Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli
Abstract
1. Introduction
2. Results
2.1. Platelet Count During Storage
2.2. P-Selectin Exposure on the Platelet Surface Membrane
2.3. Procoagulant Platelet Subpopulation
2.4. Annexin-V-Positive Extracellular Vesicles
2.5. Soluble Factor Concentration in Platelet Components
2.6. Correlation Between Platelet Count and Soluble Factor Release
2.7. The Concentration of EGF, RANTES, PF4, and CD62P
2.8. The Concentration of IL-27, CD40L, TNF-α, and OX40L
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Platelet Stimulation
4.3. Imaging Flow Cytometry
4.4. Soluble Factor Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleinveld, D.J.; van Amstel, R.B.; Wirtz, M.R.; Geeraedts, L.M.; Goslings, J.C.; Hollmann, M.W.; Juffermans, N.P. Platelet-to-red blood cell ratio and mortality in bleeding trauma patients: A systematic review and meta-analysis. Transfusion 2021, 61, S243–S251. [Google Scholar] [CrossRef] [PubMed]
- White, S.K.; Schmidt, R.L.; Walker, B.S.; Metcalf, R.A. Bacterial contamination rate of platelet components by primary culture: A systematic review and meta-analysis. Transfusion 2020, 60, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Ketter, P.M.; Kamucheka, R.; Arulanandam, B.; Akers, K.; Cap, A.P. Platelet enhancement of bacterial growth during room temperature storage: Mitigation through refrigeration. Transfusion 2019, 59, 1479–1489. [Google Scholar] [CrossRef]
- Johnson, L.; Tan, S.; Wood, B.; Davis, A.; Marks, D.C. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: A comparison with conventional platelet storage conditions. Transfusion 2016, 56, 1807–1818. [Google Scholar] [CrossRef]
- Shea, S.M.; Reisz, J.A.; Mihalko, E.P.; Rahn, K.C.; Rassam, R.M.; Chitrakar, A.; Gamboni, F.; D’Alessandro, A.; Spinella, P.C.; Thomas, K.A. Cold-stored platelet hemostatic capacity is maintained for three weeks of storage and associated with taurine metabolism. J. Thromb. Haemos. 2024, 22, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Tan, S.; Jenkins, E.; Wood, B.; Marks, D.C. Characterization of biologic response modifiers in the supernatant of conventional, refrigerated, and cryopreserved platelets. Transfusion 2018, 58, 927–937. [Google Scholar] [CrossRef]
- Ferrer, F.; Rivera, J.; Lozano, M.L.; Corral, J.; Garcia, V.V. Effect of cold-storage in the accumulation of bioreactive substances in platelet concentrates treated with second messenger effects. Haematologica 2001, 86, 530–536. [Google Scholar]
- Hamzeh-Cognasse, H.; Damien, P.; Nguyen, K.A.; Arthaud, C.-A.; Eyraud, M.-A.; Chavarin, P.; Absi, L.; Osselaer, J.-C.; Pozzetto, B.; Cognasse, F.; et al. Immune-reactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion 2014, 54, 613–625. [Google Scholar] [CrossRef]
- Marcoux, G.; Magron, A.; Sut, C.; Laroche, A.; Laradi, S.; Hamzeh-Cognasse, H.; Allaeys, I.; Cabon, O.; Julien, A.S.; Garraud, O.; et al. Platelet-derived extracellular vesicles convey mitochondrial DAMPs in platelet concentrates and their levels are associated with adverse reactions. Transfusion 2019, 59, 2403–2414. [Google Scholar] [CrossRef]
- Sut, C.; Tariket, S.; Aloui, C.; Arthaud, C.A.; Eyraud, M.A.; Fagan, J.; Chavarin, P.; Hamzeh-Cognasse, H.; Laradi, S.; Garraud, O. Soluble CD40L and CD62P levels differ in single-donor apheresis platelet concentrates and buffy coat–derived pooled platelet concentrates. Transfusion 2019, 59, 16–20. [Google Scholar] [CrossRef]
- Sandgren, P.; Shanwell, A.; Gulliksson, H. Storage of buffy coat-derived platelets in additive solutions: In vitro effects of storage at 4 degrees C. Transfusion 2006, 46, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Gardner, F.H. Platelet preservation: Effect of storage temperature on maintenance of platelet viability—Deleterious effect of refrigerated storage. N. Engl. J. Med. 1969, 280, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Pelzl, L.; Tamamushi, Y.; Maettler, C.T.; Witzemann, A.; Althaus, K.; Nowak-Harnau, S.; Seifried, E.; Bakchoul, T. Inhibition of GPIb-α-mediated apoptosis signaling enables cold storage of platelets. Haematologica 2023, 108, 2959–2971. [Google Scholar] [CrossRef]
- Baghdadi, V.; Yari, F.; Nikougoftar, M.; Rafiee, M.H. Platelets apoptosis and clearance in the presence of sodium octanoate during storage of platelet concentrate at 4 °C. Cell J. 2020, 22, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Vostal, J.G.; Gelderman, M.P.; Skripchenko, A.; Xu, F.; Li, Y.; Ryan, J.; Cheng, C.; Whitley, P.; Wellington, M.; Sawyer, S.; et al. Temperature cycling during platelet cold storage improves in vivo recovery and survival in healthy volunteers. Transfusion 2018, 58, 25–33. [Google Scholar] [CrossRef]
