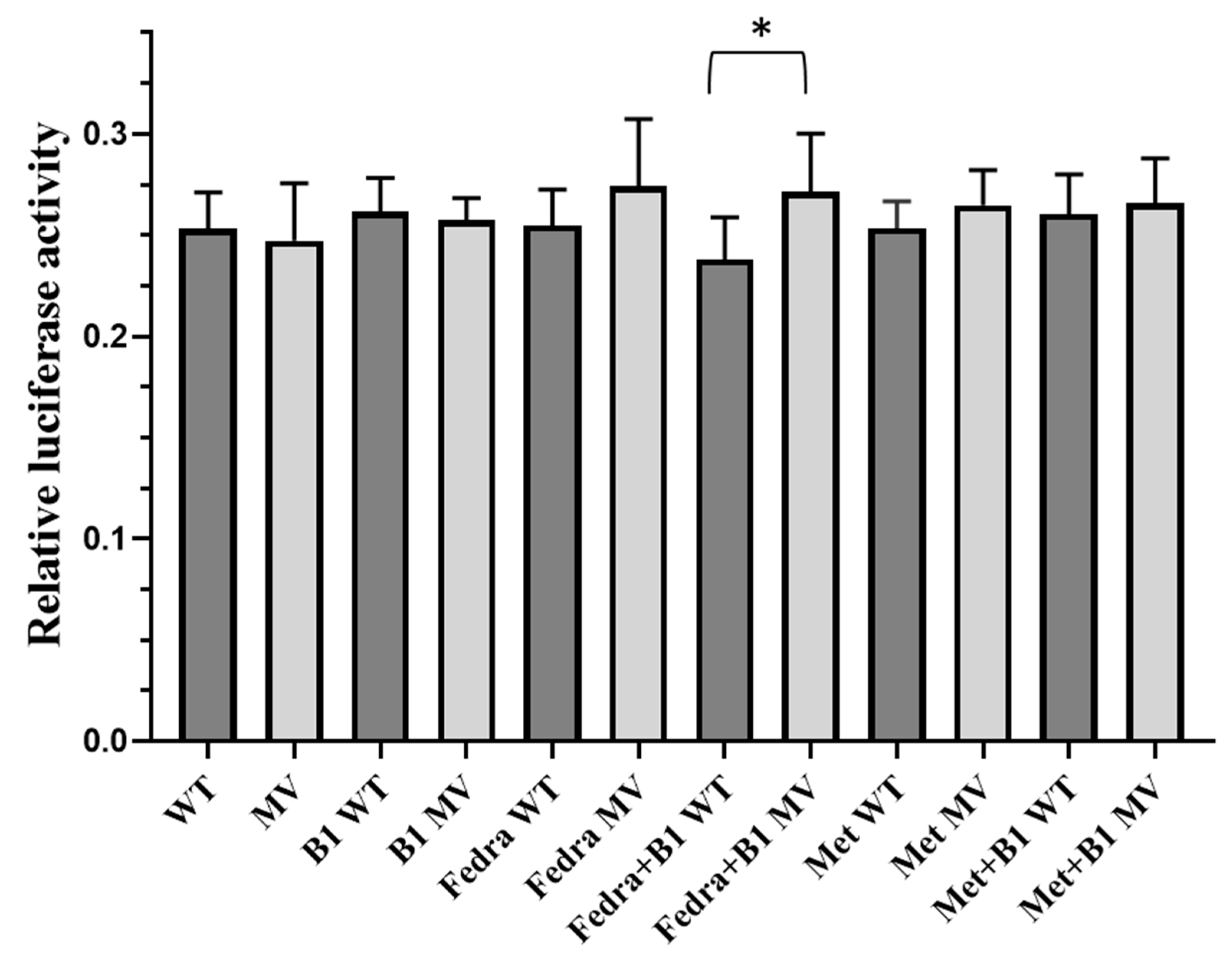
2.4. Analysis of the Effects of the Selected Haplotypes on Gene Expression in a Luciferase Reporter Assay
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis of Candidate SLC19A3 SNPs in T2DM
4.2. Analysis of Genetic Samples from Patients in the Study
4.3. Genetic Analysis and Vector Construction
| 1868 For | 5′-CGT CAG AAT TCT TTC CTT CAG TCA TTA TTG C-3′ |
| 1868 Rev | 5′-CGT CGG ATC CCT GAT CTA CGA ATG TAC CC-3 |
| 119 For | 5′-AGC TGA ATT CCA TCT ATC CAG TGA CAA CC-3′ |
| 119 Rev | 5′-CGC TGG ATC CTG AGA CCT AGG ACT ATG C-3′ |
4.4. Cell Lines and Dual Luciferase Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1000 G | 1000 Genomes |
| ABCG2 | ATP binding cassette G2 |
| ADA | American Diabetes Association |
| Arid5a | AT-Rich Interaction Domain 5A |
| AU | arthritis urica, gout |
| BMI | body mass index |
| CTRL | control |
| DDIT3 | DNA damage-inducible transcript 3 |
| DNA | deoxyribonucleic acid |
| EBF genes | early B-cell factors |
| ESR | erythrocyte sedimentation rates |
| ESR2 | Estrogen Receptor 2 |
| Fedra | fedratinib |
| FoxB1 | Forkhead Box B1 |
| GATA-1 | GATA-BINDING PROTEIN 1 |
| HbA1c | hemoglobin A1C |
| HOMA | Homeostasis Model Assessment |
| HOX genes | subset of homeobox genes |
| HWE | Hardy–Weinberg equilibrium |
| IRF9 | Interferon regulatory factor 9 |
| JAK | Janus kinase |
| KLF13 | Kruppel-like transcription factor 13 |
| LD | linkage disequilibrium |
| MAF | minor allele frequency |
| met | metformin |
| MV | minor variant |
| NR1H4 | nuclear receptor subfamily 1 group H member 4 |
| NR2C1 | nuclear receptor subfamily 2, group C, member 1 |
| NR3C1 | nuclear receptor subfamily 3, group C, member 1 |
| NR3C2 | nuclear receptor subfamily 3, group C, member 2 |
| PROX1 | Prospero homeobox protein 1 |
| PWM | position weight matrices |
| RAX2 | Retina And Anterior Neural Fold Homeobox 2 |
| RBC | red blood cells |
| RFX1 | Regulatory Factor X1 |
| RORC | RAR-related orphan receptor gamma |
| SD | standard deviation |
| SLC19A3 | solute carrier 19A3 |
| SNP | single nucleotide polymorphisms |
| SP1 | specificity protein 1* |
| STAT1 | Signal transducer and activator of transcription 1 |
| STAT5 | Signal transducer and activator of transcription 5 |
| T2DM | type 2 diabetes |
| TF | transcription factor |
| TFBS | transcription factor binding sites |
| THTR2 | thiamine transporter-2 |
| UA | uric acid |
| URAT1 | urate transporter 1 |
| WBC | white blood cells |
| WT | wild type |
References
