Molecular Signatures of Aeroallergen Sensitization in Pediatric Populations: A Comparative Study Across Spanish Cities
Abstract
1. Introduction
2. Results
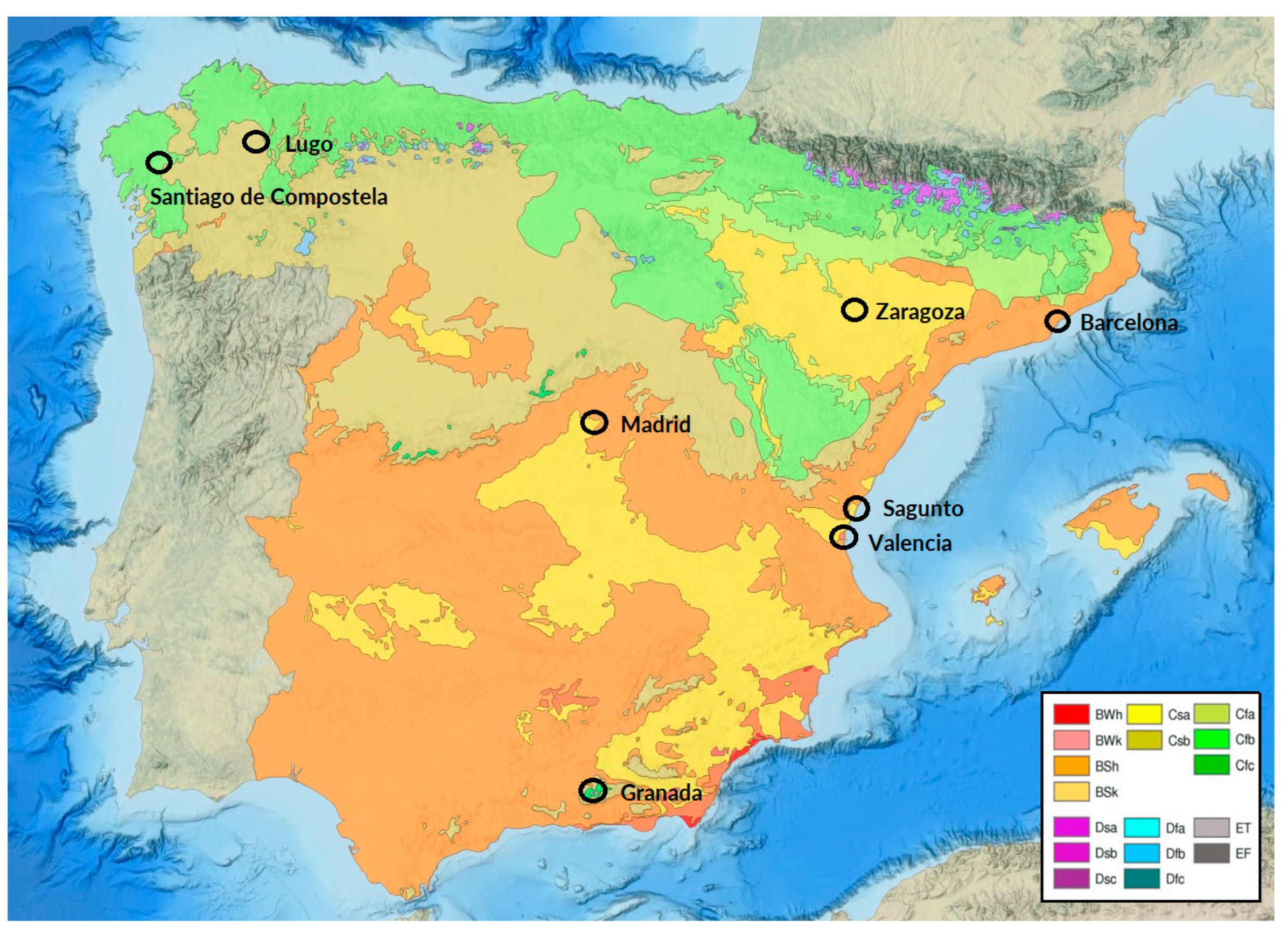
2.1. Demographic Features of Investigated Patients
2.2. Prevalence, sIgE Reactivity and Individual Molecular Profile According to Atopic Disease
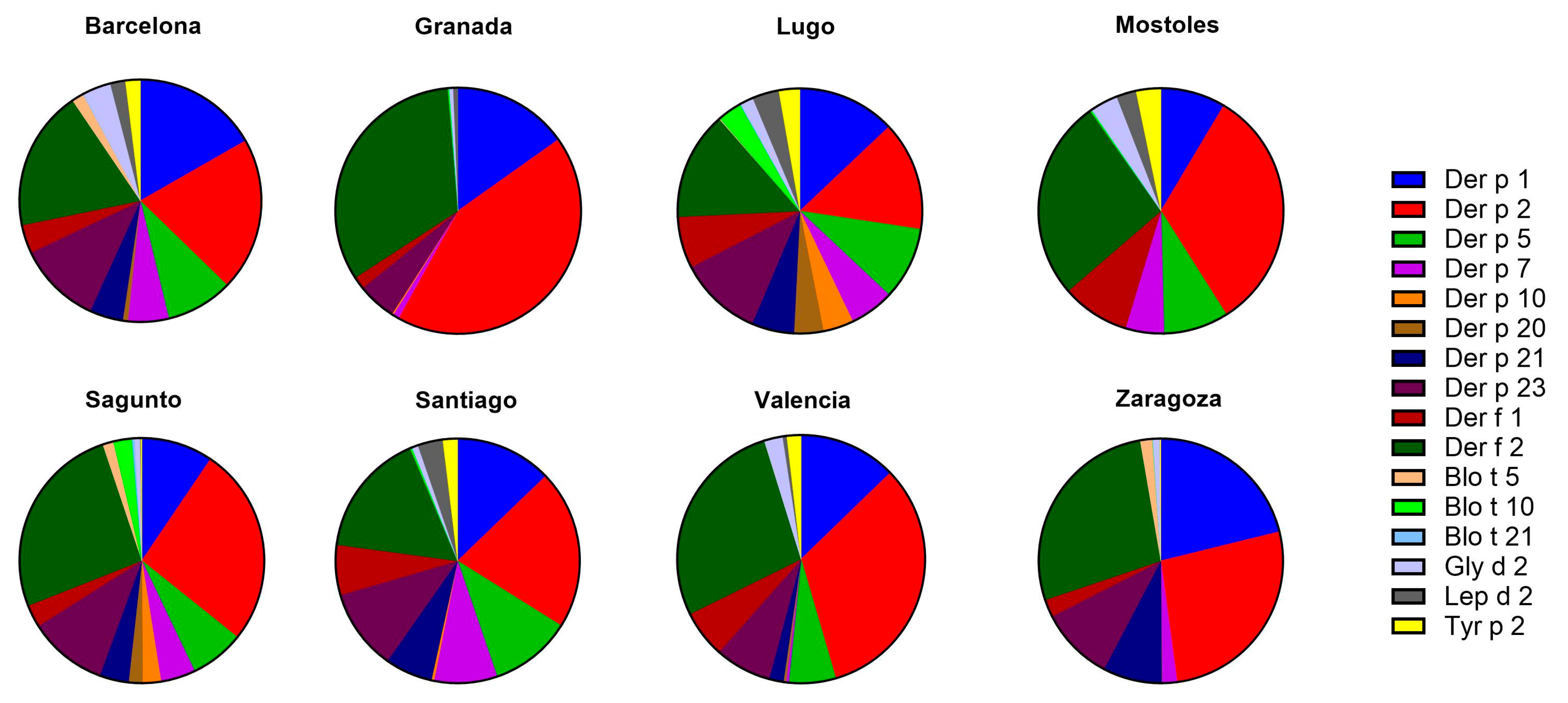
2.3. Mites
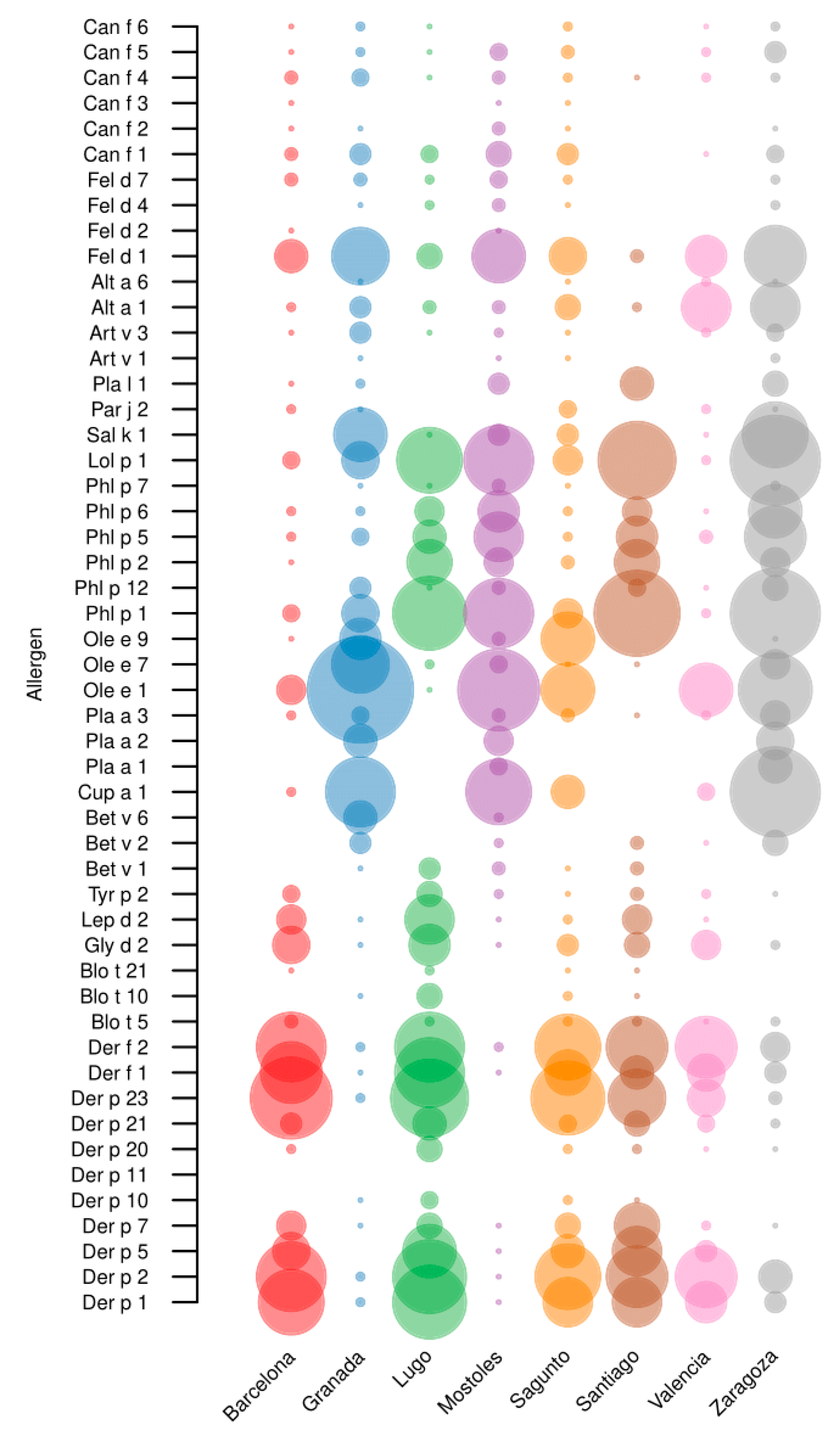
2.3.1. sIgE Reactivity Profiles by Geographic Location
2.3.2. Age and sIgE Reactivity Profiles
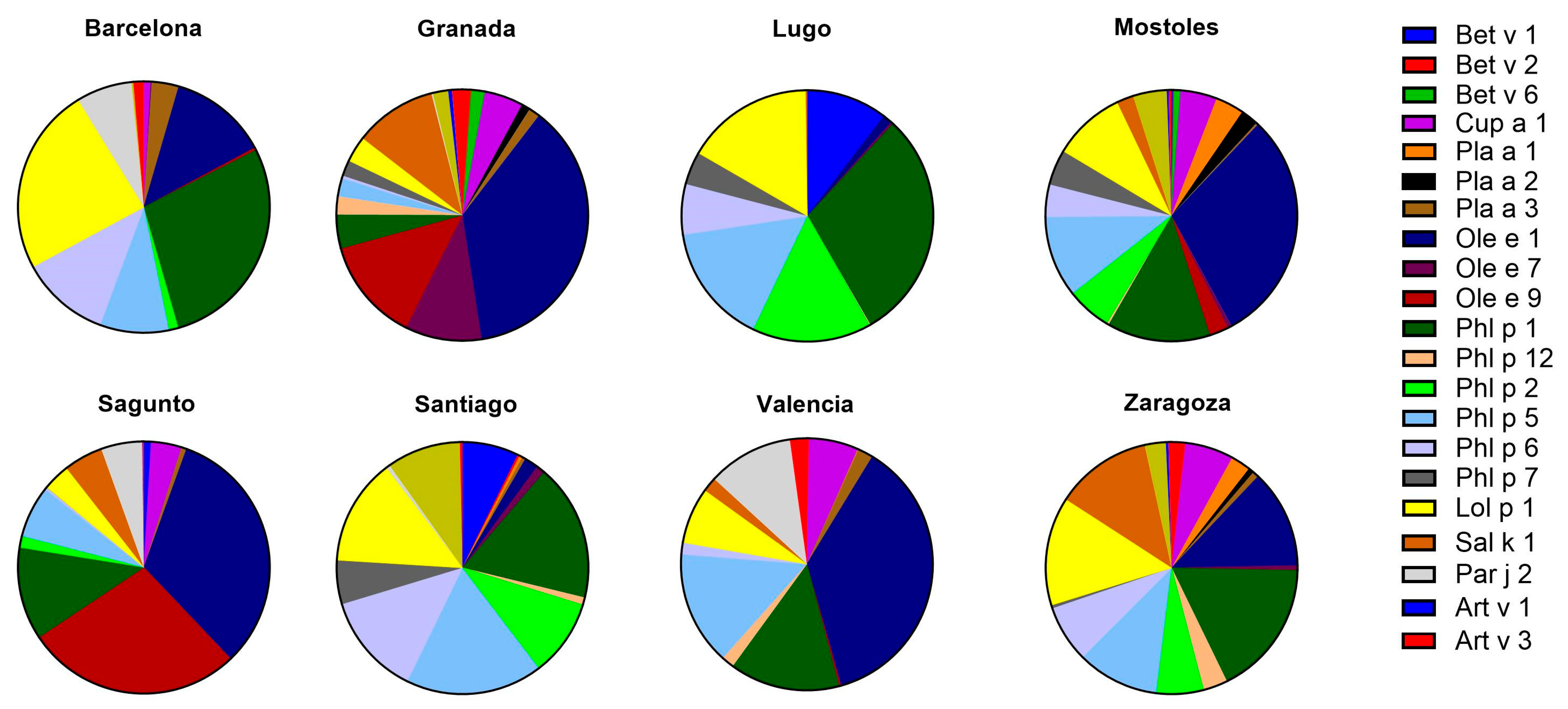
2.4. Pollens
2.4.1. sIgE Reactivity Profiles by Geographic Location
2.4.2. Age and sIgE Reactivity Profiles
2.5. Cat and Dog Epithelia
2.6. Molds
2.7. IgE Western Blot
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Skin Prick Test
4.3. Serological Analysis
4.4. ELISA Assay
4.5. IgE Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-de Paz, L.; Valdesoiro-Navarrete, L.; Roma, J.; Blat-Guimerà, E.; Benavent-Areu, J.; Bartra, J.; Sisó-Almirall, A. Prevalence and Impact of Asthma and Allergy on Daily Life, Health Outcomes and Use of Healthcare Services in Children: A Population-Based Study. Arch. Bronconeumol. 2023, 59, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Czarnobilska, M.; Dyga, W.; Czarnobilska, E. Trends in the Epidemiology of Allergic Diseases of the Airways in Children Growing Up in an Urban Agglomeration. J. Clin. Med. 2022, 11, 2188. [Google Scholar] [CrossRef]
- Zhang, L.; Akdis, C.A. Environmental Exposures Drive the Development of Allergic Diseases. Allergy 2024, 79, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Kiewiet, M.B.G.; Lupinek, C.; Vrtala, S.; Wieser, S.; Baar, A.; Kiss, R.; Kull, I.; Melén, E.; Wickman, M.; Porta, D.; et al. A Molecular Sensitization Map of European Children Reveals Exposome- and Climate-Dependent Sensitization Profiles. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 2007–2018. [Google Scholar] [CrossRef]
- Lejeune, S.; Bouazza, N.; Nicaise, P.R.; Jolaine, V.; Roditis, L.; Marguet, C.; Amat, F.; Berger, P.; Fayon, M.; Dubus, J.C.; et al. COBRAPed Cohort: Do Sensitization Patterns Differentiate Children with Severe Asthma from Those with a Milder Disease? Pediatr. Allergy Immunol. 2024, 35, e14112. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, A.; Yamamoto, Y.; Fukutomi, Y.; Shiraishi, Y.; Tanaka, J.; Oguma, T.; Taniguchi, M.; Nagai, T.; Asano, K. Sensitization Pattern to Environmental Allergens in a Japanese Population. J. Allergy Clin. Immunol. Glob. 2022, 2, 30. [Google Scholar] [CrossRef]
- Diaconu, I.D.; Gheorman, V.; Grigorie, G.A.; Gheonea, C.; Tenea-Cojan, T.S.; Mahler, B.; Voropanov, I.A.; Firoiu, M.C.; Pîrvu, A.S.; Popescu, A.B.; et al. A Comprehensive Look at the Development of Asthma in Children. Children 2024, 11, 581. [Google Scholar] [CrossRef]
- Portnoy, J.; Miller, J.D.; Williams, P.B.; Chew, G.L.; Miller, J.D.; Zaitoun, F.; Phipatanakul, W.; Kennedy, K.; Barnes, C.; Grimes, C.; et al. Environmental Assessment and Exposure Control of Dust Mites: A Practice Parameter. Ann. Allergy Asthma Immunol. 2013, 111, 465–507. [Google Scholar] [CrossRef]