- Skripchenko, A.; Gelderman, M.P.; Awatefe, H.; Turgeon, A.; Thompson-Montgomery, D.; Cheng, C.; Vostal, J.G.; Wagner, S.J. Automated cold temperature cycling improves in vitro platelet properties and in vivo recovery in a mouse model compared to continuous cold storage. Transfusion 2016, 56, 24–32. [Google Scholar] [CrossRef]
- Aurich, K.; Wesche, J.; Ulbricht, M.; Otto, O.; Greinacher, A.; Palankar, R. Divalent magnesium restores cytoskeletal storage lesions in cold-stored platelet concentrates. Sci. Rep. 2022, 12, 6229. [Google Scholar] [CrossRef]
- Rumjantseva, V.; Grewal, P.K.; Wandall, H.H.; Josefsson, E.C.; Sørensen, A.L.; Larson, G.; Marth, J.D.; Hartwig, J.H.; Hoffmeister, K.M. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat. Med. 2009, 15, 1273–1280. [Google Scholar] [CrossRef]
- Babic, A.M.; Josefsson, E.C.; Bergmeier, W.; Wagner, D.D.; Kaufman, R.M.; Silberstein, L.E.; Stossel, T.P.; Hartwig, J.H.; Hoffmeister, K.M. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion 2007, 47, 442–451. [Google Scholar] [CrossRef]
- Hoffmeister, K.M.; Felbinger, T.W.; Falet, H.; Denis, C.; Bergmeier, W.; Mayadas, T.N.; von Andrian, U.H.; Wagner, D.D.; Stossel, T.P.; Hartwig, J.H. The clearance mechanism of chilled blood platelets. Cell 2003, 112, 87–97. [Google Scholar] [CrossRef]
- Gammon, R.R.; Hebert, J.; Min, K.; O’Connor, J.J.; Ipe, T.; Razatos, A.; Reichenberg, S.; Stubbs, J.; Waltman, E.; Wu, Y. Cold stored platelets—Increasing understanding and acceptance. Transfus. Apher. Sci. 2023, 62, 103639. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.T.; Krok, E.; Pavenski, K.; Pambrun, C.; Petraszko, T. The operational impact of introducing cold stored platelets. Transfusion 2023, 63, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; Tran, S.A.; Emery, R.L.; Hammel, S.A.; Haugen, D.A.L.; Zielinski, M.D.; Zietlow, S.P.; Jenkins, D. Cold platelets for trauma-associated bleeding: Regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg”. Transfusion 2017, 57, 2836–2844. [Google Scholar]
- Brown, B.L.; Wagner, S.J.; Hapip, C.A.; Fischer, E.; Getz, T.M.; Thompson-Montgomery, D.; Turgeon, A. Time from apheresis platelet donation to cold storage: Evaluation of platelet quality and bacterial growth. Transfusion 2022, 62, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Winskel-Wood, B.; Padula, M.P.; Marks, D.C.; Johnson, L. Cold storage alters the immune characteristics of platelets and potentiates bacterial-induced aggregation. Vox Sang. 2022, 117, 1006–1015. [Google Scholar] [CrossRef]
- Nair, P.M.; Meledeo, M.A.; Wells, A.R.; Wu, X.; Bynum, J.A.; Leung, K.P.; Liu, B.; Cheeniyil, A.; Ramasubramanian, A.K.; Weisel, J.W. Cold-stored platelets have better preserved contractile function in comparison with room temperature-stored platelets over 21 days. Transfusion 2021, 61, S68–S79. [Google Scholar] [CrossRef]
- Wood, B.; Padula, M.P.; Marks, D.C.; Johnson, L. Refrigerated storage of platelets initiates changes in platelet surface marker expression and localization of intracellular proteins. Transfusion 2016, 56, 2548–2559. [Google Scholar] [CrossRef]
- Winokur, R.; Hartwig, J.H. Mechanism of shape change in chilled human platelets. Blood 1995, 85, 1796–1804. [Google Scholar]
- Sandgren, P.; Hansson, M.; Gulliksson, H.; Shanwell, A. Storage of buffy-coat-derived platelets in additive solutions at 4 °C and 22 °C: Flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007, 93, 27–36. [Google Scholar]
- Zeller-Hahn, J.; Bittl, M.; Kuhn, S.; Koessler, A.; Weber, K.; Koessler, J.; Kobsar, A. Influence of short-term refrigeration on collagen-dependent signalling mechanisms in stored platelets. Cell. Signal. 2024, 122, 111306. [Google Scholar] [CrossRef]
- Reddoch, K.M.; Pidcoke, H.F.; Montgomery, R.K.; Fedyk, C.G.; Aden, J.K.; Ramasubramanian, A.K.; Cap, A.P. Hemostatic function of apheresis platelets stored at 4 °C and 22 °C. Shock 2014, 41, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, P.; Gillespie, S.; Armstrong, P.C.; Allan, H.E.; Warner, T.D.; Brohi, K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc. Natl. Acad. Sci. USA 2019, 116, 17444–17449. [Google Scholar] [CrossRef]
- Johnson, L.; Lei, P.; Waters, L.; Padula, M.P.; Marks, D.C. Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry. Sci. Rep. 2023, 13, 1221. [Google Scholar] [CrossRef]