- Sechi, G.; Serra, A. Wernicke’s Encephalopathy: New Clinical Settings and Recent Advances in Diagnosis and Management. Lancet Neurol. 2007, 6, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Aradi, G.; Várallyay, G.; Arányi, Z.; Bereczki, D.; Vastagh, I. Wernicke’s Encephalopathy Induced by the Use of Diet Pills and Unbalanced Diet. Orv. Hetil. 2014, 155, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Reiners, K.; Strom, A.; Obeid, R. Association between Diabetes and Thiamine Status—A Systematic Review and Meta-Analysis. Metabolism 2023, 144, 155565. [Google Scholar] [CrossRef] [PubMed]
- Marcé-Grau, A.; Martí-Sánchez, L.; Baide-Mairena, H.; Ortigoza-Escobar, J.D.; Pérez-Dueñas, B. Genetic Defects of Thiamine Transport and Metabolism: A Review of Clinical Phenotypes, Genetics, and Functional Studies. J. Inherit. Metab. Dis. 2019, 42, 581–597. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Beltramo, E.; Mazzeo, A.; Porta, M. Thiamine and Diabetes: Back to the Future? Acta Diabetol. 2021, 58, 1433–1439. [Google Scholar] [CrossRef]
- Zera, K.; Sweet, R.; Zastre, J. Role of HIF-1α in the Hypoxia Inducible Expression of the Thiamine Transporter, SLC19A3. Gene 2016, 595, 212–220. [Google Scholar] [CrossRef]
- Porta, M.; Toppila, I.; Sandholm, N.; Hosseini, S.M.; Forsblom, C.; Hietala, K.; Borio, L.; Harjutsalo, V.; Klein, B.E.; Klein, R.; et al. Variation in SLC19A3 and Protection From Microvascular Damage in Type 1 Diabetes. Diabetes 2016, 65, 1022–1030. [Google Scholar] [CrossRef]
- Mazzeo, A.; Barutta, F.; Bellucci, L.; Trento, M.; Gruden, G.; Porta, M.; Beltramo, E. Reduced Thiamine Availability and Hyperglycemia Impair Thiamine Transport in Renal Glomerular Cells through Modulation of Thiamine Transporter 2. Biomedicines 2021, 9, 385. [Google Scholar] [CrossRef]
- Beltramo, E.; Mazzeo, A.; Lopatina, T.; Trento, M.; Porta, M. Thiamine Transporter 2 Is Involved in High Glucose-Induced Damage and Altered Thiamine Availability in Cell Models of Diabetic Retinopathy. Diabetes Vasc. Dis. Res. 2020, 17, 147916411987842. [Google Scholar] [CrossRef]
- Liang, X.; Chien, H.-C.; Yee, S.W.; Giacomini, M.M.; Chen, E.C.; Piao, M.; Hao, J.; Twelves, J.; Lepist, E.-I.; Ray, A.S.; et al. Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol. Pharm. 2015, 12, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Giacomini, K.M. Transporters Involved in Metformin Pharmacokinetics and Treatment Response. J. Pharm. Sci. 2017, 106, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, T.; Yasujima, T.; Said, H.M.; Yuasa, H. PH-Dependent Pyridoxine Transport by SLC19A2 and SLC19A3: Implications for Absorption in Acidic Microclimates. J. Biol. Chem. 2020, 295, 16998–17008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Xing, Y.; Cheng, T.; Ren, S.; Ma, H. Diabetes Mellitus Is Associated with a Lower Risk of Gout: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 2020, 5470739. [Google Scholar] [CrossRef]
