PI3K and PINK1 Immunoexpression as Predictors of Survival in Patients Undergoing Resection of Brain Metastases from Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Features of Cases
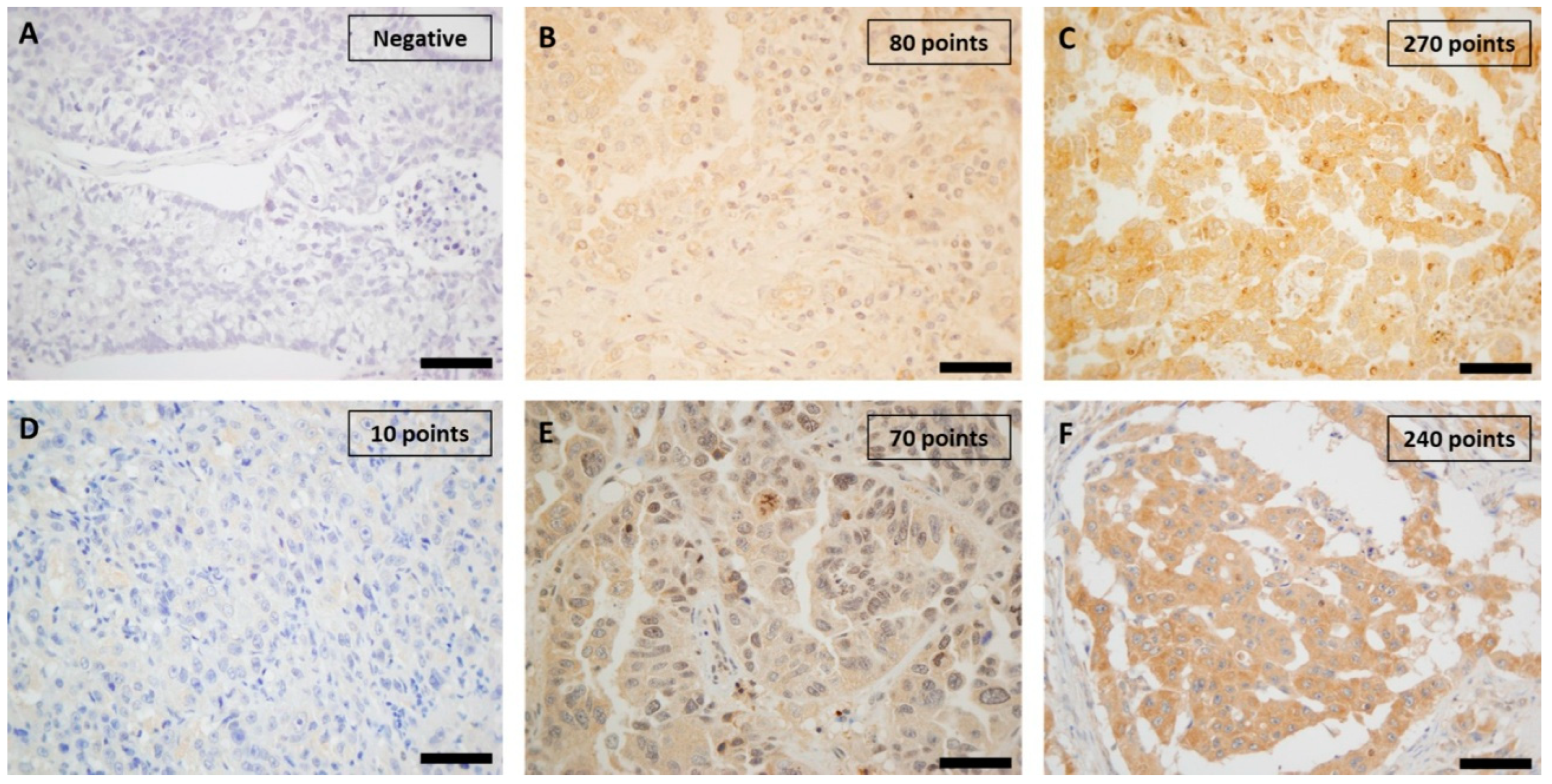
2.2. Immunohistochemical Study of Proteins
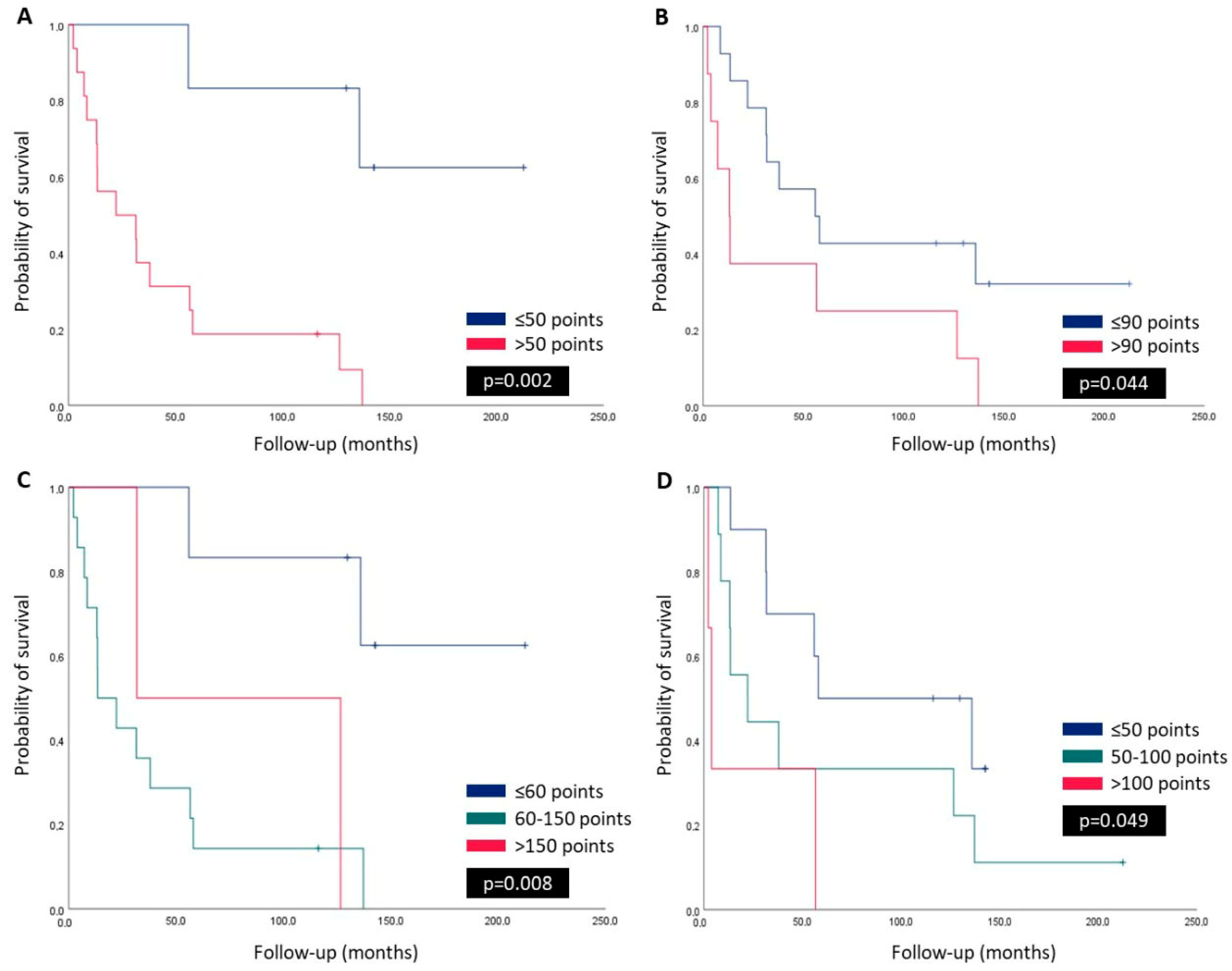
2.3. Survival Curves, and Univariate and Multivariate Analysis
3. Discussion
4. Materials and Method
4.1. Patients and Samples
4.2. Histopathologic Evaluation
4.3. Tissue Microarray Construction
4.4. Immunohistochemistry
4.5. Immunohistochemistry Assessment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Polanco, D.; Pinilla, L.; Gracia-Lavedan, E.; Mas, A.; Bertran, S.; Fierro, G.; Seminario, A.; Gómez, S.; Barbé, F. Prognostic value of symptoms at lung cancer diagnosis: A three-year observational study. J. Thorac. Dis. 2021, 13, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, Y.; Rumgay, H.; Morgan, E.; Langselius, O.; Vignat, J.; Colombet, M.; Bray, F. Estimated worldwide variation and trends in incidence of lung cancer by histological subtype in 2022 and over time: A population-based study. Lancet Respir. Med. 2025. [Google Scholar] [CrossRef]
- Myers, D.J.; Wallen, J.M. Lung Adenocarcinoma; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ha, S.Y.; Choi, S.J.; Cho, J.H.; Choi, H.J.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef]
- Sumiyoshi, K.; Yatsushige, H.; Shigeta, K.; Aizawa, Y.; Fujino, A.; Ishijima, N.; Hayakawa, T. Survival prognostic factors in nonsmall cell lung cancer patients with simultaneous brain metastases and poor performance status at initial presentation. Heliyon 2024, 10, e38128. [Google Scholar] [CrossRef]
- Waqar, S.N.; Samson, P.P.; Robinson, C.G.; Bradley, J.; Devarakonda, S.; Du, L.; Govindan, R.; Gao, F.; Puri, V.; Morgensztern, D. Non-small-cell Lung Cancer with Brain Metastasis at Presentation. Clin. Lung Cancer 2018, 19, e373–e379. [Google Scholar] [CrossRef]
- Yen, C.T.; Wu, W.J.; Chen, Y.T.; Chang, W.C.; Yang, S.H.; Shen, S.Y.; Su, J.; Chen, H.Y. Surgical resection of brain metastases prolongs overall survival in non-small-cell lung cancer. Am. J. Cancer Res. 2021, 11, 6160–6172. [Google Scholar]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 115891. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Myall, N.J.; Yu, H.; Soltys, S.G.; Wakelee, H.A.; Pollom, E. Management of brain metastases in lung cancer: Evolving roles for radiation and systemic treatment in the era of targeted and immune therapies. Neurooncol. Adv. 2021, 3 (Suppl. 5), v52–v62. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, M.J.; Razi, S.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl. Oncol. 2022, 18, 101364. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Chourasia, A.H.; Boland, M.L.; Macleod, K.F. Mitophagy and cancer. Cancer Metab. 2015, 3, 4. [Google Scholar] [CrossRef]
- Drake, L.E.; Springer, M.Z.; Poole, L.P.; Kim, C.J.; Macleod, K.F. Expanding perspectives on the significance of mitophagy in cancer. Semin. Cancer Biol. 2017, 47, 110–124. [Google Scholar] [CrossRef]
- Gladkova, C.; Maslen, S.L.; Skehel, J.M.; Komander, D. Mechanism of parkin activation by PINK1. Nature 2018, 559, 410–414. [Google Scholar] [CrossRef]
- Gan, Z.Y.; Callegari, S.; Cobbold, S.A.; Cotton, T.R.; Mlodzianoski, M.J.; Schubert, A.F.; Geoghegan, N.D.; Rogers, K.L.; Leis, A.; Dewson, G.; et al. Activation mechanism of PINK1. Nature 2022, 602, 328–335. [Google Scholar] [CrossRef]
- Bernardini, J.P.; Lazarou, M.; Dewson, G. Parkin and mitophagy in cancer. Oncogene 2017, 36, 1315–1327. [Google Scholar] [CrossRef]
- Celis-Pinto, J.C.; Fernández-Velasco, A.A.; Corte-Torres, M.D.; Santos-Juanes, J.; Blanco-Agudín, N.; Piña Batista, K.M.; Merayo-Lloves, J.; Quirós, L.M.; Fernández-Vega, I. PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. Int. J. Mol. Sci. 2023, 24, 6506. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef]
- Blazquez, R.; Wlochowitz, D.; Wolff, A.; Seitz, S.; Wachter, A.; Perera-Bel, J.; Bleckmann, A.; Beissbarth, T.; Salinas, G.; Riemenschneider, M.J.; et al. PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia 2018, 66, 2438–2455. [Google Scholar] [CrossRef]
- Chen, C.; Xiang, A.; Lin, X.; Guo, J.; Liu, J.; Hu, S.; Rui, T.; Ye, Q. Mitophagy: Insights into its signaling molecules, biological functions, and therapeutic potential in breast cancer. Cell Death Discov. 2024, 10, 457. [Google Scholar] [CrossRef]