- Obydennyy, S.I.; Sveshnikova, A.N.; Ataullakhanov, F.I.; Panteleev, M.A. Dynamics of calcium spiking, mitochondrial collapse and phosphatidylserine exposure in platelet subpopulations during activation. J. Thromb. Haemost. 2016, 14, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.J.; Rai, A.; Sharma, P.; Fang, H.; McFadyen, J.D.; Greening, D.W.; Peter, K. Differential effects of physiological agonists on the proteome of platelet-derived extracellular vesicles. Proteomics 2024, 24, 2300391. [Google Scholar] [CrossRef]
- Berthet, J.; Damien, P.; Hamzeh-Cognasse, H.; Arthaud, C.-A.; Eyraud, M.-A.; Zéni, F.; Pozzetto, B.; McNicol, A.; Garraud, O.; Cognasse, F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 2012, 145, 189–200. [Google Scholar] [CrossRef]
- Nyam-Erdene, A.; Nebie, O.; Delila, L.; Buée, L.; Devos, D.; Chou, S.-Y.; Blum, D.; Burnouf, T. Characterization and chromatographic isolation of platelet extracellular vesicles from human platelet lysates for applications in neuroregenerative medicine. ACS Biomat. Sci. Eng. 2021, 7, 5823–5835. [Google Scholar] [CrossRef]
- Khan, S.Y.; Kelher, M.R.; Heal, J.M.; Blumberg, N.; Boshkov, L.K.; Phipps, R.; Gettings, K.F.; McLaughlin, N.J.; Silliman, C.C. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood 2006, 108, 2455–2462. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.J.; Van Schilfgaarde, M.; Van Oerle, R.; Renné, T.; ten Cate, H.; Spronk, H.M.H. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef]
- Nash, J.; Davies, A.; Saunders, C.V.; George, C.E.; Williams, J.O.; James, P.E. Quantitative increases of extracellular vesicles in prolonged cold storage of platelets increases the potential to enhance fibrin clot formation. Transfus. Med. 2023, 33, 467–477. [Google Scholar] [CrossRef]
- Johnson, L.; Vekariya, S.; Wood, B.; Tan, S.; Roan, C.; Marks, D.C. Refrigeration of apheresis platelets in platelet additive solution (PAS-E) supports in vitro platelet quality to maximize the shelf-life. Transfusion 2021, 61, S58–S67. [Google Scholar] [CrossRef] [PubMed]
- Getz, T.M.; Montgomery, R.K.; Bynum, J.A.; Aden, J.K.; Pidcoke, H.F.; Cap, A.P. Storage of platelets at 4 °C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion 2016, 56, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Battinelli, E.M.; Thon, J.N.; Okazaki, R.; Peters, C.G.; Vijey, P.; Wilkie, A.R.; Noetzli, L.J.; Flaumenhaft, R.; Italiano, J.E., Jr. Megakaryocytes package contents into separate α-granules that are differentially distributed in platelets. Blood Adv. 2019, 3, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Eckly, A.; Rinckel, J.-Y.; Proamer, F.; Ulas, N.; Joshi, S.; Whiteheart, S.W.; Gachet, C. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood 2016, 128, 2538–2549. [Google Scholar] [CrossRef]
- Cognasse, F.; Aloui, C.; Anh Nguyen, K.; Hamzeh-Cognasse, H.; Fagan, J.; Arthaud, C.-A.; Eyraud, M.-A.; Sebban, M.; Fromont, E.; Pozzetto, B.; et al. Platelet components associated with adverse reactions: Predictive value of mitochondrial DNA relative to biological response modifiers. Transfusion 2016, 56, 497–504. [Google Scholar] [CrossRef]
- Reddoch-Cardenas, K.M.; Peltier, G.C.; Chance, T.C.; Nair, P.M.; Meledeo, M.A.; Ramasubramanian, A.K.; Cap, A.P.; Bynum, J.A. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion 2021, 61, 178–190. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Thomas, K.A.; Stefanoni, D.; Gamboni, F.; Shea, S.M.; Reisz, J.A.; Spinella, P.C. Metabolic phenotypes of standard and cold-stored platelets. Transfusion 2020, 60, S96–S106. [Google Scholar]
- Zhao, H.W.; Serrano, K.; Stefanoni, D.; D’Alessandro, A.; Devine, D.V. In Vitro characterization and metabolomic analysis of cold-stored platelets. J. Proteome Res. 2021, 20, 2251–2265. [Google Scholar] [CrossRef]
- Keuren, J.F.; Cauwenberghs, S.; Heeremans, J.; de Kort, W.; Heemskerk, J.W.; Curvers, J. Platelet ADP response deteriorates in synthetic storage media. Transfusion 2006, 46, 204–212. [Google Scholar] [CrossRef]
- Nurden, A.T. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front. Biosci. 2018, 23, 726–751. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Ekhlak, M.; Sonkar, V.K.; Dash, D. Mitochondrial ATP generation in stimulated platelets is essential for granule secretion but dispensable for aggregation and procoagulant activity. Haematologica 2022, 107, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Okonkwo, D.O.; Guyette, F.X.; Luther, J.F.; Vincent, L.E.; Puccio, A.M.; Harner, A.M.; Agnone, A.G.; Brubaker, D.P.; Love, E.T.; et al. Early cold stored platelet transfusion following traumatic brain injury: A randomized clinical trial. Ann. Surg. 2025, 10.1097/SLA.0000000000006640. [Google Scholar] [CrossRef] [PubMed]
- Strandenes, G.; Sivertsen, J.; Bjerkvig, C.K.; Fosse, T.K.; Cap, A.P.; Del Junco, D.J.; Kristoffersen, E.K.; Haaverstad, R.; Kvalheim, V.; Braathen, H. A pilot trial of platelets stored cold versus at room temperature for complex cardiothoracic surgery. Anesthesiology 2020, 133, 1173–1183. [Google Scholar] [CrossRef]