- Abel-Fernández, E.; Martínez, M.J.; Galán, T.; Pineda, F. Going over Fungal Allergy: Alternaria alternata and Its Allergens. J. Fungi 2023, 9, 582. [Google Scholar] [CrossRef]
- Schramm, P.J.; Brown, C.L.; Saha, S.; Conlon, K.C.; Manangan, A.P.; Bell, J.E.; Hess, J.J. A Systematic Review of the Effects of Temperature and Precipitation on Pollen Concentrations and Season Timing, and Implications for Human Health. Int. J. Biometeorol. 2021, 65, 1615. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, K.S.; Oh, J.W. The Impact of Climate Change on Pollen Season and Allergic Sensitization to Pollens. Immunol. Allergy Clin. N. Am. 2021, 41, 97–109. [Google Scholar] [CrossRef]
- González-Díaz, S.N.; Arias-Cruz, A.; Macouzet-Sánchez, C.; Partida-Ortega, A.B. Impact of Air Pollution in Respiratory Allergic Diseases. Med. Univ. 2016, 18, 212–215. [Google Scholar] [CrossRef]
- Zhang, H.; Kaushal, A.; Merid, S.K.; Melén, E.; Pershagen, G.; Rezwan, F.I.; Han, L.; Ewart, S.; Arshad, S.H.; Karmaus, W.; et al. DNA Methylation and Allergic Sensitizations: A Genome-Scale Longitudinal Study during Adolescence. Allergy 2019, 74, 1166. [Google Scholar] [CrossRef] [PubMed]
- Dor-Wojnarowska, A.; Liebhart, J.; Miecielica, J.; Rabski, M.; Fal, A.; Samoliński, B.; Nittner-Marszalska, M. The Impact of Sex and Age on the Prevalence of Clinically Relevant Sensitization and Asymptomatic Sensitization in the General Population. Arch. Immunol. Ther. Exp. 2016, 65, 253. [Google Scholar] [CrossRef]
- Khurram, T.; Missous, G.; Van Panhuys, N.; Karim, M.Y. Aeroallergen Sensitivity Patterns in Gulf Countries: A Systematic Review. Clin. Transl. Allergy 2025, 15, e70033. [Google Scholar] [CrossRef]
- Sheehan, W.J.; Rangsithienchai, P.A.; Baxi, S.N.; Gardynski, A.; Bharmanee, A.; Israel, E.; Phipatanakul, W. Age-Specific Prevalence of Outdoor and Indoor Aeroallergen Sensitization in Boston. Clin. Pediatr. 2010, 49, 579–585. [Google Scholar] [CrossRef]
- Ogershok, P.R.; Warner, D.J.; Hogan, M.B.; Wilson, N.W. Prevalence of Pollen Sensitization in Younger Children Who Have Asthma. Allergy Asthma Proc. 2007, 28, 654–658. [Google Scholar] [CrossRef]
- Torres-Borrego, J.; Molina-Terán, A.B.; Montes-Mendoza, C. Prevalence and Associated Factors of Allergic Rhinitis and Atopic Dermatitis in Children. Allergol. Immunopathol. 2008, 36, 90–100. [Google Scholar] [CrossRef]
- González-Pérez, R.; Galván-Calle, C.A.; Galán, T.; Poza-Guedes, P.; Sánchez-Machín, I.; Enrique-Calderón, O.M.; Pineda, F. Molecular Signatures of Aeroallergen Sensitization in Respiratory Allergy: A Comparative Study Across Climate-Matched Populations. Int. J. Mol. Sci. 2024, 26, 284. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Moral, L.; Roig, M.; Garde, J.; Alós, A.; Toral, T.; Fuentes, M.J. Allergen Sensitization in Children with Asthma and Rhinitis: Marked Variations Related to Age and Microgeographical Factors. Allergol. Immunopathol. 2008, 36, 128–133. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, S.Y.; Kim, H.B.; Lee, E.; Hong, S.J. Environmental Changes, Microbiota, and Allergic Diseases. Allergy Asthma Immunol. Res. 2014, 6, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; D’Amato, G.; Annesi-Maesano, I. External Exposome and Allergic Respiratory and Skin Diseases. J. Allergy Clin. Immunol. 2018, 141, 846–857. [Google Scholar] [CrossRef] [PubMed]