- Zheng, F.; Zhong, J.; Chen, K.; Shi, Y.; Wang, F.; Wang, S.; Tang, S.; Yuan, X.; Shen, Z.; Tang, S.; et al. PINK1-PTEN axis promotes metastasis and chemoresistance in ovarian cancer via non-canonical pathway. J. Exp. Clin. Cancer Res. 2023, 42, 295. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Agnihotri, S.; Golbourn, B.; Huang, X.; Remke, M.; Younger, S.; Cairns, R.A.; Chalil, A.; Smith, C.A.; Krumholtz, S.L.; Mackenzie, D.; et al. PINK1 Is a Negative Regulator of Growth and the Warburg Effect in Glioblastoma. Cancer Res. 2016, 76, 4708–4719. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Huang, H.; Li, L.; Chen, J.; Ding, Y.; Ping, J. Pink1 promotes cell proliferation and affects glycolysis in breast cancer. Exp. Biol. Med. 2022, 247, 985–995. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, W.; Jiang, S.; Yu, S.; Yan, Y.; Wang, K.; He, J.; Ren, Y.; Wang, B. Pan-Cancer Analysis of the Mitophagy-Related Protein PINK1 as a Biomarker for the Immunological and Prognostic Role. Front. Oncol. 2020, 10, 569887. [Google Scholar] [CrossRef]
- Heavey, S.; O’Byrne, K.J.; Gately, K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat. Rev. 2014, 40, 445–456. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Cao, M.; Sun, D.; Yang, F.; Yan, X.; Zhang, S.; He, Y.; Du, L.; Sun, X.; et al. Survival of 7311 lung cancer patients by pathological stage and histological classification: A multicenter hospital-based study in China. Transl. Lung Cancer Res. 2022, 11, 1591–1605. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.H.; Lee, M.K.; Eom, J.S. Recent Advances in Adjuvant Therapy for Non-Small-Cell Lung Cancer. Tuberc. Respir. Dis. 2024, 87, 31–39. [Google Scholar] [CrossRef]
- Martin, B.; Paesmans, M.; Mascaux, C.; Berghmans, T.; Lothaire, P.; Meert, A.P.; Lafitte, J.J.; Sculier, J.P. Ki-67 expression and patients survival in lung cancer: Systematic review of the literature with meta-analysis. Br. J. Cancer 2004, 91, 2018–2025. [Google Scholar] [CrossRef]
- Moghbeli, M. PI3K/AKT pathway as a pivotal regulator of epithelial-mesenchymal transition in lung tumor cells. Cancer Cell Int. 2024, 24, 165. [Google Scholar] [CrossRef]
- Li, H.; Wen, X.; Ren, Y.; Fan, Z.; Zhang, J.; He, G.; Fu, L. Targeting PI3K family with small-molecule inhibitors in cancer therapy: Current clinical status and future directions. Mol. Cancer 2024, 23, 164. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Fares, J.; Ulasov, I.; Timashev, P.; Lesniak, M.S. Emerging principles of brain immunology and immune checkpoint blockade in brain metastases. Brain 2021, 144, 1046–1066. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, J.; Chen, J.; Ni, J.; Hung, J.; Wang, Z.; Zhang, X.; Feng, J.; Ji, L. High expression of PINK1 promotes proliferation and chemoresistance of NSCLC. Oncol. Rep. 2017, 37, 2137–2146. [Google Scholar] [CrossRef]