- Stahl, A.L. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood 2006, 108, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Mbotwe, S.; Bester, J.; Robinson, C.J.; Kell, D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J. R. Soc. Interface 2016, 13, 20160539. [Google Scholar] [CrossRef]
- Chen, A.Y.; Ha, J.N.; Delano, F.A.; Schmid-Schönbein, G.W. Receptor cleavage and P-selectin-dependent reduction of leukocyte adhesion in the spontaneously hypertensive rat. J. Leukoc. Biol. 2012, 92, 183–194. [Google Scholar] [CrossRef]
- Thomas, K.A.; Srinivasan, A.J.; McIntosh, C.; Rahn, K.; Kelly, S.; McGough, L.; Clayton, S.; Perez, S.; Smith, A.; Vavro, L.; et al. Comparison of platelet quality and function across apheresis collection platforms. Transfusion 2023, 63 (Suppl. S3), S146–S158. [Google Scholar] [CrossRef]
- Johnson, L.; Roan, C.; Lei, P.; Spinella, P.C.; Marks, D.C. The role of sodium citrate during extended cold storage of platelets in platelet additive solutions. Transfusion 2023, 63, S126–S137. [Google Scholar] [CrossRef]
- Lam, F.W.; Cruz, M.A.; Leung, H.C.; Parikh, K.S.; Smith, C.W.; Rumbaut, R.E. Histone induced platelet aggregation is inhibited by normal albumin. Thromb. Res. 2013, 132, 69–76. [Google Scholar] [CrossRef]
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The median fluorescence intensity (MFI) of CD62P-PE is shown for (a) unstimulated platelets or samples activated with (b) A23187, (c) TRAP-6, (d) Histone-H4, or (e) LPS. Data represent the mean ± standard deviation (error bars, n = 6). The CD62P MFI from (a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. The median fluorescence intensity (MFI) of CD62P-PE is shown for (a) unstimulated platelets or samples activated with (b) A23187, (c) TRAP-6, (d) Histone-H4, or (e) LPS. Data represent the mean ± standard deviation (error bars, n = 6). The CD62P MFI from (a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) has been overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) has been overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The median fluorescence intensity (MFI) of CD62P-PE is shown for (a) unstimulated platelets or samples activated with (b) A23187, (c) TRAP-6, (d) Histone-H4, or (e) LPS. Data represent the mean ± standard deviation (error bars, n = 6). The CD62P MFI from (a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. The median fluorescence intensity (MFI) of CD62P-PE is shown for (a) unstimulated platelets or samples activated with (b) A23187, (c) TRAP-6, (d) Histone-H4, or (e) LPS. Data represent the mean ± standard deviation (error bars, n = 6). The CD62P MFI from (a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) has been overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) has been overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. (a) Representative brightfield, fluorescence, and darkfield images are shown for the major platelet subpopulations present in day 1 samples. The percentage of procoagulant platelets present in (b) unstimulated or following activation with (c) A23187 (10 µM), (d) TRAP-6 (10 µM), (e) Histone-H4 (30 µg/mL), or (f) LPS (20 µg/mL) is shown over storage. The percentage of procoagulant platelets present in (a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. (a) Representative brightfield, fluorescence, and darkfield images are shown for the major platelet subpopulations present in day 1 samples. The percentage of procoagulant platelets present in (b) unstimulated or following activation with (c) A23187 (10 µM), (d) TRAP-6 (10 µM), (e) Histone-H4 (30 µg/mL), or (f) LPS (20 µg/mL) is shown over storage. The percentage of procoagulant platelets present in (a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) are overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) are overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. (a) Representative brightfield, fluorescence, and darkfield images are shown for the major platelet subpopulations present in day 1 samples. The percentage of procoagulant platelets present in (b) unstimulated or following activation with (c) A23187 (10 µM), (d) TRAP-6 (10 µM), (e) Histone-H4 (30 µg/mL), or (f) LPS (20 µg/mL) is shown over storage. The percentage of procoagulant platelets present in (a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. (a) Representative brightfield, fluorescence, and darkfield images are shown for the major platelet subpopulations present in day 1 samples. The percentage of procoagulant platelets present in (b) unstimulated or following activation with (c) A23187 (10 µM), (d) TRAP-6 (10 µM), (e) Histone-H4 (30 µg/mL), or (f) LPS (20 µg/mL) is shown over storage. The percentage of procoagulant platelets present in (a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) are overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) are overlaid on the corresponding (b–e) stimulated samples for comparison. Platelets were stained with PAC-1-FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Platelet events were gated based on size (area and aspect ratio) and scatter (darkfield). Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.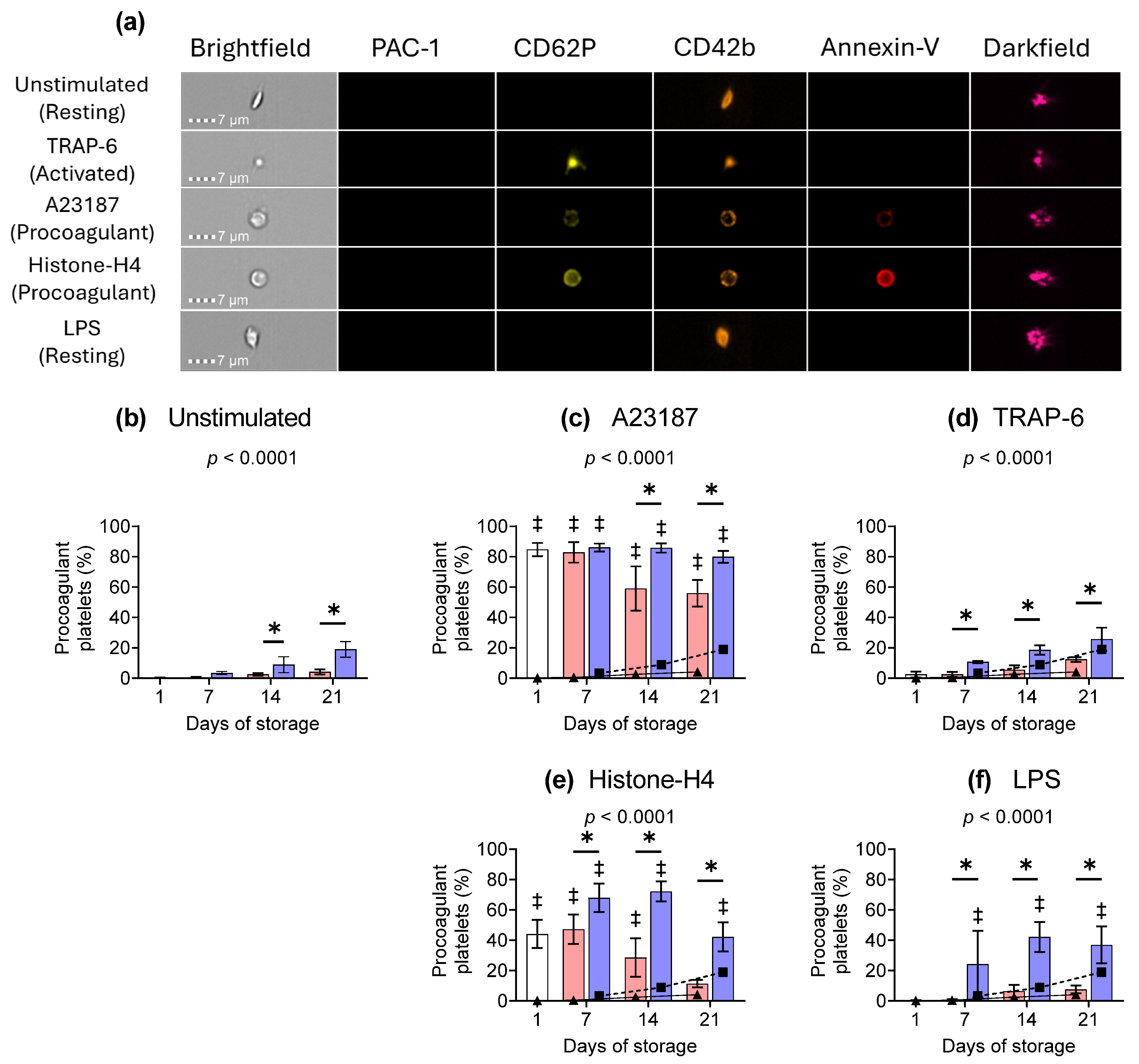
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The concentration of extracellular vesicles in (a) unstimulated platelets or following activation with (b) A23187 (10 µM), (c) TRAP 6 (10 µM), (d) Histone-H4 (30 µg/mL), or (e) LPS (20 µg/mL). Data represent the mean ± standard deviation (error bars, n = 6). The number of EVs from a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. The concentration of extracellular vesicles in (a) unstimulated platelets or following activation with (b) A23187 (10 µM), (c) TRAP 6 (10 µM), (d) Histone-H4 (30 µg/mL), or (e) LPS (20 µg/mL). Data represent the mean ± standard deviation (error bars, n = 6). The number of EVs from a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) is overlaid on the corresponding (b–e) stimulated samples for comparison. Platelet samples were stained with PAC 1 FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Extracellular vesicle events were gated based on size (area and aspect ratio), scatter (darkfield), and dual positivity for both GPIbα (CD42b) and phosphatidylserine (PS, annexin-V). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) is overlaid on the corresponding (b–e) stimulated samples for comparison. Platelet samples were stained with PAC 1 FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Extracellular vesicle events were gated based on size (area and aspect ratio), scatter (darkfield), and dual positivity for both GPIbα (CD42b) and phosphatidylserine (PS, annexin-V). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
 ) or following room-temperature (
) or following room-temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The concentration of extracellular vesicles in (a) unstimulated platelets or following activation with (b) A23187 (10 µM), (c) TRAP 6 (10 µM), (d) Histone-H4 (30 µg/mL), or (e) LPS (20 µg/mL). Data represent the mean ± standard deviation (error bars, n = 6). The number of EVs from a) unstimulated samples at RT (
) on days 7, 14, and 21 post-collection. The concentration of extracellular vesicles in (a) unstimulated platelets or following activation with (b) A23187 (10 µM), (c) TRAP 6 (10 µM), (d) Histone-H4 (30 µg/mL), or (e) LPS (20 µg/mL). Data represent the mean ± standard deviation (error bars, n = 6). The number of EVs from a) unstimulated samples at RT ( ) or 4 °C (
) or 4 °C ( ) is overlaid on the corresponding (b–e) stimulated samples for comparison. Platelet samples were stained with PAC 1 FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Extracellular vesicle events were gated based on size (area and aspect ratio), scatter (darkfield), and dual positivity for both GPIbα (CD42b) and phosphatidylserine (PS, annexin-V). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
) is overlaid on the corresponding (b–e) stimulated samples for comparison. Platelet samples were stained with PAC 1 FITC, CD62P-PE, CD42b-PE-Dazzle 594, and annexin-V-APC and analysed using imaging flow cytometry at 60× magnification. Extracellular vesicle events were gated based on size (area and aspect ratio), scatter (darkfield), and dual positivity for both GPIbα (CD42b) and phosphatidylserine (PS, annexin-V). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. ‡ = p < 0.05 compared to unstimulated platelets at the same time point.
 ) and cold-stored (
) and cold-stored ( ) platelet components are plotted against the concentration of soluble factors in the supernatant for (a) EGF, (b) RANTES, (c) PF4, (d) CD62P, (e) IL-27, (f) CD40L, (g) TNF-α, and (h) OX40L. Pearson test was used to determine the correlation (r-value), and linear regression was used to apply a line of best fit.
) platelet components are plotted against the concentration of soluble factors in the supernatant for (a) EGF, (b) RANTES, (c) PF4, (d) CD62P, (e) IL-27, (f) CD40L, (g) TNF-α, and (h) OX40L. Pearson test was used to determine the correlation (r-value), and linear regression was used to apply a line of best fit.
