Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis
Abstract
1. Introduction
- Unanswered questions from previous research.While our initial studies provided promising evidence that U-359 could enhance the effects of Tx, they also raised critical questions:
- Does U-359 act specifically against microtubule-targeting drugs like Taxol, or does it have broader anticancer potential?
- Would U-359 have similar synergistic effects with other widely used different chemotherapeutic agents?
- The need for more effective strategies to overcome MDR in cancer cells.
- Ox resistance often develops due to DNA repair mechanisms and anti-apoptotic signaling.
- 5-FU resistance is frequently linked to the up-regulation of NF-κB and ABC transporters.
- Both drugs suffer from declining efficacy over time, limiting their long-term clinical success.
- Changing and expanding the possible therapeutic potential of U-359.If U-359 only worked with Taxol, its use would be limited to certain cancer types. However, if it could also enhance Ox and 5-FU therapy, it could be used in a much wider range of treatments, including the following:
- Breast cancer (where Ox and 5-FU are standard treatments).
- Colorectal cancer (5-FU is a first-line therapy).
- Ovarian cancer (Ox is widely used).
- Optimizing our strategies by reducing toxicity and increasing efficacy.Traditional chemotherapy is associated with severe toxicity due to the high doses required for effectiveness. If U-359 could enhance the efficacy of Ox and 5-FU, it could allow for the following:
- Lower chemotherapy doses, reducing side effects such as nausea, immune suppression, and organ toxicity.
- More effective cancer cell killing at lower drug concentrations, improving patient outcomes.
- A safer treatment alternative for patients who cannot tolerate high-dose chemotherapy.
- The need for mechanistic validation.While our previous studies showed that U-359 had anticancer effects, we needed to validate the precise molecular mechanisms by which it enhances chemotherapy.Without this additional research, we would not have fully understood how U-359 works, limiting its clinical potential.Continuing this research was not just beneficial—it was essential to do the following:
- Determine whether U-359’s effects were limited to Taxol or had broader applications.
- Validate its ability to overcome drug resistance in Ox- and 5-FU-treated cancer cells.
- Confirm that U-359 allows for chemotherapy dose reduction, improving safety.
- Provide mechanistic insights into how U-359 enhances apoptosis and suppresses MDR. For this purpose, we studied the influence of U-359, used alone or in combination with 5-FU or Ox, on apoptosis induction and the inhibition of MCF-7 cell proliferation. We also analyzed the potential of U-359 as an ABC transporter inhibitor and NF-κB modulator.
2. Results
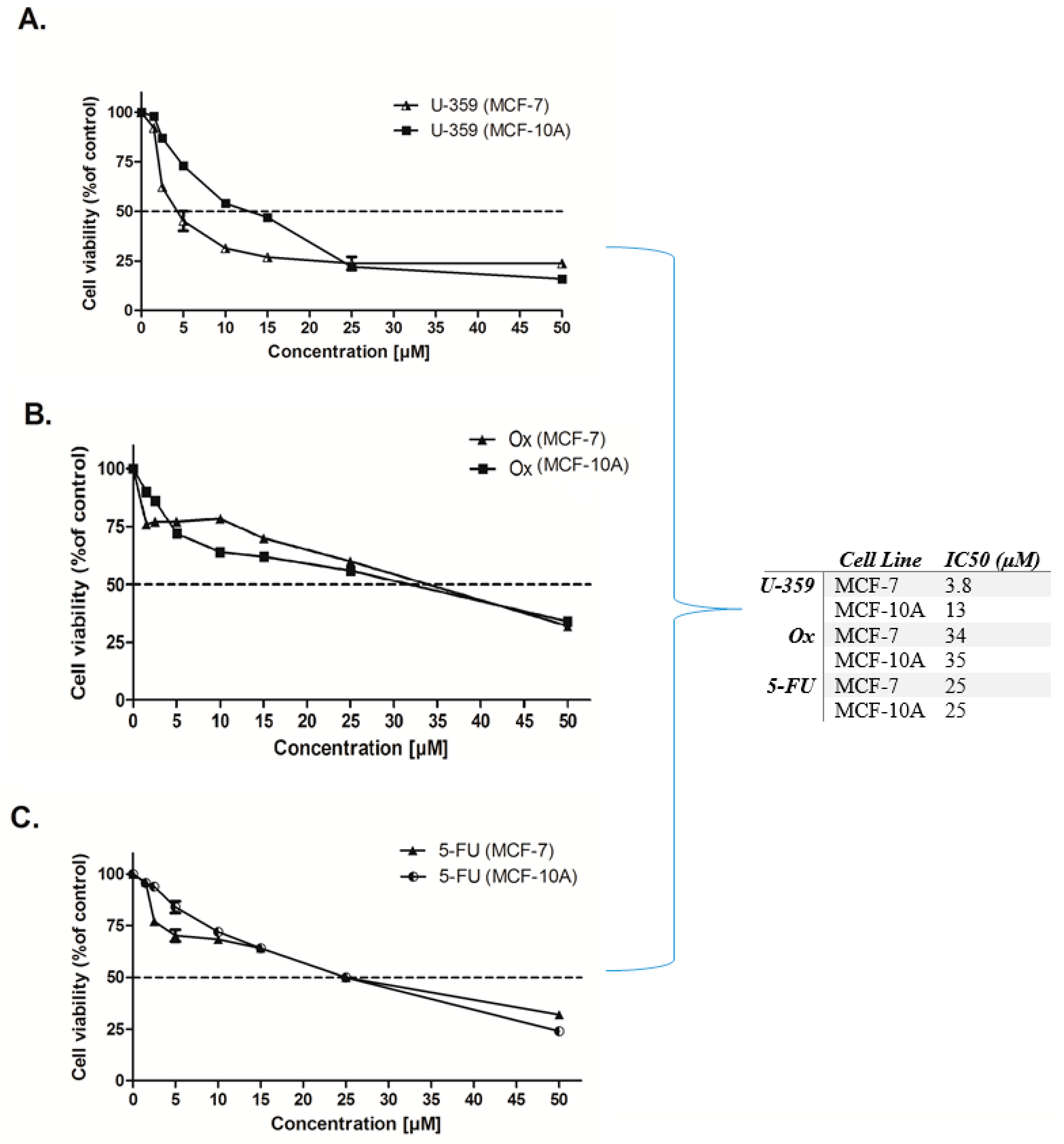
2.1. Cytotoxic Activity