- Fu, D.; Hu, Z.; Xu, X.; Dai, X.; Liu, Z. Key signal transduction pathways and crosstalk in cancer: Biological and therapeutic opportunities. Transl. Oncol. 2022, 26, 101510. [Google Scholar] [CrossRef]
- Wang, M.; Luan, S.; Fan, X.; Wang, J.; Huang, J.; Gao, X.; Han, D. The emerging multifaceted role of PINK1 in cancer biology. Cancer Sci. 2022, 113, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Armocida, D.; Ius, T.; Zancana, G.; Bianconi, A.; Cofano, F.; Tartara, F.; Frati, A.; Garbossa, D.; Salvati, M. Anamnestic radiological metastases outcome surgical score (ARMO-S). A purpose of a predictive surgical scoring system for brain metastases. J. Clin. Neurosci. 2024, 125, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bragstad, S.; Flatebo, M.; Natvig, G.K.; Eide, G.E.; Skeie, G.O.; Behbahani, M.; Pedersen, P.H.; Enger, P.O.; Skeie, B.S. Predictors of quality of life and survival following Gamma Knife surgery for lung cancer brain metastases: A prospective study. J. Neurosurg. 2018, 129, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Gan, H.X.; Xu, J.X.; Liu, J.Y. Metformin improves survival in lung cancer patients with type 2 diabetes mellitus: A meta-analysis. Med. Clin. 2019, 152, 291–297. [Google Scholar] [CrossRef]
- Zhang, B.; Leung, P.C.; Cho, W.C.; Wong, C.K.; Wang, D. Targeting PI3K signaling in Lung Cancer: Advances, challenges and therapeutic opportunities. J. Transl. Med. 2025, 23, 184. [Google Scholar] [CrossRef]
- Tambo, Y.; Sone, T.; Nishi, K.; Shibata, K.; Kita, T.; Araya, T.; Shirasaki, H.; Shimizu, T.; Yoneda, T.; Matsuoka, H.; et al. Five-year efficacy and safety of pembrolizumab as first-line treatment in patients with non-small cell lung cancer with PD-L1 tumor proportion score ≥50%: A multicenter observational study. Lung Cancer 2025, 201, 108422. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Camacho-Urkaray, E.; Santos-Juanes, J.; Gutierrez-Corres, F.B.; Garcia, B.; Quiros, L.M.; Guerra-Merino, I.; Aguirre, J.J.; Fernandez-Vega, I. Establishing cut-off points with clinical relevance for bcl-2, cyclin D1, p16, p21, p27, p53, Sox11 and WT1 expression in glioblastoma—A short report. Cell. Oncol. 2018, 41, 213–221. [Google Scholar] [CrossRef]