 ) and cold-stored (
) and cold-stored ( ) platelet components are plotted against the concentration of soluble factors in the supernatant for (a) EGF, (b) RANTES, (c) PF4, (d) CD62P, (e) IL-27, (f) CD40L, (g) TNF-α, and (h) OX40L. Pearson test was used to determine the correlation (r-value), and linear regression was used to apply a line of best fit.
) platelet components are plotted against the concentration of soluble factors in the supernatant for (a) EGF, (b) RANTES, (c) PF4, (d) CD62P, (e) IL-27, (f) CD40L, (g) TNF-α, and (h) OX40L. Pearson test was used to determine the correlation (r-value), and linear regression was used to apply a line of best fit.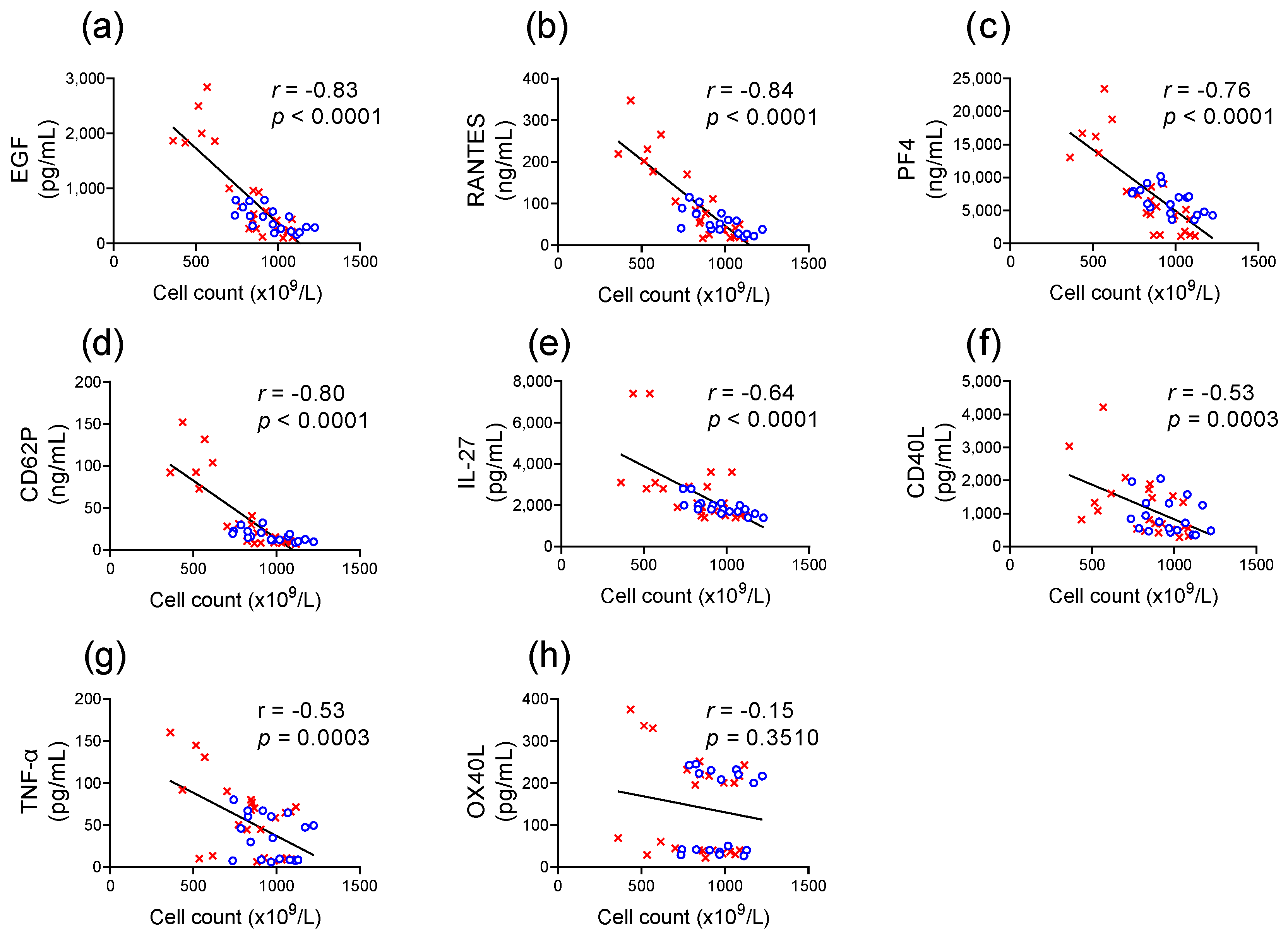
 ) or following room temperature (
) or following room temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection (x-axis). The concentration of (a) EGF, (b) RANTES, (c) PF4, and (d) CD62P was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP 6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
) on days 7, 14, and 21 post-collection (x-axis). The concentration of (a) EGF, (b) RANTES, (c) PF4, and (d) CD62P was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP 6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
 ) or following room temperature (
) or following room temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection (x-axis). The concentration of (a) EGF, (b) RANTES, (c) PF4, and (d) CD62P was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP 6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
) on days 7, 14, and 21 post-collection (x-axis). The concentration of (a) EGF, (b) RANTES, (c) PF4, and (d) CD62P was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP 6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.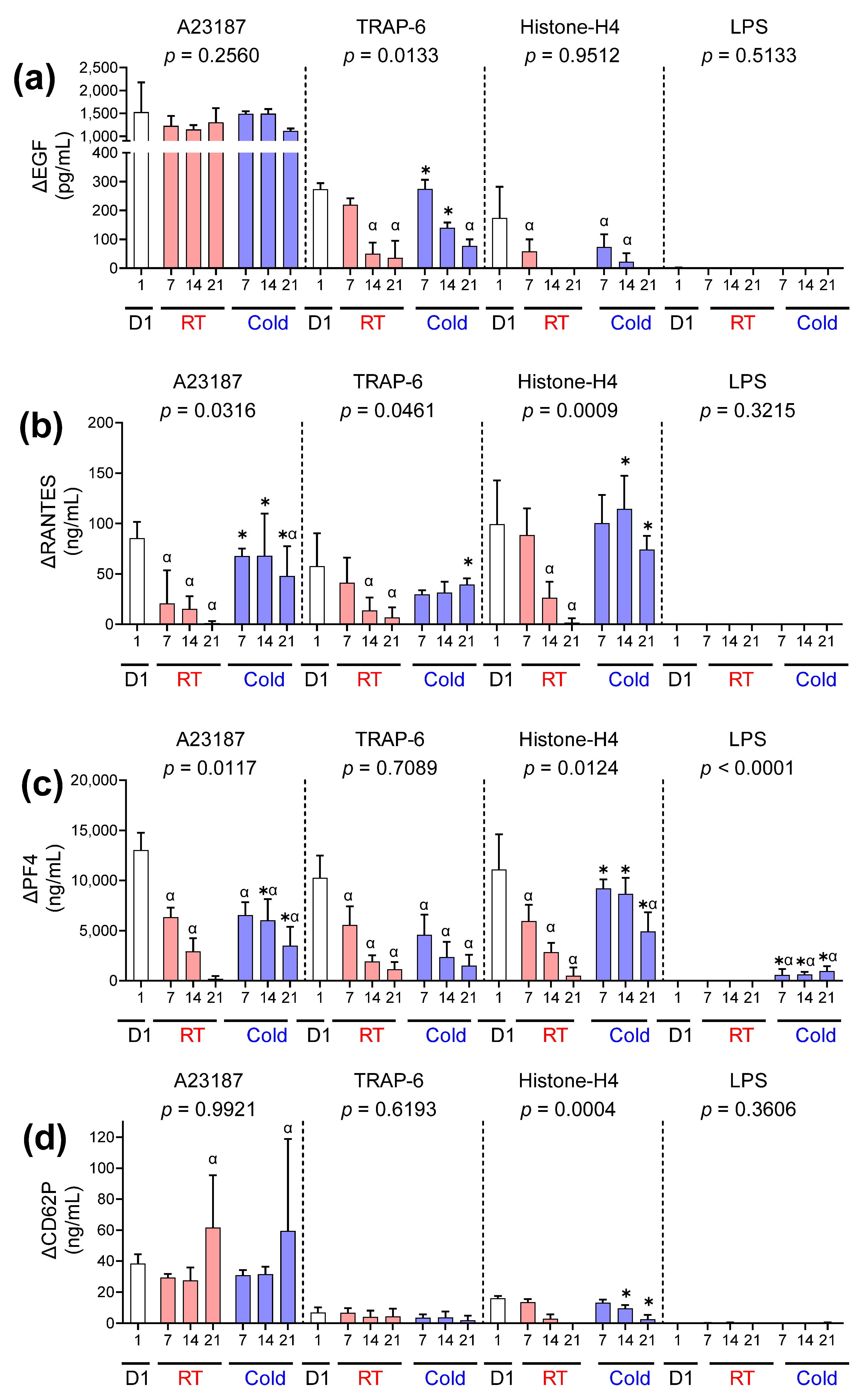
 ) or following room temperature (