2.2. Analysis of Cell Morphology Using Light Microscopy
2.3. Effect of Single and Combined Treatments on MCF-7 and MCF-10A Cell Viability
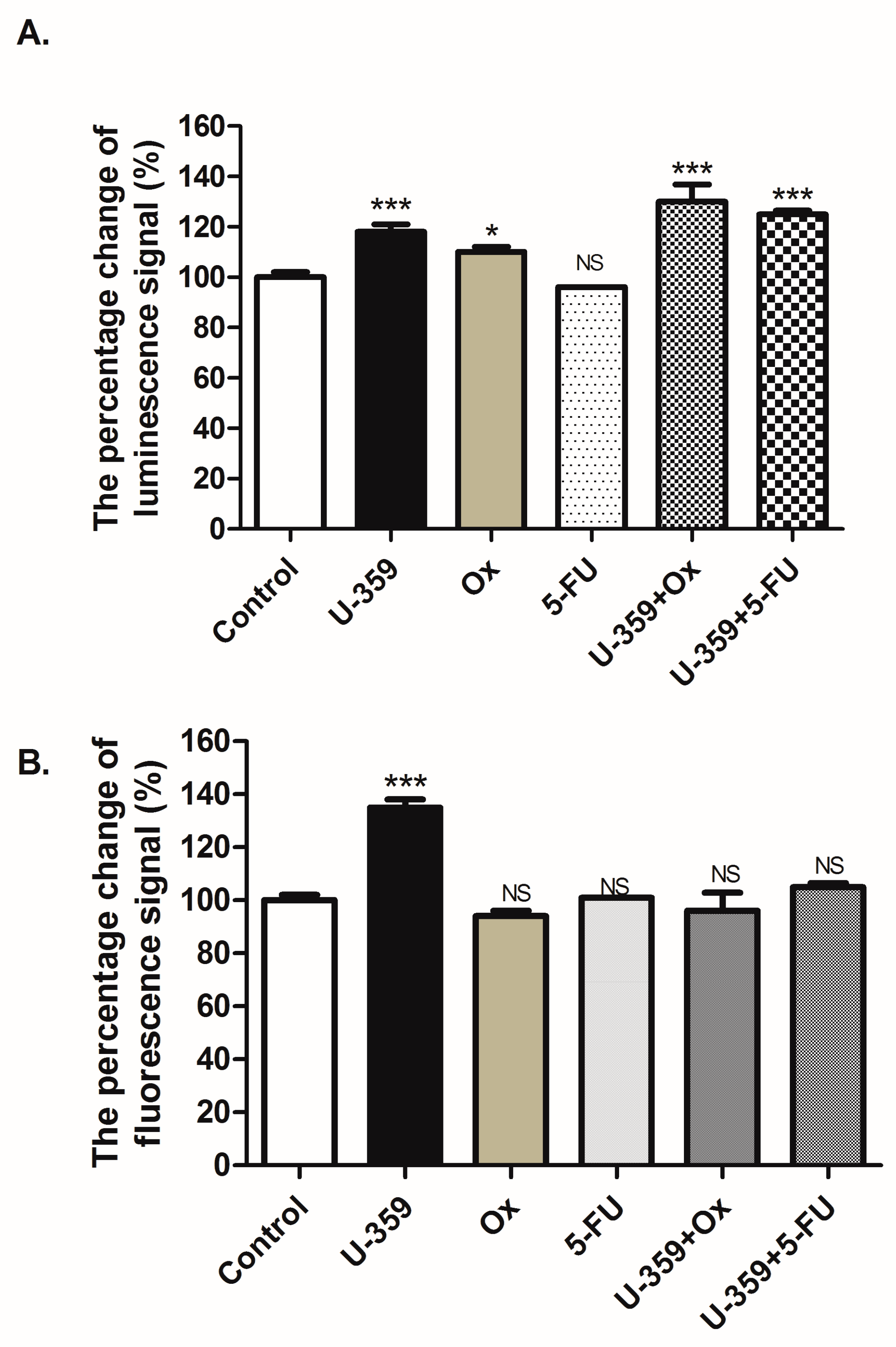
2.4. Apoptosis Analysis
2.4.1. Analysis of Apoptosis-Related Genes Expression
2.4.2. Assessment of Apoptosis and Necrosis
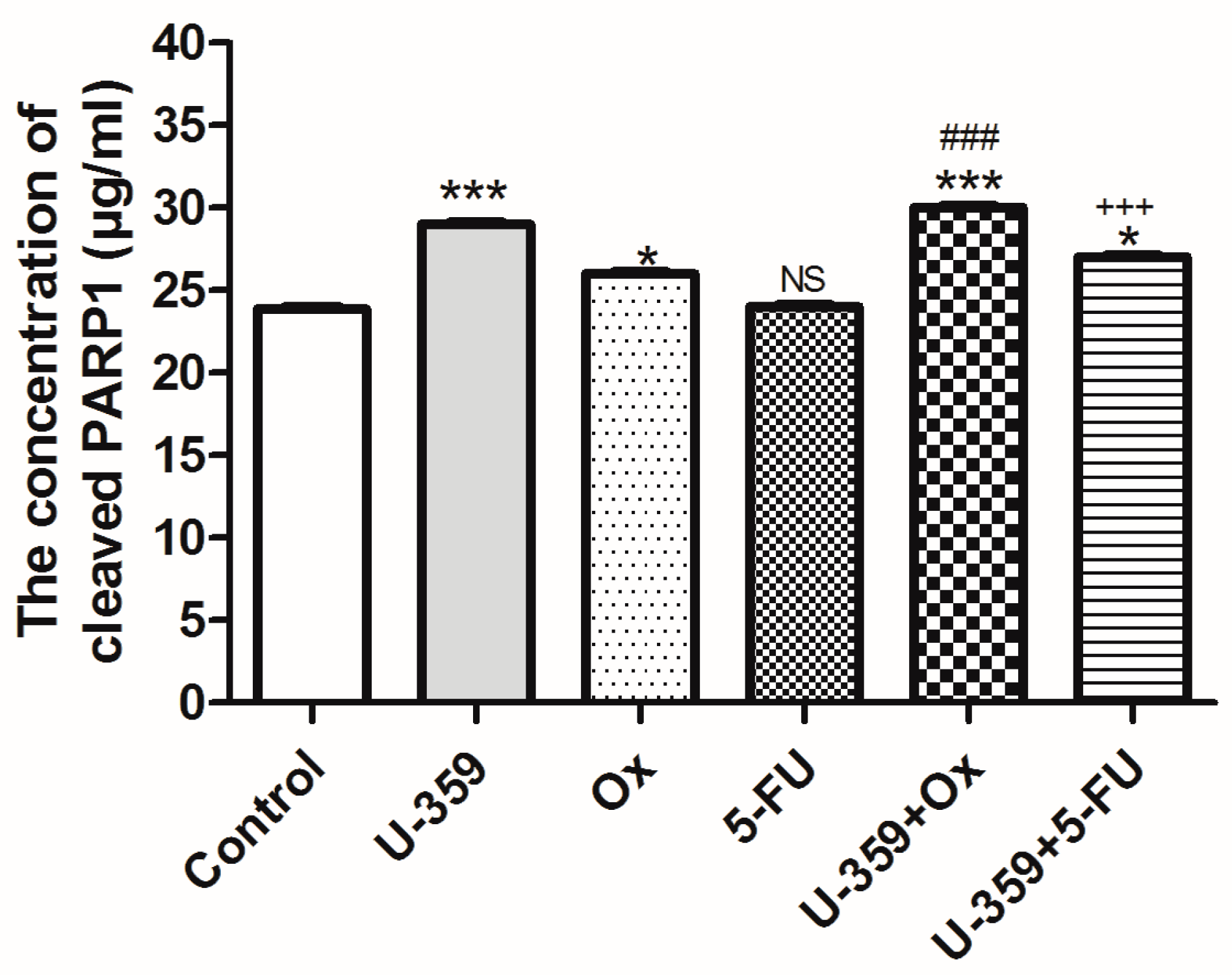
2.4.3. Evaluation of Human PARP1 Protein Levels Using an ELISA-Based Approach
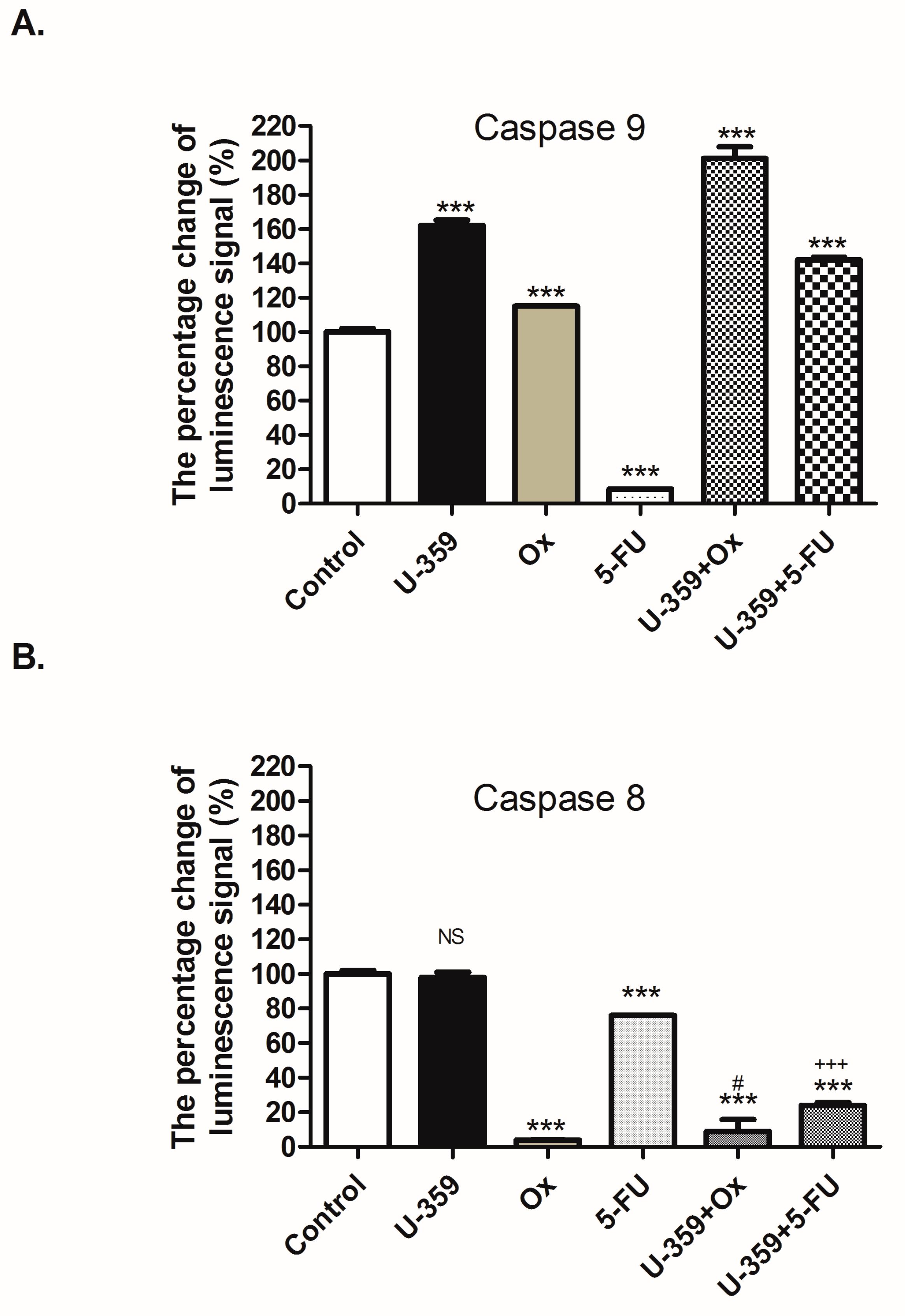
2.4.4. The Caspase 8 and 9 Activity
2.5. Multidrug Resistance Phenotype
2.5.1. Analysis of Multidrug Resistance-Related Genes Expression
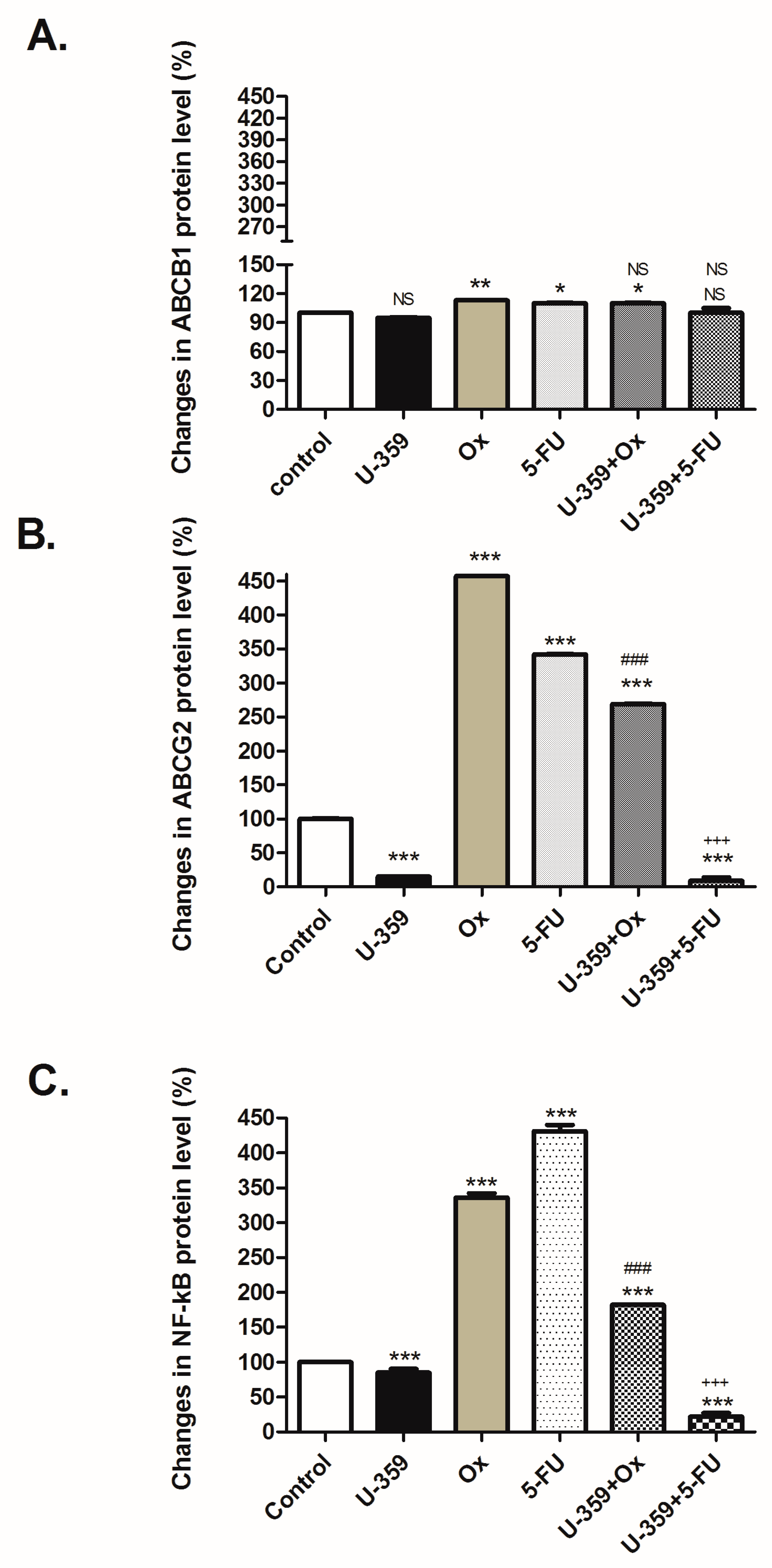
2.5.2. Regulation of ABCB1, ABCG2, and NF-κB p65 Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT-Assay
4.4. Determination of Cell Morphology Using Light Microscopy
4.5. RealTime-Glo MT Cell Viability Assay
4.6. Assessment of Cell Death Type
4.7. Assessment of Human PARP1 Protein Levels by ELISA-Based Method
4.8. The Caspase-Glo® 8 and 9 Assay
4.9. Quantitative Real-Time PCR Assay
4.10. Assessment of ABCB1, ABCG2, and NF-κB/p65 Protein Levels by ELISA-Based Method
4.11. The Bliss Independence Model
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ATP-binding cassette subfamily B member 1 (P-glycoprotein) |
| ABCG2 | ATP-binding cassette subfamily G member 2 (breast cancer resistance protein, BCRP) |
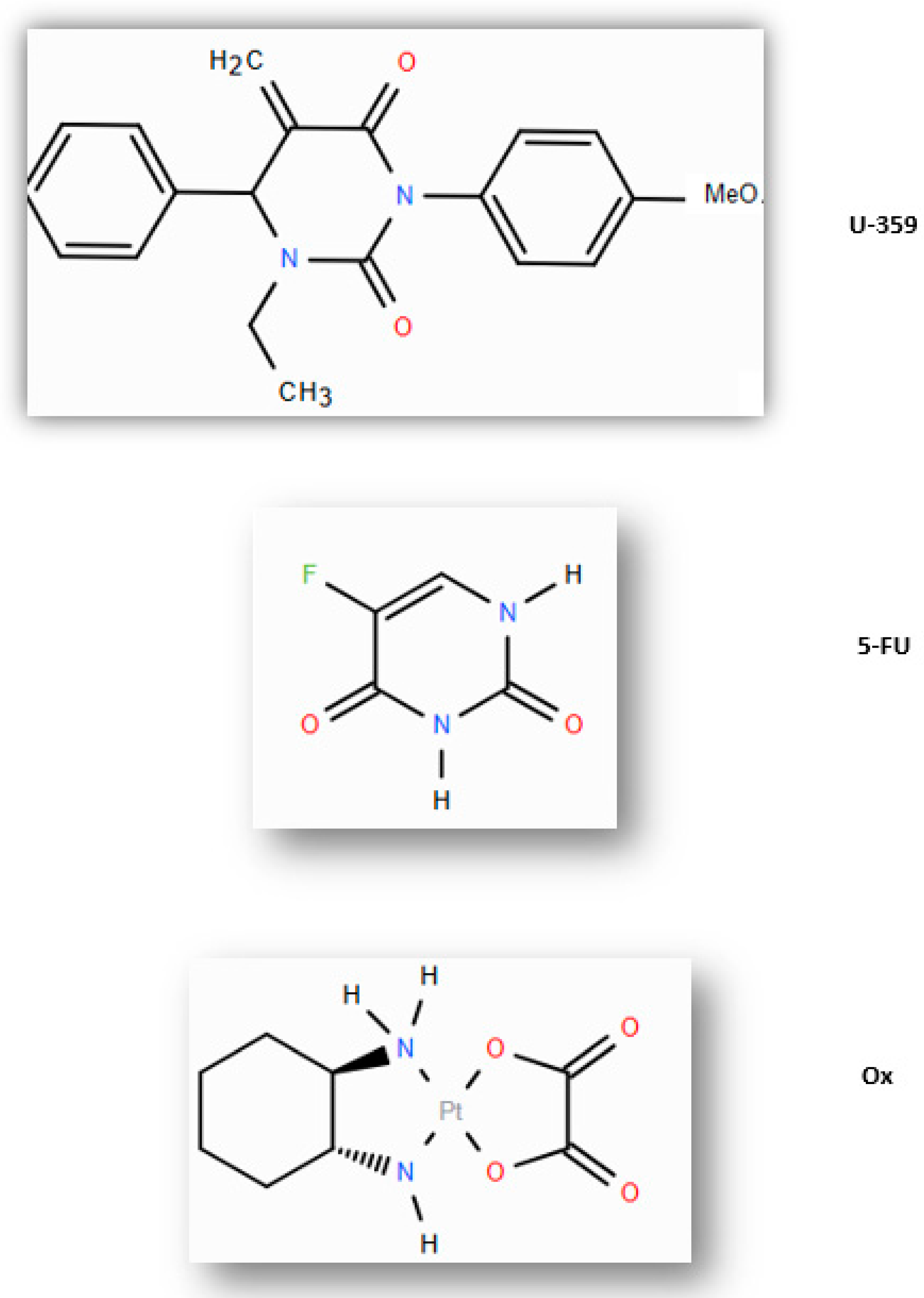
| 5-FU | 5-Fluorouracil |
| MDR | Multidrug resistance |