- El clima en España|Clima (ESO). Available online: https://educativo.ign.es/atlas-didactico/clima-eso/el_clima_en_espaa.html (accessed on 27 February 2025).
- Organización Mundial de la Salud (OMS). WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021; pp. 1–360. [Google Scholar]
- Datos Oficiales Calidad del Aire 2023. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/atmosfera-y-calidad-del-aire/calidad-del-aire/evaluacion-datos/datos/datos-oficiales-2023.html (accessed on 27 February 2025).
- New Pollution Rules Come into Effect for Cleaner Air by 2030—European Commission. Available online: https://environment.ec.europa.eu/news/new-pollution-rules-come-effect-cleaner-air-2030-2024-12-10_en (accessed on 27 February 2025).
- Boquete, M.; Iraola, V.; Fernández-Caldas, E.; Arenas Villaroel, L.; Carballada, F.J.; De La González Cuesta, C.; López-Rico, M.R.; Núñez Orjales, R.; Parra, A.; Soto-Mera, M.T.; et al. House Dust Mite Species and Allergen Levels in Galicia, Spain: A Cross-Sectional, Multicenter, Comparative Study. J. Investig. Allergol. Clin. Immunol. 2006, 16, 169–176. [Google Scholar]
- Pagán, J.A.; Huertas, A.J.; Iraola, V.; Pinto, H.; Martínez, R.; Ramírez, M.; Martos, M.D.; Carnés, J. Mite Exposure in a Spanish Mediterranean Region. Allergol. Immunopathol. 2012, 40, 92–99. [Google Scholar] [CrossRef]
- Vrtala, S. Allergens from House Dust and Storage Mites. Allergo J. Int. 2022, 31, 267–271. [Google Scholar] [CrossRef]
- Villalta, D.; Scala, E.; Asero, R.; Da Re, M.; Conte, M.; Buzzulini, F. Evaluation and Predictive Value of IgE Responses toward a Comprehensive Panel of House Dust Mite Allergens Using a New Multiplex Assay: A Real-Life Experience on an Italian Population. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 117–122. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Wang, J.; Guo, Y.; Wang, Y.; Zhang, L.; Wu, Z.; Zhu, M.; Yang, X.; Xu, P.; et al. Serological Analysis of Allergic Components of House Dust Mite Provides More Insight in Epidemiological Characteristics and Clinical Symptom Development in North China. Front. Immunol. 2023, 14, 1083755. [Google Scholar] [CrossRef]
- Amaral AF, S.; Newson, R.B.; Abramson, M.J.; Antó, J.M.; Bono, R.; Corsico, A.G.; De Marco, R.; Demoly, P.; Forsberg, B.; Gislason, T.; et al. Changes in IgE Sensitization and Total IgE Levels over 20 Years of Follow-Up. J. Allergy Clin. Immunol. 2016, 137, 1788. [Google Scholar] [CrossRef]
- Lau, S.; Falkenhorst, G.; Weber, A.; Werthmann, I.; Lind, P.; Buettner-Goetz, P.; Wahn, U. High Mite-Allergen Exposure Increases the Risk of Sensitization in Atopic Children and Young Adults. J. Allergy Clin. Immunol. 1989, 84, 718–725. [Google Scholar] [CrossRef]
- Jarvinen-Seppo, K.; Lajoie, S.; Wojno, E.T.; León, B. Understanding the Development of Th2 Cell-Driven Allergic Airway Disease in Early Life. Front. Allergy 2023, 3, 1080153. [Google Scholar] [CrossRef]