) or following room temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The concentration of (a) IL-27, (b) CD40L, (c) TNF-α, and (d) OX40L was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP-6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
) on days 7, 14, and 21 post-collection. The concentration of (a) IL-27, (b) CD40L, (c) TNF-α, and (d) OX40L was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP-6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
 ) or following room temperature (
) or following room temperature ( ) or cold storage (
) or cold storage ( ) on days 7, 14, and 21 post-collection. The concentration of (a) IL-27, (b) CD40L, (c) TNF-α, and (d) OX40L was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP-6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.
) on days 7, 14, and 21 post-collection. The concentration of (a) IL-27, (b) CD40L, (c) TNF-α, and (d) OX40L was measured in the supernatant of unstimulated platelets or following activation with A23187 (10 µM), TRAP-6 (10 µM), Histone-H4 (30 µg/mL), or LPS (20 µg/mL) using ELISA. The change (Δ) in soluble factor concentration was calculated by subtracting the concentration of soluble factors obtained from unstimulated supernatant from the corresponding stimulated samples. Data represent the mean ± standard deviation (error bars, n = 6). Significance was determined using two-way ANOVA comparing the effects of temperature (RT vs. 4 °C) and stimulation on platelet samples over time, with the interaction p-value presented. * = p < 0.05 compared to room temperature at the same time point. α = p < 0.05 compared to day 1 platelets.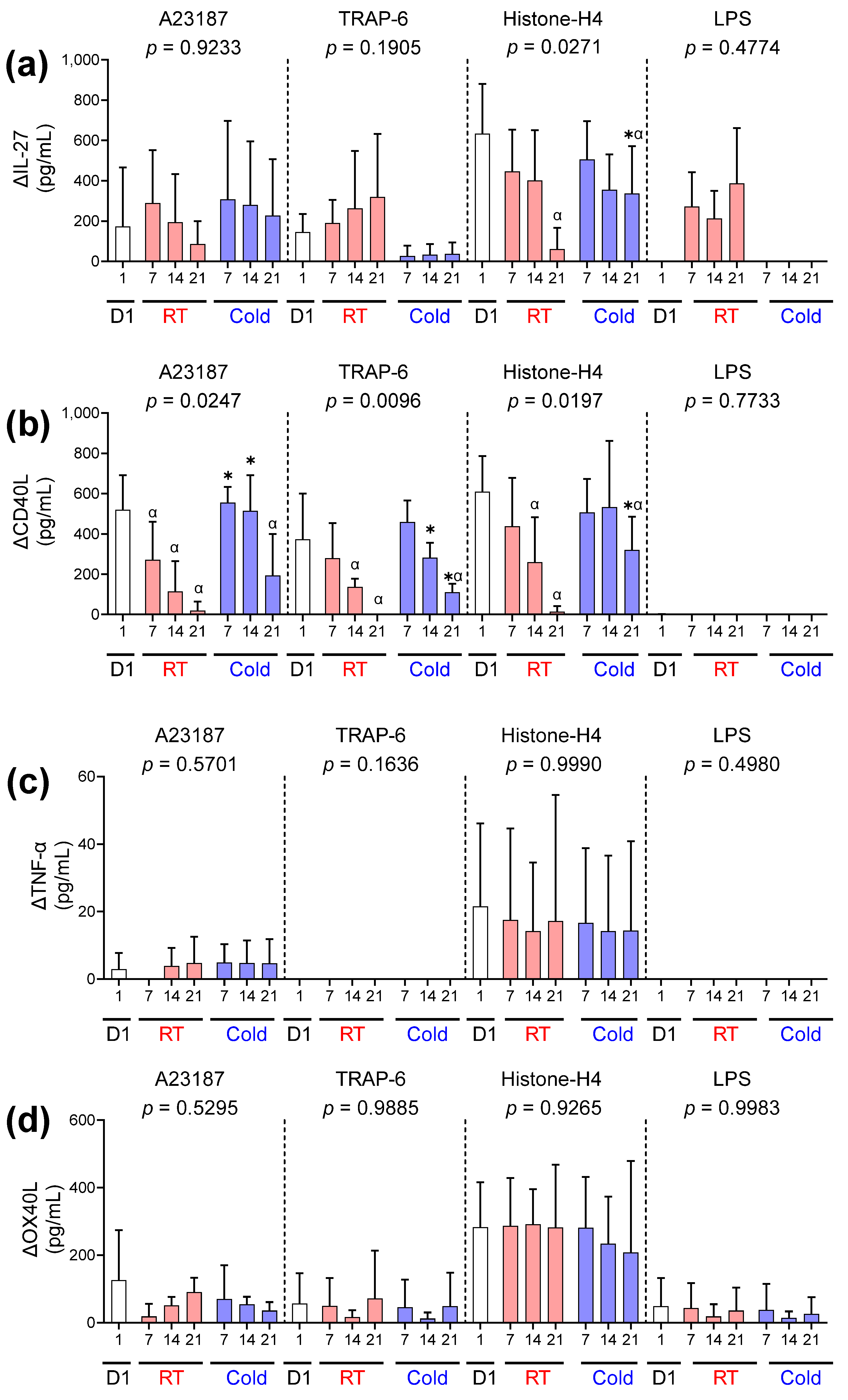
| Day of Storage | ||||||
|---|---|---|---|---|---|---|
| Soluble Factor | Storage Method | Day 1 (Baseline) | Day 7 | Day 14 | Day 21 | p-Value |