- Fernandez-Vega, I.; Santos-Juanes, J.; Camacho-Urkaray, E.; Lorente-Gea, L.; Garcia, B.; Gutierrez-Corres, F.B.; Quiros, L.M.; Guerra-Merino, I.; Aguirre, J.J. Miki (Mitotic Kinetics Regulator) Immunoexpression in Normal Liver, Cirrhotic Areas and Hepatocellular Carcinomas: A Preliminary Study with Clinical Relevance. Pathol. Oncol. Res. POR 2020, 26, 167–173. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]
| Total | Stage IV | Stages I–III | p Value | |
|---|---|---|---|---|
| Patients | 22 | 15 (68.2%) | 7 (31.8%) | - |
| Age (years) range | 58.7 ± 8.7 36–77 | 60.7 ± 5.4 52–71 | 54.4 ± 12.7 36–77 | 0.113 |
| Gender male female | 11 (50%) 11 (50%) | 8 (53.3%) 7 (46.7%) | 3(42.9%) 4 (57.1%) | 0.975 |
| Status alive deceased | 5 (22.7%) 17 (77.3%) | 2 (13.3%) 13 (86.7%) | 3 (42.9%) 4 (57.1%) | 0.321 |
| Primary tumor location Superior lobes Other sites | 12 (54.5%) 10 (45.5%) | 9 (60%) 6 (40%) | 3 (42.9%) 4 (57.1%) | 0.361 |
| Brain metastases location Fronto-temporal lobes Other sites | 17 (77.3%) 5 (22.7%) | 11 (73.3%) 4 (26.7%) | 6 (85.7%) 1 (14.3%) | 0.348 |
| Tumor size in primary tumor (cm) | 3.5 ± 1.4 | 3.6 ± 1.4 | 3.1 ± 1.6 | 0.449 |
| Survival (months) OS | 68.1 ± 62.4 | 50.0 ± 52.5 | 106.9 ± 68.0 | 0.031 |
| Adjuvant therapy No RT and/or CT RT and CT | 3 (13.6%) 14 (63.7%) 5 (22.7%) | 0 12 (80%) 3 (20%) | 3 (42.8%) 2 (28.6%) 2 (28.6%) | 0.014 |
| Grade in primary tumor Well Moderate Poor | 7(31.8%) 8(36.4%) 7 (31.8%) | 4 (26.7%) 6 (40%) 5 (33.3%) | 3 (42.8%) 2 (28.6%) 2 (28.6%) | 0.741 |
| Grade in brain metastasis Well Moderate Poor | 3 (13.6%) 1 (4.6%) 18 (81.8%) | 2 (13.3%) 0 13 (86.7%) | 1 (14.3%) 1 (14.3%) 5 (71.4%) | 0.447 |
| Necrosis in primary tumor No <25% 25%/50% 50%/75% >75% | 4 (18.2%) 7 (31.8%) 7 (31.8%) 3 (13.6%) 1 (4.6%) | 6 (40%) 3(20%) 4 (26.6%) 1 (6.7%) 1 (6.7%) | 3 (42.8%) 2 (28.6%) 1 (14.3%) 1 (14.3%) 0 | 0.294 |
| Necrosis in brain metastasis No <25% 25%/50% 50%/75% >75% | 11 (50%) 5 (22.7%) 4 (18.2%) 0 2(9.1%) | 6 (40%) 3 (20%) 4 (26.6%) 0 2 (13.4%) | 5 (71.4%) 2 (28.6%) 0 0 0 | 0.420 |
| Mitotic activity (per 10 HPF) Primary tumor Brain metastases | 18.0 ± 18.4 20.7 ± 16.5 | 22.8 ± 19.8 23.0 ± 18.6 | 7.7 ± 9.5 15.7 ± 10.2 | 0.343 0.026 0.285 |
| PI3K (H-Score): Primary tumor Brain metastases | 96.8 ± 57.9 43.5 ± 62.3 | 113.3 ± 56.3 54.3 ± 70.2 | 61.4 ± 47.1 18.3 ± 28.6 | 0.003 0.043 0.319 |
| PINK1 (H-Score): Primary tumor Brain metastases | 76.8 ± 40.0 77.5 ± 44.8 | 79.3 ± 39.9 75.7 ± 45.4 | 71.4 ± 43.0 81.7 ± 47.5 | 0.793 0.677 0.412 |