- Jenmalm, M.C. Childhood Immune Maturation and Allergy Development: Regulation by Maternal Immunity and Microbial Exposure. Am. J. Reprod. Immunol. 2011, 66 (Suppl. S1), 75–80. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Skevaki, C. Early Life Microbial Exposures and Allergy Risks: Opportunities for Prevention. Nat. Rev. Immunol. 2020, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Bønnelykke, K.; Stokholm, J. Immune-Mediated Diseases and Microbial Exposure in Early Life. Clin. Exp. Allergy 2014, 44, 475–481. [Google Scholar] [CrossRef]
- Nunes, I.; Loureiro, G.; Tavares, B.; Todo-Bom, A.; Cunha, R. Sensitization to Genuine Markers of Timothy Grass Pollen (Phleum Pratense) in the North-Central Region of Portugal. Eur. Ann. Allergy Clin. Immunol. 2024, 56, 65–70. [Google Scholar] [CrossRef]
- Tagliaferro, S.; Adani, M.; Pepe, N.; Briganti, G.; D’Isidoro, M.; Bonini, M.; Piersanti, A.; Finardi, S.; Marchetti, P.; Domenichini, F.; et al. The Impact of the Spatial Resolution of Vegetation Cover on the Prediction of Airborne Pollen Concentrations over Northern Italy. Agric. For. Meteorol. 2024, 355, 110153. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M.; Boyd, S.D.; Sampath, V.; Galli, S.J.; Nadeau, K.C. Allergy: Mechanistic Insights into New Methods of Prevention and Therapy. Sci. Transl. Med. 2023, 15, eadd2563. [Google Scholar] [CrossRef]
- Alarcón, M.; Periago, C.; Pino, D.; Mazón, J.; del Casas-Castillo, M.C.; Ho-Zhang, J.J.; De Linares, C.; Rodríguez-Solà, R.; Belmonte, J. Potential Contribution of Distant Sources to Airborne Betula Pollen Levels in Northeastern Iberian Peninsula. Sci. Total Environ. 2022, 818, 151827. [Google Scholar] [CrossRef]
- Sánchez, P.; Vélez-Del-burgo, A.; Suñén, E.; Martínez, J.; Postigo, I. Fungal Allergen and Mold Allergy Diagnosis: Role and Relevance of Alternaria Alternata Alt a 1 Protein Family. J. Fungi 2022, 8, 277. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martínez, J. Alternaria alternata Allergens: Markers of Exposure, Phylogeny and Risk of Fungi-Induced Respiratory Allergy. Environ. Int. 2016, 89–90, 71–80. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Probst, G.; Kajava, A.V.; Oberkofler, H.; Susani, M.; Crameri, R.; Ferreira, F.; Ebner, C.; Breitenbach, M. IgE-Binding Epitopes of Enolases, a Class of Highly Conserved Fungal Allergens. J. Allergy Clin. Immunol. 2000, 106, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Mo, E.K.; Lee, J.Y.; Kim, J.H.; Kim, C.H.; Hyun, I.G.; Choi, J.H. Association Between Pet Ownership and the Sensitization to Pet Allergens in Adults With Various Allergic Diseases. Allergy Asthma Immunol. Res. 2013, 5, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Satyaraj, E.; Wedner, H.J.; Bousquet, J. Keep the Cat, Change the Care Pathway: A Transformational Approach to Managing Fel d 1, the Major Cat Allergen. Allergy 2019, 74 (Suppl. S107), 5–17. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, C.; Egmar, A.C.; Hedlin, G.; Lundqvist, M.; Nordvall, S.L.; Pershagen, G.; Svartengren, M.; Van Hage-Hamsten, M.; Wickman, M. Direct and Indirect Exposure to Pets—Risk of Sensitization and Asthma at 4 Years in a Birth Cohort. Clin. Exp. Allergy 2003, 33, 1190–1197. [Google Scholar] [CrossRef]
- Cacheiro-Llaguno, C.; Mösges, R.; Calzada, D.; González-de la Fuente, S.; Quintero, E.; Carnés, J. Polysensitisation Is Associated with More Severe Symptoms: The Reality of Patients with Allergy. Clin. Exp. Allergy 2024, 54, 607–620. [Google Scholar] [CrossRef]