| NF-κB | Nuclear factor kappa B |
| Tx | Taxol |
| U-359 | 3-p-bromophenyl-1-ethyl-5-methylidenedihydrouracil |
| MCF-7 | Michigan Cancer Foundation-7 (breast cancer cell line) |
| Ox | Oxaliplatin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Pectasides, M.; Farmakis, D.; Bountouroglou, N.; Nikolaou, M.; Koumpou, M.; Mylonakis, N.; Kosmas, C. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: A phase II study. Ann. Oncol. 2003, 14, 537–542. [Google Scholar] [PubMed]
- Oshaughnessy, J.A.; Blum, J.; Moiseyenko, V.; Jones, S.E.; Miles, D.; Bell, D.; Rosso, R.; Mauriac, L.; Osterwalder, B.; Burger, H.U.; et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann. Oncol. 2001, 12, 1247–1254. [Google Scholar] [CrossRef]
- Bajetta, E.; Procopio, G.; Celio, L.; Gattinoni, L.; Della Torre, S.; Mariani, L.; Catena, L.; Ricotta, R.; Longarini, R.; Zilembo, N.; et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J. Clin. Oncol. 2005, 23, 2155–2161. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, T.; Kim, K.; George, S.L. Admissible two-stage designs for phase II cancer clinical trials. Stat. Med. 2004, 23, 561–569. [Google Scholar] [CrossRef]
- Zamora, P.; Ad, M.M.; Calvo, L.; Jara, C.; Virizuela, J.A.; Yubero, A.; Chacon, J.I.; Mira, J.; Gonzalez Baron, M. Capecitabine (X) as single agent in elderly patients (p) with metastatic breast cancer (MBC). J. Clin. Oncol. 2005, 23, 843. [Google Scholar]