| PDL1 (positive >1% tumor): Primary tumor Brain metastases | 9 (40.9%) 8 (36.4) | 7(46.7%) 8(53.3) | 2 (28.6%) 0 | 0.985 0.307 0.035 |
| Age | Tumor Size | OS | Mitosis Primary Tumor | Mitosis Brain Metastases | PI3K Primary Tumor | PI3K Brain Metastases | PIKN1 Primary Tumor | |
|---|---|---|---|---|---|---|---|---|
| Tumor size | r = −0.117 p = 0.665 | - | - | - | - | - | - | - |
| OS | r = −0.590 p = 0.016 | r = −0.109 p = 0.687 | - | - | - | - | - | - |
| Mitosis primary tumor | r = −0.351 p = 0.183 | r = 0.673 p = 0.004 | r = −0.032 p = 0.908 | - | - | - | - | - |
| Mitosis brain metastases | r = −0.486 p = 0.056 | r = 0.173 p = 0.521 | r = 0.193 p = 0.474 | r = 0.594 p = 0.015 | - | - | - | - |
| PI3Kprimary tumor | r = 0.402 p = 0.122 | r = 0.041 p = 0.880 | r = −0.468 p = 0.068 | r = 0.179 p = 0.508 | r = −0.009 p = 0.975 | - | - | - |
| PI3Kbrain metastases | r = 0.013 p = 0.963 | r = −0.180 p = 0.505 | r = −0.289 p = 0.277 | r = 0.107 p = 0.692 | r = 0.246 p = 0.358 | r = 0.556 p = 0.025 | - | - |
| PINK1 primary tumor | r = 0.180 p = 0.505 | r = 0.223 p = 0.406 | r = −0.385 p = 0.140 | r = 0.410 p = 0.115 | r = 0.145 p = 0.593 | r = 0.372 p = 0.156 | r = 0.023 p = 0.934 | - |
| PINK1brain metastases | r = 0.149 p = 0.582 | r = 0.135 p = 0.617 | r = −0.185 p = 0.492 | r = −0.284 p = 0.287 | r = −0.134 p = 0.620 | r = 0.117 p = 0.667 | r = 0.247 p = 0.356 | r = 0.045 p = 0.869 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age, years | 1.074 | 1.014–1.138 | 0.015 | |||
| Age, years (≤55 vs. >55) | 7.014 | 1.485–33.139 | 0.014 | 2.502 | 0.302–20.750 | 0.395 |
| Gender (male vs. female) | 2.009 | 0.758–5.326 | 0.161 | |||
| Primary tumor location (superior lobes vs. others) | 0.653 | 0.226–1.889 | 0.431 | |||
| Brain metastases location (frontotemporal lobes vs. others) | 1.243 | 0.460–3.361 | 0.668 | |||
| Tumour size (≤3 cm vs. >3 cm) | 1.552 | 0.585–4.116 | 0.377 | |||
| Stage (IV vs. others) | 2.902 | 0.923–9.128 | 0.068 | 4.896 | 0.890–26.941 | 0.065 |
| Adjuvant therapy (yes vs. no) | 1.161 | 0.538–2.505 | 0.704 | |||
| Mitotic activity primary tumor (≤15 vs. >15) | 0.716 | 0.270–1.901 | 0.502 | |||
| Mitotic activity brain metastases (≤15 vs. >15) | 0.795 | 0.287–2.202 | 0.660 | |||
| PI3K primary tumor | 1.008 | 1.000–1.015 | 0.038 | |||