- Bonnet, B.; Messaoudi, K.; Jacomet, F.; Michaud, E.; Fauquert, J.L.; Caillaud, D.; Evrard, B. An Update on Molecular Cat Allergens: Fel d 1 and What Else? Chapter 1: Fel d 1, the Major Cat Allergen. Allergy Asthma Clin. Immunol. 2018, 14, 14. [Google Scholar] [CrossRef]
- Konradsen, J.R.; Fujisawa, T.; Van Hage, M.; Hedlin, G.; Hilger, C.; Kleine-Tebbe, J.; Matsui, E.C.; Roberts, G.; Rönmark, E.; Platts-Mills, T.A.E. Allergy to Furry Animals: New Insights, Diagnostic Approaches, and Challenges. J. Allergy Clin. Immunol. 2015, 135, 616–625. [Google Scholar] [CrossRef]
- Dávila, I.; Domínguez-Ortega, J.; Navarro-Pulido, A.; Alonso, A.; Antolín-Amerigo, D.; González-Mancebo, E.; Martín-García, C.; Núñez-Acevedo, B.; Prior, N.; Reche, M.; et al. Consensus Document on Dog and Cat Allergy. Allergy 2018, 73, 1206–1222. [Google Scholar] [CrossRef]
- Özuygur Ermis, S.S.; Borres, M.P.; Basna, R.; Ekerljung, L.; Malmhäll, C.; Goksör, E.; Wennergren, G.; Rådinger, M.; Lötvall, J.; Lundbäck, B.; et al. Sensitization to Molecular Dog Allergens in an Adult Population: Results from the West Sweden Asthma Study. Clin. Exp. Allergy 2023, 53, 88–104. [Google Scholar] [CrossRef]
- Obando, S.U.; Domínguez, J.S. Clinical Impact of Molecular Diagnosis in Dog Allergy. Clin. Transl. Allergy 2014, 4 (Suppl. S2), 52. [Google Scholar] [CrossRef]
- Yoon, Y.; Chun, Y.; Zhang, L.; Grishina, G.; Grishin, A.; Bunyavanich, S. Nasal Transcriptomics Reveals Molecular Mechanisms of Aeroallergen Sensitization and Allergic Rhinitis. J. Allergy Clin. Immunol. 2025, 155, AB271. [Google Scholar]
- D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Al-Enezi, F.; Al-Muhsen, S.; Ansotegui, I.; Baena-Cagnani, C.E.; et al. Meteorological Conditions, Climate Change, New Emerging Factors, and Asthma and Related Allergic Disorders. A Statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.D.; Barney, T.P.; Crandall, J.H.; Brown, M.A.; Westover, T.R.; Paulson, S.M.; Smith, M.S.; Weber, K.S. Prevalence of House Dust Mite Allergens in Low-Income Homes with Evaporative Coolers in a Semiarid Climate. Arch. Environ. Occup. Health 2018, 73, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Subiza, J.; Jerezb, M.; Jiméneza, J.A.; Narganes, M.J.; Cabrera, M.; Varela, S.; Subiza, E. Allergenic Pollen and Pollinosis in Madrid. J. Allergy Clin. Immunol. 1995, 96, 15–23. [Google Scholar] [CrossRef]
- Subiza Garrido-Lestache, J. Allergenic Pollens in Spain. Allergol. Immunopathol. 2004, 32, 121–124. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Rook, G.A.W.; Scott, E.A.; Shanahan, F.; Stanwell-Smith, R.; Turner, P. Time to Abandon the Hygiene Hypothesis: New Perspectives on Allergic Disease, the Human Microbiome, Infectious Disease Prevention and the Role of Targeted Hygiene. Perspect. Public Health 2016, 136, 213–224. [Google Scholar] [CrossRef]
- Miller, R.L.; Peden, D.B. Environmental Effects on Immune Responses in Patients with Atopy and Asthma. J. Allergy Clin. Immunol. 2014, 134, 1001–1008. [Google Scholar] [CrossRef]
- Inmunotek, Allergy and Immunology|Pharmaceutical Company. Available online: https://www.inmunotek.com/en/ (accessed on 27 February 2025).