- Pálinkás, M.; Szabó, E.; Kulin, A.; Mózner, O.; Rásonyi, R.; Juhász, P.; Nagy, K.; Várady, G.; Vörös, D.; Zámbó, B.; et al. Genetic Polymorphisms and Decreased Protein Expression of ABCG2 Urate Transporters Are Associated with Susceptibility to Gout, Disease Severity and Renal-Overload Hyperuricemia. Clin. Exp. Med. 2022, 23, 1277–1284. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Hubert, K.A.; Wellik, D.M. Hox Genes in Development and Beyond. Development 2023, 150, dev192476. [Google Scholar] [CrossRef]
- Kloetzli, J.M.; Fontaine-Glover, I.A.; Brown, E.R.; Kuo, M.; Labosky, P.A. The Winged Helix Gene, Foxb1, Controls Development of Mammary Glands and Regions of the CNS That Regulate the Milk-Ejection Reflex. Genesis 2001, 29, 60–71. [Google Scholar] [CrossRef]
- De Souza, F.S.J.; Placzek, M. Conserved Roles of Rax/Rx3 Genes in Hypothalamus and Pituitary Development. Int. J. Dev. Biol. 2021, 65, 195–205. [Google Scholar] [CrossRef]
- Medina, K.L.; Singh, H. Genetic Networks That Regulate B Lymphopoiesis. Curr. Opin. Hematol. 2005, 12, 203–209. [Google Scholar] [CrossRef]
- Ducoli, L.; Detmar, M. Beyond PROX1: Transcriptional, Epigenetic, and Noncoding RNA Regulation of Lymphatic Identity and Function. Dev. Cell 2021, 56, 406–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Shin, H.Y. Genomic Mutations of the STAT5 Transcription Factor Are Associated with Human Cancer and Immune Diseases. Int. J. Mol. Sci. 2022, 23, 11297. [Google Scholar] [CrossRef] [PubMed]
- Issac, J.; Raveendran, P.S.; Das, A. V RFX1: A Promising Therapeutic Arsenal against Cancer. Cancer Cell Int. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Qi, Z.; Pang, Y.; Li, H.; Xie, H.; Wu, J.; Huang, Y.; Zhu, Y.; Shen, Y.; Zhu, Y.; et al. Retinoic Acid–Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019, 79, 2604–2618. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of Farnesoid X Receptor and Its Role in Bile Acid Metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef]
- Wachowski, N.A.; Pippin, J.A.; Boehm, K.; Lu, S.; Leonard, M.E.; Manduchi, E.; Parlin, U.W.; Wabitsch, M.; Chesi, A.; Wells, A.D.; et al. Implicating Type 2 Diabetes Effector Genes in Relevant Metabolic Cellular Models Using Promoter-Focused Capture-C. Diabetologia 2024, 67, 2740–2753. [Google Scholar] [CrossRef]
- Sun, Y.; Demagny, H.; Schoonjans, K. Emerging Functions of the Nuclear Receptor LRH-1 in Liver Physiology and Pathology. Biochim. Biophys. Acta-Mol. Basis Dis. 2021, 1867, 166145. [Google Scholar] [CrossRef]
- Daniel, S.; Kim, K.-H. Sp1 Mediates Glucose Activation of the Acetyl-CoA Carboxylase Promoter. J. Biol. Chem. 1996, 271, 1385–1392. [Google Scholar] [CrossRef]