- Wang, Z.; Sun, X.; Feng, Y.; Wang, Y.; Zhang, L.; Wang, Y.; Li, Q. Dihydromyricetin reverses MRP2-induced multidrug resistance by preventing NF-κB-Nrf2 signaling in colorectal cancer cell. Phytomedicine 2021, 82, 153414. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Magdy, T.; Schwab, M.; Zanger, U.M. Role of ABC transporters in fluoropyrimidine-based chemotherapy response. Adv. Cancer Res. 2015, 125, 217–243. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Wamhoff, H.; Dzenis, J.; Hirota, K. Uracils: Versatile starting materials in heterocyclic synthesis. Adv. Inorg. Chem. 1992, 55, 129–259. [Google Scholar]
- Koehn, E.M.; Fleischmann, T.; Conrad, J.A.; Palfey, B.A.; Lesley, S.A.; Mathews, I.I.; Kohen, A. An unusual mechanism of thymidylate biosynthesis in organisms containing the thyX gene. Nature 2009, 458, 919–930. [Google Scholar] [CrossRef]
- Pięta, M.; Kędzia, J.; Kowalczyk, D.; Wojciechowski, J.; Wolf, W.M.; Janecki, T. Enantioselective synthesis of 5-methylidenedihydrouracils as potential anticancer agents. Tetrahedron 2019, 75, 2495–2505. [Google Scholar] [CrossRef]
- Długosz-Pokorska, A.; Drogosz, J.; Pięta, M.; Janecki, T.; Krajewska, U.; Mirowski, M.; Janecka, A. New uracil analogs with exocyclic methylidene group as potential anticancer agents. Anticancer Agents Med. Chem. 2020, 20, 359–368. [Google Scholar] [PubMed]
- Długosz-Pokorska, A.; Perlikowska, R.; Janecki, T.; Janecka, A. New uracil analog with exocyclic methylidene group can reverse resistance to Taxol in MCF-7 cancer cells. Biol. Targets Ther. 2023, 69, 69–83. [Google Scholar] [CrossRef]