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The Skin Prick Test—European Standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Antunes, J.; Borrego, L.; Romeira, A.; Pinto, P. Skin Prick Tests and Allergy Diagnosis. Allergol. Immunopathol. 2009, 37, 155–164. [Google Scholar] [CrossRef]
- Macro Array Diagnostics. Available online: https://www.macroarraydx.com/es (accessed on 27 February 2025).
- Jakob, T.; Forstenlechner, P.; Matricardi, P.; Kleine-Tebbe, J. Molecular Allergy Diagnostics Using Multiplex Assays: Methodological and Practical Considerations for Use in Research and Clinical Routine: Part 21 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 320–332. [Google Scholar] [CrossRef]
- Nösslinger, H.; Mair, E.; Oostingh, G.J.; Ahlgrimm-Siess, V.; Ringauf, A.; Lang, R. Multiplex Assays in Allergy Diagnosis: Allergy Explorer 2 versus ImmunoCAP ISAC E112i. Diagnostics 2024, 14, 976. [Google Scholar] [CrossRef]
- Greiner Bio-One|Making a Difference. Available online: https://www.gbo.com/en-int/ (accessed on 27 February 2025).
- HRP Anti-Human IgE Fc, B3102E8|SouthernBiotech. Available online: https://www.southernbiotech.com/mouse-anti-human-ige-fc-hrp-b3102e8-9160-05 (accessed on 27 February 2025).
- Thermo Fisher Scientific—ES. Available online: https://www.thermofisher.com/es/es/home.html (accessed on 27 February 2025).
- Leading Life Science Research & Clinical Diagnostics|Bio-Rad. Available online: https://www.bio-rad.com/ (accessed on 27 February 2025).
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Home—GraphPad. Available online: https://www.graphpad.com/ (accessed on 27 February 2025).
- R: The R Foundation. Available online: https://www.r-project.org/foundation/ (accessed on 27 February 2025).

| Barcelona, Cataluña | Granada, Andalucía | Lugo, Galicia | Móstoles, Madrid | Sagunto, Valencia | Santiago, Galicia | Valencia, Valencia | Zaragoza, Aragón | |
|---|---|---|---|---|---|---|---|---|
| n = 236 | 27 | 30 | 30 | 29 | 30 | 30 | 30 | 30 |
| Age (y.o.) median | 9, 15 | 10, 23 | 10, 33 | 10, 34 | 9, 60 | 9, 33 | 10, 27 | 10, 60 |
| <11 y.o. (n = 111) | 18 | 15 | 15 | 15 | 17 | 19 | 15 | 15 |
| ≥11 y.o. (n = 125) | 9 | 15 | 15 | 14 | 13 | 11 | 15 | 15 |
| Sex (F/M) | 9/18 | 16/14 | 12/18 | 11/18 | 10/20 | 9/21 | 11/19 | 10/20 |
| Allergic Rhinitis (n = 205, %) | 23 (85.2) | 28 (93.3) | 29 (96.7) | 29 (100) | 30 (100.0) | 30 (100.0) | 30 (100.0) | 29 (96.7) |
| Allergic Asthma (n = 105, %) | 15 (55.6) | 23 (76.7) | 15 (50.0) | 15 (51.7) | 17 (56.7) | 10 (33.3) | 9 (30.0) | 16 (53.3) |
| SPT+ to any aeroallergen (%) | 27 (100.0) | 28 (93.3) | 30 (100.0) | 29 (100.0) | 30 (100.0) | 30 (100.0) | 30 (100.0) | 30 (100.0) |
| Total IgE (IU/mL) median | 776.15 | 390.66 | 631.91 | 406.76 | 463.51 | 809.37 | 463.23 | 862.69 |
| Positive SPT (n = 236) | Barcelona, Cataluña | Granada, Andalucía | Lugo, Galicia | Móstoles, Madrid | Sagunto, Valencia | Santiago, Galicia | Valencia, Valencia | Zaragoza, Aragón |
|---|---|---|---|---|---|---|---|---|
| HDM and/or SM (%) | 26 (96.3) | 2 (6.7) | 28 (93.3) | 7 (24.1) | 20 (66.7) | 19 (63.3) | 22 (73.3) | 10 (33.3) |
| Cat and/or dog dander (%) | 9 (33.3) | 14 (46.7) | 9 (30.0) | 16 (55.2) | 15 (50.0) | 9 (30.0) | 8 (26.7) | 24 (80.0) |
| Pollen (%) | 15 (13.1) | 28 (15.8) | 16 (53.3) | 28 (96.6) | 23 (76.7) | 23 (76.7) | 16 (53.3) | 30 (100.0) |
| Molds (%) | 2 (7.4) | 3 (2.2) | 4 (13.3) | 2 (6.9) | 4 (13.3) | 2 (6.7) | 9 (30.0) | 14 (46.7) |
| Allergen | Barcelona | Granada | Lugo | Mostoles | Sagunto | Santiago | Valencia | Zaragoza |
|---|---|---|---|---|---|---|---|---|
| Der p 1 | 14.9 ± 18.7 (16) | 0.9 ± 4.5 (2) | 15.2 ± 20.0 (18) | 0.4 ± 2.3 (1) | 6.7 ± 12.6 (12) | 11.0 ± 17.1 (14) | 7.4 ± 13.6 (10) | 4.5 ± 11.6 (5) |