- Yang, S.; Xiang, J.; Ma, C.; Yang, G.; Wang, X.; Liu, H.; Fan, G.; Kang, L.; Liang, Z. Sp1-like Protein KLF13 Acts as a Negative Feedback Regulator of TGF-β Signaling and Fibrosis. Cell Rep. 2023, 42, 112367. [Google Scholar] [CrossRef]
- Frank, A.; Brown, L.M.; Clegg, D.J. The Role of Hypothalamic Estrogen Receptors in Metabolic Regulation. Front. Neuroendocrinol. 2014, 35, 550–557. [Google Scholar] [CrossRef]
- Morales, M.; Martín-Vasallo, P.; Ávila, J. Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment. Biomedicines 2022, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C. Transcription Factor GATA-1 and Erythroid Development. Exp. Biol. Med. 1993, 202, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Berastegui, N.; Ainciburu, M.; Romero, J.P.; Garcia-Olloqui, P.; Alfonso-Pierola, A.; Philippe, C.; Vilas-Zornoza, A.; San Martin-Uriz, P.; Ruiz-Hernández, R.; Abarrategi, A.; et al. The Transcription Factor DDIT3 Is a Potential Driver of Dyserythropoiesis in Myelodysplastic Syndromes. Nat. Commun. 2022, 13, 7619. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Cavalli, A.; Cascio, A. STAT1 and Its Crucial Role in the Control of Viral Infections. Int. J. Mol. Sci. 2022, 23, 4095. [Google Scholar] [CrossRef]
- Nyati, K.K.; Zaman, M.M.-U.; Sharma, P.; Kishimoto, T. Arid5a, an RNA-Binding Protein in Immune Regulation: RNA Stability, Inflammation, and Autoimmunity. Trends Immunol. 2020, 41, 255–268. [Google Scholar] [CrossRef]
- Paul, A.; Tang, T.H.; Ng, S.K. Interferon Regulatory Factor 9 Structure and Regulation. Front. Immunol. 2018, 9, 1831. [Google Scholar] [CrossRef]
- Minematsu, T.; Giacomini, K.M. Interactions of Tyrosine Kinase Inhibitors with Organic Cation Transporters and Multidrug and Toxic Compound Extrusion Proteins. Mol. Cancer Ther. 2011, 10, 531–539. [Google Scholar] [CrossRef]
- Mofo Mato, E.P.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic Polymorphisms of Organic Cation Transporter 1 (OCT1) and Responses to Metformin Therapy in Individuals with Type 2 Diabetes: A Systematic Review. Medicine 2018, 97, e11349. [Google Scholar] [CrossRef]
- Yu, H.; Xue, W.; Yu, H.; Gu, H.; Qin, L.; Peng, A. Joint Application of Multiple Inflammatory Cytokines in Diagnosis of Gout Flare. J. Inflamm. Res. 2023, 16, 1771–1782. [Google Scholar] [CrossRef]
- England, J.T.; Gupta, V. Fedratinib: A Pharmacotherapeutic Option for JAK-Inhibitor Naïve and Exposed Patients with Myelofibrosis. Expert Opin. Pharmacother. 2022, 23, 1677–1686. [Google Scholar] [CrossRef]
- Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. Fedratinib; 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/34633785/ (accessed on 23 June 2020).