- Długosz-Pokorska, A.; Janecki, T.; Janecka, A.; Gach-Janczak, K. New uracil analog as inhibitor/modulator of ABC transporters or/and NF-κB in taxol-resistant MCF-7/Tx cell line. J. Cancer Res. Clin. Oncol. 2024, 150, 328. [Google Scholar] [CrossRef]
- Kim, S.L.; Kim, S.H.; Trang, K.T.; Kim, I.H.; Lee, S.O.; Lee, S.T. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett. 2013, 335, 479–486. [Google Scholar] [CrossRef]
- Marchetti, P.; Galla, D.A.; Russo, F.P.; Ricevuto, E.; Flati, V.; Porzio, G. Apoptosis induced by oxaliplatin in human colon cancer HCT15 cell line. Anticancer Res. 2004, 24, 219–226. [Google Scholar]
- Sedeta, E.T.; Jobre, B.; Avezbakiyev, B. Breast cancer: Global patterns of incidence, mortality, and trends. J. Clin. Oncol. 2023, 41, 10528. [Google Scholar] [CrossRef]
- Shahlaei, M.; Asl, S.M.; Derakhshani, A.; Kurek, L.; Karges, J.; Macgregor, R. Platinum-based drugs in cancer treatment: Expanding horizons and overcoming resistance. J. Mol. Struct. 2023, 137366, 137366. [Google Scholar]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A. 5-Fluorouracil: A narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [PubMed]
- Winer, J.; Jung, C.K.S.; Shackel, I.; Williams, P.M. Development and validation of real-time quantitative reverse transcriptase–polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [PubMed]