| EGF (pg/mL) | RT | 172 ± 71 | 347 ± 95 | 783 ± 203 | 2150 ± 421 | 0.0133 |
| Cold | 248 ± 75 | 397 ± 146 * | 668 ± 139 * | |||
| RANTES (ng/mL) | RT | 19 ± 3 | 50 ± 18 | 104 ± 38 | 241 ± 60 | <0.0001 |
| Cold | 39 ± 22 | 61 ± 27 * | 68 ± 31 * | |||
| PF4 (ng/mL) | RT | 1324 ± 276 | 4318 ± 549 | 7487 ± 1275 | 16,990 ± 3788 | <0.0001 |
| Cold | 4202 ± 488 | 6423 ± 691 | 8691 ± 982 * | |||
| CD62P (ng/mL) | RT | 8 ± 1 | 12 ± 2 | 29 ± 8 | 108 ± 29 | <0.0001 |
| Cold | 11 ± 1 | 15 ± 3 * | 25 ± 5 * | |||
| IL-27 (pg/mL) | RT | 2167 ± 1111 | 1733 ± 288 | 2167 ± 575 | 4433 ± 2302 | 0.0341 |
| Cold | 1600 ± 179 | 1933 ± 186 | 2200 ± 473 * | |||
| CD40L (pg/mL) | RT | 695 ± 557 | 898 ± 572 | 1123 ± 684 | 2018 ± 1329 | 0.5250 |
| Cold | 697 ± 445 | 857 ± 474 | 1185 ± 656 | |||
| TNF-α (pg/mL) | RT | 45 ± 26 | 45 ± 30 | 50 ± 35 | 92 ± 66 | 0.4530 |
| Cold | 35 ± 22 | 30 ± 27 | 46 ± 31 | |||
| OX40L (pg/mL) | RT | 129 ± 100 | 119 ± 93 | 135 ± 110 | 200 ± 163 | 0.8727 |
| Cold | 121 ± 96 | 133 ± 101 | 138 ± 111 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winskel-Wood, B.; Marks, D.C.; Johnson, L. Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli. Int. J. Mol. Sci. 2025, 26, 2944. https://doi.org/10.3390/ijms26072944
Winskel-Wood B, Marks DC, Johnson L. Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli. International Journal of Molecular Sciences. 2025; 26(7):2944. https://doi.org/10.3390/ijms26072944
Chicago/Turabian StyleWinskel-Wood, Ben, Denese C. Marks, and Lacey Johnson. 2025. "Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli" International Journal of Molecular Sciences 26, no. 7: 2944. https://doi.org/10.3390/ijms26072944
APA StyleWinskel-Wood, B., Marks, D. C., & Johnson, L. (2025). Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli. International Journal of Molecular Sciences, 26(7), 2944. https://doi.org/10.3390/ijms26072944