- Larkin, J.R.; Zhang, F.; Godfrey, L.; Molostvov, G.; Zehnder, D.; Rabbani, N.; Thornalley, P.J. Glucose-Induced Down Regulation of Thiamine Transporters in the Kidney Proximal Tubular Epithelium Produces Thiamine Insufficiency in Diabetes. PLoS ONE 2012, 7, e53175. [Google Scholar] [CrossRef]
- Szabó, E.; Kulin, A.; Mózner, O.; Korányi, L.; Literáti-Nagy, B.; Vitai, M.; Cserepes, J.; Sarkadi, B.; Várady, G. Potential Role of the ABCG2-Q141K Polymorphism in Type 2 Diabetes. PLoS ONE 2021, 16, e0260957. [Google Scholar] [CrossRef]
- Szabó, E.; Kulin, A.; Korányi, L.; Literáti-Nagy, B.; Cserepes, J.; Somogyi, A.; Sarkadi, B.; Várady, G. Alterations in Erythrocyte Membrane Transporter Expression Levels in Type 2 Diabetic Patients. Sci. Rep. 2021, 11, 2765. [Google Scholar] [CrossRef]
- Bartáková, V.; Pleskačová, A.; Kuricová, K.; Pácal, L.; Dvořáková, V.; Bělobrádková, J.; Tomandlová, M.; Tomandl, J.; Kaňková, K. Dysfunctional Protection against Advanced Glycation Due to Thiamine Metabolism Abnormalities in Gestational Diabetes. Glycoconj. J. 2016, 33, 591–598. [Google Scholar] [CrossRef]

| SNP | Assay ID | Context Sequence [VIC/FAM] |
|---|---|---|
| rs6436729 | C__26173496_10 | GCATGGTGGTATATGCCTGTAGTCC[T/A]AGCTACTGAGAAGCTGAGGCAGGAA |
| rs34241868 | C__32730488_10 | AGAATATACCCCACACCTGAACAGA[C/A]CCATTCACAAGATAATGTAGCTTAT |
| rs55975119 | C__88465680_10 | TCTCACTTCCTAGCTGCATGCCTTA[A/C]AGGACAGAAAGGTCCTCAGATCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, E.; Pálinkás, M.; Bohár, B.; Literáti-Nagy, B.; Korányi, L.; Poór, G.; Várady, G.; Sarkadi, B. Genetic Variants of the Human Thiamine Transporter (SLC19A3, THTR2)—Potential Relevance in Metabolic Diseases. Int. J. Mol. Sci. 2025, 26, 2972. https://doi.org/10.3390/ijms26072972
Szabó E, Pálinkás M, Bohár B, Literáti-Nagy B, Korányi L, Poór G, Várady G, Sarkadi B. Genetic Variants of the Human Thiamine Transporter (SLC19A3, THTR2)—Potential Relevance in Metabolic Diseases. International Journal of Molecular Sciences. 2025; 26(7):2972. https://doi.org/10.3390/ijms26072972
Chicago/Turabian StyleSzabó, Edit, Márton Pálinkás, Balázs Bohár, Botond Literáti-Nagy, László Korányi, Gyula Poór, György Várady, and Balázs Sarkadi. 2025. "Genetic Variants of the Human Thiamine Transporter (SLC19A3, THTR2)—Potential Relevance in Metabolic Diseases" International Journal of Molecular Sciences 26, no. 7: 2972. https://doi.org/10.3390/ijms26072972
APA StyleSzabó, E., Pálinkás, M., Bohár, B., Literáti-Nagy, B., Korányi, L., Poór, G., Várady, G., & Sarkadi, B. (2025). Genetic Variants of the Human Thiamine Transporter (SLC19A3, THTR2)—Potential Relevance in Metabolic Diseases. International Journal of Molecular Sciences, 26(7), 2972. https://doi.org/10.3390/ijms26072972