- Liu, Q.; Yin, X.; Languino, L.R.; Altieri, D.C. Evaluation of drug combination effect using a bliss independence dose–response surface model. Stat. Biopharm. Res. 2018, 10, 112–122. [Google Scholar] [CrossRef]


| Gene | |||||
|---|---|---|---|---|---|
| Compounds | Bax | Bcl-2 | p21 | p53 | Caspase3 |
| U-359 | 2.9 ± 0.3 *** | 0.003 ± 0.00004 *** | 60.5 ± 0.3 *** | 1.2 ± 0.08 * | 10.0 ± 0.4 *** |
| Ox | 0.14 ± 0.008 *** | 2.4 ± 0.2 * | 0.021 ± 0.002 *** | 0.56 ± 0.004 *** | 1.3 ± 0.04 * |
| 5-FU | 0.17 ± 0.01 *** | 1.5 ± 0.004 *** | 0.31 ± 0.02 *** | 0.5 ± 0.001 *** | 0.3 ± 0.04 *** |
| Ox+U-359 | 2.5 ± 0.09 *** | 0.02 ± 0.0001 *** | 2.4 ± 0.2 *** | 19.3 ± 0.7 *** | 8.1 ± 0.4 *** |
| 5-FU+U-359 | 1.9 ± 0.2 *** | 0.14 ± 0.008 *** | 16 ± 0.1 *** | 13.9 ± 0.5 *** | 5.04 ± 0.3 *** |
| Gene | |||
|---|---|---|---|
| Compounds | ABCB1 | ABCG2 | NF-kb |
| U-359 | 0.01 ± 0.001 *** | 0.01 ± 0.002 *** | 0.8 ± 0.03 *** |
| Ox | 38.0 ± 4.8 *** | 5.3 ± 0.3 *** | 21.3 ± 0.2 *** |
| 5-FU | 1.28 ± 0.004 * | 1.45 ± 0.2 *** | 1.9 ± 0.02 *** |
| Ox+U-359 | 0.007 ± 0.0 *** | 0.16 ± 0.001 *** | 0.003 ± 0.0 *** |
| 5-FU+U-359 | 0.25 ± 0.01 *** | 0.03 ± 0.004 *** | 0.39 ± 0.04 *** |
| Protein | Combination | Synergy Index (SI) | Interpretation |
|---|---|---|---|
| ABCB1 | Ox+U-359 | - | U-359 did not influence the level of ABCB1 protein |
| 5-FU+U-359 | - | U-359 did not influence the level of ABCB1 protein | |