| Der p 2 | 18.2 ± 18.7 (17) | 2.6 ± 9.9 (2) | 16.9 ± 21.6 (16) | 1.6 ± 8.6 (1) | 18.5 ± 21.0 (16) | 18.1 ± 21.1 (18) | 18.9 ± 22.3 (15) | 5.6 ± 12.3 (8) |
| Der p 5 | 8.0 ± 14.3 (9) | 0.0 ± 0.0 (0) | 11.3 ± 19.2 (12) | 0.4 ± 2.3 (1) | 5.1 ± 11.6 (8) | 9.3 ± 16.9 (11) | 3.5 ± 9.4 (5) | 0.0 ± 0.0 (0) |
| Der p 7 | 4.9 ± 11.5 (7) | 0.0 ± 0.3 (1) | 6.9 ± 15.4 (6) | 0.2 ± 1.3 (1) | 3.3 ± 8.2 (6) | 7.2 ± 15.2 (7) | 0.2 ± 0.9 (2) | 0.4 ± 2.3 (1) |
| Der p 10 | 0.0 ± 0.0 (0) | 0.0 ± 0.1 (1) | 4.7 ± 12.8 (4) | 0.0 ± 0.0 (0) | 1.7 ± 9.1 (2) | 0.3 ± 1.2 (2) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Der p 11 | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Der p 20 | 0.6 ± 2.2 (2) | 0.0 ± 0.0 (0) | 4.6 ± 11.8 (5) | 0.0 ± 0.0 (0) | 1.3 ± 5.7 (2) | 0.0 ± 0.0 (0) | 0.2 ± 1.1 (1) | 0.0 ± 0.1 (1) |
| Der p 21 | 4.0 ± 10.8 (5) | 0.0 ± 0.0 (0) | 6.6 ± 13.1 (8) | 0.0 ± 0.0 (0) | 2.7 ± 9.6 (4) | 5.4 ± 14.5 (6) | 1.1 ± 4.0 (4) | 1.6 ± 7.7 (2) |
| Der p 23 | 9.9 ± 11.6 (20) | 0.3 ± 1.3 (2) | 12.9 ± 17.8 (16) | 0.0 ± 0.0 (0) | 7.4 ± 12.7 (18) | 9.2 ± 13.3 (20) | 4.3 ± 7.7 (9) | 2.1 ± 7.1 (3) |
| Der f 1 | 3.4 ± 8.2 (15) | 0.1 ± 0.5 (1) | 8.0 ± 14.0 (12) | 0.4 ± 2.4 (1) | 2.1 ± 3.4 (11) | 5.7 ± 11.5 (10) | 3.6 ± 7.4 (9) | 0.5 ± 1.4 (5) |
| Der f 2 | 16.7 ± 18.5 (17) | 2.0 ± 7.9 (2) | 16.8 ± 21.3 (17) | 1.3 ± 6.9 (2) | 18.2 ± 21.5 (16) | 14.2 ± 18.6 (18) | 15.9 ± 19.7 (15) | 5.8 ± 13.3 (7) |
| Blo t 5 | 1.4 ± 6.0 (3) | 0.0 ± 0.0 (0) | 0.2 ± 0.6 (2) | 0.0 ± 0.0 (0) | 1.0 ± 5.1 (2) | 0.0 ± 0.1 (1) | 0.0 ± 0.1 (1) | 0.3 ± 1.7 (2) |
| Blo t 10 | 0.0 ± 0.0 (0) | 0.0 ± 0.1 (1) | 3.7 ± 10.3 (4) | 0.0 ± 0.0 (0) | 1.7 ± 9.1 (2) | 0.2 ± 1.0 (1) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Blo t 21 | 0.1 ± 0.5 (1) | 0.0 ± 0.0 (0) | 0.1 ± 0.5 (2) | 0.0 ± 0.0 (0) | 0.3 ± 1.4 (1) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Gly d 2 | 3.3 ± 10.0 (9) | 0.0 ± 0.1 (1) | 2.0 ± 6.2 (7) | 0.2 ± 1.0 (1) | 0.5 ± 1.3 (5) | 0.7 ± 1.9 (6) | 1.4 ± 5.0 (7) | 0.2 ± 0.7 (2) |
| Lep d 2 | 1.8 ± 5.4 (7) | 0.0 ± 0.2 (1) | 4.2 ± 11.5 (9) | 0.1 ± 0.6 (1) | 0.1 ± 0.3 (2) | 2.9 ± 9.0 (7) | 0.3 ± 1.7 (1) | 0.0 ± 0.1 (0) |
| Tyr p 2 | 1.8 ± 6.8 (4) | 0.0 ± 0.0 (0) | 3.2 ± 11.2 (5) | 0.2 ± 0.6 (2) | 0.1 ± 0.5 (1) | 1.7 ± 7.3 (5) | 1.1 ± 4.3 (2) | 0.0 ± 0.1 (1) |
| Allergen | Barcelona | Granada | Lugo | Mostoles | Sagunto | Santiago | Valencia | Zaragoza |
|---|---|---|---|---|---|---|---|---|
| Bet v 1 | 0.0 ± 0.0 (0) | 0.0 ± 0.2 (1) | 4.1 ± 11.9 (5) | 0.2 ± 0.7 (3) | 0.3 ± 1.9 (1) | 4.3 ± 13.6 (4) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Bet v 2 | 0.0 ± 0.0 (0) | 0.7 ± 3.3 (5) | 0.0 ± 0.0 (0) | 0.1 ± 0.2 (2) | 0.0 ± 0.0 (0) | 0.2 ± 0.5 (4) | 0.0 ± 0.1 (1) | 1.9 ± 5.1 (6) |
| Bet v 6 | 0.0 ± 0.0 (0) | 1.2 ± 2.7 (8) | 0.0 ± 0.0 (0) | 0.7 ± 3.5 (2) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Cup a 1 | 0.1 ± 0.2 (2) | 3.2 ± 6.0 (17) | 0.0 ± 0.0 (0) | 3.6 ± 4.7 (16) | 1.5 ± 4.0 (8) | 0.0 ± 0.0 (0) | 0.6 ± 2.2 (4) | 6.5 ± 11.0 (22) |
| Pla a 1 | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 2.9 ± 10.0 (4) | 0.0 ± 0.0 (0) | 0.2 ± 1.1 (1) | 0.0 ± 0.0 (0) | 2.6 ± 7.8 (8) |
| Pla a 2 | 0.0 ± 0.0 (0) | 0.7 ± 1.9 (8) | 0.0 ± 0.0 (0) | 1.8 ± 7.6 (7) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.8 ± 1.7 (9) |
| Pla a 3 | 0.3 ± 1.1 (2) | 0.9 ± 4.4 (4) | 0.0 ± 0.0 (0) | 0.2 ± 0.5 (3) | 0.2 ± 1.0 (3) | 0.3 ± 1.0 (2) | 0.2 ± 0.8 (2) | 0.8 ± 2.1 (7) |
| Ole e 1 | 1.1 ± 3.7 (7) | 24.4 ± 19.8 (26) | 0.5 ± 2.5 (1) | 23.4 ± 22.0 (20) | 11.9 ± 18.8 (13) | 1.1 ± 5.5 (1) | 3.7 ± 9.2 (13) | 13.2 ± 16.4 (18) |
| Ole e 7 | 0.0 ± 0.0 (0) | 6.5 ± 12.3 (14) | 0.1 ± 0.6 (2) | 0.4 ± 1.5 (4) | 0.0 ± 0.1 (1) | 0.7 ± 2.3 (4) | 0.0 ± 0.0 (0) | 0.6 ± 1.4 (7) |