| PI3K primary tumor; score (≤50 vs. >50) | 7.791 | 1.718–36.432 | 0.008 | 0.864 | 0.058–12.786 | 0.915 |
| PI3K primary tumor; score (<60 vs. 60–150 vs. >150) | 2.295 | 1.135–4.638 | 0.021 | |||
| PINK1 primary tumor | 1.013 | 1.000–1.026 | 0.042 | |||
| PINK1 primary tumor; score (≤90 vs. >90) | 2.589 | 0.992–6.759 | 0.052 | |||
| PINK1 primary tumor; score (<50 vs. 50–100 vs. >100) | 2.236 | 1.109–4.508 | 0.025 | 4.328 | 1.264–14.814 | 0.020 |
| PDL1 primary tumor; score (negative vs. positive) | 2.497 | 0.947–6.585 | 0.064 | 3.210 | 0.768–13.408 | 0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiera-Valdés, M.; Corte-Torres, M.D.; Navarro-López, A.; Blanco-Agudín, N.; Fernández-Menéndez, S.; Piña-Batista, K.M.; Santos-Juanes, J.; Merayo-Lloves, J.; Quirós, L.M.; Fernández-Velasco, A.A.; et al. PI3K and PINK1 Immunoexpression as Predictors of Survival in Patients Undergoing Resection of Brain Metastases from Lung Adenocarcinoma. Int. J. Mol. Sci. 2025, 26, 2945. https://doi.org/10.3390/ijms26072945
Rubiera-Valdés M, Corte-Torres MD, Navarro-López A, Blanco-Agudín N, Fernández-Menéndez S, Piña-Batista KM, Santos-Juanes J, Merayo-Lloves J, Quirós LM, Fernández-Velasco AA, et al. PI3K and PINK1 Immunoexpression as Predictors of Survival in Patients Undergoing Resection of Brain Metastases from Lung Adenocarcinoma. International Journal of Molecular Sciences. 2025; 26(7):2945. https://doi.org/10.3390/ijms26072945
Chicago/Turabian StyleRubiera-Valdés, Miriam, Mª Daniela Corte-Torres, Andrea Navarro-López, Noelia Blanco-Agudín, Santiago Fernández-Menéndez, Kelvin M. Piña-Batista, Jorge Santos-Juanes, Jesús Merayo-Lloves, Luis M. Quirós, Adela A. Fernández-Velasco, and et al. 2025. "PI3K and PINK1 Immunoexpression as Predictors of Survival in Patients Undergoing Resection of Brain Metastases from Lung Adenocarcinoma" International Journal of Molecular Sciences 26, no. 7: 2945. https://doi.org/10.3390/ijms26072945
APA StyleRubiera-Valdés, M., Corte-Torres, M. D., Navarro-López, A., Blanco-Agudín, N., Fernández-Menéndez, S., Piña-Batista, K. M., Santos-Juanes, J., Merayo-Lloves, J., Quirós, L. M., Fernández-Velasco, A. A., & Fernández-Vega, I. (2025). PI3K and PINK1 Immunoexpression as Predictors of Survival in Patients Undergoing Resection of Brain Metastases from Lung Adenocarcinoma. International Journal of Molecular Sciences, 26(7), 2945. https://doi.org/10.3390/ijms26072945