| ABCG2 | Ox+U-359 | 0.29 *** | U-359 partially reversed Ox-induced up-regulation of ABCG2, indicating moderate synergy (SI < 1) |
| 5-FU+U-359 | 0.07 *** | U-359 significantly reduced ABCG2 expression in the presence of 5-FU, demonstrating moderate synergy (SI < 1) | |
| NF-κB | Ox+U-359 | 0.69 *** | U-359 significantly reduced NF-κB expression in the presence of Ox, demonstrating moderate synergy (SI < 1) |
| 5-FU+U-359 | 0.17 *** | U-359 enhanced the efficacy of 5-FU by reducing NF-κB level, reflecting moderate synergy (SI < 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Długosz-Pokorska, A.; Janecki, T.; Janecka, A.; Gach-Janczak, K. Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis. Int. J. Mol. Sci. 2025, 26, 2964. https://doi.org/10.3390/ijms26072964
Długosz-Pokorska A, Janecki T, Janecka A, Gach-Janczak K. Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis. International Journal of Molecular Sciences. 2025; 26(7):2964. https://doi.org/10.3390/ijms26072964
Chicago/Turabian StyleDługosz-Pokorska, Angelika, Tomasz Janecki, Anna Janecka, and Katarzyna Gach-Janczak. 2025. "Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis" International Journal of Molecular Sciences 26, no. 7: 2964. https://doi.org/10.3390/ijms26072964
APA StyleDługosz-Pokorska, A., Janecki, T., Janecka, A., & Gach-Janczak, K. (2025). Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis. International Journal of Molecular Sciences, 26(7), 2964. https://doi.org/10.3390/ijms26072964