| Ole e 9 | 0.0 ± 0.1 (1) | 8.8 ± 14.3 (10) | 0.0 ± 0.0 (0) | 1.9 ± 7.2 (3) | 10.2 ± 17.3 (13) | 0.0 ± 0.0 (0) | 0.0 ± 0.1 (0) | 0.1 ± 0.2 (1) |
| Phl p 1 | 2.5 ± 8.0 (4) | 2.8 ± 7.9 (9) | 11.7 ± 17.0 (18) | 10.3 ± 15.3 (17) | 4.4 ± 11.3 (7) | 10.4 ± 18.1 (13) | 1.4 ± 6.7 (2) | 18.2 ± 18.8 (22) |
| Phl p 12 | 0.0 ± 0.0 (0) | 1.5 ± 6.4 (5) | 0.1 ± 0.3 (1) | 0.2 ± 0.7 (3) | 0.0 ± 0.0 (0) | 0.6 ± 1.4 (4) | 0.2 ± 0.8 (1) | 3.3 ± 8.7 (6) |
| Phl p 2 | 0.1 ± 0.5 (1) | 0.0 ± 0.0 (0) | 6.0 ± 11.7 (11) | 4.5 ± 11.2 (7) | 0.5 ± 2.2 (3) | 5.9 ± 12.7 (10) | 0.0 ± 0.0 (0) | 6.3 ± 14.7 (7) |
| Phl p 5 | 0.8 ± 2.9 (2) | 1.6 ± 6.9 (4) | 6.1 ± 13.6 (8) | 8.2 ± 14.2 (12) | 2.5 ± 9.7 (2) | 10.5 ± 19.3 (8) | 1.5 ± 7.6 (3) | 11.0 ± 16.5 (15) |
| Phl p 6 | 1.0 ± 4.9 (2) | 0.2 ± 0.8 (2) | 2.5 ± 9.4 (7) | 3.2 ± 8.6 (10) | 0.1 ± 0.3 (2) | 7.8 ± 16.7 (7) | 0.2 ± 0.8 (1) | 7.6 ± 14.2 (13) |
| Phl p 7 | 0.0 ± 0.0 (0) | 1.3 ± 7.4 (1) | 1.7 ± 9.1 (1) | 3.6 ± 11.0 (3) | 0.0 ± 0.1 (1) | 3.3 ± 12.3 (2) | 0.0 ± 0.0 (0) | 0.4 ± 1.7 (2) |
| Lol p 1 | 2.2 ± 6.3 (4) | 2.2 ± 6.5 (9) | 6.4 ± 10.8 (16) | 7.3 ± 12.3 (17) | 1.3 ± 3.2 (7) | 8.1 ± 14.8 (11) | 0.7 ± 3.4 (2) | 14.5 ± 14.2 (22) |
| Sal k 1 | 0.0 ± 0.0 (0) | 7.0 ± 13.4 (13) | 0.1 ± 0.4 (1) | 1.7 ± 5.6 (5) | 1.9 ± 6.1 (5) | 0.0 ± 0.0 (0) | 0.2 ± 0.9 (1) | 13.0 ± 17.5 (16) |
| Par j 2 | 0.6 ± 3.2 (2) | 0.1 ± 0.6 (1) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 1.9 ± 7.7 (4) | 0.3 ± 1.6 (1) | 1.1 ± 4.8 (2) | 0.0 ± 0.1 (1) |
| Pla l 1 | 0.0 ± 0.1 (1) | 1.3 ± 6.7 (2) | 0.0 ± 0.0 (0) | 3.4 ± 10.0 (5) | 0.0 ± 0.0 (0) | 5.6 ± 13.8 (8) | 0.0 ± 0.0 (0) | 2.8 ± 7.6 (6) |
| Art v 1 | 0.0 ± 0.0 (0) | 0.3 ± 1.7 (1) | 0.0 ± 0.0 (0) | 0.2 ± 1.1 (1) | 0.0 ± 0.1 (1) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.4 ± 1.7 (2) |
| Art v 3 | 0.1 ± 0.6 (1) | 0.9 ± 2.5 (5) | 0.0 ± 0.1 (1) | 0.3 ± 1.1 (2) | 0.1 ± 0.3 (1) | 0.2 ± 0.9 (2) | 0.2 ± 1.0 (2) | 0.4 ± 1.2 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cañavate, A.; Mesa-Del-Castillo, M.; Carballada, F.; Rivas-Juesas, C.; Porto, J.Á.; Blasco, C.; Álvaro-Lozano, M.; Lozano, J.; Manrique, J.A.; Martínez, M.J.; et al. Molecular Signatures of Aeroallergen Sensitization in Pediatric Populations: A Comparative Study Across Spanish Cities. Int. J. Mol. Sci. 2025, 26, 2963. https://doi.org/10.3390/ijms26072963
Martínez-Cañavate A, Mesa-Del-Castillo M, Carballada F, Rivas-Juesas C, Porto JÁ, Blasco C, Álvaro-Lozano M, Lozano J, Manrique JA, Martínez MJ, et al. Molecular Signatures of Aeroallergen Sensitization in Pediatric Populations: A Comparative Study Across Spanish Cities. International Journal of Molecular Sciences. 2025; 26(7):2963. https://doi.org/10.3390/ijms26072963
Chicago/Turabian StyleMartínez-Cañavate, Ana, María Mesa-Del-Castillo, Francisco Carballada, Cristina Rivas-Juesas, José Ángel Porto, Cristina Blasco, Montserrat Álvaro-Lozano, Jaime Lozano, Julián Andrés Manrique, María José Martínez, and et al. 2025. "Molecular Signatures of Aeroallergen Sensitization in Pediatric Populations: A Comparative Study Across Spanish Cities" International Journal of Molecular Sciences 26, no. 7: 2963. https://doi.org/10.3390/ijms26072963
APA StyleMartínez-Cañavate, A., Mesa-Del-Castillo, M., Carballada, F., Rivas-Juesas, C., Porto, J. Á., Blasco, C., Álvaro-Lozano, M., Lozano, J., Manrique, J. A., Martínez, M. J., Galán, T., Domingo, G., Marín, L., Vega, P., López-Rodríguez, R., Galán, P. S., Aliaga, Y., Pineda, F., & Tortajada-Girbés, M. (2025). Molecular Signatures of Aeroallergen Sensitization in Pediatric Populations: A Comparative Study Across Spanish Cities. International Journal of Molecular Sciences, 26(7), 2963. https://doi.org/10.3390/ijms26072963







